Abstract
Introduction
J-DISCOVER aims to research the treatment reality of Japanese patients with type 2 diabetes mellitus who begin second-line treatment. Here we report baseline characteristics and factors associated with selection of second-line treatment.
Methods
J-DISCOVER is a prospective, observational, multicenter, cohort study in patients with type 2 diabetes (aged ≥ 20 years) beginning second-line treatment after first-line oral monotherapy. Baseline characteristics and treatment patterns were descriptively summarized. Logistic regression models were used to identify factors associated with specific second-line treatments.
Results
A total of 1806 patients (mean age 61.7 years) were enrolled between September 2014 and December 2015. Mean ± standard deviation of baseline glycated hemoglobin (HbA1c) and body mass index (BMI) were 7.7 ± 1.3% and 25.5 ± 4.6 kg/m2, respectively. The most prescribed medication as first-line treatment was dipeptidyl peptidase 4 inhibitors (53.7% of patients) followed by biguanides (21.4%), sulfonylureas (7.2%), and alpha-glucosidase inhibitors (6.8%). Second-line treatments included dipeptidyl peptidase 4 inhibitors (31.0%), biguanides (27.9%), sodium–glucose cotransporter 2 inhibitors (12.2%), and sulfonylureas (10.9%). First- and second-line treatments had different modes of action in 76.3% of patients. Those receiving first-line dipeptidyl peptidase 4 inhibitors were more likely to receive second-line biguanides and vice versa. Selection of second-line treatment was also associated with age, BMI, HbA1c, and renal function.
Conclusions
This study showed the treatment reality and factors associated with choice of second-line treatment in Japanese patients with type 2 diabetes mellitus. The choice of second-line treatment was associated with age, BMI, HbA1c, renal function, and the mode of action of the first-line treatment.
Trial Registration
ClinicalTrials.gov NCT02226822.
Electronic Supplementary Material
The online version of this article (10.1007/s13300-020-00846-6) contains supplementary material, which is available to authorized users.
Keywords: Antidiabetic drugs, Physician prescribing patterns, Type 2 diabetes mellitus
Key Summary Points
| Why carry out this study? |
| There is no clear recommendation on the choice of oral glucose-lowering agents in Japanese guidelines. |
| Because there have been no large prospective studies to analyze treatment patterns by physicians (including primary care physicians who are not specialized in diabetes) since the introduction of dipeptidyl peptidase 4 inhibitors (DPP4is) and sodium–glucose cotransporter 2 inhibitors (SGLT2is), it would be beneficial to better understand the treatment reality for Japanese patients with type 2 diabetes (T2DM). |
| J-DISCOVER aimed to prospectively research treatment patterns and long-term glucose control of patients with T2DM who were being initiated on second-line diabetes treatment at sites across Japan since the launch of SGLT2is so that reference data on contemporary treatment patterns of patients with T2DM would be provided. This manuscript aimed to report baseline characteristics and factors associated with the selection of second-line treatment in the patients enrolled in the J-DISCOVER study. |
| What was learned from the study? |
| The most prescribed medication as first-line treatment was DPP4is followed by metformin, reflecting the absence of specific treatment recommendation in Japanese guidelines compared with US and European guidelines that explicitly specify metformin as first-line treatment. The choice of second-line treatment was associated with age, body mass index, glycated hemoglobin, renal function, and the mode of action of the first-line treatment. |
| The results of this study indicate that physicians chose second-line treatment by considering the mode of action of first-line treatment, as well as patients’ background characteristics and clinical conditions. |
Introduction
In 2017, the number of adults (aged 20–79 years) with diabetes worldwide was estimated to be 425 million and approximately 90% of these had type 2 diabetes (T2DM) [1]. In Japan, approximately 7.2 million adults have diabetes and there are around 70,000 diabetes-related deaths annually [1].
While US and European guidelines specify first-line metformin [2, 3], current Japanese guidelines do not recommend a specific therapy for patients with T2DM; instead, it is recommended that treatment is chosen according to the patient’s background and clinical conditions [4, 5]. Diabetes is a progressive disease and, after initial monotherapy, the majority of patients will require combination therapy. By analyzing Japanese prescription data from 2011, Fujihara et al. [6] reported that 26% of adult patients with T2DM (N = 33,251) were taking oral monotherapy; 18% received insulin monotherapy, while the rest (56%) were treated with combination therapy. Japanese guidelines recommend that the selection of second-line medication for patients who are not achieving glycated hemoglobin (HbA1c) targets should be based on the individual patient’s disease state, age, weight, comorbidities, and the modes of action of each therapy [4].
