Abstract
Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors are a promising source of cell therapy. However, their effectiveness in cancer remains less known. This study is the first to evaluate the quality of BM-MSCs obtained from young and elderly healthy volunteers (KNT cells). The KNT cells had normal karyotypes and were positive for MSC markers (CD90, CD73, CD105). When cultured under appropriate conditions, they showed adipogenic or osteogenic potential. Hence, the anti-neoplastic effects of secretory factors [supernatant or extracellular vesicles (EV)] from KNT cells were verified using several neoplastic cells (three multiple myeloma, three myeloid leukemia, and three lymphoma cell lines). The conditioned medium (CM), but not EV, of KNT cells derived from young healthy donors significantly inhibited myeloma and lymphoma cell proliferation, but enhanced myeloid leukemia proliferation. Anti-angiogenesis effect of CM and EV derived from young KNT against hematologic neoplasia-induced angiogenesis was evident and more prominent in CM than in EV but not evident in elderly KNT-derived EV. These findings indicate that the anti-tumor effect of KNT cells depends on the types of hematologic neoplasia, with elements existing in the supernatant and not in EVs. Therefore, BM-MSC may produce soluble factors that affect cell proliferation of neoplasia, causing cell-to-cell communication. The anti-angiogenesis effect of KNT cells depends on the age of BM-MSC donors.
Keywords: Bone marrow stromal cells, Donor age, Growth inhibitory effect, Conditioned medium, Angiogenesis
Introduction
Mesenchymal stem/stromal cells (MSCs), which are found in various tissues, including the bone marrow [1, 2], umbilical cord [3], adipose tissue [4], and dental pulp [5], are a source of multipotent somatic stem cells that differentiate into bone, cartilage, and fats. Recently, MSCs have become a source of cell therapy for tissue regeneration. In addition to their multilineage potential, MSCs have gained attention because of the immunomodulatory functions of the cytokines and chemokines that they secrete. As immunomodulation by MSCs has been shown to alleviate symptoms of treatment-resistant refractory autoimmune diseases, cell transplantation therapy is increasingly being indicated for these diseases [6]: for example, treatment with bone marrow MSCs (BM-MSCs) has also been performed in acute graft versus host disease (GvHD) after hematopoietic stem cell transplantations [7]. In addition to immunoregulation by the fluid elements released by MSCs, intercellular interactions in the niches formed by cells surrounding MSCs are likely to be intricately involved in symptom alleviation [8]. Understanding these complex mechanisms is expected to lead to the development of safer and more efficient cell therapy. Recently, in addition to the above MSC-derived cytokines and chemokines, the extracellular vesicles (EVs) released by MSCs have also garnered attention [9].
Exosomes, a type of EV, are approximately 30–200 nm vesicles with a lipid bilayer membrane structure. In the late 2000s, not only proteins but also genetic materials, such as microRNA (miRNA) and mRNA, were demonstrated to be transported between cells through exosome receival [10, 11]. A recent study demonstrated the therapeutic effects of MSC-derived exosomes on GvHD, similar to MSCs [9]. However, the full scope of the biological actions of exosomes has not been well understood. A major concern with exosome use is the risk of adverse reactions. In fact, some reports revealed that exosomes secreted by abnormal MSCs can cause a cancer to be more malignant [12].
To develop safe and high-quality cell therapy with BM-MSCs, we characterized the BM-MSCs obtained from two age groups (i.e., young and elderly). Because only limited information (donor age and whether the source is from single or multiple donors) is provided on commercially available BM-MSCs, this study was performed to evaluate the quality of BM-MSCs derived from our new original source. We then assessed their growth inhibitory effect on various types of hematologic malignancies for future therapeutic application.
Materials and methods
Isolation of human BM-MSCs
BM-MSCs obtained from healthy 22- and 25-year-old male and 80- and 86-year-old female volunteers (young KNT cells: KNT_D_170714, KNT_L_170714; elderly KNT cells: KNT_80_170825 and KNT_86_170825, Kintaro Cells Power Co., Tokyo, Japan) were used in this study (Table 1). These cells were collected under the control of the Federal Research and Clinical Center of the Federal Medical Biological Agency of Russia (FRCC FMBA, Moscow, Russia) after approval from the institutional ethical committee (approval 5-11-03-2013 of March 03, 2013).
Table 1.
