Abstract
Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP) had been suggested as a possible glycobiomarker for assessing liver fibrosis. Here, we conducted this updated meta-analysis to systematically investigate the predictive accuracy of WFA+-M2BP for diagnosing liver fibrosis and hepatocellular carcinoma (HCC) by comparing with multiple non-invasive indicators. We searched relevant literatures from Pubmed, Web of Science, EMBASE and Cochrane Library and enrolled 36 eligible studies involving 7,362 patients. Summary results were calculated using bivariate random effects model. The pooled sensitivities, specificities and areas under the summary receiver operating characteristic curves (AUSROCs) of WFA+-M2BP for identifying mild fibrosis, significant fibrosis, advanced fibrosis, cirrhosis, and HCC were 0.70/0.68/0.75, 0.71/0.75/0.79, 0.75/0.76/0.82, 0.77/0.86/0.88, and 0.77/0.80/0.85, respectively. The accuracy of WFA+-M2BP was strongly affected by etiology and it was not better than other non-invasive indicators for predicting early fibrosis. It showed similar diagnostic performance to hyaluronic acid and FibroScan for cirrhosis, but was equivalent to α-fetoprotein for HCC. In conclusion, WFA+-M2BP was suitable to diagnose late stage of liver fibrosis, especially cirrhosis. Individual cutoff value of WFA+-M2BP could be used to grade liver fibrosis in different etiology. Combined diagnostic model was suggested to improve its predictive accuracy for HCC.
Subject terms: Diagnostic markers, Liver fibrosis, Tumour biomarkers
Introduction
Chronic liver diseases (CLDs) are major health problems that cause significant economic burdens worldwide1. Globally, CLDs affect 360 per 100,000 persons, cause more than 1.75 million deaths annually, and are ranked as the 12th leading cause of deaths1,2. A wide range of etiologies, which include viral hepatitis (hepatitis B and C), alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), autoimmune hepatitis (AIH), primary biliary cirrhosis (PBC), biliary atresia (BA), and other metabolic disorders, can cause CLDs3,4. In CLDs, liver fibrogenesis could be initiated due to dysfunctions of multiple cell types: (1) in diseases with bile ductular proliferation (i.e. PBC, BA), destruction of cholangiocytes leads to loss of bile ducts, resulting in cholestasis followed by hepatic damage and hepatic failure5,6; (2) in viral hepatitis (i.e. hepatitis B, hepatitis C), quiescent hepatic stellate cells (HSCs) can be activated by cytokines and chemokines secreted from infected hepatocytes or virus-exposed Kupffer cells, causing the deposition of extracellular matrix (ECM)7; (3) in NAFLD and NASH, excessive lipid accumulation promotes lipotoxicity, triggering hepatocytes death and inflammation8. Continuous destruction and regeneration of hepatocytes could generate distortions of normal hepatic architecture and replication-related mutations, bringing about liver cirrhosis and, even worse, hepatocellular carcinoma (HCC)9,10.
The severity of liver fibrosis is an important aspect for the management of patients with CLDs, both for predicting clinical outcomes and guiding therapies11,12. Liver biopsy remains the gold standard for the accurate assessment of fibrosis. However, its use is limited due to its invasiveness. Imaging technologies such as ultrasonography, magnetic resonance imaging, hepatic arteriography, and transient elastography had also been developed and widely used for diagnosis of liver fibrosis and HCC. For example, FibroScan is an imaging-based method that has been most studied13. In recent years, with the growing interest in the use of non-invasive methods for accurate assessment of fibrosis, serum biomarkers such as hyaluronic acid (HA), procollagen III, platelet count (PLT), Fibrotest, aspartate aminotransferase-to-platelet ratio index (APRI), and fibrosis-4 index (FIB-4) had been discussed2,14.
More recently, the possible use of Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP) as a novel non-invasive serum biomarker to predict disease severity of CLDs had also been suggested. Mac-2-binding protein (M2BP) is a secretory glycoprotein which contains seven N-glycans per monomer15. In the serum, 10–16 monomers of M2BP form a “doughnut-shaped” polymer which presents 70–112 N-glycans16. It has been found that alterations of M2BP happen during the progression of liver disease and fibrosis due to the changes in N-glycosylation (i.e. sialylation or extension of polylactosamine)16, however, the underlying mechanism is unclear. As a robust lectin that binds the GalNAc residue of N-glycans and O-glycans and the clustered LacNAc structure, Wisteria floribunda agglutinin (WFA) can recognize the altered N-glycans of M2BP specifically17. Thus, this specific glycoprotein was described as WFA+-M2BP and renamed as M2BPGi (Mac-2-binding protein glycosylation isomer) after the commercialization of the diagnostic reagent. In liver, WFA+-M2BP could promote fibrogenesis by acting as an important messenger between HSCs (secrete WFA+-M2BP) and Kupffer cells (express the ligand of M2BP, Mac-2)18,19. Additionally, WFA+-M2BP could be increased by TGF-β1 in LX-2 cell and it correlates with serum IP-10 and sICAM-1 levels in patients with AIH20,21. Those evidences suggested serum WFA+-M2BP has a great potential to serve as a biomarker for reflecting the liver status of CLD patients 16,22.
The clinical application of WFA+-M2BP has been widely promoted after Japanese public health insurance supported its diagnosis expense since 201515. Increased publications have been carried out to estimate the clinical performance of WFA+-M2BP. One meta-analysis reported the possible utility of WFA+-M2BP for liver fibrosis staging caused by various etiologies23, however, it did not compare the predictive accuracy of WFA+-M2BP with multiple widely used non-invasive biomarkers. To systematically examine the performance of WFA+-M2BP for diagnosing liver fibrosis and HCC, we conducted an updated meta-analysis by including more literatures and more evaluation parameters. Our results indicated that WFA+-M2BP could be a satisfactory biomarker for staging cirrhosis, and its combined use with AFP may further improve its predictive accuracy for HCC. By elaborating on the advantages and limitations of its diagnostic accuracy in liver fibrosis and HCC, we hope our study could help clinicians make cautious and accurate diagnosis.
Results
Basic characteristics of studies
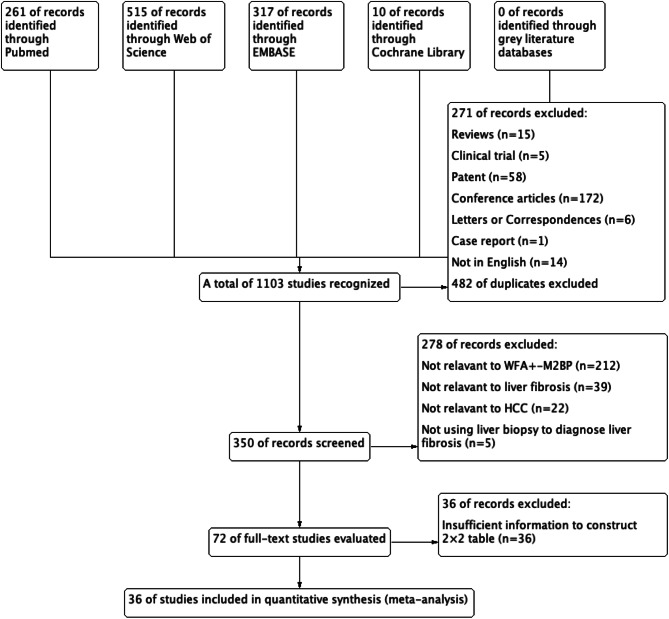
As shown in Fig. 1, after excluding duplicates and non-experimental studies, 350 references were identified. Full-text review on 72 original articles eligible for detailed evaluation were conducted after we excluded non-relevant references. A total of 36 articles were further removed because of insufficient information to construct a 2 × 2 table. Ultimately, the remaining 36 articles were selected for meta-analysis.
Figure 1.
The flow chart of the meta-analysis conducted.
We listed the main features of the included studies in Table 1 and 2. Overall, 7,362 participants were included. Among the 36 included articles, 29 articles studied the diagnostic accuracy of WFA+-M2BP on liver fibrosis and 8 articles were on HCC. For the studies on fibrosis, we noticed that 3 articles enrolled both training group and validation group24–26, and 2 articles recruited patients with 2 different etiologies27,28. Thus, we considered them as individual studies when the calculation of diagnostic accuracy was conducted. Overall, 7 kinds of etiologies of liver fibrosis that include HBV (n = 12), HCV (n = 10), NAFLD (n = 3), NASH (n = 3), AIH (n = 1), BA (n = 2), and PBC (n = 2), as well as mixed etiologies (n = 1) were discussed. HCC was mainly caused by 3 etiologies here: HBV, HCV and NAFLD. All studies employed retrospective design and used lectin-Ab sandwich immunoassay to detect serum WFA+-M2BP levels.
