Abstract
Purpose
Although biomarkers for patients with metastatic colorectal cancer exist, the benefit patients with RAS mutated tumors derive from established regimens is unclear.
Methods
Efficacy of therapeutic strategies available for RAS mutated patients (addition of chemotherapeutic agents and/or anti angiogenic agents) were investigated in fourteen randomized controlled phase III trials at trial level by meta-analysing individual study hazard ratios and 95% confidence intervals (95% CI) for overall survival (OS) and progression free survival (PFS).
Results
6810 of 10,748 patients (63.3%) were available (48.5% RAS wildtype, 51.5% RAS mutated). Across all treatment lines, additional treatment efficacy (chemotherapy and/or anti angiogenic agents) was significantly smaller in RAS mutated compared to wildtype tumors for OS and PFS. In detail, patients with RAS mutated metastatic colorectal cancer derived significant benefit in PFS but not in OS by the addition of either chemotherapy or anti angiogenic agents to the respective comparator. In patients with RAS wildtype metastatic colorectal cancer, PFS and OS were improved by the addition of chemotherapy or anti angiogenic agent.
Conclusion
The therapeutic benefit of additional substances is less distinct in patients with RAS mutated as compared to RAS wildtype metastatic colorectal cancer, especially with regard to OS.
Electronic supplementary material
The online version of this article (10.1007/s00432-020-03290-y) contains supplementary material, which is available to authorized users.
Keywords: RAS, Angiogenic, Chemotherapy, Metastatic, Colorectal cancer
Background
The RAS protein is a member of the G protein family and involved in signal transduction within the mitogen activated protein kinases (MAPK) pathway. Genetic alterations lead to constitutive activation of the RAS protein with a high oncogenic potential in metastatic colorectal cancer (mCRC) (Benvenuti et al. 2007; Vogelstein et al. 1988). RAS mutations (MUT) are detected in about 50% of all patients (Cunningham et al. 2004; Jonker et al. 2007; Sobrero et al. 2008). Since 2013, RAS wildtype (WT) status is required for the use of anti-EGFR (epidermal growth factor receptor) agents like cetuximab or panitumumab (Douillard et al. 2013; Heinemann et al. 2014).
As EGF receptor inhibition is ineffective because of constitutive oncogenic signalling, (Benvenuti et al. 2007) systemic treatment option in patients with a RAS MUT tumor currently include chemotherapy (fluoropyrimidines, irinotecan, oxaliplatin) with or without anti angiogenic agents for two treatment lines, followed by later-line treatment such as trifluridine/tipiracil and regorafenib (Grothey et al. 2013; Kubicka et al. 2013; Van Cutsem et al. 2018). For maintenance strategies following induction treatment, a combination of fluoropyrimidine and bevacizumab is usually recommended (Goey et al. 2017; Hegewisch-Becker et al. 2015; Van Cutsem et al. 2016).
Unlike anti-EGFR treatment, predictive biomarkers for the use of cytotoxic and anti angiogenic agents are still missing. A comprehensive efficacy analysis of these treatment strategies in RAS MUT tumors is currently not available.
We, therefore, performed a systematic review and meta-analysis of randomized controlled phase III trials with EMA/FDA approved cytotoxic and anti angiogenic agents to evaluate efficacy of the addition of chemotherapeutics and anti angiogenic agents when distinguished for RAS status, treatment line and investigated agents.
Methods
Trial identification
Our search strategy included trial identification by systematic literature review using the following terms: “metastatic colorectal cancer”, “randomized”, “phase III”, “NOT phase II”, “NOT meta”, “NOT pooled”. First search was performed in February 2019 and last search in November 2019. Only trials with available molecular subgroup analysis regarding (K)RAS status (KRAS exon 2–4, NRAS exon 2–4) were included. Hence, we included randomized controlled phase III trials with available subgroup data for (K)RAS status in mCRC evaluating the addition of chemotherapeutic or anti angiogenic treatment to a randomised control arm with FDA/EMA approved agents. As treatment efficacy should be evaluated according to (K)RAS status, trials with anti-EGFR treatment requiring (K)RAS wildtype status (cetuximab, panitumumab) were excluded. Patients with BRAF mutations were excluded from this analysis if indicated.
Following trials were identified in Pubmed, EMBASE, Web Of Science and the Cochrane Central Register of Controlled Trials (CENTRAL): TRIBE (Cremolini et al. 2015), AVG2107g (Hurwitz et al. 2009), FOCUS (Richman et al. 2009), ML22011 (Modest et al. 2018), AGITG MAX (Price et al. 2015), ML18147 (Kubicka et al. 2013), RAISE (Obermannova et al. 2016), VELOUR (Wirapati et al. 2017), CORRECT (Grothey et al. 2013), CONCUR (Li et al. 2015), RECOURSE (Van Cutsem et al. 2018), AIOKRK0207 (Hegewisch-Becker et al. 2015), CAIRO3 (Goey et al. 2017), PRODIGE 9 (Aparicio et al. 2018). Data were based on publications and/or poster presentations at congress meetings.
