Abstract
Driven by the broad diversity of species and physiologies and by reproduction-related bottlenecks in aquaculture, the field of fish reproductive biology has rapidly grown over the last five decades. This review provides my perspective on the field during this period, integrating fundamental and applied developments and milestones. Our basic understanding of the brain-pituitary–gonadal axis led to overcoming the failure of farmed fish to ovulate and spawn in captivity, allowing us to close the fish life cycle and establish a predictable, year-round production of eggs. Dissecting the molecular and hormonal mechanisms associated with sex determination and differentiation drove technologies for producing better performing mono-sex and reproductively-sterile fish. The growing contingent of passionate fish biologists, together with the availability of innovative platforms such as transgenesis and gene editing, as well as new models such as the zebrafish and medaka, have generated many discoveries, also leading to new insights of reproductive biology in higher vertebrates including humans. Consequently, fish have now been widely accepted as vertebrate reproductive models. Perhaps the best testament of the progress in our discipline is demonstrated at the International Symposia on Reproductive Physiology of Fish (ISRPF), at which our scientific family has convened every four years since the grandfather of the field, the late Ronald Billard, organized the inaugural 1977 meeting in Paimpont, France. As the one person who has been fortunate enough to attend all of these meetings since their inception, I have witnessed first-hand the astounding evolution of our field as we capitalized on the molecular and biotechnological revolutions in the life sciences, which enabled us to provide a higher resolution of fish reproductive and endocrine processes, answer more questions, and dive into deeper comprehension. Undoubtedly, the next (five) decades will be similarly exciting as we continue to integrate physiology with genomics, basic and translational research, and the small fish models with the aquacultured species.
Keywords: Fish reproductive biology, Fish reproductive endocrinology, Fundamental and applied research in fish reproduction, Fish reproduction and aquaculture, Spawning induction, Reproductive sterility, Gonadotropin-releasing hormones, Gonadotropins, Reproductive steroids
1. The rationale and the early days
Growing up I was fascinated by the sea, and I always wanted to be a marine biologist engaged in research that would explore ocean life to the benefit of society. Following this childhood passion, I studied biology as an undergraduate and in 1974 I enrolled in an Oceanography MSc program, both at the Hebrew University of Jerusalem. At that time, we already knew that the world was headed for a major fishery crisis, as overfishing was rampant and marine/coastal pollution was adversely impacting the spawning grounds of many marine species. When it was time to choose my Master’s thesis research topic, I decided to pursue marine aquaculture, which back then was in its infancy. The first bottleneck to be resolved in finfish aquaculture was the inability of many commercially important marine fish to reproduce reliably when raised in captivity. The budding mariculture industry had to rely on harvesting wild juvenile fish or broodstock, transporting them to the farming operation and growing them to harvest size (for juveniles) or stripping them to obtain eggs and sperm for the production of fertilized eggs, larvae and juveniles (for broodstock). These were, of course, very unreliable and unpredictable practices, as wild juveniles/broodstock were only available seasonally for a limited time, and in some years they could not be found at all. This led me (and several others in the field) to study fish reproductive biology and endocrinology, with the eventual goal of enabling predictable reproduction in captive fish and closing their life cycles. We hypothesized that fish do not reproduce in captivity because they do not experience the conditions of a spawning ground, and that the absence of these environmental conditions, which are difficult/impossible to simulate in captivity, causes a hormonal failure that is responsible for the lack of captive reproduction. The focus of the field during these early years (the 1970′s and early ‘80′s) was to study gametogenesis and develop assays for the hormones along the brain-pituitary–gonadal axis, so the entire brain-reproductive organization could be depicted. In 1974, I started my graduate research at the National Center for Mariculture in Eilat, Israel, joining the group that pioneered the development of aquaculture technologies for several important and already overfished marine fish. My initial model species was the gilthead seabream (Sparus aurata), a species that at the time was of major interest to the aquaculture industry in the Red Sea and Mediterranean regions.
In those days, seabream did not spawn at all in captivity. We started with the simple tool of histology and studied gametogenesis and reproductive cycles in this species. We found that while spermatogenesis is completed in captivity, ovaries develop to the final stages of vitellogenesis but do not undergo final oocyte maturation (FOM) and ovulation (Zohar et al., 1978). Instead, the vitellogenic oocytes undergo rapid atresia. While studying gonadal development in the seabream, we documented the fascinating process of sex reversal in this protandrous hermaphrodite (Zohar et al., 1984, Zohar et al., 1978). We also demonstrated that the seabream has an asynchronous ovarian development and thus is a batch spawner (Zohar et al., 1978, Gordin and Zohar, 1978, Zohar and Gordin, 1979). Both hermaphroditism and sequential spawning have important implications to broodstock management and egg production in the culture of this species (Zohar et al., 1995, Mylonas et al., 2011). In an effort to induce FOM, ovulation and spawning, we started to treat the females with the most effective hormonal spawning inducer at the time – human chorionic gonadotropin (hCG) (see for reviews Donaldson and Hunter, 1983, Zohar and Billard, 1978, Zohar, 1988, Zohar, 1989a, Zohar and Mylonas, 2001). Using hCG certainly induced spawning (Gordin and Zohar, 1978) and demonstrated that gilthead seabream undergoes daily cycles of FOM, ovulation and spawning that can last for up to 4 months (Zohar and Gordin, 1979). However, very soon it became apparent that seabream broodstock would not respond to hCG if treated again in subsequent years. This lack of responsiveness was first hypothesized (Lam, 1982, Donaldson and Hunter, 1983, Zohar et al., 1984), and later demonstrated (Zohar and Mylonas, 2001), to be due to an adaptive immune response with antibodies to the large hCG protein and thus the development of refractoriness to further hCG treatment.
The success of using pituitary extracts, hCG or other gonadotropins to induce spawning in captive fish, which was initiated by the pioneering work of Bernard Houssay (1930) and later implemented abundantly in a large number of species (see above reviews), indicated that in captivity the fish’s own gonadotropin (LH) is not released from the pituitary, which is the reason for their failure to undergo FOM, ovulation and spawning. This was later conclusively demonstrated in a number of studies that measured pituitary and plasma levels of luteinizing hormone (LH) in fish raised in captivity and comparing them to fish sampled on their spawning grounds (Zohar, 1989a, Mylonas et al., 1997a; Fig. 1 ). This understanding, together with the discovery of the hypothalamic releasing hormones in mammals (Amoss et al., 1971, Burgus et al., 1971, Matsuo et al., 1971), drove the fish reproductive biology community, including myself, to focus on basic and applied studies of fish gonadotropin hormone-releasing hormones (GnRHs) and the brain-reproductive axis. Very soon after the discovery of the luteinizing hormone-releasing hormone (LHRH), a few pioneers in our field used in vivo and in vitro approaches to demonstrate that fish brains possess gonadotropin-releasing potency and that synthetic GnRHs can stimulate gonadotropin release. Initial studies were conducted by the French INRA group of Bernard Breton, Roland Billard and Claudine Weil (Breton et al., 1971, Breton and Weil, 1973) and were soon followed by a wonderful publication co-authored by three of the trailblazers in our field – Larry Crim, Dick Peter and Roland Billard (Crim et al., 1976). The confirmation of GnRH activity in the fish brain led researchers and hatcheries to shift from gonadotropin-based spawning induction therapies to using GnRHs to stimulate the release of the fish’s own LH from the pituitary and, in turn, ovulation and spawning (see for reviews Donaldson and Hunter, 1983, Zohar, 1989a, Crim and Bettles, 1997, Peter and Yu, 1997, Zohar and Mylonas, 2001, Mylonas et al., 2010). Additionally, being small decapeptides, GnRHs do not induce immune responses in the treated fish, thus allowing repeated treatments.
Fig. 1.

Yoni Zohar holds a live striped bass (Morone saxatilis) electro-fished on its spawning ground in the Chesapeake Bay before taking a blood sample for studying the level of hormonal failure that is responsible for the lack of FOM, ovulation and spawning in captive held fish. Maryland, USA, 1995.
2. The glorious seventies – The birth of our family
The seventies were exciting times to be in the field of fish reproductive biology. The growing interest in aquaculture and the focus on reproductive biology of farmed fish led to a surge of basic and applied research in the reproductive endocrinology of a number of species. This research covered every aspect of fish reproductive biology, from environmental control of gametogenesis to the study of the brain-pituitary–gonadal axis and the development of aquaculture applications. A wide range of approaches were utilized, with the exception of – hard to believe – molecular biology, which was not very advanced at the time. A milestone in the field was the inaugural International Symposium on Reproductive Physiology of Fish (ISRPF) in 1977 held in Paimpont, France and organized by the scientist who has been referred to by many of us as the grandfather of the field, Roland Billard (Fig. 2 ). People were upbeat about the field and happy to be part of it. As a beginning PhD student attending his first international meeting, I was astonished to see my idols in the field imbibing freely, dancing on the tables and throwing pieces of baguette at each other. In this meeting, all the big names and pioneers in the field demonstrated its diversity and interdisciplinarity, which were already apparent back then. At the time, a debate was raging regarding the number of fish gonadotropins (GtHs), i.e., whether fish possessed one or two gonadotropins, if a “vitellogenic” GtH-I and “maturational” GtH-II existed and, if so, whether both of them (or only GtH-II) were glycoproteins? Elizabeth Burzawa-Gerard, Yves-Alain Fontaine, Bernard Breton, David Idler, John Sumpter and Madelaine Olivereau were arguing in Paimpont about the nature of fish gonadotropins. Dick Peter, Larry Crim, Pete Van-Oordt, Henk Goos, Maurice Dubois and Claudine Weil presented the early status of the fish GnRHs, their brain distribution and functions. Yoshi Nagahama, along with Bill Hoar, unveiled novel and exciting data on the cellular sources of gonadal steroids. Alexis Fostier, Bernard Jalabert and Zvi Yaron discussed the roles of steroids in gametogenesis and final oocyte maturation. Martin Schreibman described the genetic control of fish maturation. Ed Donaldson and others proposed aquaculture applications of fish reproductive physiology and myself, totally intimidated by the grandeur of the event, made my first appearance in an international conference presenting my early basic and applied work on the gilthead seabream. This meeting, in my opinion, was the birth of a tight community of fish reproductive biologists who met every four years in different countries and continents. I was very lucky to attend all of the eleven Symposia on Reproductive Physiology of Fish and witnessed the growth and intensification of the field. I often wrote about and referred to the fact that fish became a prime vertebrate reproductive model, a model for primates and human reproduction (see more below) and that in my opinion our field was driven to such depth, breadth and excellence by the reproduction-related bottlenecks in aquaculture and the need to resolve them. But this viewpoint is in no way intended to undermine the exceptional group of scientists who have worked in the field, or their friendships and close collaborations, which I have experienced from the very early days of my career until the present day. In 1977, I started my PhD with the highly prolific, collaborative and integrated INRA team in France, where I was exposed to the passion, enthusiasm, creativity and friendship of these early days in our field, working with outstanding scientists such as Roland Billard, Bernard Breton, Alexis Fostier and Bernard Jalabert.
Fig. 2.

Thirty years after the 1st ISRPF (1977) in Paimpont France, Roland Billard (center) and a few of us who attended that meeting were photographed at the 8th ISRPF (2007) in St. Malo, France. From right to left: Bernard Jalabert, Partick Prunet, Yoni Zohar, Roland Billard, Claudine Weil, Irina Barannikova and Alexis Fostier.
In 2014, I made some short opening remarks at the 9th ISRPF meeting in Faro, Portugal, organized by another pillar in the field, Adelino Canario. I reflected on why ISRPFs are the one meeting I have insisted on attending since 1977. The reason is that this community of fish reproductive biologists feels like a close family, which is not something you take for granted in science. I grew up in this community, learned from its distinguished members (including how to tie a necktie, which Martin Schreibman and Dick Peter desperately and mostly unsuccessfully tried to teach me), collaborated and became friends with many of them, and have now grown to be one of its ‘old guard’. Unfortunately, over the years our family has lost a few of its most influential members, among whom are Aubrey Gorbman, Howard Bern, Jimmy Dodd, Roland Billard, Dick Peter, Niall Bromage, Henk Goos (who succumbed to Covid-19) and, a few days ago, Zvi Yaron. The rest of us will always fondly remember their scientific contributions, kindness, mentorship and friendship.
Obviously, since its inception at the 1977 Paimpont meeting, the newly formed family/community of reproductive physiologists has continued to grow and has welcomed many younger scientists, a good number of whom were “spawned” by the above-mentioned pioneers in the field (i.e., graduated from their labs).
3. From then onward
3.1. The Gonadotropins
The controversy about the number and nature of fish gonadotropins continued for over a decade until Hiroshi Kawauchi and his team clearly demonstrated that chum salmon has two glycoproteic gonadotropins, each consisting of two subunits, that are similar to FSH and LH in other vertebrates (Kawauchi et al., 1986, Van Der Kraak et al., 1987; Suzuki et al., 1988). From that point on, we started to refer to fish gonadotropins, similar to other vertebrates, as FSH and LH. Interestingly (and somewhat ironically), these findings were first presented in 1987 at the 3rd ISRPF in Newfoundland, Canada, the very ISRPF that was hosted by David Idler – thus resolving the controversy surrounding his theory of non-traditional GtH-I and more traditional GtH-II (Idler and Ng, 1983, Crim et al., 1982, Idler and Campbell, 1980, Ng and Idler, 1980). To me, this important milestone was another strong testimonial to the collaborative nature of the family. From then on, led by young (and now young in spirit) stars like Penny Swanson, Sylvie Dufour, Abigail Elizur and Berta Sivan, several groups purified and characterized fish FSH and LH and cloned their genes, a process that was rapidly applied to multiple fish species once PCR amplification technology was implemented (Elizur et al., 1996), negating the need for the collection of thousands of pituitaries for the synthesis and screening of the traditional cDNA libraries. This progress, together with the production of recombinant LH and FSH hormones, paved the way for studying the GtHs’ respective functions, evolution, annual and daily patterns, receptors, structure–function, mechanisms of action, and regulation of their synthesis and secretion at the hormonal and molecular levels (Dufour et al., 2020, Guzmán et al., 2014, Levavi-Sivan et al., 2010, Rocha et al., 2009, Swanson et al., 2003, Dickey and Swanson, 2000, Swanson et al., 1991). In vivo and in vitro studies demonstrated the importance of FSH in the control of earlier stages of gametogenesis, spermatogenesis in males, and oogenesis in females. LH was implicated in the regulation of the final stages of gametogenesis, FOM and ovulation in the female and spermiation in the males (see above reviews). As mentioned earlier, it is LH that is not released from the pituitary in broodstock of many commercially important fish held in captivity, which is the reason for their failure to spawn. Although, as explained in section 1 above and section 4 below, the field of spawning induction has moved from the use of gonadotropins to the use of GnRHs, advances in the domain of gonadotropin biotechnology, primarily the work of Ana Gomez (Mazón et al., 2013, Mazón et al., 2015, Peñaranda et al., 2017) and Abigail Elizur (Palma et al., 2019), opened new avenues to using FSH and LH plasmids, somatic FSH/LH gene transfer and recombinant FSH and LH therapies for inducing gametogenesis in farmed fish.