Information of bone marrow-MSC donors
| Donors | Gender | Age (years) | KNT cell ID | Doubling time (h) |
|---|---|---|---|---|
| UDN1 | Male | 22 | KNT_D_170714 | 108 |
| UDN2 | Male | 25 | KNT_L_170714 | 108 |
| UDN3 | Female | 80 | KNT_80_170825 | 96 |
| UDN4 | Female | 86 | KNT_86_170825 | 108 |
Cell culture
We seeded BM-MSCs in αMEM (Gibco, Carlsbad, CA, US) supplemented with 5% PLTMax Human Platelet Lysate (Millipore, Temecula, CA, US) and 2 U/ml Heparin (Sigma, St. Louis, MO, US) at 37 °C in a humidified atmosphere containing 5% CO2. Adherent cells were harvested by trypsinization and either passaged (passages 1–4) for expansion or subjected to analyses for subsequent cytogenetic analysis. The following cell lines derived from hematologic malignancies were purchased from the Health Science Research Resource Bank, JCRB Cell Bank, and ATCC: three lymphoma cell lines (SUDHL4, CRL-2957; DL40, JCRB1334; Pfeiffer, CRL-2632), three lines with multiple myeloma (RPMI8226, JCRB0034; KMS-11, JCRB1179; U266, TIB-196), and three lines with leukemia (K562, JCRB0019; HL-60, IFO50022; U937, JCRB9021). These cells were cultured in RPMI1640 (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, UT, US) at 37 °C in a humidified atmosphere containing 5% CO2.
Flow cytometry and cytogenetic analysis
To characterize KNT cells using their surface antigen, the following monoclonal antibodies were used: CD90-FITC (Cat. No. 555595), CD73-APC (Cat. No. 560847), CD105-PerCPCy5.5 (Cat. No. 560819), CD34-FITC (Cat. No. 555821), CD45-PE (Cat. No. 555483), HLA-DR-PE (Cat. No. 555812), and CD271-PE (Cat. No. 557196) (BD Biosciences, San Jose, CA, US). The KNT cells were incubated with these antibodies and analyzed by an Accuri C6 cytometer (BD Biosciences), according to the manufacturer’s instructions. Chromosome analysis was performed with a conventional Giemsa-banding method after colcemid exposure. A further cytogenetic study was performed using commercially available spectral karyotyping (SKY) from SRL Inc. (Hachioji, Hino, Tokyo, Japan).
Osteoblast and adipocyte differentiation
To confirm the adipogenic potential of young and elderly KNT cells, these cells were incubated in αMEM with 5% PLTMax until cells were confluent. Thereafter, KNT cells were cultured with adipogenic induction medium (Lonza, Basel, Switzerland). After 3 days, maintenance medium was added to cells, and three cycles of induction and maintenance media were completed. Cells were fixed with 10% formalin (Sigma-Aldrich) and stained with 0.5% Oil Red O (Sigma-Aldrich) in methanol (Sigma-Aldrich). To confirm the osteogenic potential of KNT cells, they were incubated in αMEM with 5% PLTMax until a confluent layer was achieved. Thereafter, osteogenic differentiation medium (Lonza) was added. Medium was changed every 3–4 days. After 17 days, cells were fixed in 10% formalin and stained with 10% alizarin red (Sigma-Aldrich).
Collection of conditioned medium (CM) and EV
Young and elderly KNT cells were cultured in maintenance medium (αMEM with 5% PLTMax) which was replaced with serum-free MSCBM basal medium (Lonza). The KNT cell-derived conditioned medium (KNT cell-CM) was collected after 48 h. The collected CM was centrifuged for 15 min at 2500 rpm to remove cell and cell debris, and then concentrated (approximately tenfold concentration) using Amicon Ultra-15 3 kDa MW cut-off filter units (MilliporeSigma, Burlington, MA, US) to exclude possible contamination from lipids and metabolites. KNT cells (4 × 104 cells/cm2) were cultured in 5 ml of MSCBM basal medium (Lonza) in a T-25 flask. The culture supernatants were harvested after 48 h of incubation, and the EV fraction was purified with Exoquick-TC reagent (System Biosciences, Palo Alto, CA, US) according to the manufacturer’s instructions. EV pellets were resuspended in 500 µl of MSCBM basal medium.
Evaluation of the anti-neoplastic activity of various hematologic malignant cells
To evaluate the proliferative effect of KNT cells, lymphoma cells (SUDHL4, DL40, and Pfeiffer), multiple myeloma cells (RPMI8226, KMS-11, and U266), or myeloid leukemia cells (K562, HL-60, and U937) were used. Neoplastic cells (2 × 105 cells/mL in maintenance medium) were cultured with or without concentrated CM or EV from KNT cells. Thirty microliters of concentrated CM or EV fractions from the KNT cells were then added to the cultured hematological malignant cell lines. Cell proliferation rates were measured using the IncuCyte Zoom live-cell analysis system (Essen Biosciences, Ann Arbor, MI, US).