Trials
TRIBE and ML22011 investigated chemotherapeutic (de-)escalation strategies on bevacizumab based treatment arms in previously untreated mCRC. The MRC FOCUS trial compared 5-fluorouracil monotherapy to the combination regimes irinotecan/5-fluorouracil (IrFU) and oxaliplatin/5-fluorouracil (OxFU) as first-line therapy of mCRC. In AVG2107g and AGITG MAX, bevacizumab was used additionally to chemotherapy in untreated mCRC. All second-line trials (ML18147, RAISE, VELOUR) investigated the role of additional anti angiogenic agents to chemotherapy in previously treated patients with mCRC. CORRECT and CONCUR compared regorafenib vs. placebo treatment in mCRC. The RECOURSE trial compared single-agent chemotherapy with TAS102 to best supportive care. In maintenance, most trials investigated treatment with angiogenic inhibition compared to no treatment (AIOKRK0207: bevacizumab ± fluoropyrimidine vs. no treatment; CAIRO3: capecitabine + bevacizumab; PRODIGE 9: bevacizumab).
Data items, data collection process and summary measures
Retrospective data (hazard ratio with confidence interval) regarding overall survival (OS) and progression-free survival (PFS) were collected to compare outcome of chemotherapeutic and non-chemotherapeutic treatment addition strategies by (K)RAS status in patients with previously untreated and treated mCRC and by treatment lines at trial level. Control arms were used as reference, meaning that hazard ratios smaller than 1 indicated benefit of the addition of the respective drug to the treatment protocol.
Risk of bias in individual studies
To ascertain the validity of eligible randomized trials, two authors (AS, DPM) determined independently the adequacy of trials regarding phase of trial, presence of molecular subgroup analysis and strategies of additional treatment.
Risk of bias across studies
Primary tumor sidedness, microsatellite instability and type of cytotoxic treatment (FOLFIRI or FOLFOX) were not considered in this analysis and might have affected the cumulative evidence.
Planned methods of analysis
Standard error estimates were deduced from the 95% confidence intervals. Meta-analyses and meta-regression analyses based on the log-hazard rate ratios were performed. Random effect meta-regression models were fitted for all trials, for each treatment line (first, second, later and maintenance lines) and treatment addition (chemotherapeutic vs. anti angiogenic therapy). Interaction effect of RAS mutation type (RAS WT vs. RAS MUT tumors) with treatment addition was assessed. Heterogeneity explained by mutation was assessed by a Wald chi-square test. Residual heterogeneity was determined by computing the Cochran’s Q test (chi-square test) and the I2 statistic with its 95% confidence interval. In case of three-armed trials, correlation of 0.5 was added to treatment effects to integrate repeated comparisons of the control group to different experimental treatment arms into results. Data analysis was structured to resolve complexity of different result layers. In a first step, benefit of therapeutic addition vs. control was investigated for all patients regardless of therapy strategy or molecular subgroups. Subsequent analyses across all treatment lines were performed separately for RAS WT patients and for RAS MUT patients, and then for RAS WT vs. MUT patients, respectively. Within the molecular subgroups, we first compared efficacy of therapeutic addition vs. control regardless of substance classes. Then, benefit of chemotherapeutic and anti angiogenic strategies were analyzed in detail. Finally, each treatment line including maintenance was stratified by RAS WT, RAS MUT and RAS WT vs. MUT and analyzed for treatment efficacy. Weight of the trials was respected by number of trial patients. All tests were two-sided and the significance level was set to 0.05. The analyses were performed using R 3.6.1, particularly packages forestplot and metafor.
Results
Study selection
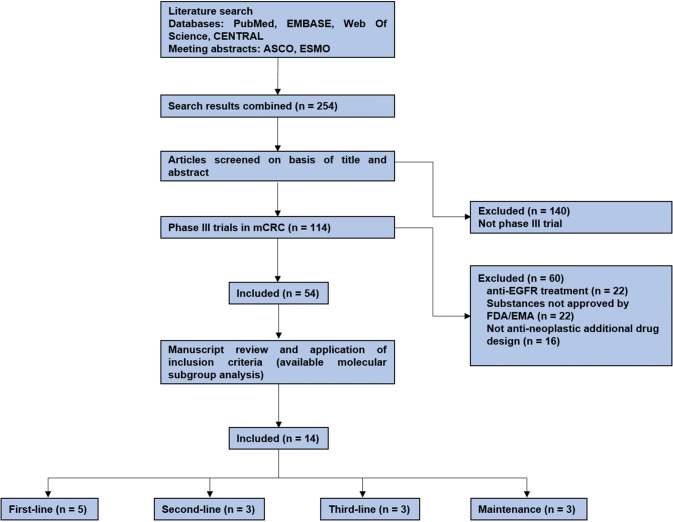
Search terms identified 114 phase III trials in total, of which 60 trials had to be excluded due to anti EGFR treatment (22 trials), testing of substances not approved by FDA/EMA for treatment of metastatic colorectal cancer (22 trials) and trial designs which did not compare an additional anti-neoplastic drug to standard treatment (16 trials). Of these remaining trials, 40 trials did not provide molecular subgroup analyses for (K)RAS status. (Fig. 1).