3.2. The gonads
Moving downstream from the pituitary to the gonads, I have watched the field make a huge leap since we started by exploring the effects of simple estrogens and androgens on fish reproduction. As long ago as the early sixties, David Idler’s group (which initially worked on steroids before moving to gonadotropins) found that fish gonads produce interesting and unique C21 steroids, specifically the progestin 17α,20 β-dihydroxy-4 pregnen-3-one (17 α,20 β-P; now referred to as 17,20 β-dihydroxypregn-4-en-3-one) that was identified in the plasma of sockeye salmon during the preovulatory period (Idler et al, 1962). Then came the exciting seventies when a number of young and creative scientists – Bernard Jalabert, Alexis Fostier, Yoshi Nagahama, Peter Thomas, Rick Goetz and others – demonstrated, using in vivo and in vitro studies, the critical role of the 17α,20 β-P in inducing final oocyte maturation in several fish species. This activity of 17α,20 β-P, which seems to be quite ubiquitous in a wide range of fish species (see reviews by Scott et al., 2010, Fostier et al., 1983, Goetz, 1983, Scott and Canario, 1987, Zohar, 1989a) led this steroid to be referred to as the maturation inducing steroid (MIS). In subsequent years, using advanced steroid biochemistry approaches, Sandy Scott, his former PhD student, Adelino Canario, David Kime, Rudiger Schultz and other labs discovered the large diversity of steroids produced by fish gonads, including additional C21 steroids that are also produced at the time of final oocyte maturation (above reviews; Scott et al., 1999, Schulz et al., 2010). In 2010, we read the inspiring review written by a trio of the field’s leaders – Sandy Scott, John Sumpter and Norman Stacey, discussing the evidence that 17α,20β-P was not only a ‘female steroid’, but also a major ‘male steroid’ involved in the initiation of meiosis during spermiation (Scott et al., 2010). And although John Sumpter started as one of us in the 1977 Paimpont meeting, he transitioned soon thereafter to a greater and higher universe, following his breakthrough discovery that river and lake waters contain estrogenic endocrine disrupters that induce vitellogenin production in the male fish inhabiting these habitats (Sumpter, 1995). We dearly missed John’s British sense of humor but fortunately enough he later returned to our community and meetings. Since then, this field has seen additional major contributions towards understanding the reproductive, hormonal and genetic impacts of environmental endocrine disrupting chemicals from John and his former PhD student, Charlie Tyler, as well as from Peter Thomas, Oliana Carnevali, Glen Van Der Kraak and Olivier Kah (Brion et al., 2012, Boxall et al., 2012, Carnevali et al., 2018, Söffker and Tyler, 2012, Tokumoto et al., 2005, Tokumoto et al., 2007).
Also, around the late seventies to the early eighties, we saw the emergence of the “two-cell type” model of steroidogenesis in fish. Led by one of our field’s luminaries, Yoshi Nagahama, classic experiments in salmonids and other fish species showed that both estradiol-17β, the steroid responsible for oocyte growth and vitellogenesis, and the MIS (17α,20β-P) are produced in two steps, with each step occurring in a different follicular cell layer. During oocyte growth, the theca cells produce testosterone that is aromatized to estradiol-17β in the granulosa cells. When fully grown vitellogenic follicles are ready to enter FOM, there is a shift in steroidogenesis, such that theca cells start to produce 17α-hydroxyprogesterone that is converted to 17α,20β-P in the granulosa cells. Detailed in vitro and in vivo studies by Yoshi and his early students Graham Young, Hirohiko Kagawa, Shinji Adachi, Hideki Tanaka and others, integrated hormonal, biochemical, molecular and functional approaches to demonstrate the details of the drastic and very fast enzymatic changes occurring in the follicular cells during this transition (see reviews by Rajakumar and Senthilkumaran, 2020, Tokarz et al., 2015, Pandian, 2013, Nagahama and Yamashita, 2008, Kobayashi et al., 2013, Nagahama, 2003, Nagahama, 1997, Nagahama et al., 1993, Nagahama et al., 1994, Nagahama et al., 1995). Yoshi has been a pillar in our field. We have always eagerly waited to listen to his inspirational talks. He has been highly collaborative and a real friend and welcomed everyone to his lab (although they had to work long shifts!); a real sensei in our field.
Another example of the concept of “fish as an endocrine vertebrate model” came from Peter Thomas’s innovative work in fish showing, for the first time in any vertebrate, that gonadal steroids can drive rapid actions initiated at the cell surface membrane receptors (as opposed to the nuclear receptors), which circumvent the relatively slow classic gene-mediated mechanism of steroid hormone action. This novel mechanism is non-genomic and involves rapid activation of intracellular signal transduction pathways within only a few minutes (see for review Thomas, 2012, Thomas et al., 2017). Peter and his colleagues presented evidence demonstrating cross-talk between the traditional, genomic slow-acting pathway and the alternative, cell membrane-mediated, non-genomic hormonal pathway during the above-described fast transition in fish ovaries from estradiol-17β to 17α, 20β-P production towards the end of oogenesis, regulating the onset of oocyte maturation. These findings had major fundamental implications, not only to the field of comparative endocrinology but also to the biomedical sciences such as in the field of cancer. As Peter was once quoted saying “Fish are excellent models for examining many aspects of vertebrate physiology because the results can often be translated to humans and impact biomedical research. For example, we found that the huge numbers of eggs in fish ovaries provide an abundant source of tissue for purifying steroid membrane receptors on vertebrate eggs. In addition, the characteristics of the fish and human steroid membrane receptors are very similar”.
3.3. Reproductive pheromones
Back to the MIS – several of those who worked at the time on these steroids wondered why they display such high levels in the fish circulation in some species (e.g., salmonids) but not in others (e.g., sparids), after all they are produced and act at the gonad. At the same time, intense steroid reduction and glucuronide and sulfate conjugation processes were discovered, rendering the MIS and other sex steroids present in plasma hydrophilic. These polar and conjugated steroids are excreted to the water mainly through fish urine, while free steroids are preferentially released through the gills (Vermeirssen and Scott, 1996). Taken together, these observations led to the hypothesis that these key gonadal steroids may end up in the water (in conjugated or free form) and act as pheromones that facilitate successful reproduction and spawning (Stacey and Sorensen, 1987, Scott and Canario, 1987, Scott and Canario, 1992). A series of very elegant studies ensued, totally unique to fish, confirming this hypothesis. Norman Stacey, and his former student Peter Sorensen, Sandy Scott, Adelino Canario, Makito Kobayashi, Lorenzo Colombo and others, integrated platforms of fish behavior, olfaction and electrophysiology, endocrine assays and gonad biology, to clearly demonstrate that several male and female gonadal steroids indeed act as pheromonal cues to elicit final hormonal and gametogenic changes in their counterpart gender, as well as attract each other to engage in spawning behavior. Moreover, the same researchers demonstrated that pheromones and their olfactory ability are species-specific, generally males and females of one species respond only to conspecific individuals and will ignore heterospecific pheromones. This explains how in the wild, different species of fish can reproduce at the same time while keeping their mating conspecific. This fascinating field of reproductive biology, very unique to fish, has been abundantly reviewed over the years (Sorensen and Stacey, 1999, Stacey and Sorensen, 2002, Stacey and Sorensen, 2009; Stacey, 2010, Stacey, 2014, Keller-Costa et al., 2014, Keller-Costa et al., 2015, Munakata and Kobayashi, 2010). Looking at the authorship lists of publications in this field once again is a testimony to the close collaborations between scientists working in our field. The competitive rivalries so common in the life sciences simply did not exist in our fish reproductive physiology family.
3.4. Gonadal feedback to the brain-pituitary axis
One of the early papers authored by Roland Billard used gonadectomy to demonstrate, for the first time in fish, gonadal feedback on pituitary gonadotropin secretion (Billard, 1978). In collaboration with Dick Peter, the two pioneers showed that this gonadal feedback is exerted by sex steroids (Billard and Peter, 1977). The crosstalk between the gonads and the brain-pituitary axis has since been studied in detail by many labs and in multiple fish species. Researchers like Vance Trudeau and Gustavo Somoza, both of whom graduated from or trained in Dick Peter’s lab (Trudeau, 1997, Trudeau and Somoza, 2020), and Olivier Kah (Kah et al., 1993, Diotel et al., 2011) integrated the study of hormones, receptors and their genes with high resolution microscopy to dissect the details of the feedback dialog between the gonads, the brain and the pituitary, at the level of the GnRHs, GtHs, other neurohormones and their receptors involved in the control of reproduction. Olivier and his former post-doc, Jose-Antonio Munoz-Cueto, took the field to the next level via their outstanding imaging and mapping of the brain and pituitary sites involved in these communications, as well as other endocrine factors involved in this axis. To this end, I am very proud that the three of us (along with Carmen Sarasquete) joined forces in the early 2000′s to publish a detailed atlas of the brain of the commercially important gilthead seabream (Sparus aurata) (Munoz-Cueto et al., 2001).
3.5. Sex determination and differentiation
The large diversity of fish species represents a wide range of gonadal developmental patterns and physiologies. While most fish are gonochoristic, many are hermaphroditic with protandrous, protogynous and even simultaneous patterns of bisexual gonadal development. As I mentioned above, hermaphroditism in fish captured my interest from my early days, and over the years, as my group occasionally pursued social and endocrine mechanisms involved in sex reversal (e.g., Wong and Zohar, 2006, Reyes-Tomassini et al., 2017), I witnessed the excellent work of Ching-Fong Chang, whose group significantly contributed to our understanding of endocrine, molecular and epigenetic processes regulating sex reversal in fish (e.g., Wu et al., 2015, Wu et al., 2016, Wu et al., 2017, Lin et al., 2019).
Also, fish display a variety of mechanisms of gender determination, ranging from genetic to environmental, with homogametic males or females (see review by Penman and Piferrer, 2008). Fundamental biology and aquaculture interests drove intensive research into the mechanisms and processes controlling sex determination and differentiation in fish. Again, starting in the seventies, several groups from around the world began describing the intricate network of hormones and other factors, both brain and gonadal, that interact to determine gender in a large number of fish. Early studies from one of our field’s founders, Edward Donaldson (Donaldson, 1986, Donaldson and Hunter, 1982, Hunter and Donaldson, 1983), and his former student, Francesc Pifferer (Piferrer, 2001), combined the basic and translational aspects of this topic. Working in parallel on the same species but on two separate continents, Yoshi Nagahama and Manfred Schartl and their groups conducted thorough and very elegant studies that revealed and characterized the first sex-determining genes in a fish, the medaka (Nanda et al., 2002, Matsuda et al., 2002 reviewed by Kottler et al., 2020). Yoshi Nagahama and his many colleagues went on to dissect and elucidate the ensuing sex differentiation cascade that determines the gender of fish (Devlin and Nagahama, 2002, Nagahama, 2005, Zhou et al., 2012, Nakamura, 2013, Kobayashi et al., 2013, Chakraborty et al., 2016, Chakraborty et al., 2019, Nakamoto et al., 2018).
3.6. Monosex and sterility
With the growth and intensification of aquaculture, it became apparent that quite often one gender performs better than the other, reaches sexual maturity at a different age, and that achieving sexual maturity is associated with reduced growth rate, deterioration of flesh quality and in some species, mortality. This led to a growing interest in producing monosex populations or reproductively sterile fish, thereby overcoming these hurdles. The urgency of producing reproductively sterile fish significantly increased with the recognition that aquaculture may cause “biological pollution” in the environment via farmed escapees interbreeding and displacing wild fish stocks. Sterile fish will provide a means to biologically/genetically contain farmed fish, in addition to resulting in better performance. Starting with steroid treatments combined with hybridization to induce sex reversal and sterility in cichlids, cyprinids and salmonids, the field very quickly moved to chromosome set manipulations, gynogenesis and polyploidy. During the late seventies and into the eighties, we saw the emergence of studies and technologies whereby eggs are activated to start development by sperm, in which the genetic content has been destroyed, thus resulting in animals possessing only maternal inheritance (gynogenesis). In this process, shortly after activation the eggs are exposed to environmental shock (pressure or temperature) that leads to diploidization of the embryos, generating all-female offspring. Using intact sperm to fertilize the eggs prior to shocking them results in triploid individuals, which are often sterile. This technical breakthrough, together with androgenesis (to produce all-male diploids in fish with homogametic males) and sex-reversing homogametic individuals to obtain broodstock of the opposite phenotypic sex, led to aquaculture applications generating monosex or sterile fish (see for reviews Chourrot, 1982, Thorgaard, 1986, Donaldson and Benfey, 1987, Felip et al., 2001, Benfey, 2016). Optimizing polyploidy technologies (e.g., Smedley et al. 2016) led to scaling up and commercializing genotypically all-female triploids, phenotypically sterile fish for a number of species. The booming Atlantic salmon industry, which has been blamed for significant biological pollution (Glover et al., 2017), started to introduce sterile triploid fish into floating net-pens, mainly in Norway and Scotland. However, very quickly it became apparent that triploid fish may under-perform their diploid counterparts. For instance, in suboptimal conditions they may display morphological deformities, cataract symptoms (Taylor et al., 2013, Taylor et al., 2015) and possibly compromised immune systems and increased susceptibility to disease (Benfey, 2016).
Potential obstacles with scaling up the triploidy technology drove a search for alternative approaches to produce reproductively sterile fish. Many of us in the field brainstormed possible strategies to induce sterility and, I am proud to say, an excellent former PhD student of mine (they were all outstanding!), Ten-Tsao Wong, was one of the first to explore a very promising approach – eliminating primordial germ cells (PGCs) during early development (see for reviews Wong and Zohar, 2015a, Wong and Zohar, 2018). In fish, PGCs become established immediately after fertilization and undergo guided migration to their final destination in the gonads during a short window of time very early in development (Braat et al., 1999, Weidinger et al., 2003, Herpin et al., 2007). The hypothesis was that if the PGCs are prevented from making it to the gonads, the fish will become sterile. Indeed, using transgenic approaches to alter the signaling pathways involved in the PGC migration ultimately led to sterility (Knaut et al., 2003, Wong and Collodi, 2013). In a series of inspiring studies, a young star in our field, Anna Wargelius, used gene editing to knockout one of the key genes involved in the development of PGCs – the deadend gene (dnd) (Weidinger et al., 2003) in Atlantic salmon. The mutated fish had no germ cells in the gonad, were 100% sterile, and performed as well as the wild-type salmon (Wargelius et al., 2016, Wargelius, 2019). However, with the uncertainty about authorizing the use of gene-edited fish in aquaculture, the implementation of this approach in the industry is still remote.
In the meantime, Ten-Tsao Wong and I worked on an alternative technology for sterility that avoids genetic modifications. Our concept is to use transitional knockdown gene-silencing (morpholino) to prevent the production of the dnd protein during the short window of time of PGC migration in early development. Also, with the objective of making this technology scalable, we set out to immerse fish eggs, before or after fertilization, in the gene silencing agent (dnd morpholino) together with endocytosis-enhancing factors to boost uptake of the morpholino into the eggs. We started with the zebrafish model. Indeed, a 5-hour immersion of zebrafish eggs in these compounds disrupted the migration of PGCs and led to 100% sterility with no germ cells in the gonads (Wong and Zohar, 2015b, Wong and Zohar, 2018). Since then, we have been engaged in collaborative efforts with industry, as well as academic colleagues, to implement and optimize this strategy primarily in Atlantic salmon and rainbow trout, with increased confidence of imminent success. At the same time, we have been testing this strategy in other farmed species such as tilapia, sablefish and kingfish. The history of the effort to develop sterile fish is another example of the close synergism between basic and translational research in our field of fish reproductive biology.