Table 1.
Characteristics of the included studies on liver fibrosis.
| Study | Region | Number of patients | Diagnostic indicators | Median age (year) | Male % | Etiology | Histological system | Blind | Liver biopsy length (mm) | Interval between biopsy and blood test | Fibrosis (0-1/2/3/4) | WFA+-M2BP optimal cutoff values (COI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kuno 201316 | Japan | 160 | WFA+-M2BP & FIB-4 & HA | 54.9 | 26.9 | HCV | Unclear | Yes | Unclear | Unclear | 66/41/33/20 | NA |
| Abe 201529 | Japan | 289 | WFA+-M2BP & APRI & AST/ALT & FIB-4 & HA & PLT | 54.8 | 55.0 | NAFLD | Brunt | Yes | > 15 | Same day or within 2 months | 148/49/41/51 | ≥ F1: 0.59; ≥ F2: 0.90; ≥ F3: 0.94; F4: 1.46 |
| Toshima 201522 | Japan | 200 | WFA+-M2BP & APRI & HA | 64.0 | 67.5 | Mixed (HBV, HCV, alcoholism, and non-infection) | METAVIR | Yes | Unclear | Same day | 129/21/16/34 | ≥ F1: 1.00; ≥ F2: 1.86; ≥ F3: 2.21; F4: 2.64 |
| Umemura 201530 | Japan | 137 | WFA+-M2BP & APRI & AST/ALT & FIB-4 | 57.0 | 19.0 | PBC | METAVIR | Yes | > 15 | Same day | 81/27/18/11 | ≥ F1: 0.70; ≥ F2: 1.00; ≥ F3: 1.40; F4: 2.00 |
| Heo 201631 | South Korea | 95 | WFA+-M2BP | 51.0 | 72.6 | HBV | Batts and Ludwig | Yes | Unclear | Same day | 16/29/10/40 | ≥ F2: 0.80; ≥ F3: 1.60; F4: 2.00 |
| Ichikawa 201632 | Japan | 112 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 47.0 | 64.3 | HBV | Revised Inuyama | Yes | > 15 | Same day | 40/26/24/22 | ≥ F2: 0.94; ≥ F3: 1.26; F4: 1.26 |
| Ishii 201633 | Japan | 189 | WFA+-M2BP & APRI & AST/ALT& FIB-4 & HA & PLT | 44.0 | 62.4 | HBV | METAVIR | Unclear | Unclear | Unclear | 108/37/28/16 | ≥ F2: 1.40; ≥ F3: 1.40; F4: 1.90 |
| Nishikawa_a 20162 | Japan | 84 | WFA+-M2BP & APRI & AST/ALT & FIB-4 & HA & PLT | 64.0 | 17.9 | AIH | METAVIR | No | Unclear | Unclear | 18/24/24/18 | ≥ F3: 3.70; F4: 3.90 |
| Nishikawa_b 201634 | Japan | 57 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 59.0 | 14.0 | PBC | METAVIR | Unclear | Unclear | Unclear | 24/17/11/5 | ≥ F3: 3.40; F4: 3.70 |
| Nishikawa_c HBV 201627 | Japan | 249 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 45.6 | 62.2 | HBV | METAVIR | Unclear | Unclear | Unclear | 138/51/41/19 | ≥ F2: 1.37; ≥ F3: 1.42; F4: 1.86 |
| Nishikawa_c HCV 201627 | Japan | 386 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 60.9 | 46.6 | HCV | METAVIR | Unclear | Unclear | Unclear | 111/63/90/122 | ≥ F2: 2.42; ≥ F3: 2.03; F4: 2.42 |
| Nishikawa_d 201635 | Japan | 134 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 51.7 | 48.5 | NASH | Brunt | Unclear | Unclear | Unclear | 28/68/25/13 | ≥ F2: 1.00; ≥ F3: 1.10; F4: 1.60 |
| Nishikawa_e Tr 201624 | Japan | 125 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 45.9 | 59.2 | HBV | METAVIR | Unclear | Unclear | Unclear | 73/27/14/11 | ≥ F3: 1.42 |
| Nishikawa_e Va 201624 | Japan | 124 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 45.3 | 65.3 | HBV | METAVIR | Unclear | Unclear | Unclear | 65/24/27/8 | ≥ F3: 1.42 |
| Nishikawa_f Tr 201625 | Japan | 210 | WFA+-M2BP & APRI & AST/ALT & FIB-4 & HA & PLT | 59.9 | 49.0 | HCV | Unclear | Unclear | > 15 | Unclear | 70/34/46/60 | ≥ F3: 1.82 |
| Nishikawa_f Va 201625 | Japan | 176 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | 62.2 | 43.8 | HCV | Unclear | Unclear | > 15 | Unclear | 41/29/44/62 | ≥ F3: 1.82 |
| Shigefuku_NAFLD 201628 | Japan | 58 | WFA+-M2BP & APRI & AST/ALT & FIB-4 & HA & PLT | NA | NA | NAFLD | Brunt | Yes | > 15 | Same day | NA | ≥ F3: 1.06 |
| Shigefuku_HCV 201628 | Japan | 72 | WFA+-M2BP & APRI & AST/ALT & FIB-4 & HA & PLT | NA | NA | HCV | Desmet | Yes | > 15 | Same day | NA | ≥ F3: 3.28 |
| Ura 201636 | Japan | 146 | WFA+-M2BP | NA | 44.5 | HCV | METAVIR | Unclear | Unclear | Unclear | 91/18/19/18 | ≥ F2: 2.14; F3: 2.17 |
| Yamada 201637 | Japan | 64 | WFA+-M2BP & APRI & HA | 1.1 | 25.0 | BA | METAVIR | Unclear | Unclear | Same day | 1/1/11/51 | F4: 3.53 |
| Zou Tr 201626 | China | 221 | WFA+-M2BP & APRI & AST/ALT & FIB-4 | 38.0 | 68.3 | HBV | METAVIR | Unclear | Unclear | Same day | 132/42/23/24 | ≥ F2: 1.06 |
| Zou Va 201626 | China | 76 | WFA+-M2BP | 37.0 | 61.8 | HBV | METAVIR | Unclear | Unclear | Same day | 39/17/10/10 | ≥ F2: 1.06 |
| Huang 201738 | Taiwan | 229 | WFA+-M2BP | 52.8 | 52.8 | HCV | METAVIR | Yes | > 15 | Unclear | 85/56/38/50 | ≥ F1:1.42; ≥ F2:1.61; ≥ F3:1.42; F4:2.67 |
| Lai 201739 | Malaysia | 220 | WFA+-M2BP | 50.1 | 51.8 | NASH | Unclear | Yes | Unclear | Same day | 161/16/36/7 | ≥ F1:0.57; ≥ F2:0.66; ≥ F3:0.69; F4:0.7 |
| Noguchi 201740 | Japan | 70 | WFA+-M2BP & APRI & FIB-4 & PLT | 48.6 | 52.87 | HBV | METAVIR | Unclear | Unclear | Same day | 34/17/13/6 | ≥ F2:0.81; ≥ F3: 0.82 |
| Fujita 201841 | Japan | 122 | WFA+-M2BP & APRI & FIB-4 | 53 | 67.2 | HCV | METAVIR | Unclear | Unclear | Unclear | 27/66/20/9 | ≥ F3: 2.19 |
| Jekarl 201842 | South Korea | 151 | WFA+-M2BP & APRI & FIB-4 & FibroScan | 44.6 | 66.9 | HBV | Knodell | Unclear | > 15 | Same day | 8/86/42/15 | ≥ F3:0.76; F4: 0.71 |
| Mak 201843 | China | 327 | WFA+-M2BP | 38.1 | 70.0 | HBV | Ishak | Yes | > 10 | Within 90 days | 292/206/50/6 | ≥ F2:0.25; ≥ F3:0.45; F4:0.96 |
| Matsuura 201844 | Japan | 84 | WFA+-M2BP & APRI & FIB-4 & FibroScan | 63 | 57.1 | HCV | METAVIR | Unclear | Unclear | Unclear | 20/20/19/25 | ≥ F4: 2.66 |
| Ogawa 201845 | Japan | 165 | WFA+-M2BP & APRI & AST/ALT & FIB-4 & FibroScan | 54.2 | 58.2 | NAFLD | Brunt | Unclear | > 15 | Same day or within 2 months | 83/24/47/11 | ≥ F2: 0.83 |
| Ueno 201846 | Japan | 37 | WFA+-M2BP & APRI & AST/ALT & FIB-4 & PLT | 18 | 32.4 | BA | METAVIR | Unclear | Unclear | Unclear | 16/6/4/11 | ≥ F2:1.59; ≥ F3:1.67; F4:1.84 |
| Kanno 201947 | Japan | 85 | WFA+-M2BP & APRI & FIB-4 & HA & PLT | NA | 47.1 | NASH | Brunt | Unclear | Unclear | Within 1 month | 12/14/31/28 | ≥ F4: 3.11 |
| Yeh 201948 | Taiwan | 160 | WFA+-M2BP | 40 | 76.9 | HBV | METAVIR | Unclear | Unclear | Same day | 72/37/25/26 | ≥ F1:0.96; ≥ F2:1.345; ≥ F3:1.535; F4:1.665 |
| Nagata 201649 | Japan | 108 | WFA+-M2BP & APRI & FIB-4 & HA | NA | NA | HCV | Desmet | Unclear | Unclear | Unclear | 13/49/25/21 (F0/1/2/3–4) | ≥ F3: 2.2 |
WFA+-M2BP, wisteria floribunda agglutinin-positive Mac-2-binding protein; APRI, Aspartate aminotransferase-to-platelet ratio index; FIB-4, Fibrosis-4 index; AST/ALT, AST to ALT ratio; HA, hyaluronic acid; PLT, platelet count; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; AIH, autoimmune hepatitis; PBC, primary biliary cirrhosis; BA, biliary atresia; COI, cutoff index; CI, confidence interval; PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio; AUSROC, area under the summary receiver operating characteristic curve; Tr, training group; Va, validation group; NA, not available.