Fig. 1.
Workflow of trial identification process; ASCO American Society of Clinical Oncology, ESMO European Society of Medical Oncology
Patients
Fourteen trials comprising 10,748 patients were included into the analysis. 6 810 patients (63.3%) were evaluated according to molecular status. (Table 1) Detailed outcome results for each trial in each treatment line according to RAS status were presented in supplementary data. (Online resources 1–4).
Table 1.
Trial characteristics of the analyzed randomized controlled trials
| Trial name | Trial characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase | Treatment line | Control arm | Escalation arm(s) | Target | Randomized | Tumors collected | With RAS wildtype | With RAS mutated | |
| TRIBE | III | First line | FOLFIRI + Bevacizumab | FOLFOXIRI + Bevacizumab | Chemotherapy | 508 | 391 | 93 | 236 |
| AVG2107g | III | First line | IFL | IFL + Bevacizumab | Anti angiogenic | 813 | 230 | 152 | 78 |
| FOCUS | III | First line | 5-FU | IrFU; OxFU | Chemotherapy | 2 135 | 711 | 389 | 300 |
| ML22011 | III | First line | FP + Bevacizumab + Irinotecan | FP + Bevacizumab followed by Irinotecan | Chemotherapy | 421 | 374 | 158 | 194 |
| AGITG-MAX | III | First line | Capecitabine | Capecitabine + Bevacizumab(+ Mitomycin) | Anti angiogenic | 471 | 280 | 171 | 109 |
| ML18147 | III | Second line | Chemotherapy | Chemotherapy + Bevacizumab | Anti angiogenic | 820 | 616 | 316 | 300 |
| RAISE | III | Second line | FOLFIRI | FOLFIRI + Ramucirumab | Anti angiogenic | 1 076 | 1 072 | 542 | 530 |
| VELOUR | III | Second line | FOLFIRI | FOLFIRI + Aflibercept | Anti angiogenic | 1 226 | 482 | 218 | 264 |
| CORRECT | III | Later line | Placebo | Regorafenib | Anti angiogenic | 753 | 729 | 299 | 430 |
| CONCUR | III | Later line | Placebo | Regorafenib | Anti angiogenic | 204 | 143 | 79 | 64 |
| RECOURSE | III | Later line | Best supportive care | Best supportive care + TAS102 | Chemotherapy | 800 | 800 | 393 | 407 |
| AIOKRK0207 | III | Maintenance | No treatment | Bevacizumab | Anti angiogenic | 472 | 335 | 141 | 172 |
| CAIRO3 | III | Maintenance | No treatment | Capecitabine + Bevacizumab | Chemotherapy | 558 | 420 | 140 | 240 |
| PRODIGE 9 | III | Maintenance | No treatment | Bevacizumab | Anti angiogenic | 491 | 375 | 202 | 173 |
RAS rat sarcoma, FOLFIRI 5-fluorouracil/folinic acid/irinotecan, FOLFOXIRI 5-fluorouracil/folinic acid/oxaliplatin/irinotecan, IFL irinotecan/5-fluorouracil/folinic acid, VEGF vascular endothelial growth factor, 5-FU 5-fluorouracil, IrFU irinotecan/5-fluorouracil, OxFU oxaliplatin/5-fluorouracil, FP fluoropyrimidine, TAS102 trifluridin/tipiracil, EGFR epidermal growth factor receptor, ctDNA circulating tumor DNA
Effect of additional treatment agent (chemotherapy and/or anti angiogenic agent)
Across all trials the benefit in overall survival (OS) (HR 0.83 (95% CI 0.78–0.89), p < 0.0001, p for heterogeneity = 0.25) and PFS (HR: 0.60 (95% CI 0.54–0.67), p < 0.0001, p for heterogeneity < 0.0001) was significant.