The availability of dipeptidyl peptidase 4 inhibitors (DPP4is) from 2009 and sodium–glucose cotransporter 2 inhibitors (SGLT2is) from 2014 has given physicians increased choice of prescriptions for T2DM treatment. However, there have been no large prospective studies to analyze treatment patterns since the introduction of these drugs. Furthermore, large-scale studies in Japanese patients with T2DM have been conducted mainly in hospitals or clinics with diabetes specialists [7–9], whereas in reality many patients are treated by physicians (including primary care physicians) without expertise in diabetes.
As part of the multinational DISCOVER study [10, 11], J-DISCOVER aimed to prospectively research treatment patterns and long-term glucose control of patients with T2DM who were being initiated on second-line diabetes treatment at sites across Japan since the launch of SGLT2is so that reference data on contemporary treatment patterns of patients with T2DM would be provided [12]. Importantly, the study was not limited to physicians specializing in diabetes. Because Japanese guidelines recommend physicians use their own judgment to choose first-line or later treatments, it would be beneficial to study factors associated with treatment patterns, and to better understand the treatment reality for Japanese patients with T2DM. In this paper, we report the baseline characteristics and factors associated with the selection of second-line treatment in the patients enrolled in the J-DISCOVER study.
Methods
Study Design
The J-DISCOVER study design and methods have been published previously [12]. Briefly, J-DISCOVER (ClinicalTrials.gov NCT02226822) was a 3-year, single-country, multicenter, prospective, observational, longitudinal, cohort study in patients with T2DM who were initiating second-line treatment. The primary objective of J-DISCOVER was to describe the long-term disease management patterns, including number and type of medications prescribed and other management approaches used, and the clinical evolution of these patients, including the clinical course of their diabetes (HbA1c levels and frequency of hypoglycemia), the development and outcomes of any diabetes-related complications, and their quality of life. Secondary objectives included assessment of treatment response (overall and specific to individual classes of medication), treatment changes, and diabetes-associated complications. As this was a non-interventional study, investigators did not proactively collect safety data. Adverse drug reactions were reported as per local regulations.
J-DISCOVER enrolled male and female patients (aged ≥ 20 years) with a diagnosis of T2DM who were initiating a second oral or parenteral antidiabetic medication (adding or switching) after first-line oral monotherapy. Patients were excluded from the study if they were prescribed an injectable agent first line; however, patients who initially received short-term insulin treatment followed by oral treatment were eligible if the treatment with insulin lasted no more than 2 weeks and occurred at least 6 months before initiation of second-line treatment. Because all patients were already receiving treatment, there were no strict inclusion criteria based on HbA1c. A full explanation of the definitions and diagnostic criteria for T2DM, as well full inclusion and exclusion criteria and treatment targets, are outlined in the Supplementary Methods (online supporting information).
All procedures followed will be in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients included in the study. The full list of institutional review boards/ethics committees that approved this study can be found in the Supplementary Material.
In this study, the patients were enrolled from hospitals and clinics. According to the Health Care Act in Japan, a “hospital” is defined as “a medical institution with an inpatient facility of 20 beds or more” and “clinics” have no beds or not more than 19 beds. Although endocrine/diabetes specialists can work both in clinics and hospitals in Japan, hospitals are mainly responsible for the treatment of serious diseases and for precise examinations. On the other hand, the clinics focus on treating common diseases, minor injuries, and chronic diseases. Additionally, the physicians included diabetes specialists and general physicians (GPs) without expertise in diabetes. Japan provides universal coverage of insurance and patients can visit any medical institution (hospital or clinic) as they wish. Therefore, patients with diabetes are treated by both specialists and GPs. In general, diabetes specialists see patients who have difficulty in glycemic control or have multiple complications and are referrals from GPs. On the other hand, patients whose conditions are stable are often referred back to GPs.