Effect on neoplastic cell-induced tube formation
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza and cultured in maintenance medium [EBM-2 medium supplemented with EGM-2 bulletKit (Lonza)]. HUVECs (300 µL of 4 × 105 cells/mL in EBM-2 basal medium) were placed on a 260-µl Matrigel (BD Biosciences) in a 24-well plate and incubated overnight at 37 °C. Neoplastic cell (SUDHL4, RPMI8226, or K562)-derived concentrated CM (20 µL/well) with or without KNT cell-derived CM (20 µL/well) or KNT cell-derived EV was subsequently added. KNT cells used were KNT_D_176714 (as young KNT cells) or KNT_86_170825 (elderly KNT). After 24 h, cells were stained with calcein AM (BD Biosciences) and visualized under a fluorescence microscope. Total tube area was quantified as the mean pixel density obtained from image analysis of five random microscopic fields using Image-J software [13].
Statistical analyses
Data are expressed as mean ± SD. Two treatment groups were compared using Student’s t test. Multiple group comparisons were performed by ANOVA. GraphPad Prism version 5c for Macintosh (GraphPad Inc., La Jolla, CA, US) was used for statistical analyses. Results were considered statistically significant when P was < 0.05.
Results
Characterization of KNT cells
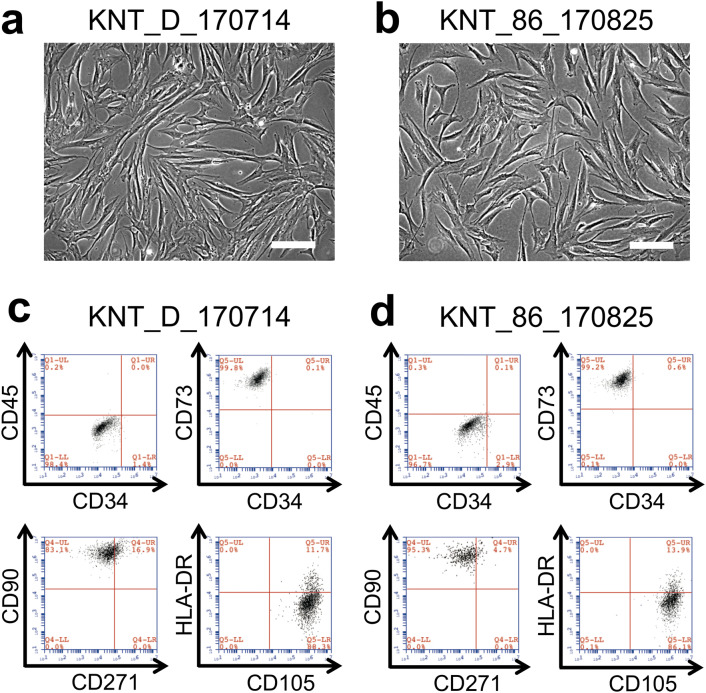
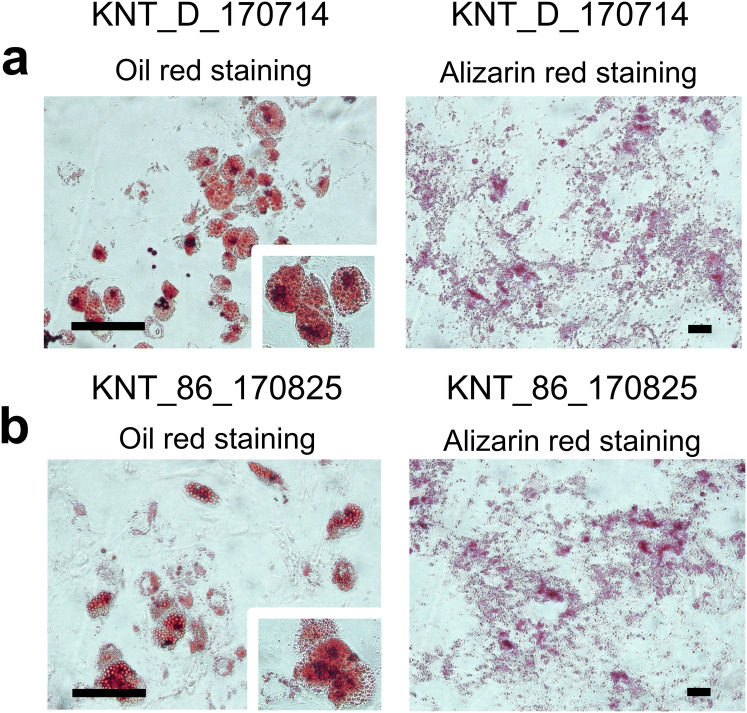
KNT cells derived from young donors and those from elderly donors were microscopically compared to elucidate their morphology. KNT cells from both groups had a fibroblast-like morphology with no difference between the age groups (Fig. 1a, b). Flow cytometric analysis of the expression of CD90, CD73, and CD105, which are known as general MSC surface markers, demonstrated that KNT cells from the young and elderly groups were positive for CD90, CD73, and CD105 (Fig. 1c, d), but negative for the expression of the hematopoietic markers CD34, CD45, and HLA-DR (Fig. 1c, d). Chromosome analysis revealed normal karyotypes for both young and elderly donor-derived cells (data not shown). KNT cells from the young and elderly individuals were, respectively, analyzed for their multilineage potential using specific differentiation media. Oil Red O-stained droplets and alizarin red-stained calcium deposition were observed, confirming similar levels of differentiation to adipocytes and osteoblasts regardless of donor age (Fig. 2a, b).