3.7. Puberty
Another approach to control the age-at-first maturity in farmed fish is through the manipulation of puberty. The idea is to be able to advance the onset of puberty in potential broodstock of species that reach late sexual maturity (sturgeon, striped bass), and delay pubertal onset in species that reach sexual maturity before harvest (gilthead seabream, salmon and many others). Two of our community’s most prolific members, Manuel Carrillo and Silvia Zanuy (whose recent retirements have left a huge void in the ISRPF community), made major contributions to the understanding of environmental and hormonal control of puberty, focusing on the commercially important European seabass (reviewed by Carrillo et al., 2015, Carrillo et al., 2009). Parallel studies were performed in other farmed fish and all the main contributors to this field, from eight institutions and four countries, joined forces in publishing several seminal reviews on the control of puberty in farmed fish (Taranger et al., 2010, Andersson et al., 2018, Crespo et al., 2019) – again reflecting the strong spirit of collaboration in our field. As explained in these reviews, simple environmental manipulations have already been implemented by the industry, such as adding lights to prolong days and delay puberty in large scale net-pen production of Atlantic salmon and Atlantic cod. One of the major contributors to this field was Niall Bromage (e.g., Bromage et al., 2001, Guerrero-Tortolero and Bromage, 2008), a friend, a collaborator and a teacher who was taken from our community much too early. More recently, and since the discovery that brain kisspeptins also play a role in regulating puberty in fish (reviewed Zohar et al., 2010, Carrillo et al., 2015), studies which involved collaborations between Abigail Elizur, Berta Sivan and my group used sustained administration of kisspeptins to advance puberty in the commercially important yellowtail kingfish (Nocillado et al., 2013).
4. GnRHs – The basic and the applied
As mentioned in section 1 above, the discovery of brain GnRH, together with the recognition that the lack of FOM, ovulation and spawning in farmed fish is a result of a failure of LH release from the pituitary, prompted the field to focus on studying the fish GnRHs and using them to induce LH release and in turn oocyte maturation, ovulation and spawning. Many excellent reviews were written on both basic (Kah et al., 2007, Zohar et al., 2010, Munoz-Cueto et al., 2001, Roch et al., 2011, Roch et al., 2014, Kah, 2020, Trudeau and Somoza, 2020) and applied (Zohar, 1988, Zohar, 1989a, Yaron and Zohar, 1993, Zohar and Mylonas, 2001, Mylonas and Zohar, 2007, Mylonas and Zohar, 2008, Mananos et al., 2008) aspects of this research. Therefore, herein I will simply review the general evolution of the field and some milestones.
4.1. From discovery to the industry
A major breakthrough in the field was the discovery in 1983 of the first fish GnRH by Nancy Sherwood and colleagues (Sherwood et al., 1983). This decapeptide was first isolated from brains of chum salmon and was therefore named salmon (s) GnRH. The same sGnRH was then found in a few additional fish species. At the same time, several groups began to use mammalian LHRH or sGnRH to induce LH release, ovulation and spawning in a number of farmed species. Very soon, it became apparent that although the GnRHs indeed induced pituitary LH release, the duration of the blood LH surge was very short and a single GnRH injection was not sufficient to induce FOM and ovulation (Omeljaniuk et al., 1987, Crim et al., 1988, Zohar, 1989a, Zohar, 1989b, Zohar et al., 1989a, Zohar et al., 1989b). Our group then showed that this short-lived action of the native GnRHs was the result of quick degradation, and thus inactivation, of the administered native GnRHs by specific peptidases that cleave the decapeptide between positions 5–6 and 9–10 (Zohar et al., 1990). Substituting amino acids in positions 6 and 10 of the peptide led to the production of synthetic GnRH analogues (GnRHa), which were shown to be resistant to enzymatic degradation (Goren et al., 1990, Weil et al., 1991, Weil et al., 1992). These cleavage-resistant GnRHa displayed higher affinity to the pituitary GnRH receptors (De Leeuw et al., 1988, Habibi et al., 1989, Pagelson and Zohar, 1992) and induced a much stronger LH release from the pituitary, in terms of both amplitude and duration of the LH surge (Peter et al., 1988, Zohar, 1988, Zohar, 1989a, Zohar et al., 1989a, Crim and Bettles, 1997). However, although at a slower rate than the native peptides, even these “super-potent” GnRHa were still cleared relatively rapidly from the fish circulation (Gothilf and Zohar, 1991), probably due to the fact that being short peptides they were processed and excreted by the fish as intact molecules. In some fish species, a single injection of super-potent GnRHa was sufficient to induce FOM, ovulation and spawning. However, in others, especially in serial/batch spawners, such as our seabream and European seabass models, a single GnRHa injection was not sufficient and multiple injections of GnRHa were required. The repeated injections and handling were labor intensive and stressful to the fish, often leading to mortalities.
To overcome this shortcoming, Larry Crim, Dick Peter, Nancy Sherwood and my lab all searched for ways to prolong the presence of GnRHa analogues in the fish circulation. To achieve that goal, we experimented with the use of polymer-based, controlled-release delivery systems that provided a sustained presence of GnRHa in the blood and a prolonged release of LH from the pituitary, which in turn successfully induced FOM, ovulation and spawning. I started by treating female seabream with commercially available GnRHa implants that were used to down-regulate gonadotropin release in humans with hormonally-dependent cancers, such as breast or prostate cancer. The fact that, in fish, prolonged presence of GnRHa induced continuous release of LH rather than downregulating it, was surprising and scientifically very intriguing to many of us, but also enabled us to use this strategy to induce spawning in captive broodstocks. To be able to develop our own, fish-specific GnRHa delivery systems, I joined for one year (1988) the MIT lab of the guru of controlled-release technology Robert Langer (see for example Langer 2019). While at MIT, I developed several GnRHa polymeric delivery systems and worked with commercial hatcheries in Maine to test their potency in synchronizing ovulation in Atlantic salmon (Fig. 3 ). A variety of polymers and delivery systems (implants and microspheres) have since been used to enable optimal patterns of GnRHa/LH release, in most cases tailored to the ovarian physiology of the fish of interest (i.e., one-time spawner, batch spawner, etc.). This resulted in a generic GnRHa-based spawning induction technology, which has been successfully tested and optimized in a wide range of aquaculture species and abundantly implemented in the industry over the years (see reviews listed above, Fig. 4 ). The GnRHa controlled release delivery systems were found to be highly effective in solving another broodstock problem in aquaculture – the insufficiency of sperm production in males (Zohar and Mylonas, 2001, Mylonas et al., 1997b, Mylonas et al., 2017). The technology proved to be very efficient in enhancing sperm production in captive male broodstock, also enabling the use of fewer males for the spawning operations and thereby saving space, labor and money. The person who conducted many of the studies and contributed the most to the development, optimization, testing and industrial implementation of the GnRHa controlled-release technology was Constantinos (Dinos) Mylonas, who started the work as my brilliant PhD student and later flourished as an independent and highly productive scientist (e.g., Zohar and Mylonas, 2001, Mylonas and Zohar, 2001a, Mylonas and Zohar, 2001b, Mylonas and Zohar, 2007, Mylonas and Zohar, 2008, Mylonas et al., 2010, Mylonas et al., 2017).
Fig. 3.
Yoni Zohar strip-spawns an Atlantic salmon which was induced to ovulate using GnRHa implant. Maine, USA, 1988.
Fig. 4.
Yoni Zohar administers GnRHa implants to Atlantic salmon held in floating net-pens in a commercial broodstock operation in Chile, 1993.
Although the use of the GnRHa alone (injections or delivery systems) efficiently induced ovulation, spermiation and spawning in many farmed species, in some other species GnRHa alone is not enough. In the 1977 Paimpont 1st ISRPF meeting, a very productive collaboration between Dick Peter, Larry Crim and Henk Goos provided the first evidence for the existence of a gonadotropin release inhibitory factor (GRIF) in the brain of goldfish (Peter and Crim, 1979, Peter et al., 1978). Many follow-up studies led by Dick Peter, his past graduate students John Chang and Hamid Habibi, as well as Olivier Kah, Sylvie Dufour and others, have identified GRIF as dopamine, which was later confirmed in all studied cyprinid fish and a handful of other fish species (reviewed by Dufour et al., 2020). As presented by Dick Peter, Hao-Ren Lin and Glen Van Der Kraak at the 3rd ISRPF in 1987, in broodstock of such fish, LH release, FOM, ovulation and spawning can only be induced by the administration of both GnRHa and dopamine antagonists (Peter et al., 1988). More recently, we found out that the situation is more complex (which is not surprising), as other inhibitory factors have been shown to be involved in modulating pituitary gonadotropin release, primarily the gonadotropin inhibitory hormone – GnIH (e.g., Ogawa and Parhar, 2014, Spicer et al., 2017; Zhang et al., 2019). It still remains to be seen if this discovery will have practical implications related to the control of reproduction in farmed fish.
4.2. The biotechnological approach
As the GnRH spawning induction technology became widespread, more and more groups applied it to additional fish species. One fish of special interest is the bluefin tuna (BFT), for which closing the life cycle became a priority in the development of its aquaculture, especially in light of dwindling BFT abundance in the oceans. Indeed, our GnRHa sustained release technology has been optimized and successfully used for induction of BFT spawning (Mylonas et al., 2007, Rosenfeld et al., 2012) and has since been implemented in the industry (Zohar et al., 2016). However, keeping the giant bluefin tuna broodstock in captivity is not a trivial task. This quandary drove one of the brightest minds in our field, Goro Yoshizaki, to originate the idea of germ cell transplantation and surrogate broodstock technology. In 2002, Baltimore (where I have been working for the last 30 years) hosted the annual meeting of the Society for the Study of Reproduction and I was asked to co-organize a session dedicated to fish reproduction. When we reviewed submitted abstracts, I was mystified and intrigued by one submission that described manipulating fish primordial germ cells (PGCs) so they could be visualized for harvesting from one fish species (donor) and transplanted into another (recipient). This is how I met Goro Yoshizaki, which led to a wonderful decades-long friendship.
Goro’s rationale was totally out-of-the-box – yet very simple. If it is so difficult to maintain and spawn bluefin tuna (which we all know is of huge importance in Japan) in captivity, why not transfer PGC of bluefin tuna to a much smaller closely related surrogate, such as mackerel, and generate mackerel broodstock that produce tuna gametes and offspring? Sounds like science-fiction? Well, for the past 20 years Goro and his colleagues have worked diligently to make this concept a reality. Starting with salmonids, they were indeed able to microinject rainbow trout PGCs into masu salmon. The transplanted PGCs colonized the gonads of the recipient masu salmon and differentiated into functional eggs and sperm, which led to the production of trout offspring from salmon (Takeuchi et al., 2004). In follow-up studies, Goro demonstrated the extraordinary plasticity in fish gamete differentiation, as both oogonia and spermatogonia transplanted into surrogate fish were able to develop into either mature eggs or sperm in the recipient fish, and were successfully used to generate offspring (Okutsu et al., 2007, Yoshizaki et al., 2010, Lee et al., 2013). His group later implemented the surrogate broodstock technology in a number of other commercially important fish, such as yellowtail (Morita et al., 2015) and the Japanese fugu (Tiger puffer, Hamasaki et al., 2017). Goro also used the germ cell transplantation concept to generate reproductively sterile fish (Nagasawa et al., 2019). For more details on his groundbreaking basic and translational work, the readers are referred to recent reviews by Yoshizaki and Lee, 2018, Yoshizaki and Yazawa, 2019. A couple of years ago, I visited Goro’s lab in Tokyo and saw juvenile surrogate mackerel carrying bluefin tuna germ cells. In March 2020, Goro wrote to me that although recipient male mackerel produced functional tuna sperm, the effort is still underway for the females. Stay tuned, everyone!
4.3. Back to the GnRH bench
The success in resolving the spawning bottleneck in aquaculture led us all back to the bench, trying to better understand why the endogenous GnRH ‘malfunctions’ in the captive broodstock of many species. While I cannot say that we have answered that question, we have certainly learned a great deal about the very unique fish GnRH system (reviewed by Kah et al., 2007, Kah and Dufour, 2011, Zohar et al., 2010, Chang and Pemberton, 2018, Choi, 2018, Kah, 2020, Munoz-Cueto et al., 2001). Following the above-mentioned discovery of sGnRH in salmonids and many other species of fish, a second form of GnRH was found to be present in fish brains, the ubiquitous chicken (c) GnRH-II (as it was referred to at the time) (Amano et al., 1991). Around the same time, my group collaborated with Nancy Sherwood trying to identify the nature of the GnRHs present in the gilthead seabream (Fig. 5 ). To achieve this goal, we collected 10,000 (!) seabream brains and pituitaries and very carefully shipped them on dry ice to Nancy’s lab, where her team used HPLC combined with GnRH immunoreactivity and amino acid analysis to characterize the seabream GnRHs. A couple of months later Nancy informed me that something must have gone wrong during the collection and/or shipment of the brains and pituitaries. She had detected 3 GnRH peaks in the HPLC chromatogram (we expected only two, sGnRH and cGnRH-II), which she thought may have indicated that the GnRHs had degraded to smaller, yet still immunoreactive, fragments. A bit frustrated, but still determined, we went back to the fish farm and collected and shipped another 5,000 brains and pituitaries to Nancy, who again saw the same three peaks. It then dawned on us that we had just discovered, for the first time in any vertebrate, that the seabream brain possesses three forms of GnRH and, adding to the excitement, that one of the three forms was a novel GnRH isoform that we named seabream (sb) GnRH (Powell et al., 1994). The other two forms were the previously known sGnRH and cGnRH-II. Using molecular approaches, we then confirmed the presence of three GnRHs via the isolation and characterization of three corresponding cDNAs in the seabream brain (Gothilf et al., 1996, Gothilf et al., 1997, Chow et al., 1998). Several very exciting years in the GnRH field ensued, where more and more species of fish, mostly more-evolved fish such as many of the perciforms, were shown to possess three forms of GnRH. This indicated that our 3-GnRH discovery in seabream, which was initially thought to be the exception, had suddenly become the rule. In an effort to understand the functional significance of GnRH multiplicity and the roles of each of the isoforms, our lab and others mapped the sites of expression of the three GnRHs in the brain, tracked their projection to the pituitary, and studied their biological activities and affinities to GnRH receptors. In addition, we monitored levels of the three GnRHs and their mRNAs in the brain and pituitaries of several species (e.g., Gothilf et al., 1997, Holland et al., 1998 and above reviews). All of these studies demonstrated that in fish with three GnRHs, the novel GnRH (sbGnRH in our case) is the hypophysiotropic form that is most relevant to gonadotropin release and gametogenesis. Most of the less-evolved fish species (e.g., salmonids and cyprinids), however, possess only two forms of GnRH and, in these species, sGnRH (the first fish GnRH discovered) is the hypophysiotropic peptide.
Fig. 5.
Yoni Zohar standing by a broodstock gilthead seabream tank at the Aquaculture Research Center, Institute of Marine and Environmental Technology, University of Maryland. 2019.