Efficacy analysis in RAS WT vs. MUT tumors across all treatment lines
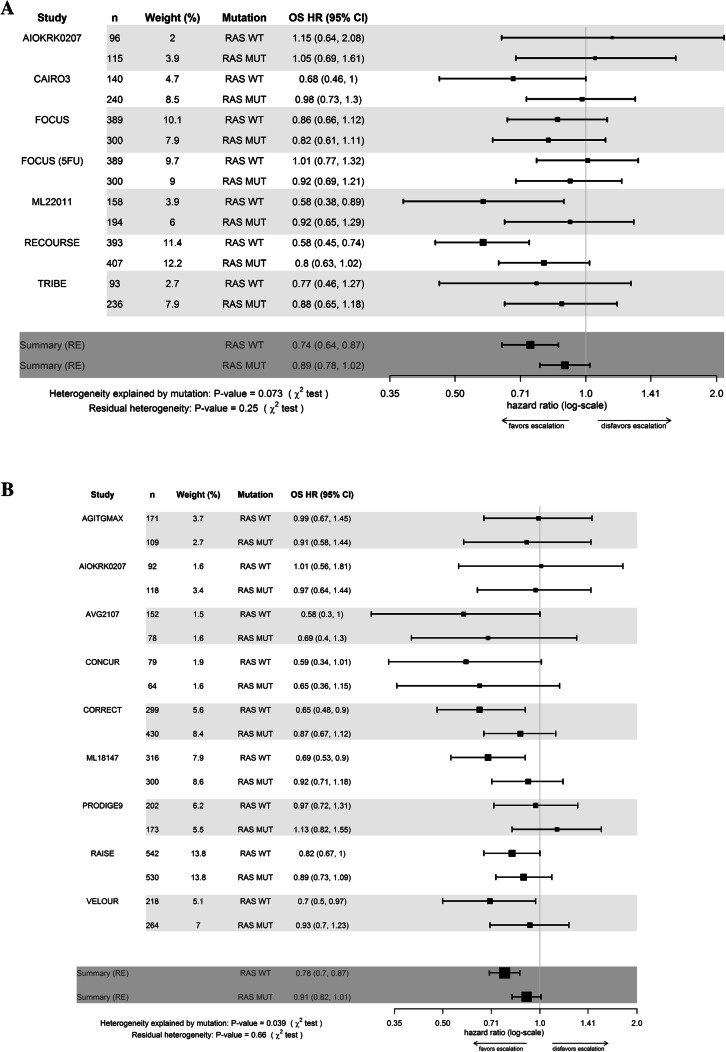
The benefit in OS with the addition of chemotherapeutic and/or anti angiogenic agents was significantly greater in RAS WT tumors as compared to RAS MUT tumors when all studies were analysed together (p for interaction = 0.003). In detail, the effect of the addition of a chemotherapeutic agent was less pronounced in patients with RAS MUT mCRC (WT: HR = 0.74, 95%CI 0.64–0.87; MUT: HR = 0.89, 95% CI 0.78–1.02), p for interaction = 0.07) and the addition of anti angiogenic treatment was significantly less efficient in RAS MUT compared to WT tumors. Interaction of anti angiogenic treatment and RAS status was significant (WT: HR = 0.78, 95% CI 0.70–0.87; MUT: HR = 0.91, 95%CI 0.82–1.01; p for interaction = 0.039).
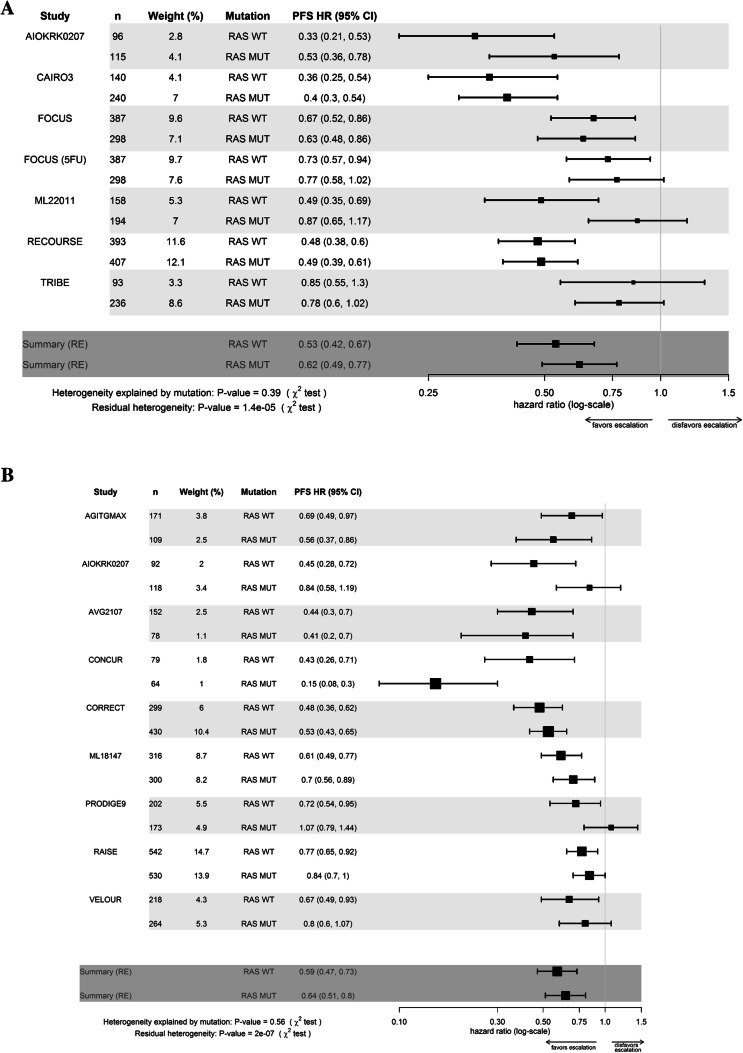
Regarding PFS, the effect of addition of chemotherapeutic and/or anti angiogenic agents was comparable in patients with RAS WT and MUT tumors.. However, heterogeneity was significant when analysing all trials and the subsets of additional chemotherapeutic or anti angiogenic agents (p < 0.0001).]. (Figs. 2, 3, Table 2).