Fig. 1.
Phase contrast morphology (a, b) and cell surface markers (c, d) of BM-MSCs derived from young and elderly donors. Flow cytometric analysis of cell surface markers of KNT cells. The results confirmed that KNT cells lacked the expression of the hematopoietic markers CD34, CD45, or HLA-DR, but expressed CD90, CD73, and CD105 relative to their isotype controls (c, d). Scale bar 100 µm. Young KNT (KNT_D_176714) and elderly KNT (KNT_86_170825)
Fig. 2.
Oil red O staining reveals adipogenic differentiation, while alizarin red staining reveals osteogenic differentiation. Young KNT cells (a, KNT_D_170714) and elderly KNT cells (b, KNT_86_170825) can differentiate into adipocytes and osteoblasts. Scale bar 200 µm
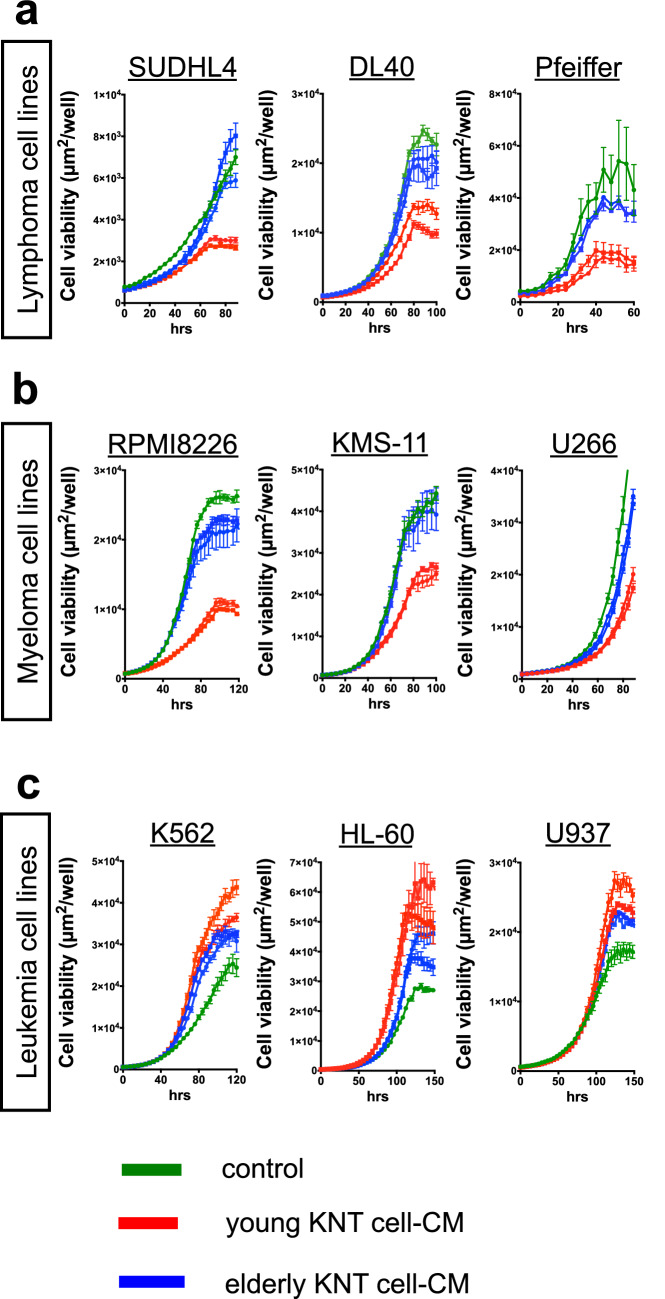
Effect of cell proliferation in the KNT cell-conditioned media (KNT cell-CM) varies among hematologic malignant cells
We investigated the growth of cultured hematopoietic malignant cells after the addition of concentrated KNT cell-CM to elucidate their anti-neoplastic effect. In the lymphoma cell lines (SUDHL4, DL40, and Pfeiffer), the addition of young KNT cell-CM suppressed cell growth (red line, Fig. 3a) compared to the control (green line, no KNT cell-CM). Similarly, in the multiple myeloma cell lines (RPMI8226, KMS-11, and U266), adding young KNT cell-CM suppressed cell growth in a time-dependent manner (red line, Fig. 3b). In contrast, when young KNT cell-CM was added to the myeloid leukemia cell lines (K562, HL-60, and U937), cell growth was enhanced (red line, Fig. 3c). Unlike young KNT cell-CM, however, elderly KNT cell-CM did not affect tumor growth of the lymphoma and myeloma cell lines. Instead, it enhanced the growth of the myeloid leukemia cell lines (blue lines, Fig. 3a–c).