During the nineties, the field also witnessed the discovery of several additional forms of GnRH, specifically in fish possessing three GnRHs, where a species-specific form is usually the hypophysiotropic one. With the increasing number of GnRH variants discovered, and in order to standardize GnRH nomenclature and avoid confusion, a new classification of the GnRH forms was adopted based on the phylogenetic analysis of known sequences and their respective sites of expression (Fernald and White, 1999, Kah et al., 2007, Zohar et al., 2010, Munoz-Cueto et al., 2001). According to this new nomenclature, the three GnRHs are now referred to as GnRH1, 2 and 3. In all fish, GnRH2 is the ubiquitous cGnRH-II. In fish with three GnRHs, GnRH1 is the form that expresses in the preoptic area (POA) of the brain and whose neurons project to the pituitary (ergo, the hypophysiotropic GnRH). GnRH3 is the form that, in fish with 3 GnRHs, expresses in the olfactory bulb and terminal nerve (OB/TN) areas of the brain. In all fish, regardless of the number of GnRHs, GnRH2 expresses in the midbrain tegmentum and projects to several brain areas. Fish with two GnRHs do not possess GnRH1, and subsequently GnRH3 is expressed in the OB/TN, ventral telencephalon and the POA, and projects to the pituitary. It is thus considered to be the hypophysiotropic GnRH in these species. While this nomenclature is still somewhat confusing, it has become the standard in the field.
Whereas the hypophysiotropic GnRH has been clearly identified in many fish, the field is still trying to understand the exact roles of the non-hypophysiotropic GnRHs (either one or two forms depending on the species). Accumulating evidence indicates that GnRH2 is involved in integrating reproduction with feeding activities, and possibly also in reproductive behavior and in transducing photoperiod information processed by the pineal gland into circadian reproductive events (reviewed by Munoz-Cueto, 2020). The OB/TN GnRH3 has been implicated in transducing external cues, such as olfaction and social information (pheromones) to reproductive processes (Ueda et al., 2016; Munoz-Cueto 2020). Additionally, the multiplicity of GnRHs across fish and vertebrate species has raised many interesting evolutionary questions that were addressed by several excellent reviews (Okubo and Nagahama, 2008, Kah et al., 2007, Roch et al., 2011, Roch et al., 2014, Lovejoy et al., 2018, Choi, 2018, Umatani and Oka, 2019, Dufour et al., 2020, Kah, 2020, Munoz-Cueto et al., 2001, Trudeau and Somoza, 2020).
The story of GnRH multiplicity is another striking example of the concept of “fish as a vertebrate/primate model”. For years after the discovery of the LHRH in humans, it was believed that humans possess only one form of GnRH. However, the widespread demonstration of GnRH multiplicity in fish (and other non-mammalian vertebrates) led the neuroendocrine research community to re-visit the mammalian GnRH situation and, indeed, to discover two GnRH isoforms in the brains of primates – the initially-described LHRH (GnRH1) and the ubiquitous GnRH2.
4.4. And then came the small fish models
During the mid-nineties, when most of the major discoveries on GnRH multiplicity were made, we witnessed the emergence of the small fish models – zebrafish and medaka. Interestingly, zebrafish possess 2 GnRH isoforms while the medaka has 3 GnRHs, thus allowing comparative studies between these two GnRH system models. The fact that these fish reach sexual maturity within 3 months, together with the availability of a range of applicable genomics/post-genomics platforms such as full genome sequencing, gene transfer, fluorescence labeling, gene silencing, gene editing and others, enabled many of us in the field to reach new levels of understanding of the GnRH and HPG axis. Using transgenic fish expressing fluorescent proteins (driven by the 2/3 GnRH promotors) in the GnRH neurons enabled detailed mapping of the GnRH system and its early development. Knockdown and ablation technologies led to progress in deciphering the functions of the 2/3 GnRHs and the regulation of their development, expression and activities. This progress has been abundantly reviewed (see above reviews). It is also interesting that, following the confirmation that the zebrafish possesses only 2 forms of GnRH (Steven et al., 2003), this species immediately became a prime model for studying GnRHs in primates and humans, who, like zebrafish, possess 2 GnRHs. The fact that the traditional mammalian models (i.e., rodents) have only 1 form of GnRH, and thus are not useful as primate/human models, makes zebrafish an even more desirable surrogate species for the study of the primate GnRH system.
For years, researchers using fish models suffered from the lack of knock-out technologies, a situation that crippled our ability to gain a full understanding of processes and functions compared to mammalian species. This situation persisted until gene editing (TALEN/CRISPR-Cas9) emerged less than a decade ago (Sander et al., 2011, Hwang et al., 2013), and was quickly implemented in fish studies (reviewed by Zhu and Ge, 2018). Obviously, many fish reproductive biologists began to use this platform as a “loss-of-function” research tool, which will no doubt eventually reveal the exact functions of each of the GnRH forms. However, my team certainly did not expect the shocking result that knocking out GnRH3 in zebrafish, the hypophysiotropic form in this species, had absolutely no effect on reproduction and spawning (Spicer et al., 2016). We then thought that perhaps GnRH2 may also be involved in reproductive competence and/or may provide redundancy or some form of compensation for the lack of GnRH3. To our further surprise, fish in which GnRH2 was knocked-out (Marvel et al., 2018) or fish with a double knockout of both GnRH2 and GnRH3 (Marvel et al., 2019) continued to reproduce normally. Very interestingly, this situation may be different in fish with 3 GnRHs, as knocking out the hypophysiotropic GnRH1 in medaka results in failure of ovulation, however males are still fertile (Takahashi et al., 2016). Confirming and adding to the zebrafish finding, Liu et al (2017) found that triple knockout zebrafish, with mutations in GnRH3 and Kisspeptin 1 and 2, also displayed normal reproduction.
The lack of distinct reproductive phenotypes in GnRH knockouts led many of us to hypothesize that in the absence of the GnRHs, other factors/hormones may be upregulated and compensate for the lack of GnRHs, sustaining the activity of the HPG axis and ensuring successful reproduction. Several follow-up studies led to a number of theories regarding mechanisms of compensation/redundancy and suggested potential candidates for these roles, which were summarized in multiple recent reviews (Liu and Lin, 2017, Trudeau, 2018, Whitlock et al., 2019, Muñoz-Cueto et al., 2020). An array of brain factors known to be involved in reproduction, such as GnIH, NKBs, kisspeptins, secretoneurins and others, have been hypothesized to take over the GnRH activities in their absence, through as yet to be discovered redundancy or compensation pathways. A very recent study conducted in a collaboration that I highly enjoy, between the lab of Yoav Gothilf, my former PhD student, and my group, provided convincing data suggesting that Kiss1 (which is not believed to be involved in fish reproduction) compensates for the absence of Kiss2 (the reproductively relevant Kiss form) in Kiss2 knockout zebrafish (Etzion et al., 2020, this volume). Interestingly, Vance Trudeau’s group provided evidence for the existence of a new neuropeptide, secretoneurin (SN), that can act both independently and along with the GnRHs in regulating pituitary gonadotropin release (Trudeau et al., 2012, Trudeau and Somoza, 2020). Further studies indicated that SN is also likely involved in regulating the GnRH system (Shu et al, 2018). Vance’s group recently demonstrated that targeted mutation of the two secretogranin-2 genes involved in SN synthesis leads to significant reproductive malfunction in zebrafish (Mitchell et al., 2020), indicating a key role for SN in the endocrine control of fish reproduction.
The gene editing revolution has added mystery and challenges, not only in the study of brain reproductive peptides, but also at lower levels of the HPG axis. Studies by Wei Ge, Christopher H.K. Cheng (Univ. of Hong Kong) and one of our very early colleagues and friend, Hao-Ren Lin, showed that knocking out LH and FSH in zebrafish results in some reproductive abnormalities (Chu et al., 2015, Zhang et al., 2015a, Zhu and Ge, 2018, Li and Cheng, 2018), and double knockout of the gonadotropin receptors in the gonads results in infertility (Chu et al., 2015, Zhang et al., 2015b, Zhu and Ge, 2018, Li and Cheng, 2018). Taken together with the lack of reproductive effects in the GnRH and kisspeptin knockouts, this suggests that at the level of the brain, there is higher plasticity and functional redundancy of multiple reproductive neuropeptides, whereas downstream in the HPG axis, factors at the pituitary and gonadal level may be more specialized and less disposed to compensation. The implementation of gene editing in the study of GnRHs and, more generally, in fish reproductive biology, is still in its infancy. A broader integration of gene editing in our field will no doubt generate a flurry of exciting discoveries within the next 5–10 years.
5. A few concluding thoughts
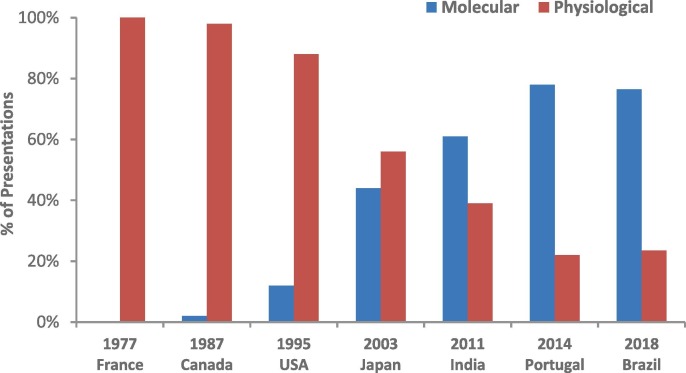
During my 46 years in basic and translational fish reproductive biology, I have seen the field evolve in parallel to the life sciences revolution and the expansion of its modern platforms. In my concluding remarks at the 2014 10th ISRPF in Faro, Portugal, I analyzed trends in our field from the first ISRPF in 1977 until that point (see also Canario and Zohar, 2015), and I have now added the 2018 11th ISRPF in Brazil to that analysis. As shown in Fig. 6 , at the 1977 1st ISRPF, there was no mention of any molecular studies, all presentations were addressing aspects of “physiology”. In every ISRPF meeting since then, we have seen an increase in the number of “molecular” studies, and at the 10th and 11th ISRPF ~80% of the presentations involved molecular aspects. The application of platforms of genomics/functional genomics and other -omics has become dominant. While this molecular revolution has driven the field to greater levels of understanding, the next generations of scientists would be well served to remember that ‘simple’ animal physiology must remain an indispensable component of our field. Future basic and translational discoveries will require the integration and synergism of physiological and molecular approaches.
Fig. 6.
The percent of total presentations at the International Symposia on Reproductive Physiology of Fish (ISRPF) that used physiological (red) or molecular (blue) platforms.
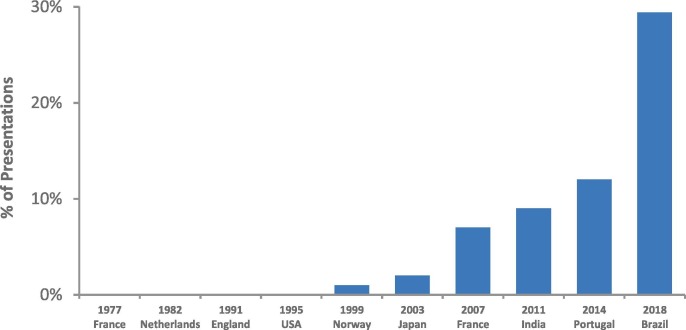
Another very striking trend is in the number of presentations using the zebrafish model (Fig. 7 ), which has increased from zero in the years from 1977 up until 1995, to 12% in 2014, and then to 29% in 2018 (more than doubling in four short years). Again, as discussed earlier in this review, while zebrafish and medaka have indeed paved the way to new avenues of tools and research, we should not abandon the larger fish models and/or the species of aquacultural importance.
Fig. 7.
The percent of total presentations at the International Symposia on the Reproductive Physiology of Fish (ISRPF) in which the zebrafish model was studied.
Before concluding this review, I would like to say a few words about the unique underlying distinctions that I have observed in our community of fish reproductive physiologists, features that set us apart from other fields. First and foremost – our people. I believe that those of us studying fish reproductive biology have not randomly stumbled into the field. We are here because we have a passion for fish and fish biology, for the oceans, for aquaculture. Therefore, we all share the same enthusiasm, and it shows. Over my many years in the field, I have enjoyed working with my colleagues, and many have become enduring friendships. Hence, the sense of family in our research community is once again a unifying factor that ultimately dictates how we interact, collaborate and work together to collectively advance the field. As I write these concluding lines, we are in the midst of the COVID-19 pandemic. It is heartwarming to see how many of us interact via email, social media, phone and video conferencing to ensure that our scientific friends and colleagues are safe and healthy.
Nowadays, the academic community at large recognizes the utmost importance of multidisciplinary and collaborative research. As indicated by a few examples given in this review, we in the field of fish reproductive biology have understood this from the beginning and developed a culture of collaborative research and integrating expertise. Such cooperation is not always the case in the life sciences and cannot be taken for granted – but for the ISRPF family, it has always been and should continue to be appreciated and fostered.
And finally, the beneficial “gene flow” in our field is quite obvious. As we all look around (and I have provided a few examples in this review), we can see that many of our ‘offspring’ (grad students, post-docs) remain in the field, amplify and improve it. These new generations will continue to ensure progress in our field during the next five decades and beyond. When I am asked what I am most proud of in my career, my answer is without a doubt the ‘spawning’ of many top-notch students and post-docs over the years (e.g., Fig. 8 ). I am very proud of them, they have all developed successful careers in the life sciences and have made an impact around the world. Many have remained in fish and reproductive biology (Fig. 9 ), and some have already produced their own ex-students who are now the F3 generation in our community. In humble appreciation of the above, I dedicate this review to all those who I have been fortunate to work with during my 46 highly rewarding years in fish reproductive biology - my students, group members, colleagues and friends.
Fig. 8.
Yoni Zohar (fourth from left) with members of his team during a (fish) break from the XII International Congress of Comparative Endocrinology in Toronto, Canada (May 1993). From right to left: Shimon Hassin (PhD student); Abigail Elizur (a close collaborator); Yoav Gothilf (PhD student); Constantinos (Dinos) Mylonas (PhD student); Claire Holland (PhD student); John Stubblefield (research associate) and Verapong Vuthiphandchai (PhD student).
Fig. 9.
Yoni Zohar (seated in center) with past and current (at the time) members of his team at the 10th ISRPF (2014) in Olhao, Portugal. From right to left, seated: Arianna Servili (former post-doc), Talya Etzion (visiting PhD student from the Gothilf Lab), Olivia Smith-Spicer (PhD student), Nilli Zmora (post-doc); Yoav Gothilf (former PhD student), Shigeho Ijiri (former post-doc); standing: Ten-Tsao Wong (former PhD student and post-doc), Constantinos (Dinos) Mylonas (former PhD student and post-doc); Evaristo Mananos (former post-doc) and John Stubblefield (research associate).
Acknowledgements
Throughout my career, I have been consistently funded by many US and international funding agencies, including NSF, NOAA, National Sea Grant, Maryland Sea Grant, USDA, US-Israel BARD, Israel Science Foundation, NOAA-SK, UDSA-SBIR, the Gudelsky Family Foundation and many others. I am very grateful for the support of my programs and I hope that I have delivered on the collective investment of the funding agencies. I would like to thank my colleagues who provided critical comments on different sections of this review – Adelino Canario, Abigail Elizur, Vance Trudeau and Goro Yoshizaki. Special thanks to John Stubblefield from my group who spent long hours editing the manuscript.