Table 2.
Characteristics of the included studies on HCC.
| Study | Region | Number of all participates | Number of HCC patients | Diagnostic indicators | Median age (year) | Male % | Etiology | WFA+-M2BP optimal cutoff values (COI) |
|---|---|---|---|---|---|---|---|---|
| Nagata 201649 | Japan | 119 | 8 | WFA+-M2BP & APRI & FIB-4 & AFP | NA | 58.8 | HCV | 2.4 |
| Cheung 201750 | China | 114 | 57 | WFA+-M2BP | NA | NA | HBV | 0.69 |
| Chuaypen 201851 | Japan | 30 | 150 | WFA+-M2BP & AFP | NA | 75.7 | HBV | 2.4 |
| Kawanaka 201852 | Japan | 331 | 51 | WFA+-M2BP | NA | 51.4 | NAFLD | 1.255 |
| Lin 201853 | Taiwan | 921 | 122 | WFA+-M2BP & AFP | NA | NA | HCV | 1.5 |
| Kim 201954 | South Korea | 170 | 64 | WFA+-M2BP | 55 | 77.6 | HBV | 2.14 |
| Mak_a 201955 | China | 207 | 14 | WFA+-M2BP | 40 | 57.0 | HBV | 0.685 |
| Mak_b 201956 | China | 78 | 39 | WFA+-M2BP | NA | NA | HBV | 1.15 |
HCC, hepatocellular carcinoma; WFA+-M2BP, wisteria floribunda agglutinin-positive Mac-2-binding protein; APRI, Aspartate aminotransferase-to-platelet ratio index; FIB-4, Fibrosis-4 index; AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; COI, cutoff index, NA, not available.
Quality assessment
On the basis of QUADAS-2 assessment, the overall quality of included studies was moderate. As shown in Supplementary Figs. 1, 2, in terms of patient selection, 13 studies had high risk of bias because of inappropriate exclusions or case–control designs. A total of 29 studies had high risk of bias in index test because of the awareness of reference standard result before conducting the index test. Five studies did not mention the use of blind method for index tests when explaining the reference standard results. Regarding flow and timing, 25 studies had high or unclear risk of bias because not all patients received the same reference standard or due to unclear interval between index test and reference standard. Moreover, we had significant concerns on 7 studies when evaluate the applicability of their patient selections.
Pooled predictive accuracy of WFA+-M2BP in liver fibrosis
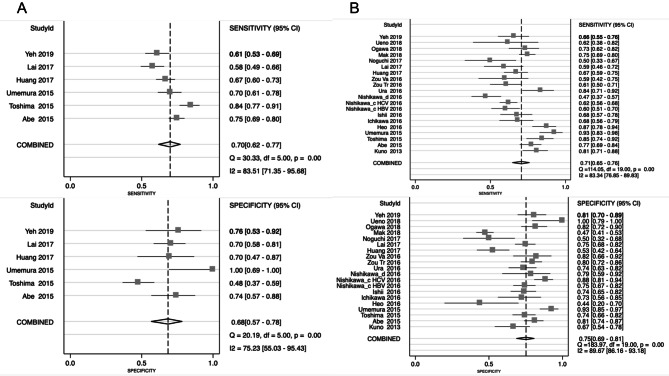
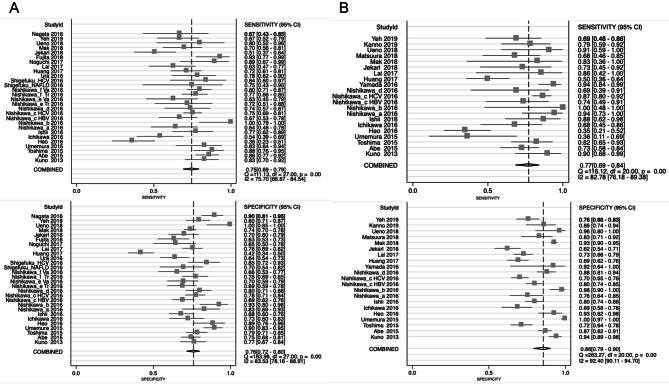
Here, we summarized the predictive accuracy of WFA+-M2BP in each liver fibrosis. A total of 6 studies with 1,235 patients were evaluated for the performance of WFA+-M2BP on predicting mild fibrosis. The pooled sensitivity and specificity were 0.70 (95% CI 0.62–0.77) and 0.68 (95% CI 0.57–0.78), respectively (Fig. 2A). Besides, the pooled AUSROC was 0.75 (95% CI 0.71–0.78). Twenty studies with 3,602 patients were included in significant fibrosis. The pooled sensitivity, specificity and AUSROC were 0.71 (95% CI 0.65–0.76), 0.75 (95% CI 0.69–0.81), and 0.79 (95% CI 0.75–0.82), respectively (Fig. 2B). For predicting advanced fibrosis, 28 studies involving 4,427 patients were assessed. The pooled sensitivity, specificity and AUSROC were 0.75 (95% CI 0.69–0.79), 0.76 (95% CI 0.72–0.80), and 0.82 (95% CI 0.78–0.85), respectively (Fig. 3A). For cirrhosis, 21 studies with 3,449 patients were identified. As displayed in Fig. 3B, the pooled sensitivity and specificity were 0.77 (95% CI 0.69–0.84) and 0.86 (95% CI 0.79–0.90), respectively. The pooled AUSROC was 0.88 (95% CI 0.85–0.91). Those pooled results demonstrated that the predictive accuracy of WFA+-M2BP greatly increased with the progression of liver fibrosis. Its level could nicely reflect the presence of late fibrosis especially cirrhosis. High AUSROC indicated WFA+-M2BP could be applied as an alternative biomarker for biopsy when diagnosing cirrhosis.
Figure 2.
Forest plots of sensitivity and specificity of WFA+-M2BP for the diagnosis of mild fibrosis (A) and significant fibrosis (B).
Figure 3.
Forest plots of sensitivity and specificity of WFA+-M2BP for the prediction of advanced fibrosis (A) and cirrhosis (B).
Heterogeneity analysis, threshold effect and meta-regression
To investigate the heterogeneities of the included studies, threshold effect and overall heterogeneity were analyzed. Significant heterogeneities existed in each stage of liver fibrosis (Q = 23.11, I2 = 91%, P < 0.001; Q = 94.75, I2 = 98%, P < 0.001; Q = 50.32, I2 = 96%, P < 0.001; Q = 64.79, I2 = 97%, P < 0.001). However, no significant threshold effect was found. In four liver fibrosis stages from mild fibrosis to cirrhosis, the spearman correlation coefficients between sensitivities and specificities were − 0.94 (P = 0.88), − 0.01 (P < 0.01), − 0.02 (P < 0.01), and − 0.16 (P = 0.03), respectively.