Fig. 3.
Cell viability of various hematologic neoplastic cells in response to the KNT cell-conditioned medium (CM). The green line represents the control (n = 3). CM from young KNT cells [red lines, (n = 3): KNT_D_170714 and KNT_L_170714] inhibited the growth of the lymphoma and myeloma cell lines, but enhanced the growth of the leukemia cell lines. The blue line shows the response to CM from elderly KNT cells [blue lines, (n = 3): KNT_80_170825 and KNT_86_170825]. Values are means ± SD of three independent experiments, with each performed on different days
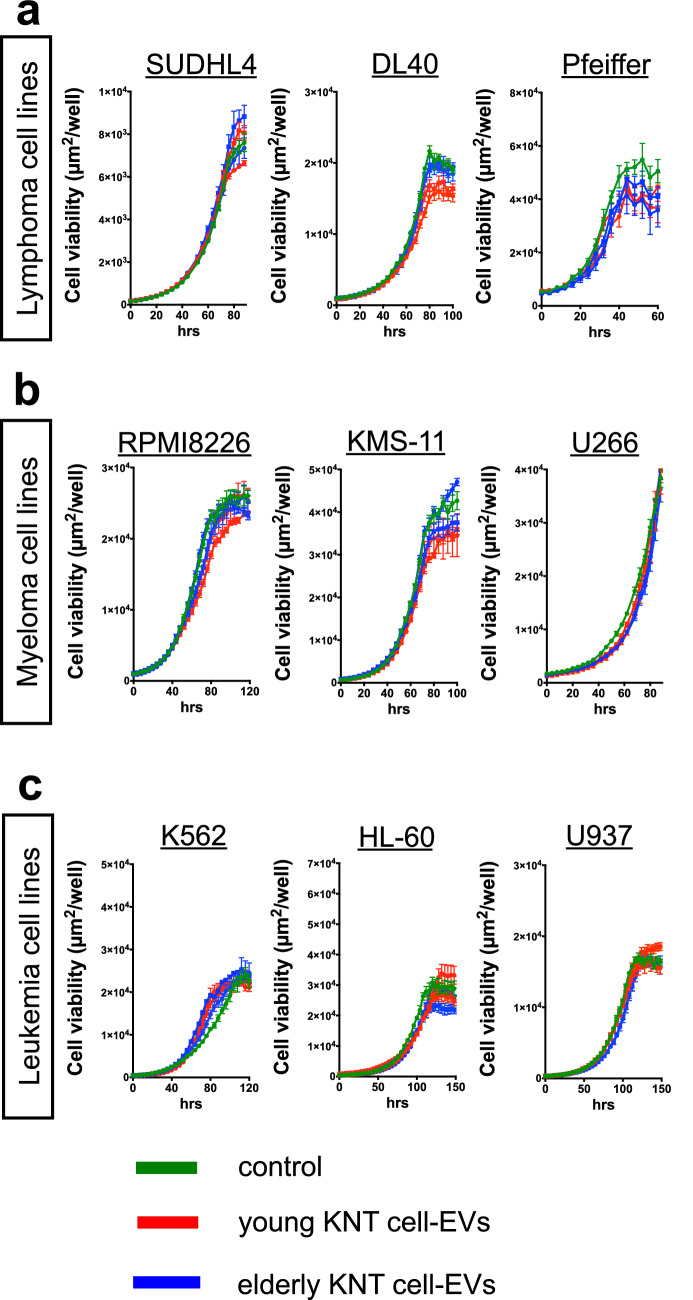
Similar to the analysis of the anti-neoplastic effects of KNT cells, hematopoietic tumor cells were cultured for 5 days after the addition of KNT cell-derived EVs. Unlike KNT cell-CM, neither the young nor the elderly donor’s KNT cell-derived EVs affected cell growth in the hematologic neoplasia cell lines (Fig. 4a–c). Therefore, CM, but not EV, from KNT cells inhibited the growth of the lymphoma and myeloma cell lines, but this effect was limited in young KNT-CM (Table 2).
Fig. 4.
Cell viability of various hematologic neoplastic cells in response to KNT cell-extracellular vesicles (EVs). The green line represents the control. CM from young KNT cells [red lines, (n = 3): KNT_D_170714 and KNT_L_170714] or elderly KNT cells [blue lines, (n = 3): KNT_80_170825 and KNT_86_170825]. Values are means ± SD of three independent experiments, with each performed on different days
Table 2.