References
- Amano M., Oka Y., Aida K., Okumoto N., Kawashima S., Hasegawa Y. Immunocytochemical demonstration of salmon GnRH and chicken GnRH-II in the brain of masu salmon, Oncorhynchus masou. J. Comp. Neurol. 1991;14:587–597. doi: 10.1002/cne.903140313. [DOI] [PubMed] [Google Scholar]
- Amoss M., Burgus R., Blackwell R., Vale W., Fellows R., Guillemin R. Purification, amino acid composition and N-terminus of the hypothalamic luteinizing hormone releasing factor (LRF) of ovine origin. Biochem. Biophys. Res. Commun. 1971;44:205–210. doi: 10.1016/s0006-291x(71)80179-1. [DOI] [PubMed] [Google Scholar]
- Andersson E., Taranger G.L., Wargelius A., Schulz R.W. Reference Module in Life Sciences - Encyclopedia of Reproduction (Second Edition) Elsevier; Amsterdam: 2018. Puberty in fish; pp. 426–429. [DOI] [Google Scholar]
- Benfey T.J. Effectiveness of triploidy as a management tool for reproductive containment of farmed fish: Atlantic salmon (Salmo salar) as a case study. Rev. Aquacult. 2016;8:264–282. [Google Scholar]
- Billard R., Peter R.E. Gonadotropin release after implantation of anti-estrogens in the pituitary and hypothalamus of goldfish, Carassius auratus. Gen. Comp. Endocrinol. 1977;32:213–220. doi: 10.1016/0016-6480(77)90154-x. [DOI] [PubMed] [Google Scholar]
- Billard R. Testicular feedback on the hypothalamo-pituitary axis in rainbow trout (Salmo gairdnery R.) Ann. Biol. Anim. Biochim. Biophys. 1978;18:813–818. [Google Scholar]
- Braat A.K., Zandbergen T., Van Der Water S., Goos H.J.Th., Zivkovic D. Characterization of zebrafish primordial germ cells: morphology and early distribution of vasa RNA. Dev Dyn. 1999;216:153–167. doi: 10.1002/(SICI)1097-0177(199910)216:2<153::AID-DVDY6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Breton B., Weil C. Effect of synthetic LH-FSH releasing hormone and hypothalamic extracts of the carp on in vivo gonadotropic hormone secretion in the carp (Cyprinus carpio L.). C R Acad. Sci. Hebd Seances Acad. Sci. D. 1973;277:2061–2064. [PubMed] [Google Scholar]
- Breton B., Jalabert B., Billard R., Weil C. In vitro stimulation of the release of pituitary gonadotropic hormone by a hypothalamic factor in the carp Cyprinus carpio L. C R Acad. Sci. Hebd Seances Acad. Sci. D. 1971;273:2591–2594. [PubMed] [Google Scholar]
- Brion F., Le Page Y., Piccini B., Cardoso O., Tong S.K., Chung B.C., Kah O. Screening estrogenic activities of chemicals or mixtures in vivo using transgenic (cyp19a1b-GFP) zebrafish embryos. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0036069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxall A.B.A., Rudd M.A., Brooks B.W., Caldwell D.J., Choi K., Hickmann S., Innes E., Ostapyk K., Staveley J.P., Verslycke T., Ankley G.T., Beazley K.F., Belanger S.E., Berninger J.P., Carriquiriborde P., Coors A., DeLeo P.C., Dyer S.D., Ericson J.F., Gagné F., Giesy J.P., Gouin T., Hallstrom L., Karlsson M.V., Joakim Larsson D.G., Lazorchak J.M., Mastrocco F., McLaughlin A., McMaster M.E., Meyerhoff R.D., Moore R., Parrott J.L., Snape J.R., Murray-Smith R., Servos M.R., Sibley P.K., Straub J.O., Szabo N.D., Topp E., Tetreault G.R., Trudeau V.L., Van Der Kraak G. Pharmaceuticals and personal care products in the environment: What are the big questions? Environ. Health Perspect. 2012;120(9):1221–1229. doi: 10.1289/ehp.1104477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromage N., Porter M., Randall C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture. 2001;197:63–98. [Google Scholar]
- Burgus R., Butcher M., Ling N., Monahan M., Rivier J., Fellows R., Amoss M., Blackwell R., Vale W., Guillemin R. Molecular structure of the hypothalamic factor (LRF) of ovine origin monitoring the secretion of pituitary gonadotropic hormone of luteinization (LH). C. R. Acad. Sci. Hebd. Seances Acad. Sci. D. 1971;273:1611–1613. [PubMed] [Google Scholar]
- Canario, A., Zohar, Y., 2015. Proceedings of the 10th International Symposium on Reproductive Physiology of Fish. (Canario, A., Zohar, Y., Eds). General and Comparative Endocrinology Special Issue: Expanding the Knowledge Base of Reproductive Success: From Genes to the Environment. Volume 221. 283 pages.
- Carnevali O., Santangeli S., Forner-Piquer I., Basili D., Maradonna F. Endocrine-disrupting chemicals in aquatic environment: what are the risks for fish gametes? Fish Physiol. Biochem. 2018;44(6):1561–1576. doi: 10.1007/s10695-018-0507-z. [DOI] [PubMed] [Google Scholar]
- Carrillo M., Espigares F., Felip A., Escobar S., Molés G., Rodríguez R., Alvarado M.V., Gómez A., Zanuy S. Updating control of puberty in male European sea bass: a holistic approach. Gen. Comp. Endocrinol. 2015;221:42–53. doi: 10.1016/j.ygcen.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Carrillo M., Zanuy S., Felip A., Bayarri M.J., Molés G., Gómez A. Hormonal and environmental control of puberty in perciform fish: the case of sea bass. Ann. N. Y. Acad. Sci. 2009;1163:49–59. doi: 10.1111/j.1749-6632.2008.03645.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Mohapatra S., Zhou L.Y., Ohta K., Matsubara T., Iguchi T., Nagahama Y. Estrogen receptor β2 oversees germ cell maintenance and gonadal sex differentiation in medaka, Oryzias latipes. Stem Cell Rep. 2019;13(2):419–433. doi: 10.1016/j.stemcr.2019.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Zhou L.Y., Chaudhari A., Iguchi T., Nagahama Y. Dmy initiates masculinity by altering Gsdf/Sox9a2/Rspo1 expression in medaka (Oryzias latipes) Sci. Rep. 2016;6 doi: 10.1038/srep19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.P., Pemberton J.G. Comparative aspects of GnRH-Stimulated signal transduction in the vertebrate pituitary-Contributions from teleost model systems. Mol. Cell. Endocrinol. 2018;463:142–167. doi: 10.1016/j.mce.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Choi D. Evolutionary viewpoint on GnRH (gonadotropin-releasing hormone) in chordata – amino acid and nucleic acid sequences. Dev. Reprod. 2018;22:119–132. doi: 10.12717/DR.2018.22.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourrot D. Tetraploidy induced by heat shocks in the rainbow trout (Salmo gairdneri R.) Reprod. Nutr. Dev. 1982;22(3):569–574. doi: 10.1051/rnd:19820412. [DOI] [PubMed] [Google Scholar]
- Chow M.M., Kight K.E., Gothilf Y., Alok D., Stubblefield J., Zohar Y. Multiple GnRHs present in a teleost species are encoded by separate genes: Analysis of the sbGnRH and cGnRH-II genes from the striped bass, Morone saxatilis. J. Mol. Endocrinol. 1998;21:277–289. doi: 10.1677/jme.0.0210277. [DOI] [PubMed] [Google Scholar]
- Chu L., Li J., Liu Y., Cheng C.H.K. Gonadotropin signaling in zebrafish ovary and testis development: Insights from gene knockout study. Mol Endocrinol. 2015;29(12):1743–1758. doi: 10.1210/me.2015-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo D., Bogerd J., Sambroni E., LaGac F., Andersson E., Edvardsen R.B., Bergman E.J., Bjornsson B.J., Taranger G.L., Schulz R.W. The initiation of puberty in Atlantic salmon brings about large changes in testicular gene expression that are modulated by the energy status. BMC Genomics. 2019;20:475. doi: 10.1186/s12864-019-5869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim, L.W., Bettles, S., 1997. Use of GnRH analogues in fish culture. In: Fingerman, M., Nagabhushanam, R., Thompson, M.F. (Eds.). Recent Advances in Marine Biotechnology, Vol. 1: Endocrinology and Reproduction. Oxford and IBH Publishing, New Delhi, pp. 369–382.
- Crim L.W., Peter R.E., Billard R., et al. Stimulation of gonadotropin secretion by intraventricular injection of hypothalamic extracts in the goldfish, Carassius auratus. Gen. Gen. Comp. Endocrinol. 1976;30(1):77–82. doi: 10.1016/0016-6480(76)90068-x. [DOI] [PubMed] [Google Scholar]
- Crim L.W., Sherwood N.M., Wilson C.E. Sustained hormone release. II. Effectiveness of LHRH analog (LHRHa) administration by either single time injection or cholesterol pellet implantation on plasma gonadotropin levels in a bioassay model fish, the juvenile rainbow trout. Aquaculture. 1988;74:87–95. [Google Scholar]
- Crim L.W., Genge P.D., Billard R., Idler D.R. The influence of immature gonads on onset of gonadotropic hormone accumulation in the juvenile rainbow trout pituitary gland. Gen. Comp. Endocrinol. 1982;48(2):161–166. doi: 10.1016/0016-6480(82)90013-2. [DOI] [PubMed] [Google Scholar]
- De Leeuw, R., Van’t Veer, C., Goos, H.J., Van Oordt, P.G., 1988. The dopaminergic regulation of gonadotropin-releasing hormone receptor binding in the pituitary of the African catfish, Clarias gariepinus. Gen. Comp. Endocrinol. 72, 408–415. [DOI] [PubMed]
- Devlin R., Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208(3–4):191–364. [Google Scholar]
- Dickey J.T., Swanson P. Effects of salmon gonadotropin-releasing hormone on follicle stimulating hormone secretion and subunit gene expression in coho salmon (Oncorhynchus kisutch) Gen. Comp. Endocrinol. 2000;118(3):436–449. doi: 10.1006/gcen.2000.7482. [DOI] [PubMed] [Google Scholar]
- Diotel N., Do-Rego J.L., Anglade I., Vaillant C., Pellegrini E., Vaudry H., Kah O. The brain of teleost fish, a source, and a target of sexual steroids. Front. Neurosci. 2011;5:137. doi: 10.3389/fnins.2011.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson, E.M. and Benfey, T.J., 1987. Current status of induced sex manipulation. In: Proc. Third Intl. Symp. Reprod. Physiol. Fish. pp. 108–119. Edited by D.R. Idler, L.W. Crim and J.M. Walsh, Memorial University Press, St. John's.
- Donaldson E.M. The integrated development and application of controlled reproduction techniques in Pacific salmonid aquaculture. Fish Physiol. Biochem. 1986;2:9–24. doi: 10.1007/BF02264070. [DOI] [PubMed] [Google Scholar]
- Donaldson, E.M., Hunter, G.A., 1983. Induced final maturation, ovulation and spermiation in cultured fishes. In: Hoar, W.S., Randall, D.J., Donaldson, E.M. (Eds.), Fish Physiology. Reproduction, Vol. IXB, Academic Press, Orlando, FL, pp. 351–403.
- Donaldson E.M., Hunter G.A. Sex control in fish with particular reference to salmonids. Can. J. Fish. Aqua. Sci. 1982;39(1):99–110. doi: 10.1139/f82-012. [DOI] [Google Scholar]
- Dufour S., Quérat B., Tostivint H., Pasqualini C., Vaudry H., Rousseau K. Origin and evolution of the neuroendocrine control of reproduction in vertebrates, with special focus on genome and gene duplications. Physiol. Rev. 2020;100:869–943. doi: 10.1152/physrev.00009. [DOI] [PubMed] [Google Scholar]
- Elizur A., Zmora N., Rosenfeld H., Meiri I., Hassin S., Gordin H., Zohar Y. ßGtH I and ßGtH II from the gilthead seabream, Sparus aurata. Gen. Comp. Endocrinol. 1996;102:39–46. doi: 10.1006/gcen.1996.0044. [DOI] [PubMed] [Google Scholar]
- Etzion, T., Zmora, N., Zohar, Y., Levavi-Sivan, B., Golan, M., Gothilf, Y., 2020. Ectopic over expression of kiss1 may compensate for the loss of kiss2. Gen. Comp. Endo. (This volume). [DOI] [PubMed]
- Felip A., Zanuy S., Carrillo M., Piferrer F. Induction of triploidy and gynogenesis in teleost fish with emphasis on marine species. Genetica. 2001;111(1–3):175–195. doi: 10.1023/a:1013724322169. [DOI] [PubMed] [Google Scholar]
- Fernald R.D., White R.B. Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front. Neuroendocrinol. 1999;20:224–240. doi: 10.1006/frne.1999.0181. [DOI] [PubMed] [Google Scholar]
- Fostier, A., Jalabert, B., Billard, R., Breton, B., Zohar, Y., 1983. The gonadal steroids. In “Fish Physiology,” Vol IX, “Reproduction” (W.S. Hoar, D.J. Randall, and E.N. Donaldson, eds.), pp.277-372. Academic Press, New York.
- Glover K.A., Solberg M.F., McGinnity P., Hindar K., Verspoor E., Coulson M.W., Hansen M.M., Araki H., Skaala Ø., Svåsand T. Half a century of genetic interaction between farmed and wild Atlantic salmon: status of knowledge and unanswered questions. Fish Fish. 2017;18:890–927. [Google Scholar]
- Goetz, F.W., 1983. Hormonal control of oocyte final maturation and ovulation in fishes. In: Fish Physiology, Volume 9, Part B (Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds). Elsevier. pp. 117–170. 10.1016/S1546-5098(08)60303-9. [DOI]
- Gordin H., Zohar Y. Induced spawning of Sparus aurata (L.) by means of hormonal treatment. Ann. Biol. Anim. Biochem. Biophys. 1978;18:985–990. [Google Scholar]
- Goren A., Zohar Y., Fridkin M., Elhanati E., Koch Y. Degradation of gonadotropin releasing hormone in the gilthead seabream, Sparus aurata: I. Cleavage of native salmon GnRH, mammalian LHRH and their analogs in the pituitary. Gen. Comp. Endocrinol. 1990;79:291–305. doi: 10.1016/0016-6480(90)90115-3. [DOI] [PubMed] [Google Scholar]
- Gothilf, Y., Zohar, Y., 1991. Clearance of different forms of GnRH from the circulation of the gilthead seabream, Sparus aurata, in relation to their degradation and bioactivities. In: “Reproductive Physiology of Fish” (A.P. Scott, J.P. Sumpter, D.E. Kime and M.S. Rolfe eds). pp. 35-37. University of East Anglia Press.