Meta-regression analysis (at least 10 studies were requested) was performed to further discuss the cause of heterogeneity in the studies reported for significant fibrosis, advanced fibrosis, and cirrhosis. We investigated 10 factors that might be the potential sources of heterogeneity: year of publication, region, median age, male proportion, number of patients, etiology, histological system, liver biopsy length, interval between biopsy and blood test, and blind method. For identifying significant fibrosis, the accuracy of WFA+-M2BP could be influenced by age, male proportion, etiology, and blind method (P < 0.01, P = 0.07, P = 0.05, and P < 0.01, respectively). For advanced fibrosis, the performance of WFA+-M2BP could be affected by age, male proportion, etiology, and region (P < 0.01, P < 0.01, P = 0.01, and P < 0.01, respectively). For cirrhosis, the heterogeneity of WFA+-M2BP for the detection might be due to the heterogeneity of age, male proportion, region, etiology, and blind method (P < 0.01, P < 0.01, P = 0.02, P = 0.07, and P = 0.08, respectively).
Predictive accuracy of WFA+-M2BP in liver fibrosis stratified by etiology
As etiology was one of the main reasons of heterogeneities based on our meta-regression analysis, we further analyzed the predictive accuracy of WFA+-M2BP in liver fibrosis caused by various etiologies. We combined studies related to NAFLD and NASH together, and combined studies related to AIH, BA, PBC and mixed etiologies into the “other etiologies” category because of limited number of references. Intriguingly, WFA+-M2BP showed diverse diagnostic accuracies in different etiology groups (Table 3). In general, for the prediction of significant fibrosis, advanced fibrosis, and cirrhosis, WFA+-M2BP owned the best diagnostic accuracies among patients with AIH, BA, PBC or mixed etiologies by reaching the highest pooled sensitivity, specificity, PLR, DOR, AUSROC and lowest NLR, when the results were compared with patients in other etiology groups. Besides, for advanced fibrosis, heterogeneities dramatically dropped in different etiology groups except for HBV and HCV. And for cirrhosis, no heterogeneity was found in the subgroup of NAFLD or NASH (Q = 3.11, I2 = 36%, P = 0.106), indicating the accuracy of WFA+-M2BP was influenced by the etiology of disease. In Table 3, different weighted mean WFA+-M2BP values were observed in different etiologies, suggesting individual cutoff value of WFA+-M2BP should be applied to grade liver fibrosis in each etiology. In addition, we noticed that compared with significant fibrosis and advanced fibrosis, WFA+-M2BP possessed the highest AUSROCs in diagnosing cirrhosis regardless of the etiology.
Table 3.
Overview of meta-analyses results for liver fibrosis stratified by etiology.
| Fibrosis stages | Number of studies | Number of patients | Weighted Mean WFA+-M2BP value (COI) | Overall Heterogeneity | Pooled sensitivity (95% CI) | Pooled specificity (95% CI) | Pooled PLR (95% CI) | Pooled NLR (95% CI) | Pooled DOR (95% CI) | Pooled AUSROC (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Q value, P value | I2 (%) | ||||||||||
| HBV | |||||||||||
| Significant fibrosis | 9 | 1,499 | 0.97 | 60.36, < 0.001 | 97 | 0.67 (0.61–0.73) | 0.68 (0.58–0.77) | 2.1 (1.6–2.7) | 0.48 (0.43–0.53) | 4 (3–6) | 0.72 (0.68–0.76) |
| Advanced fibrosis | 10 | 1,602 | 1.14 | 14.41, < 0.001 | 86 | 0.65 (0.55–0.73) | 0.73 (0.69–0.76) | 2.4 (2.1–2.7) | 0.49 (0.39–0.61) | 5 (4–7) | 0.75 (0.71–0.79) |
| Cirrhosis | 7 | 1,283 | 1.43 | 19.75, < 0.001 | 90 | 0.67 (0.52–0.79) | 0.82 (0.72–0.89) | 3.7 (2.5–5.4) | 0.40 (0.28–0.58) | 9 (5–16) | 0.81 (0.77–0.84) |
| HCV | |||||||||||
| Significant fibrosis | 4 | 921 | 2.12 | 25.71, < 0.001 | 92 | 0.73 (0.63–0.81) | 0.72 (0.57–0.84) | 2.6 (1.6–4.2) | 0.37 (0.27–0.52) | 7 (4–14) | 0.79 (0.75–0.82) |
| Advanced fibrosis | 9 | 1,609 | 1.98 | 20.67, < 0.001 | 90 | 0.78 (0.74–0.81) | 0.73 (0.63–0.81) | 2.8 (2.0–4.0) | 0.31 (0.25–0.38) | 9 (6–15) | 0.78 (0.74–0.82) |
| Cirrhosis | 4 | 859 | 2.53 | 13.20, 0.001 | 85 | 0.78 (0.57–0.90) | 0.81 (0.67–0.91) | 4.2 (1.9–9.0) | 0.28 (0.12–0.63) | 15 (3–68) | 0.87 (0.83–0.89) |
| NAFLD or NASH | |||||||||||
| Significant fibrosis | 4 | 808 | 0.84 | 4.12, 0.064 | 51 | 0.65 (0.52–0.76) | 0.78 (0.73–0.83) | 3.0 (2.1–4.3) | 0.45 (0.30–0.65) | 7 (3–14) | 0.80 (0.76–0.83) |
| Advanced fibrosis | 4 | 701 | 0.90 | 2.88, 0.119 | 30 | 0.76 (0.65–0.85) | 0.76 (0.72–0.79) | 3.1 (2.6–3.8) | 0.32 (0.21–0.48) | 10 (6–17) | 0.77 (0.73–0.81) |
| Cirrhosis | 4 | 728 | 1.45 | 3.11, 0.106 | 36 | 0.76 (0.64–0.85) | 0.84 (0.77–0.89) | 4.7 (3.3–6.8) | 0.28 (0.19–0.43) | 17 (9–30) | 0.85 (0.82–0.88) |
| Other etiologiesa | |||||||||||
| Significant fibrosis | 3 | 374 | 1.52 | 10.47, 0.003 | 81 | 0.83 (0.72–0.90) | 0.92 (0.75–0.98) | 10.8 (3.1–37.3) | 0.18 (0.11–0.31) | 58 (16–207) | 0.93 (0.90–0.95) |
| Advanced fibrosis | 5 | 515 | 2.33 | 2.95, 0.114 | 32 | 0.84 (0.70–0.92) | 0.88 (0.80–0.94) | 7.2 (3.9–13.2) | 0.18 (0.09–0.36) | 40 (13–121) | 0.93 (0.90–0.95) |
| Cirrhosis | 5 | 579 | 2.82 | 33.65, < 0.001 | 94 | 0.86 (0.67–0.95) | 0.95 (0.77–0.99) | 18.0 (3.5–93.5) | 0.15 (0.06–0.37) | 123 (25–610) | 0.95 (0.93–0.97) |
HBV, hepatitis B virus; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; AIH, autoimmune hepatitis; PBC, primary biliary cirrhosis; BA, biliary atresia; COI, cutoff index; CI, confidence interval; PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio; AUSROC, area under the summary receiver operating characteristic curve.
aOther etiologies include AIH, BA, PBC and mixed etiologies.
Predictive accuracy of WFA+-M2BP versus non-invasive indicators for grading liver fibrosis
Due to limited number of studies containing the information of other non-invasive indicators in mild fibrosis, we compared WFA+-M2BP with other non-invasive indicators for predicting significant fibrosis, advanced fibrosis and cirrhosis. As shown in Table 4, for significant fibrosis, the AUSROC of WFA+-M2BP (0.79) was only greater than that of AST/ALT (0.74, P = 0.048). For the detection of advanced fibrosis, WFA+-M2BP yielded AUSROC (0.82) similar to those of APRI (0.78, P = 0.113), FIB-4 (0.79, P = 0.235), HA (0.82, P = 1.0), and FibroScan (0.81, P = 0.831). Greater AUSROC of WFA+-M2BP was only observed when it was compared with AST/ALT (0.67, P < 0.001) and PLT (0.69, P < 0.001). However, when determining cirrhosis, WFA+-M2BP surpassed 4 indicators (WFA+-M2BP = 0.88; APRI = 0.79, P < 0.001; FIB-4 = 0.83, P = 0.034; AST/ALT = 0.79, P < 0.001, PLT = 0.83, P = 0.021) except for HA and FibroScan (HA = 0.88, P = 1.0; FibroScan = 0.87, P = 0.644). Those results indicated that WFA+-M2BP owned the best performance for diagnosing cirrhosis by exceeding most of the widely used indicators.