Fig. 2.
a Forest plot of overall treatment effect of chemotherapeutic escalation regarding overall survival for patients with RAS wildtype (WT) and mutated (MUT) tumors b Forest plot of overall treatment effect of escalation by anti angiogenic escalation regarding overall survival for patients with RAS wildtype (WT) and (MUT) tumors. OS overall survival, HR hazard ratio, 95% CI 95% confidence interval, RE random effects model. FOCUS upfront IrFU/OxFU vs. FU. FOCUS 5-FU: sequential 5-FU then IrFU/OxFU vs. FU
Fig. 3.
a Forest plot of overall treatment effect of chemotherapeutic escalation regarding progression free survival for patients with RAS wildtype (WT) and mutated (MUT) tumors b Forest plot of overall treatment effect of escalation by anti angiogenic escalation regarding progression free survival for patients with RAS wildtype (WT) and mutated (MUT) tumors. PFS progression-free survival, HR hazard ratio, 95% CI 95% confidence interval, RE random effects model. FOCUS: upfront IrFU/OxFU vs. FU. FOCUS 5-FU: sequential 5-FU then IrFU/OxFU vs. FU
Table 2.
Efficacy of escalation vs. non-escalation and escalation strategies for OS and PFS (adjusted for trial effect)
| Parameter | Therapeutic escalation vs. non-escalation | Therapeutic escalation strategy | ||||
|---|---|---|---|---|---|---|
| All escalation strategies | Chemotherapy | Anti angiogenic | ||||
| RAS WT | RAS MUT | RAS WT | RAS MUT | RAS WT | RAS MUT | |
| OS | ||||||
| HR (95% CI) [p-value] |
0.74 (0.68–0.82) [< 0.0001] |
0.89 (0.81–0.97) [0.007] |
0.74 (0.64–0.87) [0.0001] |
0.89 (0.78–1.02) [0.098] |
0.78 (0.70–0.87) [< 0.0001] |
0.91 (0.82–1.01) [0.07] |
| log(HR) | − 0.298 | − 0.12 | − 0.29 | − 0.11 | − 0.25 | − 0.095 |
| log(HRMUT)—log(HRWT) | 0.178 | 0.183 | 0.157 | |||
| p value for interaction | 0.003 | 0.07 | 0.039 | |||
| p value for heterogenity | 0.93 | 0.25 | 0.66 | |||
| PFS | ||||||
| HR (95% CI) [p-value] |
0.55 (0.50–0.61) [< 0.0001] |
0.61 (0.56–0.68) [< 0.0001] |
0.53 (0.42–0.67) [< 0.0001] |
0.62 (0.49–0.77) [< 0.001] |
0.59 (0.47–0.73) [< 0.0001] |
0.64 (0.51–0.80) [< 0.0001] |
| log(HR) | − 0.597 | − 0.487 | − 0.63 | − 0.48 | − 0.54 | − 0.443 |
| log(HRMUT)—log(HRWT) | 0.111 | 0.142 | 0.093 | |||
| p value for interaction | 0.093 | 0.39 | 0.56 | |||
| p value for residual heterogenity | 0.029 | < 0.0001 | < 0.0001 | |||
RAS rat sarcoma, WT wildtype, MUT mutated, OS overall survival, PFS progression free survival, log HR natural logarithm of hazard ratio, VEGF vascular endothelial growth factor
Efficacy according to RAS WT or MUT tumors for each treatment line
OS was improved regardless of treatment line in RAS WT patients. In patients with RAS MUT mCRC, the relative improvement of additional treatments was greater in first and later-line treatment, while patients in second-line (p for interaction = 0.07) did not benefit from additional therapy. (Table 3) PFS was improved with the addition of agents in all treatment lines.
Table 3.