Statistical Analyses
Demographic variables, patient characteristics, and treatment patterns were summarized using descriptive statistics including numbers, percentages, mean (standard deviation [SD]), and median (interquartile range [IQR]). Univariate and multivariate Firth logistic regression models were used to identify baseline characteristics associated with the choice of a specific second-line treatment [13]. Covariates were age (≥ 65 vs < 65 years), sex, baseline HbA1c (continuous variable, per 1% increment), body mass index (BMI) (< 22 and ≥ 25 vs ≥ 22 to < 25 kg/m2), renal function (estimated glomerular filtration rate [eGFR] < 60 vs ≥ 60 mL/min/1.73 m2), history of cardiovascular disease (yes vs no), and first-line antihyperglycemic medication (biguanides, sulfonylureas, alpha-glucosidase inhibitors [α-GIs], thiazolidinediones, glinides, DPP4is, SGLT2is). The odds ratio of first-line antihyperglycemic medication compared with the overall mean was calculated using effect coding, a commonly used method for coding categorical variables in regression models [14]. Cardiovascular disease history was defined as a medical history including myocardial infarction, coronary artery bypass grafting, coronary intervention, stable angina, unstable angina, heart failure, stroke, transient ischemic attack, carotid artery stenting, carotid endarterectomy, peripheral artery disease, peripheral artery bypass graft, venous thromboembolism, or thrombolytic therapy.
Results
Patient Characteristics
We analyzed data for 1806 patients (mean age 61.7 years, 61.6% male) enrolled from September 2014 to December 2015 (Fig. 1 and Table 1). Patients were enrolled from 142 sites across Japan, including hospitals (19.8%) or clinics (79.4%) and the majority of physicians in charge were endocrine/diabetes specialists (68.4%). Mean ± SD HbA1c, BMI, and eGFR were 7.7 ± 1.3%, 25.5 ± 4.6 kg/m2, and 78.8 ± 20.5 mL/min/1.73 m2, respectively, and 11.4% of patients had history of cardiovascular disease (Table 1). The proportion of patients with HbA1c ≥ 7% and ≥ 8% were 70.9% and 28.7%, respectively, while the proportions of those with a BMI of ≥ 25 kg/m2 and ≥ 22 to < 25 kg/m2 were 49.3% and 29.5%, respectively (Table 1).
Fig. 1.
Patient flow chart
Table 1.
Baseline demographic and clinical characteristics of patients enrolled in the J-DISCOVER study
| Characteristic | N = 1806 |
|---|---|
| Age, mean (SD), years | 61.7 (12.8) |
| Male, n (%) | 1113 (61.6) |
| Smoking history | |
| Non-smoking, n (%) | 780 (43.2) |
| Smoked in the past, n (%) | 507 (28.1) |
| Smoking, n (%) | 447 (24.8) |
| Unknown, n (%) | 71 (3.9) |
| Time since diagnosis, median (IQR), years | 3.1 (0.8–7.2) |
| HbA1c, mean (SD), % | 7.7 (1.3) |
| HbA1c ≥ 7%, n (%) | 1281 (70.9) |
| HbA1c ≥ 8%, n (%) | 519 (28.7) |
| Severe hypoglycemia in the past 12 months, n (%) | 9 (0.5) |
| Mild hypoglycemia in the past month, n (%) | 18 (1.0) |
| BMI, mean (SD), kg/m2 | 25.5 (4.6) |
| BMI ≥ 25 kg/m2, n (%) | 890 (49.3) |
| BMI ≥ 22 and < 25 kg/m2, n (%) | 532 (29.5) |
| BMI < 22 kg/m2, n (%) | 373 (20.7) |
| Systolic blood pressure, mean (SD), mmHg | 131.5 (15.7) |
| Diastolic blood pressure, mean (SD), mmHg | 76.8 (11.4) |
| Hypertension, n (%) | 988 (54.7) |
| Hyperlipidemia, n (%) | 1063 (58.9) |
| Liver dysfunction, n (%) | 174 (9.6) |
| Cardiovascular disease, n (%) | 205 (11.4) |
| Heart failure, n (%) | 37 (2.0) |
| Serum creatinine, mean (SD), mg/dL | 0.75 (0.25) |
| eGFR, mean (SD), mL/min/1.73 m2 | 78.8 (20.5) |
| eGFR < 60 mL/min/1.73 m2, n (%) | 210 (11.6) |
| Chronic kidney disease, n (%) | 252 (14.0) |
| Duration of first-line treatment, median (IQR), months | 11.0 (4.0–32.0) |
| Second-line treatment | |
| Added-on, n (%) | 1552 (85.9) |
| Switched, n (%) | 254 (14.1) |
BMI body mass index, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, IQR interquartile range
Treatment Patterns
The median duration of first-line treatment was 11 months (Table 1); the most commonly used class of oral first-line treatment was DPP4is (53.7% of patients), followed by biguanides (21.4%), sulfonylureas (7.2%), and α-GIs (6.8%; Table 2).
Table 2.