Table 4.
AUSROC values of seven non-invasive indicators for predicting significant fibrosis, advanced fibrosis and cirrhosis.
| Indicators | Significant fibrosis | Advanced fibrosis | Cirrhosis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Number of patients | AUSROC (95% CI) | Z value | P value | Number of studies | Number of patients | AUSROC (95% CI) | Z value | P value | Number of studies | Number of patients | AUSROC (95% CI) | Z value | P value | |
| WFA+-M2BP | 20 | 3,602 | 0.79 (0.75–0.82) | 28 | 4,427 | 0.82 (0.78–0.85) | 21 | 3,449 | 0.88 (0.85–0.91) | ||||||
| APRI | 9 | 1,563 | 0.77 (0.74–0.81) | 0.79 | 0.428 | 18 | 2,552 | 0.78 (0.74–0.81) | 1.58 | 0.113 | 11 | 1,720 | 0.79 (0.75–0.82) | 3.83 | < 0.001 |
| FIB-4 | 10 | 1,723 | 0.76 (0.72–0.79) | 1.19 | 0.235 | 18 | 2,512 | 0.79 (0.75–0.82) | 1.19 | 0.235 | 11 | 1,616 | 0.83 (0.79–0.86) | 2.13 | 0.034 |
| AST/ALT | 5 | 746 | 0.74 (0.70–0.77) | 1.98 | 0.048 | 8 | 960 | 0.67 (0.63–0.71) | 5.53 | < 0.001 | 4 | 444 | 0.79 (0.75–0.82) | 3.83 | < 0.001 |
| HA | 6 | 1,230 | 0.83 (0.79–0.86) | 1.58 | 0.113 | 15 | 2,332 | 0.82 (0.79–0.85) | 0.00 | 1.000 | 10 | 1,608 | 0.88 (0.85–0.91) | 0.00 | 1.000 |
| PLT | 7 | 1,177 | 0.73 (0.68–0.76) | 0.50 | 0.619 | 14 | 1,971 | 0.69 (0.65–0.73) | 5.53 | < 0.001 | 8 | 1,221 | 0.83 (0.80–0.86) | 2.31 | 0.021 |
| FibroScan | 1 | 165 | 0.83 (0.77–0.89) | 1.13 | 0.259 | 1 | 151 | 0.81 (0.71–0.88) | 0.21 | 0.831 | 2 | 235 | 0.87 (0.84–0.90) | 0.46 | 0.644 |
WFA+-M2BP, wisteria floribunda agglutinin-positive Mac-2-binding protein; APRI, Aspartate aminotransferase-to-platelet ratio index; FIB-4, Fibrosis-4 index; AST/ALT, AST to ALT ratio; HA, hyaluronic acid; PLT, platelet count; AUSROC, area under the summary receiver operating characteristic curve; CI, confidence interval.
Diagnostic accuracy of WFA+-M2BP for the prediction of HCC
For the prediction of HCC, a total of 8 studies with 2,240 participants were selected (Table 2). Among them, 4 studies reported the occurrence of HCC after antiviral treatment or HBeAg seroconversion49,50,55,56, one study discussed the reoccurrence of HCC after curative resection54, and 3 studies focused on the development of HCC51–53. The WFA+-M2BP levels here were pretreatment or basal levels. As several studies described the diagnostic information of APRI, FIB-4, and AFP, we compared the diagnostic accuracies of WFA+-M2BP with these three indicators for HCC. There was no significant threshold effect in included studies (r = − 0.7, P = 0.49). However, significant heterogeneity was observed (Table 5). In addition, among all the indicators, WFA+-M2BP yielded the highest pooled sensitivity (0.77, 95% CI 0.60–0.89) which surpassed APRI, FIB-4 and AFP. Although AFP had the highest pooled specificity (0.94, 95% CI 0.82–0.98), the AUSROCs of WFA+-M2BP and AFP were very similar (P = 0.671).
Table 5.
Meta-analyses results of four non-invasive markers for diagnosing HCC.
| Indicators | Number of studies | Number of patients | Heterogeneity | Sensitivity (95% CI) | Specificity (95% CI) | Weighted Mean cutoff | AUSROC (95% CI) | Z value | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Q value, P value | I2 (%) | |||||||||
| WFA+-M2BP | 8 | 2,240 | 95.18, < 0.001 | 98 | 0.77 (0.60–0.89) | 0.80 (0.71–0.86) | 1.55 (COI) | 0.85 (0.82–0.88) | ||
| APRI | 1 | 119 | NA | NA | 0.67 | 0.81 | 0.55 | 0.74 | 6.99 | < 0.001 |
| FIB-4 | 1 | 119 | NA | NA | 0.67 | 0.81 | 2.95 | 0.78 | 4.77 | < 0.001 |
| AFP | 3 | 1,340 | 63.00, < 0.001 | 97 | 0.61 (0.42–0.77) | 0.94 (0.82–0.98) | 18.54 (ng/mL) | 0.84 (0.80–0.87) | 0.43 | 0.671 |
HCC, hepatocellular carcinoma; WFA+-M2BP, wisteria floribunda agglutinin-positive Mac-2-binding protein; APRI, aspartate aminotransferase-to-platelet ratio index; FIB-4, fibrosis-4 index; AFP, α-fetoprotein; AUSROC, area under the summary receiver operating characteristic curve; CI, confidence interval; NA, not available.
Publication bias and sensitivity analysis
As displayed in Supplementary Fig. 3, Deek’s funnel plots were almost symmetric for studies that reported mild liver fibrosis, significant fibrosis, and advanced fibrosis (P values = 0.1, 0.33, and 0.09, respectively), suggesting no evidence of publication bias. However, a significant publication bias was observed in studies on cirrhosis (P = 0.03). For studies on the prediction of HCC, there was no publication bias (P = 0.83) (Supplementary Fig. 4).
Through sensitivity analysis, we observed outlier studies existed in each stage of liver fibrosis (Supplementary Fig. 5). Surprisingly, after the removal of outlier studies, the heterogeneity in mild fibrosis disappeared (Supplementary Table 1) and the publication bias in studies on cirrhosis was diminished (Supplementary Fig. 6). However, Supplementary Table 1 indicated that the summary results were not significantly affected by individual studies. Also, as displayed in Supplementary Fig. 7, no outlier study was found in HCC.
Discussion
WFA+-M2BP is a serum glycobiomarker that is receiving growing attentions. It had been reported that the elevated WFA+-M2BP level was associated with the risk of HCC57–59, the loss of HBeAg in chronic hepatitis B patients60, and the severity of liver fibrosis61,62. In our meta-analysis, we evaluated 36 articles in total, and explored the predictive accuracy of WFA+-M2BP for distinguishing liver fibrosis stages and HCC by comparing with diverse non-invasive indicators. Our results suggested WFA+-M2BP possessed satisfactory diagnostic accuracy for predicting cirrhosis and moderate diagnostic performance for detecting mild fibrosis, significant fibrosis, advanced fibrosis and HCC. The AUSROC of WFA+-M2BP was equivalent to HA and FibroScan for assessing cirrhosis, and similar to AFP for diagnosing HCC.
Previously, literatures on the diagnostic performance of WFA+-M2BP in different stages of liver fibrosis were controversial. Zou et al.26 reported WFA+-M2BP was useful to assess early stages of liver fibrosis, and Ura et al.36 showed WFA+-M2BP was more accurate in diagnosing significant fibrosis than advanced fibrosis. In contrast, several other studies indicated that WFA+-M2BP had the strongest ability to predict cirrhosis37,62. In our meta-analysis, we found that the overall AUSROCs of WFA+-M2BP for identifying mild fibrosis, significant fibrosis, advanced fibrosis and cirrhosis were 0.75, 0.79, 0.82 and 0.88, respectively. For cirrhosis, WFA+-M2BP reached the highest pooled sensitivity and specificity at 0.77 and 0.86. Since it is widely accepted that AUC between 0.85 and 0.90 is as good as liver biopsy for staging fibrosis63, our study underscores the notion that WFA+-M2BP could serve as a surrogate biomarker for biopsy when diagnosing cirrhosis.