- Gothilf Y., Meiri I., Elizur A., Zohar Y. Preovulatory changes in the levels of three gonadotropin-releasing hormone-encoding messenger ribonucleic acids (mRNAs), gonadotropin beta-subunit mRNAs, plasma gonadotropin, and steroids in the female gilthead seabream, Sparus aurata. Biol. Reprod. 1997;57:1145–1154. doi: 10.1095/biolreprod57.5.1145. [DOI] [PubMed] [Google Scholar]
- Gothilf Y., Muñoz-Cueto J.A., Sagrillo C.A., Selmanoff M., Chen T.T., Kah O., Elizur A., Zohar Y. Three forms of gonadotropin-releasing hormone in a perciform fish (Sparus aurata): complementary deoxyribonucleic acid characterization and brain localization. Biol. Reprod. 1996;55:636–645. doi: 10.1095/biolreprod55.3.636. [DOI] [PubMed] [Google Scholar]
- Guerrero-Tortolero D.A., Bromage N. Growth and maturation of Atlantic salmon (Salmo salar) populations with different grilse proportions under natural photoperiod and superimposed nighttime light. Aquaculture. 2008;285(1–4):63–66. [Google Scholar]
- Guzmán J.M., Luckenbach J.A., Yamamoto Y., Swanson P. Expression profiles of Fsh-regulated ovarian genes during oogenesis in coho salmon. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0114176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi H.R., Marchant T.A., Nahorniak C.S., Van Der Loo H., Peter R.E., Rivier J.E., Vale W.W. Functional relationship between receptor binding and biological activity for agonists of mammalian and salmon gonadotropin-releasing hormones in the pituitary of goldfish (Carassius auratus) Biol. Reprod. 1989;40:1152–1161. doi: 10.1095/biolreprod40.6.1152. [DOI] [PubMed] [Google Scholar]
- Hamasaki M., Takeuchi Y., Yazawa R., Yoshikawa S., Kadomura K., Yamada T., Miyaki K., Kikuchi K., Yoshizaki G. Production of tiger puffer, Takifugu rubripes, offspring from triploid grass puffer, Takifugu niphobles, parents. Mar. Biotechnol. 2017;19:579–591. doi: 10.1007/s10126-017-9777-1. [DOI] [PubMed] [Google Scholar]
- Herpin A., Rohr S., Riedel D., Kluever N., Raz E., Schartl M. Specification of primordial germ cells in medaka (Oryzias latipes) BMC Dev. Biol. 2007;7:3. doi: 10.1186/1471-213X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M.C., Gothilf Y., Meiri I., King J.A., Okuzawa K., Elizur A., Zohar Y. Levels of the native forms of GnRH in the pituitary of the gilthead seabream, Sparus aurata, at several characteristic stages of the gonadal cycle. Gen. Comp. Endocrinol. 1998;112:394–405. doi: 10.1006/gcen.1998.7138. [DOI] [PubMed] [Google Scholar]
- Houssay B.A. Accion sexual de la hipofisis en los peces y reptiles. Rev. Soc. Arg. Biol. 1930;106:686–688. [Google Scholar]
- Hunter, G.A., Donaldson, E.M., 1983. Chapter 5 - Hormonal Sex Control and its Application to Fish Culture. In: W.S. Hoar, W.S., Randall, D.J. and Donaldson E.M. (Eds), Fish Physiology IXB, Academic Press, London, pp. 223-303.
- Hwang W.Y., Fu Y., Reyon D., Maeder M.L., Tsai S.Q., Sander J.D., Peterson R.T., Joanna Yeh J.R., Joung J. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 2013;31:227–229. doi: 10.1038/nbt.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler D.R., Campbell C.M. Gonadotropin stimulation of estrogen and yolk precursor synthesis in juvenile rainbow trout. Gen. Comp. Endocrinol. 1980;41(3):384–391. doi: 10.1016/0016-6480(80)90083-0. [DOI] [PubMed] [Google Scholar]
- Idler D.R., Ng T.B. Teleost gonadotropins: isolation, biochemistry and function. Fish Physiol. 1983;9:187–221. doi: 10.1016/S1546-5098(08)60289-7. [DOI] [Google Scholar]
- Idler D.R., Schmidt P.J., Ronald A.P. Isolation of 20β-dihydrocortisone from sockeye salmon (Oncorhynchus nerka) plasma. Can. J. Biochem. Physiol. 1962;40(5):549–553. doi: 10.1139/o62-065. [DOI] [Google Scholar]
- Kah O. A 45-years journey within the reproductive brain of fish. Gen. Comp. Endocrinol. 2020;113370 doi: 10.1016/j.ygcen.2019.113370. [DOI] [PubMed] [Google Scholar]
- Kah, O., Dufour, S., 2011. Conserved and divergent features of reproductive neuroendocrinology in teleost fishes. In: Norris, D.O., Lopez, K.H. (Eds.), Hormones and Reproduction of Vertebrates, Volume 1: Fishes, Chapter 2. Academic Press, USA, pp. 15–42.
- Kah O., Lethimonier C., Somoza G., Guilgur L.G., Vaillant C., Lareyre J.J. GnRH and GnRH receptors in metazoa: A historical, comparative, and evolutive perspective. Gen. Comp. Endocrinol. 2007;153:346–364. doi: 10.1016/j.ygcen.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Kah O., Anglade I., Lepretre E., Dubourg P., de Monbrison D. The reproductive brain in fish. Fish Physiol. Biochem. 1993;11:85–98. doi: 10.1007/BF00004554. [DOI] [PubMed] [Google Scholar]
- Kawauchi, H., Suzuki, K., Nagahama, Y., Adachi, S., Naito, N., Nakai, Y. 1986. Occurrence of two distinct gonadotropins in chum salmon pituitary. In Pars Distalis of the Pituitary Gland. Structure, Function and Regulation (F. Yoshimura, F., Gorbman, A., Eds.) Elsevier, Amsterdam. pp. 383–390.
- Keller-Costa T., Canario A.V.M., Hubbard P.C. Chemical communication in cichlids: a mini-review. Gen. Comp. Endocrinol. 2015;221:64–74. doi: 10.1016/j.ygcen.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Keller-Costa T., Hubbard P.C., Paetz C., Nakamura Y., Da Silva J.P., Rato A., Barata E.N., Schneider B., Canario A. Identity of a tilapia pheromone released by dominant males that primes females for reproduction. Curr. Biol. 2014;24:2130–2135. doi: 10.1016/j.cub.2014.07.049. [DOI] [PubMed] [Google Scholar]
- Knaut H., Werz C., Geisler R., Nüsslein-Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421(6920):279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Nagahama Y., Nakamura M. Diversity and plasticity of sex determination and differentiation in fishes. Sex. Dev. 2013;7:115–125. doi: 10.1159/000342009. [DOI] [PubMed] [Google Scholar]
- Kottler V.A., Feron R., Nanda I., Klopp C., Du K., Kneitz S., Helmprobst F., Lopez-Roques C., Lluch J., Journot L., Parrinello H., Guiguen Y., Schartl M. Independent origin of XY and ZW sex determination mechanisms in mosquitofish sister species. Genetics. 2020;214(1):193–209. doi: 10.1534/genetics.119.302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T.J. Applications of endocrinology to fish culture. Can. J. Aquat. Fish. Sci. 1982;39:11–137. [Google Scholar]
- Langer R. Controlling the movement of molecules. Q. Rev. Biophys. 2019;52(e5):1–21. doi: 10.1017/S0033583519000040. [DOI] [Google Scholar]
- Lee, S., Iwasaki, Y., Shikina, S., Yoshizaki, G., 2013. Generation of functional eggs and sperm from cryopreserved whole testes. Proceedings of the National Academy of Sciences of the United States of America. DOI: 10.1073/pnas.1218468110. [DOI] [PMC free article] [PubMed]
- Levavi-Sivan B., Bogerd J., Mañanós E.L., Gómez A., Lareyre J.J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010;165:412–437. doi: 10.1016/j.ygcen.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Li J., Cheng C.H.K. Evolution of gonadotropin signaling on gonad development: insights from gene knockout studies in zebrafish. Biol. Reprod. 2018;99(4):686–694. doi: 10.1093/biolre/ioy101. [DOI] [PubMed] [Google Scholar]
- Lin C.-J., Wu G.-C., Dufour S., Chang C.-F. Activation of the brain-pituitary-gonadotropic axis in the black porgy Acanthopagrus schlegelii during gonadal differentiation and testis development and effect of estradiol treatment. Gen. Comp. Endo. 2019;281:17–29. doi: 10.1016/j.ygcen.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lin H. Genetic analysis of the reproductive axis in fish using genome editing nucleases. Sci. Bull. 2017;62:302–308. doi: 10.2108/zsj.21.427. [DOI] [PubMed] [Google Scholar]
- Liu Y., Tang H., Xie R., Li S., Liu X., Lin H., Zhang Y., Cheng C.H.K. Genetic evidence for multifactorial control of the reproductive axis in zebrafish. Endocrinology. 2017;158:604–611. doi: 10.1210/en.2016-1540. [DOI] [PubMed] [Google Scholar]
- Lovejoy D.A., Michalec O.M., Hogg D.W., Wosnick D.I. Role of elasmobranchs and holocephalans in understanding peptide evolution in the vertebrates: Lessons learned from gonadotropin releasing hormone (GnRH) and corticotropin releasing factor (CRF) phylogenies. Gen. Comp. Endocrinol. 2018;264:78–83. doi: 10.1016/j.ygcen.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Mananos E., Duncan N., Mylonas C.C. In: Methods in Reproductive Aquaculture: Marine and Freshwater Species. First Edition. Cabrita I., Robles V., Herraez P., editors. CRC Press; New York. 572 pages: 2008. Reproduction and control of ovulation, spermiation and spawning in cultured fish; pp. 1–40. [Google Scholar]
- Marvel M., Spicer O.S., Wong T.T., Zmora N., Zohar Y. Knockout of Gnrh2 in zebrafish reveals its roles in regulating feeding behavior and oocyte quality. Gen. Comp. Endocrinol. 2019;99(3):565–577. doi: 10.1016/j.ygcen.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Marvel M., Spicer O., Wong T.T., Zohar Y. Knockout of the Gnrh genes in zebrafish: effects on reproduction and potential compensation by reproductive and feeding-related factors. Biol. Reprod. 2018;99(3):565–577. doi: 10.1093/biolre/ioy078. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Nagahama Y., Shinomiya A., Sato T., Matsuda C., Kobayashi T., Morrey C.E., Shibata N., Asakawa S., Shimizu N., Hori H., Hamaguchi S., Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417(6888):559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- Matsuo H., Baba Y., Nair R.M., Arimura A., Schally A.V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem. Biophys. Res. Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- Mazón M.J., Molés G., Rocha A., Crespo B., Lan-Chow-Wing O., Espigares F., Muñoz I., Felip A., Carrillo M., Zanuy S., Gómez A. Gonadotropins in European sea bass: endocrine roles and biotechnological applications. Gen. Comp. Endocrinol. 2015;221:31–41. doi: 10.1016/j.ygcen.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Mazón M.J., Zanuy S., Muñoz I., Carrillo M., Gómez A. Luteinizing hormone plasmid therapy results in long-lasting high circulating Lh and increased sperm production in European sea bass (Dicentrarchus labrax) Biol. Reprod. 2013;88(2):1–7. doi: 10.1095/biolreprod.112.102640. [DOI] [PubMed] [Google Scholar]
- Mitchell K., Zhang W.S., Lu C., Tao B., Chen L., Hu W., Trudeau V.L. Targeted mutation of secretogranin-2 disrupts sexual behavior and reproduction in zebrafish. PNAS. 2020;117(23):12772–12783. doi: 10.1073/pnas.2002004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Morishima K., Miwa M., Kumakura N., Kudo S., Ichida K., Mitsuboshi T., Takeuchi Y., Yoshizaki G. Functional sperm of the yellowtail (Seriola quinqueradiata) were produced in the small-bodied surrogate, jack mackerel (Trachurus japonicus) Mar. Biotechnol. 2015;17:644–654. doi: 10.1007/s10126-015-9657-5. [DOI] [PubMed] [Google Scholar]
- Munakata A., Kobayashi M. Endocrine control of sexual behavior in teleost fish. Gen. Comp. Endocrinol. 2010;165(3):456–468. doi: 10.1016/j.ygcen.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cueto J.A., Zmora N., Paullada-Salmerón J.A., Marvel M., Mañanos E., Zohar Y. The gonadotropin-releasing hormones: lessons from fish. Gen. Comp. Endocrinol. Special Issue. 2020;291 doi: 10.1016/j.ygcen.2020.113422. [DOI] [PubMed] [Google Scholar]
- Munoz-Cueto J.A., Sarasquette C., Zohar Y., Kah O. Brain atlas of the gilthead seabream (Sparus aurata) Sea Grant Press. 2001;126 pages. [Google Scholar]
- Mylonas C.C., Zohar Y., Pankhurst N., Kagawa H., Chang C.F. In: Sparidea: Biology and Aquaculture. Pavlidis M., Mylonas C.C., editors. Blackwell Publishing Ltd.; Oxford, UK: 2011. Chapter 4 - Reproduction and broodstock management in Sparidae; pp. 95–131. [DOI] [Google Scholar]
- Mylonas C.C., Fostier A., Zanuy S. Broodstock management and hormonal manipulations of reproduction. Gen. Comp. Endocrinol. 2010;165:516–534. doi: 10.1016/j.ygcen.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Mylonas C.C., Scott A.P., Zohar Y. Plasma gonadotropin II, sex steroids, and thyroid hormones in wild striped bass (Morone saxatilis) during spermiation and final oocyte maturation. Gen. Comp. Endocrinol. 1997;108:223–236. doi: 10.1006/gcen.1997.6967. [DOI] [PubMed] [Google Scholar]
- Mylonas C.C., Scott A.P., Vermeirssen E.L.M., Zohar Y. Changes in plasma gonadotropin II and sex-steroid hormones, and sperm production of striped bass after treatment with controlled-release gonadotropin-releasing hormone agonist-delivery systems. Biol. Reprod. 1997;57:669–675. doi: 10.1095/biolreprod57.3.669. [DOI] [PubMed] [Google Scholar]
- Mylonas C.C., Zohar Y. In: New technologies in aquaculture: Improving production efficiency, quality and environmental management. Burnell G., Allan G., editors. Woodhead Publishing Ltd; Cambridge, UK: 2008. Controlling reproduction in aquaculture; pp. 110–142. [Google Scholar]
- Mylonas C.C., Zohar Y. In: The fish oocyte: from basic studies to biotechnological applications. Babin P.J., Lubzens E., editors. Springer Press; Dordrecht, The Netherlands: 2007. Promoting oocyte maturation, ovulation and spawning in farmed fish; pp. 437–474. [Google Scholar]
- Mylonas C.C., Bridges C.R., Gordin H., Belmonte Ríos A., García A., De la Gándara F., Fauvel C., Suquet M., Medina A., Papadaki M., Heinisch G., De Metrio G., Corriero A., Vassallo-Agius R., Guzmán J.M., Mañanos E., Zohar Y. Preparation and administration of gonadotropin-releasing hormone agonist (GnRHa) implants for the artificial control of reproductive maturation in captive-reared Atlantic bluefin tuna (Thunnus thynnus) Rev. Fish. Sci. 2007;15(3):183–210. [Google Scholar]
- Mylonas C.C., Zohar Y. Endocrine regulation and artificial induction of oocyte maturation and spermiation in basses of the genus Morone. Aquaculture. 2001;202:205–220. [Google Scholar]
- Mylonas C.C., Zohar Y. Use of GnRHa-delivery systems for the control of reproduction in fish. Rev. Fish Biol. Fish. 2001;10:463–491. [Google Scholar]
- Mylonas C.C., Duncan N.J., Asturiano J.F. Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture. 2017;472:21–44. [Google Scholar]
- Nagahama Y. Molecular mechanisms of sex determination and gonadal sex differentiation in fish. Fish Physiol. Biochem. 2005;31:105. doi: 10.1007/s10695-006-7590-2. [DOI] [PubMed] [Google Scholar]
- Nagahama, Y., 2003. Sex Determination and Gonadal Sex Differentiation in Fish – Special Lecture. The 3rd International Medaka Symposium, 2003. Okazaki, Japan. February 27 and 28, 2003.