First- and second-line treatment prescribed for patients enrolled in the J-DISCOVER study (N = 1806)
| Seconda | First | ||||||
|---|---|---|---|---|---|---|---|
| BG, n = 387 (21.4%) | SU, n = 130 (7.2%) | α-GI, n = 122 (6.8%) | TZD, n = 62 (3.4%) | Glinide, n = 64 (3.5%) | DPP4i, n = 970 (53.7%) | SGLT2i, n = 71 (3.9%) | |
| BG, n = 504 (27.9%) | – | 27 | 17 | 19 | 11 | 410 | 20 |
| SU, n = 196 (10.9%) | 14 | – | 1 | 1 | 0 | 180 | 0 |
| α-GI, n = 103 (5.7%) | 15 | 4 | – | 0 | 4 | 79 | 1 |
| TZD, n = 116 (6.4%) | 11 | 5 | 4 | – | 2 | 94 | 0 |
| Glinide, n = 87 (4.8%) | 5 | 0 | 6 | 0 | – | 71 | 5 |
| DPP4i, n = 560 (31.0%) | 264 | 88 | 87 | 31 | 46 | – | 44 |
| SGLT2i, n = 221 (12.2%) | 72 | 6 | 7 | 8 | 1 | 127 | – |
| GLP-1RA, n = 6 (0.3%) | 2 | 0 | 0 | 2 | 0 | 2 | 0 |
| Insulin, n = 13 (0.7%) | 4 | 0 | 0 | 1 | 0 | 7 | 1 |
α-GI alpha-glucosidase inhibitors, BG biguanides, DPP4i dipeptidyl peptidase 4 inhibitors, GLP-1RA glucagon-like peptide 1 receptor agonists, SGLT2i sodium–glucose cotransporter 2 inhibitors, SU sulfonylureas, TZD thiazolidinediones
aSecond-line treatment contains both added-on and switched treatment
Second-line treatment was initiated a median of 3.1 years after diagnosis of T2DM; in the majority of patients (85.9%), the second-line agent was added on top of first-line treatment, rather than switching from first-line (Table 1). The most frequently used second-line treatment was DPP4is (31.0%), followed by biguanides (27.9%), SGLT2is (12.2%), and sulfonylureas (10.9%; Table 2). Combining the data for first- and second-line treatment, DPP4is were prescribed the most (1530 patients, 84.7%), followed by biguanides (891 patients, 49.3%), sulfonylureas (326 patients, 18.1%), and SGLT2is (292 patients, 16.2%; Fig. 2). In patients who received DPP4is first-line (n = 970), the most common second-line agents were biguanides (410 patients, 42.3%), sulfonylureas (180 patients, 18.6%), and SGLT2is (127 patients, 13.1%; Table 2). In those who initially received biguanides (n = 387), DPP4is were prescribed second-line in 264 patients (68.2%) and SGLT2is in 72 patients (18.6%; Table 2). The most frequently used second-line treatments in patients who were prescribed sulfonylureas first-line (n = 130) were DPP4is (88 patients, 67.7%), followed by biguanides (27 patients, 20.8%; Table 2). Although there were no major differences in the prescribing patterns between hospitals and clinics, biguanides were prescribed more frequently as a first-line treatment in hospitals than clinics (33.2% vs 18.7%; Supplementary Tables S1 and S2). Diabetes specialists prescribed more first-line α-GIs than non-specialists (7.8% vs 4.1%) (Supplementary Tables S3 and S4).
Fig. 2.

Antihyperglycemic agents used first- and second-line by patients enrolled in the J-DISCOVER study (N = 1806). α-GI alpha-glucosidase inhibitors, BG biguanides, DPP4i dipeptidyl peptidase 4 inhibitors, GLP-1RA glucagon-like peptide 1 receptor agonists, SGLT2i sodium–glucose cotransporter 2 inhibitors, SU sulfonylureas, TZD thiazolidinediones
Next, we focused on the mode of action of the treatment. In Japan, oral hypoglycemic agents are divided into three groups, i.e., insulin-sensitizing agents (biguanides/thiazolidinediones), insulin secretagogues (sulfonylureas/glinides/DPP4is), and carbohydrate absorption/excretion-modulating agents (α-GIs/SGLT2is) [4]. In 76.3% of patients, first- and second-line agents had different modes of action; first/second-line combinations were an insulin-sensitizing agent and an insulin secretagogue in 48.3% of patients, an insulin secretagogue and a carbohydrate absorption/excretion-modulating agent in 20.4% of patients, and an insulin-sensitizing agent and a carbohydrate absorption/excretion-modulating agent in 7.6% of patients.