Interestingly, WFA+-M2BP exhibited different predictive accuracies for staging liver fibrosis caused by different etiologies in CLDs. In our study, for patients with AIH, BA, PBC or mixed etiologies, WFA+-M2BP exhibited excellent performance for distinguishing significant fibrosis, advanced fibrosis and cirrhosis. In general, WFA+-M2BP had lower accuracy in HBV-infected patients than in patients with HCV infection, NAFLD or NASH. Our conclusion was consistent with a previous meta-analysis23. However, our study was more extensive, as we included more publications, had different subgroup setups, and used different models for the calculation of pooled results. WFA+-M2BP has the potential to reflect hepatic fibrosis as hepatic stellate cells (HSCs) are the source of WFA+-M2BP and its level is closely associated with α-smooth-muscle actin (αSMA) expression19,21. However, HBV‐positive patients are more likely to experience quiescent hepatic inflammation, and HBV-related cirrhosis had large regenerative nodules and thin fibrous septa23,27. As a result, as shown in Table 3, minor changes of WFA+-M2BP optimal cutoffs in each liver fibrosis stage of HBV-infected patients may lead to low diagnostic accuracy. Whether better predictive performance of WFA+-M2BP is related to more severe liver damage or inflammation response, or more activation of HSCs, will need to be investigated by further studies. Here, we suggest that individual cutoff value of WFA+-M2BP should be used to stage liver fibrosis of different etiology.
Overall, WFA+-M2BP was not better than other non-invasive indicators for predicting significant fibrosis and advanced fibrosis. However, for assessing cirrhosis, the diagnostic accuracy of WFA+-M2BP was equivalent to HA and FibroScan and superior to four markers (i.e., APRI, FIB-4, AST/ALT, and PLT). First, our meta-analysis study revealed similar AUSROC values of APRI and FIB-4 in each fibrosis stage (significant fibrosis: 0.7407 and 0.7844, advanced fibrosis: 0.7347 and 0.8165, and cirrhosis: 0.7268 and 0.8448, respectively)64, indicating the reliability of our analysis. Second, we observed the similar result as a previous study, which reported that HA was more efficient than APRI and FIB-4 for fibrosis staging63,65. Although studies claimed that FibroScan could offer more promising results than HA for staging both early hepatic fibrosis and cirrhosis66,67, we were not able to tell the difference between FibroScan and HA in this study due to limited sample size. Since the accuracy of FibroScan is strongly influenced by disease etiology68,69, additional studies stratified by etiology will be necessary to draw the conclusion.
As M2BP has been shown to promote cancer progression, WFA+-M2BP may potentially be used to predict HCC development18. In our current study, WFA+-M2BP was superior to APRI and FIB-4 and equivalent to AFP for diagnosing HCC. Currently, whether AFP should be used for routine surveillance of HCC is controversial due to its limited sensitivity in early detection. In our study, WFA+-M2BP had a higher sensitivity (0.77) and a lower specificity (0.80) when it was compared with AFP (sensitivity = 0.61 and specificity = 0.94). This finding indicated the possibility of improving the diagnosis of HCC if WFA+-M2BP and AFP are used together. Besides, it had been reported the posttreatment level of WFA+-M2BP could nicely reflect HCC development for patients underwent anti-viral therapy by reaching a high sensitivity, specificity and AUROC (0.875, 0.939 and 0.973)49. As a result, combined diagnostic model or posttreatment detection of WFA+-M2BP might be helpful for the prediction of HCC.
It should be noted that there are limitations in our study: (1) Due to the limited study number, we could not evaluate the predictive accuracy of WFA+-M2BP in mild fibrosis stratified by etiology; (2) This meta-analysis is a pilot study which compared the performance of multiple indicators by analyzing results from literatures on WFA+-M2BP. We suggested future study could be performed to investigate the comparison between WFA+-M2BP and a certain non-invasive indicator for staging liver fibrosis or predicting HCC in a certain disease by analyzing more literatures from multiple databases; (3) More high-quality studies are needed for analysis. Our quality assessment demonstrated that the existence of moderate risk of bias was mainly due to the awareness of reference standard result before conducting the index test. Also, significant heterogeneities existed in studies regarding each stage of liver fibrosis and HCC. And we found that the age of the participants, male proportion, etiology, and certain outlier studies might be the source of these heterogeneities. Besides, we noticed obvious publication bias existed in studies on cirrhosis, although this bias could be diminished after the removal of three outlier studies. (4) We conducted this diagnostic meta-analysis and emphasized the diagnostic performance of WFA+-M2BP in liver fibrosis and HCC, without elaborating on the outcomes of patients with different basal levels of serum WFA+-M2BP.
In conclusion, our meta-analysis demonstrated that WFA+-M2BP could be used as a surrogate biomarker for liver biopsy to diagnose cirrhosis in chronic liver diseases. It showed modest accuracy for identifying early stage of liver fibrosis and HCC. Compared with other non-invasive indicators, the predictive performance of WFA+-M2BP was similar to HA and FibroScan in assessing cirrhosis, but was equivalent to AFP in HCC. As the accuracy of WFA+-M2BP was strongly influenced by the etiology of disease, individual cutoff value was suggested to be applied in certain etiology.
Methods
Literature search strategy
We performed this meta-analysis according to the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy70. Literatures published before September 22, 2019 in Pubmed, Web of Science, EMBASE, the Cochrane Library, and grey literature database including OpenGrey (https://www.opengrey.eu) were searched. The search terms included “Wisteria floribunda agglutinin-positive Mac-2-binding protein or WFA+-M2BP or M2BPGi or Mac-2-binding protein glycosylation isomer” and “fibrosis or cirrhosis” or “hepatocellular carcinoma or liver cancer”.
Inclusion and exclusion criteria
Inclusion criteria were: (1) study objects contained patients with liver fibrosis or hepatocellular carcinoma caused by various etiologies; (2) studies used liver biopsy as gold standard to measure the severity of liver fibrosis; (3) studies used typical imaging techniques and/or histopathology to diagnose HCC; (4) studies employed WFA+-M2BP and may also include APRI, FIB-4, aspartate aminotransferase to alanine aminotransferase ratio (AST/ALT), HA, PLT, FibroScan stiffness value or AFP to predict liver fibrosis or HCC; and (5) data on true-positive (TP), true-negative (TN), false-positive (FP), false-negative (FN) results were reported separately or could be calculated from the article.
Studies as follows were excluded: (1) duplicate studies; (2) non-experimental studies such as reviews, letters, clinical trials, correspondences, comments, case reports, and patents; (3) studies published in a language other than English; (4) non-human subjects; or (5) the relevant data were inaccessible or unclear.
Data extraction and quality assessment
Firstly, we employed the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2)71 to assess the quality of the selected studies independently. Next, the following information were extracted: first author’s name, year of publication, region, number of patients, type of biomarkers, age, proportion of male, etiology, histological system, blind method, liver biopsy length, interval between biopsy and blood test, patient number in different fibrosis stages, WFA+-M2BP cutoff values, and TP, TN, FP, and FN results. Besides, Metavir, Brunt, Batts and Ludwig or Revised Inuyama stages ≥ F1, ≥ F2, ≥ F3, and F4 were defined as mild fibrosis, significant fibrosis, advanced fibrosis, and cirrhosis, respectively.
Statistical analyses
We calculated 2 × 2 tables and performed QUADAS-2 quality assessment by using Review Manager 5.2 (The Nordic Cochrane Centre, Copenhagen, Denmark). Besides, we employed bivariate random effects model to conduct statistical analysis. Thus, “Midas” module in Stata version 14.2 (StataCorp, College Station, TX) was used to summarize test accuracy: pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), the area under the summary receiver operating characteristic curve (AUSROC). Z test was adopted to compare AUSROCs of WFA+-M2BP and other indicators. Diagnostic threshold effect would exist if the Spearman correlation coefficient > 0 and P < 0.0572. If inconsistency index (I2) ≥ 50% or P < 0.05 was observed, it suggested significant heterogeneity73. In that case, we would conduct meta-regression analysis, which evaluated potential factors to determine covariates. In joint model, factors with P < 0.1 was considered the potential source of heterogeneity74,75. Furthermore, publication bias was determined by using Deeks’s funnel plot, and P < 0.05 indicated possible bias76. Finally, we carried out sensitivity analysis to measure the robustness of the summary results.
Supplementary information
Acknowledgements
This research is supported by Sichuan Province Health and Family Planning Commission (Sichuan Health Office issued [2017] 70) and Chinese Scholarship Council (CSC) Scholarship (to S.F.). We are grateful to Dr. J.-H. James Ou (University of Southern California) for critical reading of this manuscript and for providing valuable comments to our study. We thank Dr. Zhongyi Zhao (Sichuan University) for offering helpful suggestions during major revision.