Summary of anti-neoplastic or angiogenic effect of KNT cells
| Conditioned medium from | Extracellular vesicles from | |||
|---|---|---|---|---|
| Young KNT | Elderly KNT | Young KNT | Elderly KNT | |
| Effect for neoplastic cell's proliferation | ||||
| Lymphoma cells | Suppressive | Not significant | Not significant | Not significant |
| Myeloma cells | Suppressive | Not significant | Not significant | Not significant |
| Myeloid leukemia cells | Enhance | Enhance | Not significant | Not significant |
| Effect for neoplasia-induced tube formation of human umbilical vessels | ||||
| Lymphoma cells (SUDHL4) | Suppressive (P < 0.0001) | Suppressive (P = 0.0273) | Suppressive (P = 0.0002) | Not significant (P = 0.232) |
| Myeloma cells (RPMI8226) | Suppressive (P < 0.0001) | Not significant (P = 0.0898) | Suppressive (P = 0.0007) | Not significant (P = 0.213) |
| Myeloid leukemia cells (K562) | Suppressive (P < 0.0001) | Suppressive (P = 0.0044) | Suppressive (P = 0.0001) | Not significant (P = 0.387) |
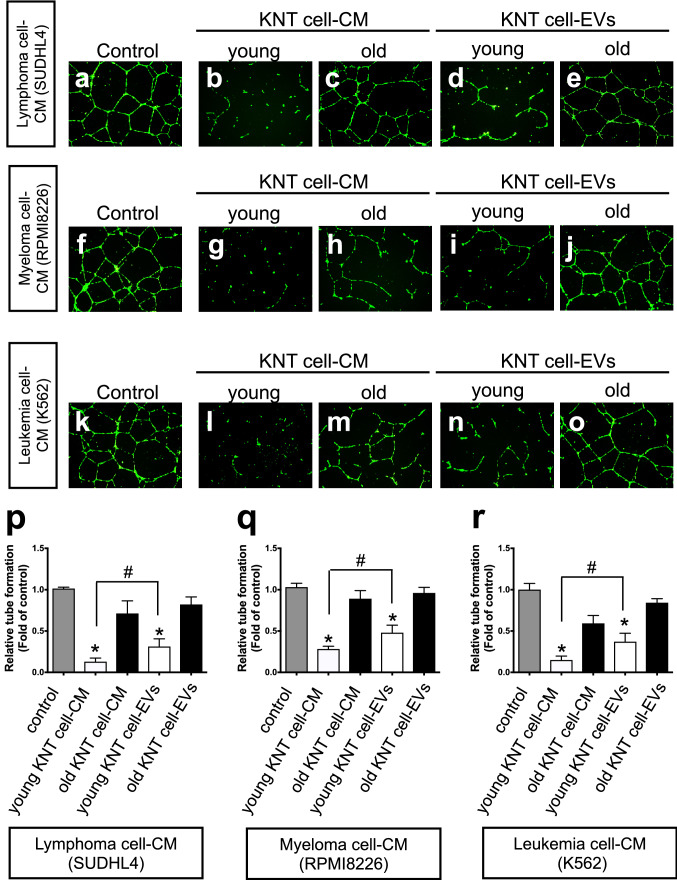
Extracellular vesicles of KNT cells derived from young donors inhibit neoplasia-induced angiogenesis
We investigated the anti-angiogenic effect using a tube-formation assay. Because of the formation of net-like vascular tubes, neoplastic cell-derived CM (control, Fig. 5a, f, k), followed by the CM or EV from KNT cells was added to evaluate angiogenesis (Matrigel + HUVEC + neoplastic cell’s CM with or without KNT-CM or KNT-EV). The addition of CM from young KNT cells, simultaneously with the start of vascular formation, profoundly inhibited tube formation compared to the controls, irrespective of the hematologic neoplastic cell type (Fig. 5b, g, p, q, r: P < 0.0001 vs control). Young KNT cell-derived EVs substantially suppressed neoplasia-induced tube formation (Fig. 5d, i, n), with a more evident suppression level in CM than in EV obtained from KNT cells (Fig. 5p, q, r, #P < 0.01). The suppressive effect was not significant for KNT-EV from elderly individuals in lymphoma (SUDHL4)-induced angiogenesis (Fig. 5p, P = 0.232), myeloma (RPMI8226)-induced angiogenesis (Fig. 5q, P = 0.213), and myeloid leukemia (K562)-induced angiogenesis (Fig. 5r, P = 0.387) (Table 2).
Fig. 5.