Efficacy of therapeutic escalation in each treatment line in RAS WT vs. mut tumors
| Parameter | Treatment lines | |||||||
|---|---|---|---|---|---|---|---|---|
| First line | Second line | Later line | Maintenance | |||||
| RAS WT | RAS MUT | RAS WT | RAS MUT | RAS WT | RAS MUT | RAS WT | RAS MUT | |
| OS | ||||||||
| HR (95% CI) [p-value] |
0.83 (0.71–0.98) [0.026] |
0.87 (0.75–1.02) [0.08] |
0.76 (0.66–0.87) [0.0001] |
0.91 (0.79–1.04) [0.17] |
0.60 (0.50–0.73) [< 0.0001] |
0.81 (0.69–0.96) [0.017] |
0.89 (0.71–1.10) [0.27] |
1.03 (0.86–1.24) [0.72] |
| log(HR) | − 0.19 | − 0.13 | − 0.28 | − 0.096 | − 0.50 | − 0.20 | − 0.12 | 0.03 |
| log(HRMUT)—log(HRWT) | 0.052 | 0.18 | 0.30 | 0.155 | ||||
| p value for interaction | 0.65 | 0.072 | 0.018 | 0.28 | ||||
| p value for heterogenity | 0.57 | 0.85 | 0.88 | 0.75 | ||||
| PFS | ||||||||
| HR (95% CI) [p-value] |
0.64 (0.55–0.73) [< 0.0001] |
0.72 (0.63–0.83) [< 0.0001] |
0.70 (0.60–0.80) [< 0.0001] |
0.78 (0.68–0.91) [0.0009] |
0.47 (0.30–0.72) [0.0005] |
0.39 (0.25–0.61) [< 0.0001] |
0.60 (0.46–0.78) [< 0.0001] |
0.87 (0.69–1.10) [< 0.0001] |
| log(HR) | − 0.45 | − 0.33 | − 0.36 | − 0.242 | − 0.76 | − 0.94 | − 0.66 | − 0.42 |
| log(HRMUT)—log(HRWT) | 0.126 | 0.12 | 0.18 | 0.249 | ||||
| p value for interaction | 0.22 | 0.24 | 0.57 | 0.066 | ||||
| p value for residual heterogenity | 0.08 | 0.38 | 0.01 | < 0.0001 | ||||
RAS rat sarcoma, WT wildtype, MUT mutated, OS overall survival, PFS progression free survival, log HR natural logarithm of hazard ratio
Maintenance options did not improve OS, but PFS with a trend towards higher efficacy in patients with RAS WT compared to MUT tumors (p for interaction = 0.066) (Table 3).
Discussion
Our analysis was motivated by the limited evidence regarding the benefit of adding further treatment to standards (control arms) in RAS MUT mCRC. One prior meta-analysis focussed on the benefit of the addition of bevacizumab to first-line treatment and found significantly prolonged PFS but not OS in currently used treatment regimen containing infusional 5-fluoruracil and irinotecan. However, molecular subgroups were not analysed. (Baraniskin et al. 2019) Therefore, we analysed data from fourteen randomized controlled phase III trials with available molecular subgroup data for RAS testing in mCRC across several treatment lines.
Mutations in KRAS and NRAS genes constitutively activate the RAS G protein with a high oncogenic potential in the MAPK signaling pathway. (Benvenuti et al. 2007) Thus, RAS mutations were often associated with worse prognosis of mCRC—both due to different biology and due to lack of anti-EGFR targeted therapy. (Andreyev et al. 2001, 1998; Barault et al. 2008; Cremolini et al. 2015; Hegewisch-Becker et al. 2018; Modest et al. 2016; Richman et al. 2009).
Generally, the addition of chemotherapeutic and/or anti angiogenic agents demonstrated a significant benefit in patients RAS WT and MUT tumors in our meta analysis in terms of OS and PFS. However, in patients with RAS MUT tumors the benefit in OS with the addition of a new agent across all trials and treatment lines was a modest relative risk reduction for death of 12%. Although statistically significant, it might be argued if 12% can be regarded as clinically meaningful improvement. Overall, the addition of agents to the comparators was significantly more effective in patients with RAS WT tumors when compared to RAS MUT tumors in OS and PFS (see Table 2). This finding may suggest that RAS WT mCRC represents a more treatment sensitive entity in as compared to RAS MUT mCRC independently from anti-EGFR antibody therapy.
When studies investigating chemotherapeutic agents were analysed separately, a trend towards limited efficacy was observed in RAS MUT tumors for OS, but not for PFS. The relative risk reduction in RAS MUT tumors in this respective setting was only 11% for OS compared to 26% in patients with RAS WT mCRC. Importantly, OS benefit from anti angiogenic treatment was significantly smaller in patients with RAS MUT tumors as compared to RAS WT tumors (see Table 2). These results overlap with our findings of less meaningful benefit in second-line treatment, as included second-line trials investigated anti angiogenic treatment only. Overactivation of the MAPK signalling pathway was shown to stimulate angiogenesis VEGF-independently and might be a reason for low efficacy of anti angiogenic treatment in patients with RAS MUT tumors. (Mehta and Besner 2007).