Author contributions
S.F. and C.T. conceived and designed the study. All studied were screened and identified by S.F. and Z.W. independently, and they extracted the data using a standardized form. Y.Z. resolved any differences in the process of study selection or data collection. S.F., Z.W., Y.Z., and C. T. analyzed the interpreted the data. S.F. prepared the manuscript and C. T. revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67471-y.
References
- 1.Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American Gastroenterological Association Institute Technical Review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152:1544–1577. doi: 10.1053/j.gastro.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa H, et al. Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol. Res. 2016;46:613–621. doi: 10.1111/hepr.12596. [DOI] [PubMed] [Google Scholar]
- 3.Noruegas MJ, Matos H, Goncalves I, Cipriano MA, Sanches C. Acoustic radiation force impulse-imaging in the assessment of liver fibrosis in children. Pediatr. Radiol. 2012;42:201–204. doi: 10.1007/s00247-011-2257-2. [DOI] [PubMed] [Google Scholar]
- 4.Stepanova M, et al. Direct and indirect economic burden of chronic liver disease in the United States. Clin. Gastroenterol. Hepatol. 2017;15:759–766.e755. doi: 10.1016/j.cgh.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi H, Gores GJ. Mechanisms of liver injury: an overview. Curr. Mol. Med. 2003;3:483–490. doi: 10.2174/1566524033479528. [DOI] [PubMed] [Google Scholar]
- 6.Glaser SS, Gaudio E, Miller T, Alvaro D, Alpini G. Cholangiocyte proliferation and liver fibrosis. Expert. Rev. Mol. Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khatun M, Ray RB. Mechanisms underlying hepatitis C virus-associated hepatic fibrosis. Cells. 2019;8:1249. doi: 10.3390/cells8101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierantonelli I, Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103:e1–e13. doi: 10.1097/TP.0000000000002480. [DOI] [PubMed] [Google Scholar]
- 9.Baglieri J, Brenner DA, Kisseleva T. The role of fibrosis and liver-associated fibroblasts in the pathogenesis of hepatocellular carcinoma. Int. J. Mol. Sci. 2019;20:1723. doi: 10.3390/ijms20071723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber A, Boege Y, Reisinger F, Heikenwalder M. Chronic liver inflammation and hepatocellular carcinoma: persistence matters. Swiss Med. Wkly. 2011;141:w13197. doi: 10.4414/smw.2011.13197. [DOI] [PubMed] [Google Scholar]
- 11.Pang JX, et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS ONE. 2014;9:e95776. doi: 10.1371/journal.pone.0095776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkes J, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245–1251. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 13.European Association for the Study of the Liver Electronic address, e. e. e. & European Association for the Study of the, L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017 doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Sebastiani G, Gkouvatsos K, Plebani M. Non-invasive assessment of liver fibrosis: it is time for laboratory medicine. Clin. Chem. Lab. Med. 2011;49:13–32. doi: 10.1515/CCLM.2011.001. [DOI] [PubMed] [Google Scholar]
- 15.Narimatsu H. Development of M2BPGi: A novel fibrosis serum glyco-biomarker for chronic hepatitis/cirrhosis diagnostics. Expert Rev. Proteomics. 2015;12:683–693. doi: 10.1586/14789450.2015.1084874. [DOI] [PubMed] [Google Scholar]
- 16.Kuno A, et al. A serum "sweet-doughnut" protein facilitates fibrosis evaluation and therapy assessment in patients with viral hepatitis. Sci. Rep. 2013;3:1065. doi: 10.1038/srep01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki K, et al. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563–1570. doi: 10.1002/hep.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirabe K, et al. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J. Gastroenterol. 2018;53:819–826. doi: 10.1007/s00535-017-1425-z. [DOI] [PubMed] [Google Scholar]
- 19.Bekki Y, et al. Hepatic stellate cells secreting WFA(+)-M2BP: Its role in biological interactions with Kupffer cells. J. Gastroenterol. Hepatol. 2017;32:1387–1393. doi: 10.1111/jgh.13708. [DOI] [PubMed] [Google Scholar]
- 20.Migita K, et al. Serum cytokine profiles and Mac-2 binding protein glycosylation isomer (M2BPGi) level in patients with autoimmune hepatitis. Medicine. 2018;97:e13450. doi: 10.1097/md.0000000000013450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada N, et al. Serum Mac-2 binding protein glycosylation isomer predicts the activation of hepatic stellate cells after liver transplantation. J. Gastroenterol. Hepatol. 2019;34:418–424. doi: 10.1111/jgh.14438. [DOI] [PubMed] [Google Scholar]
- 22.Toshima T, et al. A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J. Gastroenterol. 2015;50:76–84. doi: 10.1007/s00535-014-0946-y. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein levels and liver fibrosis: a meta-analysis. J. Gastroenterol. Hepatol. 2017;32:1922–1930. doi: 10.1111/jgh.13802. [DOI] [PubMed] [Google Scholar]
- 24.Nishikawa, H. et al. A proposed predictive model for advanced fibrosis in patients with chronic hepatitis B and its validation. Medicine 95(35), (no pagination) (2016). [DOI] [PMC free article] [PubMed]
- 25.Nishikawa H, et al. Proposal of a predictive model for advanced fibrosis containing Wisteria floribunda agglutinin-positive Mac-2-binding protein in chronic hepatitis C. Hepatol. Res. 2016;47:74–84. doi: 10.1111/hepr.12724. [DOI] [PubMed] [Google Scholar]
- 26.Zou X, et al. Serum WFA+-M2BP levels for evaluation of early stages of liver fibrosis in patients with chronic hepatitis B virus infection. Liver Int. 2016 doi: 10.1111/liv.13188. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa H, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein for patients with chronic hepatitis B and C: A comparative study. J. Viral Hepat. 2016;23:977–984. doi: 10.1111/jvh.12575. [DOI] [PubMed] [Google Scholar]
- 28.Shigefuku R, et al. Correlations of hepatic hemodynamics, liver function, and fibrosis markers in nonalcoholic fatty liver disease: Comparison with chronic hepatitis related to hepatitis C virus. Int. J. Mol. Sci. 2016;17:1545. doi: 10.3390/ijms17091545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe M, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2015;50:776–784. doi: 10.1007/s00535-014-1007-2. [DOI] [PubMed] [Google Scholar]
- 30.Umemura T, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am. J. Gastroenterol. 2015;110:857–864. doi: 10.1038/ajg.2015.118. [DOI] [PubMed] [Google Scholar]
- 31.Heo, J. Y. et al. Use of wisteria floribunda agglutinin-positive human Mac-2 binding protein in assessing risk of hepatocellular carcinoma due to hepatitis B virus. Medicine95, 10.1097/md.0000000000003328 (2016). [DOI] [PMC free article] [PubMed]
- 32.Ichikawa Y, et al. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein may predict liver fibrosis and progression to hepatocellular carcinoma in patients with chronic hepatitis B virus infection. Hepatol. Res. 2016;47:226–233. doi: 10.1111/hepr.12712. [DOI] [PubMed] [Google Scholar]
- 33.Ishii A, et al. Clinical implication of serum Wisteria floribunda Agglutinin-Positive Mac-2-binding protein in treatment naive chronic hepatitis B. Hepatol. Res. 2016;46:1065–1073. doi: 10.1111/hepr.12703. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa H, et al. Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-gamma-inducible protein-10 in primary biliary cirrhosis. Hepatol. Res. 2016;46:575–583. doi: 10.1111/hepr.12595. [DOI] [PubMed] [Google Scholar]
- 35.Nishikawa H, et al. Clinical significance of serum wisteria floribunda agglutinin-positive Mac-2-binding protein level in non-alcoholic steatohepatitis. Hepatol. Res. 2016;46:1194–1202. doi: 10.1111/hepr.12662. [DOI] [PubMed] [Google Scholar]
- 36.Ura K, et al. Serum WFA(+)-M2BP is a non-invasive liver fibrosis marker that can predict the efficacy of direct-acting anti-viral-based triple therapy for chronic hepatitis C. Aliment Pharmacol. Ther. 2016;43:114–124. doi: 10.1111/apt.13431. [DOI] [PubMed] [Google Scholar]
- 37.Yamada N, et al. Serum Mac-2 binding protein glycosylation isomer predicts grade F4 liver fibrosis in patients with biliary atresia. J. Gastroenterol. 2016;52:245–252. doi: 10.1007/s00535-016-1235-8. [DOI] [PubMed] [Google Scholar]