The neoplastic cell-induced tube-formation assays. Twenty-four hours after the addition of CM from KNT cells, endothelial cells were stained with calcein AM [green (n = 3)] and visualized under a fluorescence microscope. CM from young KNT cells inhibited angiogenesis in all cell lines. Young KNT (KNT_D_176714) and elderly KNT (KNT_86_170825). Values are means ± SD of three independent experiments, with each performed on different days
Discussion
In the current study, CM derived from KNT cells was found to affect the proliferation of hematologic neoplasia cells. In fact, CM addition suppressed the proliferation of malignant lymphoma and multiple myeloma cells, but enhanced the cell proliferation of myeloid leukemia. However, this proliferative effect on neoplastic cells was noted in CM, but not in EV. This finding indicates that the EV obtained from KNT cells, either from young or elderly donors, may not be able to affect the proliferation of hematologic neoplastic cells, which further indicates that the CM of KNT cells may contain some factors for the proliferation of neoplastic cells.
Previous studies demonstrated that MSCs differentiate into pericytes or tumor-associated fibroblasts (CAF), secreting vascular endothelial growth factor (VEGF) [14, 15], interleukin (IL)-8 [16], tumor growth factor (TGF)-β [14, 17], epidermal growth factor (EGF) [15, 18], and platelet-derived growth factor (PDGF) [14, 19], ultimately forming a tumor growth supporting microenvironment. Besides tumor progression, MSCs can suppress tumor growth through cell cycle arrest and inhibiting proliferation [20–22]. The anti-tumor properties of MSCs isolated from sources in experiments of various tumor models have been discussed [23–25]. These evidences suggest that MSCs secrete some soluble factors that influence cell growth of various types of neoplasia, with differences in proliferative potency based on target neoplastic cells.
BM-MSCs escape the rejection of heterogenous transplantation due to a deficiency in the major histocompatibility complex (MHC) [26, 27]. In addition, the EVs from BM-MSCs with a lipid bilayer display similar immunologic behavior to BM-MSCs. As EVs contain mRNA, miRNA, etc., they carry genetic elements which are transported to adjacent cells via cell-to-cell communication [28–30]. As shown here, although CM from KNT cells affects the proliferation of hematologic neoplastic cells, we cannot deny the possibility that BM-MSCs may affect the growth of minimal residual disease. Therefore, when BM-MSCs may be applied to cancer-bearing patients to control immune surveillance, the proliferative potential of neoplastic cells should be considered. Based on this study, we proposed the potential of applying BM-MSCs-derived EV to minimize the effect of neoplastic proliferation. As a result, we demonstrated that BM-MSCs derived from young donors display more potency in affecting neoplastic proliferation than those from elderly people. The age of donors for immune surveillance is, thus, a further issue that must be resolved to secure the potential of BM-MSCs for clinical application.
We assessed tube formation, representable for neoplasia-induced angiogenesis, is largely depending on donor age of KNT cells. In this study, we applied the concentrated CM derived from various hematologic neoplasias to obtain the neoplasia-induced angiogenesis control. Thereafter, we added the concentrated CM or EV from KNT cells for 24 h to evaluate their effect. Both CM and EV remarkably inhibited neoplasia-induced angiogenesis, irrespective of the types of hematologic neoplasia. Such effect of KNT cells was prominent in young-donor KNT cells, though a limited effect was notable in the CM of elderly KNT cells. Previously, we reported that a microRNA profile in EVs released by BM-MSCs is dependent on donor age [30]. We further attempted to rejuvenate EVs by introducing microRNA-340 to regain their anti-angiogenesis effect [30]. The findings indicated that the donor’s age is critical for the achievement of high therapeutic efficacy in BM-MSC cell therapy from the viewpoint of angiogenesis. In the current study, we demonstrated that young-donor-derived BM-MSC-EV and CM may be prominent anti-angiogenesis agents, irrespective of the type of hematologic neoplasia. We must consider the anti-angiogenesis effect in various types of neoplasia, including solid tumors. In addition, whether or not the EV-containing factors, such as microRNAs, are consistent among different types of neoplasia should be clarified using an enough number of BM-MSC to confirm our results. This is because the angiogenesis-targeted approach is an important issue in additional therapy for solid tumor [31, 32]. In this study, it was difficult to exclude the possibility of gender bias and obtaining enough KNT cell lines for this study proved difficult, since we could use only young males and elderly females BM-MSC due to donor’s intention.
In conclusion, we could demonstrate that the function of KNT cells is heterogenous based on donor’s age and the secreting components. Although our results did not refute the potential clinical usefulness of BM-MSC, we must consider its characteristics, especially for its use in neoplasia-bearing patients.
Acknowledgements
The Department of Advanced Cellular Therapy is an endowment department that is supported by a grant from Kintaro Cells Power Corporation (Tokyo, Japan). This study was also supported in part by the Private University Strategic Research-Based Support Project (S1511011) and in part by a Grant-in-Aid (16K07183, 18K07244) from the Ministry of Education, Culture, Sports, Science, and Technology (Tokyo, Japan).