- Nagahama Y. 17α, 20β-Dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in fish oocytes: mechanisms of synthesis and action. Steroids. 1997;62:190–196. doi: 10.1016/s0039-128x(96)00180-8. [DOI] [PubMed] [Google Scholar]
- Nagahama Y., Yamashita M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008;50(Suppl 1):S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- Nagahama Y., Yoshikuni M., Yamashita M., Tokumoto T., Katsu Y. Regulation of oocyte growth and maturation in fish. Curr. Top. Dev. Biol. 1995;30:103–145. doi: 10.1016/s0070-2153(08)60565-7. [DOI] [PubMed] [Google Scholar]
- Nagahama Y., Yamashita M., Tokumoto T. Regulation of oocyte maturation in fish. Curr. Topics Dev. Biol. 1994;30:103–145. doi: 10.1016/s0070-2153(08)60565-7. [DOI] [PubMed] [Google Scholar]
- Nagahama Y., Yoshikuni M., Yamashita M., Sakai N., Tanaka M. Molecular endocrinology of oocyte growth and maturation in fish. Fish Physiol. Biochem. 1993;11(1–6):3–14. doi: 10.1007/BF00004545. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Ishida M., Octavera A., Kusano K., Kezuka F., Kitano T., Yoshiura Y., Yoshizaki G. Novel method for mass producing genetically sterile fish from surrogate broodstock via spermatogonial transplantation. Biol. Reprod. 2019;100(2):535–546. doi: 10.1093/biolre/ioy204. [DOI] [PubMed] [Google Scholar]
- Nakamoto M., Shibata Y., Ohno K., Usami T., Kamei Y., Taniguchi Y., Todo T., Sakamoto T., Young G., Swanson P., Naruse K., Nagahama Y. Ovarian aromatase loss-of-function mutant medaka undergo ovary degeneration and partial female-to-male sex reversal after puberty. Mol. Cell. Endocrinol. 2018;460:104–122. doi: 10.1016/j.mce.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Nakamura M. Morphological and physiological studies on gonadal sex differentiation in teleost fish. Aqua-BioScience Monogr. 2013;6(1):1–47. [Google Scholar]
- Nanda I., Kondo M., Hornung U., Asakawa S., Winkler C., Shimizu A., Shan Z., Haaf T., Shimizu N., Shima A., Schmid M., Schartl M. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. U.S.A. 2002;99(18):11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T.B., Idler D.R. Gonadotropic regulation of androgen production in flounder and salmonids. Gen. Comp. Endocrinol. 1980;42(1):25–38. doi: 10.1016/0016-6480(80)90253-1. [DOI] [PubMed] [Google Scholar]
- Nocillado J.N., Zohar Y., Biran J., Levavi-Sivan B., Elizur A. Chronic kisspeptin administration stimulated gonadal development in pre-pubertal male yellowtail kingfish (Seriola lalandi; Perciformes) during the breeding and non-breeding season. Gen. Comp. Endocrinol. 2013;191:168–176. doi: 10.1016/j.ygcen.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Pagelson G., Zohar Y. Characterization of gonadotropin-releasing hormone (GnRH) receptors in the gilthead seabream (Sparus aurata) Biol. Reprod. 1992;47:1004–1008. doi: 10.1095/biolreprod47.6.1004. [DOI] [PubMed] [Google Scholar]
- Pandian T.J. CRC Press; New York: 2013. Endocrine Sex Differentiation in Fish; p. 318. [Google Scholar]
- Palma P., Nocillado J., Superio J., de Jesus-Ayson E.G., Ayson F., Takemura A., Lu M.W., Elizur A. Induction of gonadal development in protogynous grouper with orally delivered FSH DNA. Mar. Biotechnol. 2019;21(5):697–706. doi: 10.1007/s10126-019-09914-w. [DOI] [PubMed] [Google Scholar]
- Peñaranda D.S., Gallego V., Rozenfeld C., Herranz-Jusdado J.G., Pérez L., Gómez A., Giménez I., Asturiano J.F. Using specific recombinant gonadotropins to induce spermatogenesis and spermiation in the European eel (Anguilla anguilla) Theriogenology. 2017;107:6–20. doi: 10.1016/j.theriogenology.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Penman D.J., Piferrer F. Fish gonadogenesis. Part I: Genetic and environmental mechanisms of sex determination. Rev. Fish. 2008;16(Suppl. 1):13–34. [Google Scholar]
- Peter R.E., Crim L.W. Reproductive endocrinology of fishes: Gonadal cycles and gonadotropin in teleosts. Ann. Rev. Physiol. 1979;41:323–335. doi: 10.1146/annurev.ph.41.030179.001543. [DOI] [PubMed] [Google Scholar]
- Peter R.E., Yu K.L. Neuroendocrine regulation of ovulation in fishes: basic and applied aspects. Rev. Fish Biol. Fish. 1997;7:173–197. [Google Scholar]
- Peter R.E., Lin H.R., Van Der Kraak G. Induced ovulation and spawning of culture freshwater fish in China: advances in application of GnRH analogues and dopamine antagonists. Aquaculture. 1988;74:1–10. [Google Scholar]
- Peter R.E., Crim L.W., Goos H.J., Crim J.W. Lesioning studies on the gravid female goldfish: neuroendocrine regulation of ovulation. Gen. Comp. Endocrinol. 1978;35:391–401. doi: 10.1016/0016-6480(78)90133-8. [DOI] [PubMed] [Google Scholar]
- Piferrer F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture. 2001;197:229–281. doi: 10.1016/S0044-8486(01)00589-0. [DOI] [Google Scholar]
- Powell J.F.F., Zohar Y., Elizur A., Park M., Fischer W.H., Craig A.G., Rivier J.F., Lovejoy D.A., Sherwood N.M. Three forms of gonadotropin-releasing hormone characterized from brains of one species. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12081–12085. doi: 10.1073/pnas.91.25.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S., Parhar I.S. Structural and functional divergence of gonadotropin-inhibitory hormone from jawless fish to mammals. Front. Endocrinol. 2014;5(177):1–17. doi: 10.3389/fendo.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo K., Nagahama Y. Structural and functional evolution of gonadotropin releasing hormone in vertebrates. Acta Physiol. 2008;193:3–15. doi: 10.1111/j.1748-1716.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- Okutsu T., Shikina S., Kanno M., Takeuchi Y., Yoshizaki G. Production of trout offspring from triploid salmon parents. Science. 2007;317(5844):1517. doi: 10.1126/science.1145626. [DOI] [PubMed] [Google Scholar]
- Omeljaniuk R.J., Shih S.H., Peter R.E. In vivo evaluation of dopamine receptor-mediated inhibition of gonadotropin secretion from the pituitary gland of the goldfish, Carassius auratus. J. Endocrinol. 1987;144:449–458. doi: 10.1677/joe.0.1140449. [DOI] [PubMed] [Google Scholar]
- Rajakumar A., Senthilkumaran B. Steroidogenesis and its regulation in teleost - a review. Fish Physiol. Biochem. 2020;46:803–818. doi: 10.1007/s10695-019-00752-0. [DOI] [PubMed] [Google Scholar]
- Reyes-Tomassini J.J., Wong T.T., Zohar Y. Seasonal expression of arginine vasotocin mRNA and its correlations to gonadal steroidogenic enzymes and sexually dimorphic coloration during sex reversal in the gilthead seabream (Sparus aurata) Fish Physiol. Biochem. 2017;43:823–832. doi: 10.1007/s10695-017-0338-3. [DOI] [PubMed] [Google Scholar]
- Roch G.J., Busby E.R., Sherwood N.M. GnRH receptors and peptides: skating backward. Gen. Comp. Endocrinol. 2014;209:118–134. doi: 10.1016/j.ygcen.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Roch G.J., Busby E.R., Sherwood N.M. Evolution of GnRH: Diving deeper. Gen. Comp. Endocrinol. 2011;171(1):1–16. doi: 10.1016/j.ygcen.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Rocha A., Zanuy S., Carrillo M., Gómez A. Seasonal changes in gonadal expression of gonadotropin receptors, steroidogenic acute regulatory protein and steroidogenic enzymes in the European sea bass. Gen. Comp. Endocrinol. 2009;162:265–275. doi: 10.1016/j.ygcen.2009.03.023. [DOI] [PubMed] [Google Scholar]
- Rosenfeld H., Mylonas C.C., Bridges C.R., Heinisch G., Correiro A., Vessallo-Aguis R., Medina A., Belamonte A., Garcia A., De-La-Gandara F., Fauvel C., De Metrio G., Meiri I., Gordin H., Zohar Y. GnRHa-mediated stimulation of the reproductive endocrine axis in captive Atlantic bluefin tuna, Thunnus thynnus. Gen. Comp. Endocrinol. 2012;175:55–64. doi: 10.1016/j.ygcen.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Sander J.D., Cade L., Khayter C., Reyon D., Peterson R.T., Joung J.K., Yeh J.-R.J. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R.W., de Franca L.R., Lareyre J.J., LeGac F., Chiarini-Garcia H., Nobrega R.H., Miura T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010;165:390–411. doi: 10.1016/j.ygcen.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Scott A.P., Canario A.V.M. 17α, 20β, dihydroxy-4-pregnen-3-one-20-sulfate: a new metabolite of the teleost maturation-inducing steroid. Gen. Comp. Endocrinol. 1992;85:91–100. doi: 10.1016/0016-6480(92)90176-k. [DOI] [PubMed] [Google Scholar]
- Scott A.P., Canario A.V.M. Status of oocyte maturation-inducing steroids in teleosts. Gen. Comp. Endocrinol. 1987;70:91–100. doi: 10.1016/0016-6480(92)90176-k. [DOI] [PubMed] [Google Scholar]
- Scott A.P., Sumpter J.P., Stacey N. The role of the maturation-inducing steroid, 17,20 beta-dihydroxypregn-4-en-3-one, in male fishes: a review. J. Fish Biol. 2010;76(1):183–224. doi: 10.1111/j.1095-8649.2009.02483.x. [DOI] [PubMed] [Google Scholar]
- Scott A.P., Witthames P.R., Vermeirssen E.L.M., Carolsfeld J. Prolonged-release gonadotrophin-releasing hormone analogue implants enhance oocyte final maturation and ovulation, and increase plasma concentrations of sulfated C21 steroids in North Sea plaice. J. Fish Biol. 1999;55:316–328. [Google Scholar]
- Sherwood N., Eiden L., Brownstein M., Spiess J., Rivier J., Vale W. Characterization of a teleost gonadotropin-releasing hormone. Proc. Natl. Acad. Sci. U.S.A. 1983;80:2794–2798. doi: 10.1073/pnas.80.9.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H., Yang L., Zhang Y., Liu X., Lin H., Li S. Identification and functional characterization of two Secretogranin II genes in orange-spotted grouper (Epinephelus coioides) Gen. Comp. Endocrinol. 2018;261:115–126. doi: 10.1016/j.ygcen.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Smedley M.A., Clokie B.G.J., Migaud H., Campbell P., Walton J., Hunter D., Corrigan D., Taylor J.F. Dietary phosphorous and protein supplementation enhances seawater growth and reduces severity of vertebral malformation in triploid Atlantic salmon (Salmo salar L.) Aquaculture. 2016;451:357–368. doi: 10.1016/j.aquaculture.2015.10.001. [DOI] [Google Scholar]
- Söffker M., Tyler C.R. Endocrine disrupting chemicals and sexual behaviors in fish – a critical review on effects and possible consequences. Crit. Rev. Toxicol. 2012;42(8):653–668. doi: 10.3109/10408444.2012.692114. [DOI] [PubMed] [Google Scholar]
- Sorensen, P.W., Stacey, N., 1999. Evolution and specialization of fish hormonal pheromones. In: Advances in Chemical Signals in Vertebrates (Johnston, R.E., Muller-Schwarze, D., Sorensen, P.W.). pp 15-47. Kluwer Academic / Plenum Publishers, New York. DOI: 10.1007/978-1-4615-4733-4_2.
- Spicer O.S., Zmora N., Wong T.T., Golan M., Levavi-Sivan B., Gothilf Y., Zohar Y. The GNIH (Lpxrfa) system's regulation of reproduction in the brain-pituitary axis of the zebrafish (Danio rerio) Biol. Reprod. 2017;96(5):1031–1042. doi: 10.1093/biolre/iox032. [DOI] [PubMed] [Google Scholar]
- Spicer O.S., Wong T.T., Zmora N., Zohar Y. Targeted mutagenesis of the hypophysiotropic Gnrh3 in zebrafish (Danio rerio) reveals no effects on reproductive performance. PLoS ONE. 2016;11(6) doi: 10.1371/journal.pone.0158141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey N. In: Fish Pheromones and Related Cues, John Wiley & Sons Inc. Sorensen P.W., Wisenden B.D., editors. Hoboken; NJ: 2014. Chapter 3 - Hormonally derived pheromones in teleost fishes; pp. 33–88. [Google Scholar]
- Stacey, N., 2010. Hormonally derived sex pheromones in fishes. In: Hormones and Reproduction in Vertebrates: Vol. 1, Fishes, Edition: 1, Chapter: 9, Publisher: Elsevier, Editors: D.O. Norris, K. H. Lopez, pp.169-192. DOI: 10.1016/B978-0-12-375009-9.10009-8.