With a detailed view on different treatment lines, later-line treatment (as compared to control) improved OS to a greater extent in patients with RAS WT compared to patients with RAS MUT tumors. Although a certain benefit of later-line therapy was also observed RAS MUT mCRC, the hazard ratio for OS was only 0.81 (see Table 3). This limited efficacy in this treatment setting needs to be considered carefully in the context of short observation time (absolute benefit is very moderate) and resulting adverse effects and their impact on quality of life in end-stage cancer patients.
Concerning maintenance therapy, our meta-analysis included only trials that compared bevacizumab or capecitabine plus bevacizumab to best supportive care (BSC). (Aparicio et al. 2018; Goey et al. 2017; Hegewisch-Becker et al. 2015) A significant effect on overall survival was seen in neither RAS WT nor RAS MUT patients, while PFS trended to be improved in RAS WT mCRC. When stratified by substances, addition of anti angiogenic therapy alone did not improve outcome in the maintenance setting, while the combination of capecitabine and bevacizumab improved OS in patients with RAS wildtype tumors. These results might again strengthen the hypothesis of limited benefit of anti angiogenic therapy in patients with RAS MUT mCRC. Therefore, our findings raise the question if maintenance strategies (instead of treatment holidays) should be promoted in patients with RAS MUT tumors. Compared to active therapy, careful observation may provide a more quality of life friendly approach without significant impairment of outcome in patients with RAS MUT mCRC.
With 6 810 patients, our meta- analysis represents the one of the largest analyses in this setting so far and only randomized trials investigating FDA approved drugs were included. However, several limitations need to be mentioned. As no individual patient data were available, published hazard ratios and confidence intervals had to be obtained from data extraction. Additionally, two trials contained old treatment regimen (IFL and IrFU/OxFU, respectively) that are not recommended anymore (Hurwitz et al. 2009; Richman et al. 2009). Our treatment subgroups contained more anti angiogenic-based studies than chemotherapy investigating trials. In particular, data for studies with chemotherapeutic agents beyond first-line therapy are rare (only one further line trial) (Van Cutsem et al. 2018). This clear relation of treatment lines and substance classes might have biased our observation. In AIOKRK0207, outcome was distinguished between between double wildtype mutational status and any mutation only. Therefore, patients with BRAF MUT tumors might have biased AIOKRK0207 results in our analysis, although the number should be limited. As most of our investigated trials did not distinguish for primary tumor side and microsatellite (in)stability, we were not able to conduct side-related subgroup analyses. Furthermore, potential treatment interaction might have occurred, since irinotecan and oxaliplatin were used for cytotoxic treatment. Lastly, significant heterogeneity was observed for PFS evaluation in some sub-analyses.
Summary
In summary, our meta-analyses suggests that the addition of chemotherapeutic and/or anti angiogenic agents optimizes outcome in RAS WT, but not necessarily in RAS MUT mCRC. Treatment efficacy in RAS MUT compared to WT mCRC was significantly less evident with advancing treatment lines. Furthermore, in this analysis, maintenance options improved neither OS nor PFS in patients with RAS MUT tumors. Although anti angiogenic therapy is available irrespective of RAS status, our overall analysis demonstrates meaningful efficacy predominantly in RAS wildtype mCRC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Open Access funding provided by Projekt DEAL. We thank all patients and their families of the analysed trials for their contribution to our research.
Author contributions
AS and DPM performed mainly literature review, data acquisition and manuscript writing. IR performed statistical analysis of data and designed the figures VH and SS provided substantial contribution to the conception and design of this investigation, reviewed the manuscript critically and provided valuable expertise for medical writing JCE, MM, CGJ, CBW, KH, LMP and IJ provided substantial contribution to data acquisition and interpretation.
Funding
No funding was received for this work.
Data availability
All data and material are held by the authors’ institution and may be available upon request.
Compliance with ethical standards
Conflict of interest
Arndt Stahler received honoria for talks by Roche and reimbursement for travel by Roche, Amgen and MSD Sharp and Dohme. C. Benedikt Westphalen and Clemens Gießen-Jung received honoria for talks and reimbursement for travel by Roche. LMP received reimbursement for travel by Merck. Volker Heinemann, Sebastian Stintzing and Dominik Paul Modest received honoria for talks, advisory boards and reimbursement for travel by Amgen, Merck, Roche, Takeda, Servier, Pierre Fabre, Taiho, Lilly Oncology, Servier, Sanofi and Bayer Pharmaceuticals.
Ethical approval
The trials included in this analysis were approved by their respective ethics committee.
Consent to participate
All patients consented to participate in the respective clinical trials.
Consent for publication
All authors approved the final version of the manuscript for publication.