- 38.Huang CI, et al. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein expression predicts disease severity in chronic hepatitis C patients. Kaohsiung J. Med. Sci. 2017;33:394–399. doi: 10.1016/j.kjms.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai LL, et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein in non-alcoholic fatty liver disease. PLoS ONE. 2017;12:e0174982. doi: 10.1371/journal.pone.0174982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noguchi R, et al. Serum angiotensin-converting enzyme level for evaluating significant fibrosis in chronic hepatitis B. World J. Gastroenterol. 2017;23:6705–6714. doi: 10.3748/wjg.v23.i36.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujita K, et al. Fibrosis staging using direct serum biomarkers is influenced by hepatitis activity grading in hepatitis C virus infection. J. Clin. Med. 2018;7:267. doi: 10.3390/jcm7090267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jekarl DW, et al. Diagnosis of Liver fibrosis with wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA-M2BP) among chronic hepatitis B patients. Ann. Lab. Med. 2018;38:348–354. doi: 10.3343/alm.2018.38.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mak LY, et al. Role of serum M2BPGi levels on diagnosing significant liver fibrosis and cirrhosis in treated patients with chronic hepatitis B virus infection. Clin. Transl. Gastroenterol. 2018;9:163. doi: 10.1038/s41424-018-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuura K, et al. Circulating let-7 levels in serum correlate with the severity of hepatic fibrosis in chronic hepatitis C. Open Forum Infect. Dis. 2018;5:ofy268. doi: 10.1093/ofid/ofy268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogawa Y, et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2018;33:1795–1803. doi: 10.1111/jgh.14156. [DOI] [PubMed] [Google Scholar]
- 46.Ueno T, et al. Clinical implications of serum Mac-2-binding protein (M2BPGi) during regular follow-up of patients with biliary atresia. Pediatr. Surg. Int. 2018;34:1065–1071. doi: 10.1007/s00383-018-4317-2. [DOI] [PubMed] [Google Scholar]
- 47.Kanno M, et al. Serum aldo-keto reductase family 1 member B10 predicts advanced liver fibrosis and fatal complications of nonalcoholic steatohepatitis. J. Gastroenterol. 2019;54:549–557. doi: 10.1007/s00535-019-01551-3. [DOI] [PubMed] [Google Scholar]
- 48.Yeh ML, et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein in the prediction of disease severity in chronic hepatitis B patients. PLoS ONE. 2019;14:e0220663. doi: 10.1371/journal.pone.0220663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagata H, et al. Serial measurement of Wisteria floribunda agglutinin positive Mac-2-binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN-based and IFN-free therapy. Hepatol. Int. 2016;10:956–964. doi: 10.1007/s12072-016-9754-1. [DOI] [PubMed] [Google Scholar]
- 50.Cheung KS, et al. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts liver cancer development in chronic hepatitis B patients under antiviral treatment. Oncotarget. 2017;8:47507–47517. doi: 10.18632/oncotarget.17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chuaypen N, et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein level as a diagnostic marker of hepatitis B virus-related hepatocellular carcinoma. Hepatol. Res. 2018;48:872–881. doi: 10.1111/hepr.13187. [DOI] [PubMed] [Google Scholar]
- 52.Kawanaka M, et al. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts the development of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2018;48:521–528. doi: 10.1111/hepr.13054. [DOI] [PubMed] [Google Scholar]
- 53.Lin YJ, et al. A glycomarker for short-term prediction of hepatocellular carcinoma: A longitudinal study with serial measurements. Clin. Transl. Gastroenterol. 2018;9:183. doi: 10.1038/s41424-018-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim HS, et al. Serum Wisteria floribunda agglutinin-positive human Mac-2 binding protein level predicts recurrence of hepatitis B virus-related hepatocellular carcinoma after curative resection. Clin. Mol. Hepatol. 2019 doi: 10.3350/cmh.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mak L-Y, et al. Serum Mac-2 binding protein glycosylation isomer level predicts hepatocellular carcinoma development in E-negative chronic hepatitis B patients. World J. Gastroenterol. 2019;25:1398–1408. doi: 10.3748/wjg.v25.i11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mak LY, et al. Serum Mac-2-binding protein glycosylation isomer and risk of hepatocellular carcinoma in entecavir-treated chronic hepatitis B patients. J. Gastroenterol. Hepatol. 2019;34:1817–1823. doi: 10.1111/jgh.14637. [DOI] [PubMed] [Google Scholar]
- 57.Cheung KS, et al. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts liver cancer development in chronic hepatitis B patients under antiviral treatment. Oncotarget. 2017 doi: 10.18632/oncotarget.17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagata H, et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J. Hepatol. 2017;67:933–939. doi: 10.1016/j.jhep.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 59.Kim SU, et al. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts the risk of HBV-related liver cancer development. Liver Int. 2017;37:879–887. doi: 10.1111/liv.13341. [DOI] [PubMed] [Google Scholar]
- 60.Nishikawa H, et al. Clinical implication of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level on hepatitis B e-antigen loss or seroconversion in hepatitis B e-antigen positive patients. Hepatol. Res. 2016;46:1065–1073. doi: 10.1111/hepr.12655. [DOI] [PubMed] [Google Scholar]
- 61.Nakamura M, Kanda T. Serum microRNA-122 and Wisteria floribunda agglutinin-positive Mac-2 binding protein are useful tools for liquid biopsy of the patients with hepatitis B virus and advanced liver fibrosis. PLoS ONE. 2017;12:e0177302. doi: 10.1371/journal.pone.0177302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei B, et al. M2BPGi as a potential diagnostic tool of cirrhosis in Chinese patients with Hepatitis B virus infection. J. Clin. Lab. Anal. 2017;32:e22621. doi: 10.1002/jcla.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orasan OH, Ciulei G, Cozma A, Sava M, Dumitrascu DL. Hyaluronic acid as a biomarker of fibrosis in chronic liver diseases of different etiologies. Clujul Med. 2016;89:24–31. doi: 10.15386/cjmed-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 65.El Serafy MA, Kassem AM, Omar H, Mahfouz MS, El Said El Raziky M. APRI test and hyaluronic acid as non-invasive diagnostic tools for post HCV liver fibrosis: Systematic review and meta-analysis. Arab. J. Gastroenterol. 2017;18:51–57. doi: 10.1016/j.ajg.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Coco B, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 67.Obara N, et al. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J. Gastroenterol. 2008;43:720. doi: 10.1007/s00535-008-2225-2. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen-Khac E, et al. Noninvasive diagnosis of large esophageal varices by Fibroscan: strong influence of the cirrhosis etiology. Alcohol Clin. Exp. Res. 2010;34:1146–1153. doi: 10.1111/j.1530-0277.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 69.Pu K, et al. Diagnostic accuracy of transient elastography (FibroScan) in detection of esophageal varices in patients with cirrhosis: A meta-analysis. World. J. Gastroenterol. 2017;23:345. doi: 10.3748/wjg.v23.i2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Vet HCW, E. A., Riphagen, I.I., Aertgeerts, B., & Pewsner, D. Chapter 7: searching for studies. Cochrane handbook for systematic reviews of diagnostic test accuracy version 0.4 [updated September 2008]. The Cochrane Collaboration (2008).
- 71.Whiting PF, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 72.Jia MM, et al. Diagnostic accuracy of urine HE4 in patients with ovarian cancer: a meta-analysis. Oncotarget. 2017;8:9660–9671. doi: 10.18632/oncotarget.14173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen Y, et al. Diagnostic value of thyroid transcription factor-1 for pleural or other serous metastases of pulmonary adenocarcinoma: a meta-analysis. Sci. Rep. 2016;6:19785. doi: 10.1038/srep19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fillmoren CL, Rommel CA, Welch BM, Zhang M, Kawamoto K, et al. The perils of meta-regression to identify clinical decision support system success factors. J. Biomed. Inform. 2015;56:65–68. doi: 10.1016/j.jbi.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Avni T, Leibovici L, Paul M. PCR diagnosis of invasive candidiasis: systematic review and meta-analysis.PCR diagnosis of invasive candidiasis: systematic review and meta-analysis. J. Clin. Microbiol. 2011;49:665–670. doi: 10.1128/JCM.01602-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deeks JJ, MacaskillMacaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.