- Stacey N.E., Sorensen P.W. In: Pfaff D.W., Arnold A.P., Etgen A.M., Fahrbach S.E., Rubin R.T., editors. Vol. 2. Academic Press; New York: 2002. Hormonal pheromones in fish; pp. 375–434. (Hormones, Brain and Behavior). [Google Scholar]
- Stacey N.E., Sorensen P. In: Hormones, Brain and Behavior. 2nd ed. Pfaff D.W., Arnold A.P., Etgen A.M., Fahrbach S.E., Rubin R.T., editors. Academic Press; New York: 2009. Hormonal pheromones in fish; pp. 639–681. [Google Scholar]
- Stacey N.E., Sorensen P.W. 17 α,20 β-dihydroxy-4-pregnen-3-one: a steroidal primer pheromone increasing milt volume in the goldfish Carassius auratus. Can. J. Zool. 1987;64(11):2412–2417. [Google Scholar]
- Steven C., Lehnen N., Kight K., Ijiri S., Harris W.A., Zohar Y. Molecular characterization of the GnRH system in zebrafish (Danio rerio): cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. Gen. Comp. Endocrinol. 2003;133:27–37. doi: 10.1016/s0016-6480(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Sumpter J. Feminized responses in fish to environmental estrogens. Toxicol. Lett. 1995;82–83:737–742. doi: 10.1016/0378-4274(95)03517-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Kawauchi H., Nagahama Y. Isolation and characterization of two distinct gonadotropins from chum salmon pituitary glands. Gen. Comp. Endocrinol. 1988;71:292–301. doi: 10.1016/0016-6480(88)90257-2. [DOI] [PubMed] [Google Scholar]
- Swanson P., Dickey J.T., Campbell B. Biochemistry and physiology of fish gonadotropins. Fish Physiol. Biochem. 2003;28:53–59. [Google Scholar]
- Swanson P., Suzuki K., Kawauchi H., Dickhoff W. Isolation and characterization of two coho salmon gonadotropins, GTH I and GTH II. Biol. Reprod. 1991;44:29–38. doi: 10.1095/biolreprod44.1.29. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Kanda S., Abe T., Oka Y. Evolution of the hypothalamic-pituitary-gonadal axis regulation in vertebrates revealed by knockout medaka. Endocrinology. 2016;157(10):3994–4002. doi: 10.1210/en.2016-1356. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Yoshizaki G., Takeuchi T. Biotechnology: surrogate broodstock produces salmonids. Nature. 2004;430:629–630. doi: 10.1038/430629a. [DOI] [PubMed] [Google Scholar]
- Taranger G.L., Carrillo M., Schulz R.W., Fontaine P., Zanuy S., Felip A., Weltzien F.-A., Dufour S., Karlsen Ø., Norberg B., Andersson E., Hansen T. Control of puberty in farmed fish. Gen. Comp. Endocrinol. 2010;165:483–515. doi: 10.1016/j.ygcen.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Taylor J., Waagbo R., Diez-Padrisa M., Campbell P., Walton J., Hunter D., Matthew C., Migaud H. Adult triploid Atlantic salmon (Salmo salar) have higher dietary histidine requirements to prevent cataract development in seawater. Aquac. Nutr. 2015;21(1):18–32. [Google Scholar]
- Taylor J., Sambraus F., Mota-Velasco J.C., Guy D.R., Hamilton A., Hunter D., Corrigan D., Migaud H. Ploidy and family effects on Atlantic salmon (Salmo salar) growth, deformity and harvest quality during a full commercial production cycle. Aquaculture. 2013;410–411:41–50. [Google Scholar]
- Thomas P., Pang Y., Dong J. Membrane androgen receptor characteristics of human ZIP9 (SLC39A) zinc transporter in prostate cancer cells: androgen-specific activation and involvement of an inhibitory G protein in zinc and MAP kinase signaling. Mol. Cell. Endocrinol. 2017;447:23–34. doi: 10.1016/j.mce.2017.02.025. [DOI] [PubMed] [Google Scholar]
- Thomas P. Rapid steroid hormone actions initiated at the cell surface and the receptors that mediate them with an emphasis on recent progress in fish models. Gen. Comp. Endocrinol. 2012;175(3):367–383. doi: 10.1016/j.ygcen.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgaard G.H. Ploidy manipulation and performance. Aquaculture. 1986;57:57–64. doi: 10.1016/0044-8486(86)90180-8. [DOI] [Google Scholar]
- Tokarz J., Möller G., Hrabě de Angelis M., Adamski J. Steroids in teleost fish: a functional point of view. Steroids. 2015;103:123–144. doi: 10.1016/j.steroids.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Tokumoto T., Tokumoto M., Thomas P. Interactions of diethylstilbestrol (DES) and DES analogs with membrane progestin receptor-alpha and the correlation with their nongenomic progestin activities. Endocrinology. 2007;148:3459–3467. doi: 10.1210/en.2006-1694. [DOI] [PubMed] [Google Scholar]
- Tokumoto T., Tokumoto M., Nagahama Y. Induction and inhibition of oocyte maturation by EDCs in zebrafish. Reprod. Biol. Endocrinol. 2005;3:69. doi: 10.1186/1477-7827-3-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau V.L. Neuroendocrine regulation of gonadotrophin II release and gonadal growth in the goldfish, Carassius auratus. Rev. Reprod. 1997;2:55–68. doi: 10.1530/ror.0.0020055. [DOI] [PubMed] [Google Scholar]
- Trudeau, V.L., 2018. Facing the challenges of neuropeptide gene knockouts: why do they not inhibit reproduction in adult teleost fish? Front. Neurosci. 12, 302. https://doi. org/10.3389/ fnins.2018.00302. [DOI] [PMC free article] [PubMed]
- Trudeau V.L., Somoza G.M. Multimodal hypothalamo-hypophysial communication in the vertebrates. Gen. Comp. Endocrinol. 2020;293 doi: 10.1016/j.ygcen.2020.113475. [DOI] [PubMed] [Google Scholar]
- Trudeau V.L., Martyniuk C.J., Zhao E., Hu H., Volkoff H., Decatur W.A., Basak A. Is secretoneurin a new hormone? Gen. Comp. Endocrinol. 2012;175:10–18. doi: 10.1016/j.ygcen.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Ueda H., Nagahama S., Nakamura S., Inada K., Okubo T., Furukawa N., Murakami R., Tsuchida S., Zohar Y., Konno K., Watanabe M. Involvement of hormones in olfactory imprinting and homing in chum salmon. Sci. Rep. 2016;6:21102. doi: 10.1038/srep21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umatani C., Oka Y. Multiple functions of non-hypophysiotropic gonadotropin releasing hormone neurons in vertebrates. Zoological Lett. 2019;22:5–23. doi: 10.1186/s40851-019-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kraak, G., Peter, R. E., Suzuki, K., Kawauchi, H. 1987. Immunological characteristics of two glycoprotein gonadotropins from the carp pituitary. In ‘Proceedings of the 3rd International Symposium of Reproductive Physiology in Fish” (Idler, D.R., Crim, L.W. Walsh, J.M. Eds.), pp. 78. Memorial University Press.
- Vermeirssen E.L.M., Scott A.P. Excretion of free and conjugated steroids in rainbow trout (Oncorhynchus mykiss): evidence for branchial excretion of the maturation-inducing steroid, 17,20β-Dihydroxy-4-pregnen-3-one. Gen. Comp. Endocrinol. 1996;101(2):180–194. doi: 10.1006/gcen.1996.0020. [DOI] [PubMed] [Google Scholar]
- Wargelius A. Application of genome editing in aquatic farm animals: Atlantic salmon. Transgenic Res. 2019;28(2):101–105. doi: 10.1007/s11248-019-00163-0. [DOI] [PubMed] [Google Scholar]
- Wargelius A., Leininger S., Skaftnesmo K.O., Kleppe L., Andersson E., Taranger G.L., Schulz R.W., Edvardsen R.B. Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci. Rep. 2016;6(1):1–8. doi: 10.1038/srep21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidinger G., Stebler J., Slanchev K., Dumstrei K., Wise C., Lovell-Badge R., Thisse C., Thisse B., Raz E. Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr. Biol. 2003;13(16):1429–1434. doi: 10.1016/s0960-9822(03)00537-2. [DOI] [PubMed] [Google Scholar]
- Weil C., Breton B., Sambroni E., Zmora N., Zohar Y. In vitro bioactivities of various forms of GnRH in relation to their resistance to degradation at the pituitary level in the rainbow trout, Oncorhynchus mykiss. Gen. Comp. Endocrinol. 1992;87:33–43. doi: 10.1016/0016-6480(92)90147-c. [DOI] [PubMed] [Google Scholar]
- Weil, C., Breton, B., Sambroni, E., Zmora, N., Zohar, Y., 1991. Bioactivity of various forms of GnRH in relation to their resistance to degradation at the pituitary level in the rainbow trout, Oncorhynchus mykiss. In: “Reproductive Physiology of Fish” (A.P. Scott, J.P. Sumpter, D.E. Kime and M.S. Rolfe eds). pp. 51-53. University of East Anglia Press.
- Whitlock K.E., Postlethwait J., Ewer J. Neuroendocrinology of reproduction: is gonadotropin-releasing hormone (GnRH) dispensable? Front. Neuroendocrinol. 2019;53 doi: 10.1016/j.yfrne.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, T.T., Zohar, Y., 2018. Reproductive technology: sex control and sterilization in fish. In: Encyclopedia of Reproduction, 2nd Ed, Elsevier. pp. 796-801. DOI: 10.1016/B978-0-12-809633-8.20633-X.
- Wong T.T., Zohar Y. Production of reproductively sterile fish: A mini-review of germ cell elimination technologies. Gen. Comp. Endocrinol. 2015;221:3–8. doi: 10.1016/j.ygcen.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Wong T.T., Zohar Y. Production of reproductively sterile fish by a non-transgenic silencing technology. Sci. Rep. 2015;5:15822. doi: 10.1038/srep15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.T., Collodi P. Inducible Sterilization of Zebrafish by Disruption of Primordial Germ Cell Migration. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0068455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.T., Zohar Y. Molecular biology of ovarian aromatase in sex reversal: cDNA and 5’-flanking region isolation and differential expression of ovarian aromatase in the gilthead seabream, Sparus aurata. Biol. Reprod. 2006;74:857–864. doi: 10.1095/biolreprod.105.045351. [DOI] [PubMed] [Google Scholar]
- Wu, G.C., Li, H.W., Tey, W.G., Lin, C.L., and Chang, C.F., 2017. Expression profile of amh/Amh during bi-directional sex change in the protogynous orange-spotted grouper Epinephelus coioides. PLoS ONE 12(10), e0185864. [DOI] [PMC free article] [PubMed]
- Wu, G.C., Li, H.W., Huang, C.H., Lin, H.J., Lin, C.J., Chang, C.F., 2016. The testis is a primary factor that contributes to epigenetic modifications in the ovaries of the protandrous black porgy, Acanthopagrus schlegelii. Biol. Reprod. 94(6), 132, 1-13. [DOI] [PubMed]
- Wu G.C., Trey W.G., Li H.W., Chang C.F. Sexual fate reprogramming in the steroid-induced bi-directional sex change in the protogynous orange-spotted grouper Epinephelus coioide. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron Z., Zohar Y. In: Recent Advances in Aquaculture. Muir J.M., Roberts R.J., editors. Blackwell Scientific Publications, London, Vol; IV: 1993. Applications of comparative endocrinology to fish culture, an overview; pp. 3–10. [Google Scholar]
- Yoshizaki G., Ichikawa M., Hayashi M., Iwasaki Y., Miwa M., Shikina S., Okutsu T. Sexual plasticity of ovarian germ cells in rainbow trout. Development. 2010;137:1227–1230. doi: 10.1242/dev.044982. [DOI] [PubMed] [Google Scholar]
- Yoshizaki G., Lee S. Production of live fish derived from frozen germ cells via germ cell transplantation. Stem Cell Res. 2018;29:103–110. doi: 10.1016/j.scr.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Yoshizaki G., Yazawa R. Application of surrogate broodstock technology in aquaculture. Fish. Sci. 2019;85:429–437. doi: 10.1007/s12562-019-01299-y. [DOI] [Google Scholar]
- Zhang H., Chen L., Zhang B., Lin Q. Molecular identification of GnIH and its potential role in reproductive physiology and male pregnancy of the lined seahorse (Hippocampus erectus) Gen. Comp. Endocrinol. 2019;281:196–202. doi: 10.1016/j.ygcen.2019.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhu B., Ge W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol. Endocrinol. 2015;29:76–98. doi: 10.1210/me.2014-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Lau S.W., Zhang L., Ge W. Disruption of zebrafish follicle-stimulating hormone receptor (fshr) but not luteinizing hormone receptor (lhcgr) gene by TALEN leads to failed follicle activation in females followed by sexual reversal to males. Endocrinology. 2015;156:3747–3762. doi: 10.1210/en.2015-1039. [DOI] [PubMed] [Google Scholar]
- Zhou L., Charkraborty T., Yu X., Wu L., Liu G., Mohapatra S., Wang D., Nagahama Y. R-spondins are involved in the ovarian differentiation in a teleost, medaka (Oryzias latipes) BMC Dev. Biol. 2012;12 doi: 10.1186/1471-213X-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Ge W. Genome editing in fishes and their applications. Gen. Comp. Endocrinol. 2018;257:3–12. doi: 10.1016/j.ygcen.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Zohar Y., Mylonas C.C., Rossenfeld H., De F., La, Gandara, Corriero A. In: Advances in Tuna Aquaculture. Benetti D., Partridge G.J., Buentello A., editors. Elsevier; 2016. Reproduction, broodstock management and spawning in captive Atlantic bluefin tuna; pp. 159–188. [Google Scholar]
- Zohar Y., Muñoz-Cueto J.A., Elizur A., Kah O. Neuroendocrinology of reproduction in teleost fish. Gen. Comp. Endocrinol. 2010;165:438–455. doi: 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Zohar Y., Elizur A., Sherwood N.M., Powell J.F., Rivier J.E., Zmora N. Gonadotropin-releasing activities of the three native forms of gonadotropin releasing hormone present in the brain of gilthead seabream, Sparus aurata. Gen. Comp. Endocrinol. 1995;97:289–299. doi: 10.1006/gcen.1995.1029. [DOI] [PubMed] [Google Scholar]
- Zohar Y., Goren A., Fridkin M., Elhanati E., Koch Y. Degradation of gonadotropin releasing hormone in the gilthead seabream, Sparus aurata: II. Cleavage of native salmon GnRH, mammalian LHRH and their analogs in the pituitary, kidney, and liver. Gen. Comp. Endocrinol. 1990;79:306–319. doi: 10.1016/0016-6480(90)90116-4. [DOI] [PubMed] [Google Scholar]
- Zohar Y., Goren A., Tosky M., Pagelson G., Leibovitz D., Koch Y. The bioactivity of gonadotropin-releasing hormones and its regulation in the gilthead seabream, Sparus aurata: in vivo and in vitro studies. Fish. Physiol. Biochem. 1989;7:59–67. doi: 10.1007/BF00004690. [DOI] [PubMed] [Google Scholar]
- Zohar Y., Tosky M., Pagelson G., Finkelman Y. Induction of spawning in the gilthead seabream, Sparus aurata, using [D-Ala6-Pro9NET]-LHRH: comparison with the use of HCG. Israeli J. Aquacul. 1989;41:105–113. [Google Scholar]
- Zohar Y., Billard R., Weil C. In: Aquaculture de Bar et des Sparides. Billard R., Barnabe G., editors. I.N.R.A. Press; Paris: 1984. La reproduction de la daurade et du bar: le cycle sexuel et l'induction de la ponte; pp. 3–24. [Google Scholar]
- Zohar Y., Abraham M., Gordin H. The gonadal cycle of the captivity reared hermaphroditic teleost, Sparus aurata (L.) during the first two years of life. Ann. Biol. Anim. Biochem. Biophys. 1978;18:877–882. [Google Scholar]
- Zohar Y., Mylonas C.C. Endocrine manipulations of spawning in farmed fish: from hormones to genes. Aquaculture. 2001;197:99–136. [Google Scholar]
- Zohar Y., Gordin H. Spawning kinetics in the gilthead sea bream, Spaurs aurata (L.) after low doses of human chorionic gonadotropin. J. Fish. Biol. 1979;15:665–670. [Google Scholar]
- Zohar, Y., Billard, R. 1978. New data on the possibilities of controlling reproduction in teleost fish by hormonal treatment. Actes Coll. C.N.E.X.O. 8, 111-123.
- Zohar Y. In: Fish Culture in Warm Water Systems: Problems and Trends. Shilo M., Sarig S., editors. CRC Press; Florida: 1989. Fish reproduction: its physiology and artificial manipulation; pp. 65–119. [Google Scholar]
- Zohar Y. Endocrinology and fish farming: aspects in reproduction, growth, and smoltification. Fish Physiol. Biochem. 1989;7:395–405. doi: 10.1007/BF00004734. [DOI] [PubMed] [Google Scholar]
- Zohar Y. In: Reproduction in Fish - Basic and Applied Aspects in Endocrinology and Genetics. Zohar Y., Breton B., editors. INRA Press; Paris: 1988. Gonadotropin releasing hormone in spawning induction in teleosts: basic and applied considerations; pp. 47–62. [Google Scholar]