Code availability
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA (1998) Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst 90:675–684 [DOI] [PubMed] [Google Scholar]
- Andreyev HJ et al (2001) Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer 85:692–696. 10.1054/bjoc.2001.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio T et al (2018) Bevacizumab maintenance versus no maintenance during chemotherapy-free intervals in metastatic colorectal cancer: a randomized phase III trial (PRODIGE 9). J Clin Oncol 36:674–681. 10.1200/JCO.2017.75.2931 [DOI] [PubMed] [Google Scholar]
- Baraniskin A, Buchberger B, Pox C, Graeven U, Holch JW, Schmiegel W, Heinemann V (2019) Efficacy of bevacizumab in first-line treatment of metastatic colorectal cancer: a systematic review and meta-analysis. Eur J Cancer 106:37–44. 10.1016/j.ejca.2018.10.009 [DOI] [PubMed] [Google Scholar]
- Barault L et al (2008) Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer 122:2255–2259. 10.1002/ijc.23388 [DOI] [PubMed] [Google Scholar]
- Benvenuti S et al (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643–2648. 10.1158/0008-5472.CAN-06-4158 [DOI] [PubMed] [Google Scholar]
- Cremolini C et al (2015) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16:1306–1315. 10.1016/S1470-2045(15)00122-9 [DOI] [PubMed] [Google Scholar]
- Cunningham D et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345. 10.1056/NEJMoa033025 [DOI] [PubMed] [Google Scholar]
- Douillard JY et al (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369:1023–1034. 10.1056/NEJMoa1305275 [DOI] [PubMed] [Google Scholar]
- Goey KKH et al (2017) Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Ann Oncol 28:2128–2134. 10.1093/annonc/mdx322 [DOI] [PubMed] [Google Scholar]
- Grothey A et al (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312. 10.1016/S0140-6736(12)61900-X12657 [DOI] [PubMed] [Google Scholar]
- Hegewisch-Becker S et al (2015) Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): a randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 16:1355–1369. 10.1016/S1470-2045(15)00042-X [DOI] [PubMed] [Google Scholar]
- Hegewisch-Becker S et al (2018) Impact of primary tumour location and RAS/BRAF mutational status in metastatic colorectal cancer treated with first-line regimens containing oxaliplatin and bevacizumab: Prognostic factors from the AIO KRK0207 first-line and maintenance therapy trial. Eur J Cancer 101:105–113. 10.1016/j.ejca.2018.06.015 [DOI] [PubMed] [Google Scholar]
- Heinemann V et al (2014) FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 15:1065–1075. 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- Hurwitz HI, Yi J, Ince W, Novotny WF, Rosen O (2009) The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 14:22–28. 10.1634/theoncologist.2008-0213 [DOI] [PubMed] [Google Scholar]
- Jonker DJ et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048. 10.1056/NEJMoa071834 [DOI] [PubMed] [Google Scholar]
- Kubicka S et al (2013) Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol 24:2342–2349. 10.1093/annonc/mdt231 [DOI] [PubMed] [Google Scholar]
- Li J et al (2015) Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial Lancet. Oncol 16:619–629. 10.1016/S1470-2045(15)70156-7 [DOI] [PubMed] [Google Scholar]
- Mehta VB, Besner GE (2007) HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors 25:253–263. 10.1080/08977190701773070 [DOI] [PubMed] [Google Scholar]
- Modest DP et al (2016) Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol 27:1746–1753. 10.1093/annonc/mdw261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modest DP et al (2018) Sequential versus combination therapy of metastatic colorectal cancer using fluoropyrimidines, irinotecan, and bevacizumab: a randomized, controlled study-XELAVIRI (AIO KRK0110). J Clin Oncol. 10.1200/JCO.18.00052 [DOI] [PubMed] [Google Scholar]
- Obermannova R et al (2016) Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol 27:2082–2090. 10.1093/annonc/mdw402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ et al (2015) Correlation of extended RAS and PIK3CA gene mutation status with outcomes from the phase III AGITG MAX STUDY involving capecitabine alone or in combination with bevacizumab plus or minus mitomycin C in advanced colorectal cancer. Br J Cancer 112:963–970. 10.1038/bjc.2015.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman SD et al (2009) KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol 27:5931–5937. 10.1200/JCO.2009.22.4295 [DOI] [PubMed] [Google Scholar]
- Sobrero AF et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319. 10.1200/JCO.2007.13.1193 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386–1422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- Van Cutsem E et al (2018) The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. Eur J Cancer 90:63–72. 10.1016/j.ejca.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B et al (1988) Genetic alterations during colorectal-tumor development. N Engl J Med 319:525–532. 10.1056/NEJM198809013190901 [DOI] [PubMed] [Google Scholar]
- Wirapati P et al (2017) Velour trial biomarkers update: Impact of RAS, BRAF, and sidedness on aflibercept activity. J Clin Oncol 35:3538–3538. 10.1200/JCO.2017.35.15_suppl.353828862883 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and material are held by the authors’ institution and may be available upon request.