Author contributions
NG, KO, and JHO designed the research and wrote the paper. NG, TU, and CK performed the research and analyzed the data.
Compliance with ethical standards
Conflict of interest
Nina Gladkova is an employee of Kintaro Cells Power Corporation. Kazuma Ohyashiki received research support from Celgene KK, Chugai Pharmaceutical KK, and Janssen Pharma KK. No declaration on COI for Nina Gladlpva, Tomohiro Umezu, Satoshi Imanishi, Chiaki Kawana, and Junko H. Ohyashiki.
Ethical approval
“The healthy donors BM puncture” was approved by the local ethical committee (approval 5-11-03-2013 of March 03, 2013) at the Federal Research Clinical Center of Federal Medical-Biologic Agency of Russia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nina Gladkova and Tomohiro Umezu have contributed equally as first authors.
Contributor Information
Nina Gladkova, Email: cellspowerlab@gmail.com.
Tomohiro Umezu, Email: t_umezu@tokyo-med.ac.jp.
Satoshi Imanishi, Email: s-ima@tokyo-med.ac.jp.
Chiaki Kawana, Email: chiaki-k@tokyo-med.ac.jp.
Junko H. Ohyashiki, Email: junko@hh.iij4u.or.jp
Kazuma Ohyashiki, Email: ohyashik@rr.iij4u.or.jp.
References
- 1.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 2.Beyer NN, da Silva ML. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–282. doi: 10.1007/3-540-31265-X_11. [DOI] [PubMed] [Google Scholar]
- 3.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 4.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 5.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llufriu S, Sepúlveda M, Blanco Y, et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE One. 2014;9:e113936. doi: 10.1371/journal.pone.0113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Simon JA, López-Villar O, Andreu EJ, et al. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica. 2011;96:1072–1076. doi: 10.3324/haematol.2010.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16:239–253. doi: 10.1016/j.stem.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970–973. doi: 10.1038/leu.2014.41. [DOI] [PubMed] [Google Scholar]
- 10.Bayraktar R, Van Roosbroeck K, Calin GA. Cell-to-cell communication: microRNA as hormones. Mol Oncol. 2017;11:1673–1686. doi: 10.1002/1878-0261.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343–34351. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Mesenchymal stromal cells’ role in tumor microenvironment: involvement of signaling pathways. Cancer Biol Med. 2017;14:129–141. doi: 10.20892/j.issn.2095-3941.2016.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridge SM, Sullivan FJ, Glynm SA. Mesenchymal stromal cells: key players in cancer progression. Mol Cancer. 2017;16:31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Zhou Y, Yang J, et al. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J Exp Clin Cancer Res. 2015;34:52. doi: 10.1186/s13046-015-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, Xiao CH, Tan LD, Wang QS, Li WQ, Feng YM. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signaling. Br J Cancer. 2015;110:724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr Opin Genet Dev. 2015;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Huang F, Wang M, Yang TT, et al. Gastric cancer-derived MSC-secreted PDGF-DD promotes gastric cancer progression. J Cancer Res Clin Oncol. 2014;140:1835–1848. doi: 10.1007/s00432-014-1723-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn JO, Lee HW, Seo KW, Kanfg SK, Ra JC, Youn HY. Anti-tumor effect of adipose tissue derived-mesenchymal stem cells expressing interferon-β and treatment with cicplatin in a xenograft mouse model for canine melanoma. PLoS ONE One. 2013;8:e74897. doi: 10.1371/journal.pone.0074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Block GJ, Ohkouchi S, Fung F, et al. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao L, Xu ZL, Zhao TJ, Ye LH, Zhang XD. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signaling. Cancer Lett. 2008;269:67–77. doi: 10.1016/j.canlet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Khakoo AY, Pati S, Anderson SA, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao L, Xu Z, Zhao T, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 25.Cousin B, Ravet E, Poglio S, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS ONE One. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan JM, Barry FB, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogenic rejection. J Inflamm. 2006;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotech. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci. 2013;14:14240–14269. doi: 10.3390/ijms140714240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteside TL. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin Immunol. 2018;35:69–79. doi: 10.1016/j.smim.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umezu T, Imanishi S, Azuma K, et al. Replenishing exosomes from older bone marrow stromal cells with miR-340 inhibits myeloma-related angiogenesis. Blood Adv. 2017;1:812–823. doi: 10.1182/bloodadvances.2016003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. doi: 10.1038/emm.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JK, Park SR, Jung BK, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by downregulating VEGF expression in breast cancer cells. PLoS ONE One. 2013;8:e84256. doi: 10.1371/journal.pone.0084256. [DOI] [PMC free article] [PubMed] [Google Scholar]