Factors Associated with Choice of Second-Line Treatment
An overview of baseline demographic and clinical characteristics by second-line treatment is presented in Table 3. Mean HbA1c was considerably higher in patients prescribed insulin second-line (11.7%) than in those prescribed another second-line treatment (7.3–8.3%). The percentage of patients with a BMI ≥ 25 kg/m2 was considerably higher in patients prescribed SGLT2is second-line (78.7%) and glucagon-like peptide 1 receptor agonists (GLP-1RAs) second-line (66.7%) than in those prescribed other second-line treatments (33.3–53.8%; Table 3).
Table 3.
Baseline demographic and clinical characteristics by second-line treatment
| BG (n = 504) | SU (n = 196) | α-GI (n = 103) | TZD (n = 116) | Glinide (n = 87) | DPP4i (n = 560) | SGLT2i (n = 221) | GLP-1RA (n = 6) | Insulin (n = 13) | Total (n = 1806) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, mean (SD), years | 60.2 (11.7) | 62.9 (13.6) | 64.5 (10.9) | 66.0 (11.2) | 64.5 (12.2) | 63.2 (13.4) | 55.4 (12.5) | 59.7 (11.4) | 57.7 (12.8) | 61.7 (12.8) |
| Male, n (%) | 305 (60.5) | 126 (64.3) | 62 (60.2) | 64 (55.2) | 64 (73.6) | 355 (63.4) | 125 (56.6) | 3 (50.0) | 9 (69.2) | 1113 (61.6) |
| HbA1c, mean (SD), % | 7.6 (1.3) | 8.3 (1.5) | 7.5 (1.2) | 7.3 (1.0) | 7.6 (1.1) | 7.6 (1.2) | 7.8 (1.4) | 7.5 (1.2) | 11.7 (2.0) | 7.7 (1.3) |
| BMI, mean (SD), kg/m2 | 25.4 (4.1) | 24.5 (4.7) | 24.8 (4.1) | 25.3 (3.9) | 23.8 (3.9) | 25.1 (4.5) | 29.0 (5.3) | 28.5 (8.7) | 25.3 (4.3) | 25.5 (4.6) |
| BMI ≥ 25 kg/m2, n (%) | 251 (49.8) | 74 (37.8) | 48 (46.6) | 54 (46.6) | 29 (33.3) | 249 (44.5) | 174 (78.7) | 4 (66.7) | 7 (53.8) | 890 (49.3) |
| BMI ≥ 22 to < 25 kg/m2, n (%) | 150 (29.8) | 66 (33.7) | 25 (24.3) | 42 (36.2) | 29 (33.3) | 185 (33.0) | 31 (14.0) | 1 (16.7) | 3 (23.1) | 532 (29.5) |
| BMI < 22 kg/m2, n (%) | 98 (19.4) | 56 (28.6) | 30 (29.1) | 20 (17.2) | 29 (33.3) | 123 (22.0) | 13 (5.9) | 1 (16.7) | 3 (23.1) | 373 (20.7) |
| Severe hypoglycemia in the past 12 months, n (%) | 1 (0.2) | 2 (1.0) | 2 (1.9) | 0 | 1 (1.1) | 2 (0.4) | 1 (0.5) | 0 | 0 | 9 (0.5) |
| Mild hypoglycemia in the past month, n (%) | 2 (0.4) | 2 (1.0) | 1 (1.0) | 1 (0.9) | 1 (1.1) | 9 (1.6) | 2 (0.9) | 0 | 0 | 18 (1.0) |
| Hypertension, n (%) | 238 (47.2) | 104 (53.1) | 57 (55.3) | 73 (62.9) | 55 (63.2) | 317 (56.6) | 136 (61.5) | 4 (66.7) | 4 (30.8) | 988 (54.7) |
| Hyperlipidemia, n (%) | 306 (60.7) | 96 (49.0) | 61 (59.2) | 78 (67.2) | 49 (56.3) | 328 (58.6) | 132 (59.7) | 4 (66.7) | 9 (69.2) | 1063 (58.9) |
| Liver dysfunction, n (%) | 44 (8.7) | 18 (9.2) | 9 (8.7) | 12 (10.3) | 10 (11.5) | 54 (9.6) | 27 (12.2) | 0 | 0 | 174 (9.6) |
| CVD, n (%) | 54 (10.7) | 27 (13.8) | 14 (13.6) | 11 (9.5) | 15 (17.2) | 70 (12.5) | 13 (5.9) | 1 (16.7) | 0 | 205 (11.4) |
| eGFR, mean (SD), mL/min/1.73 m2 | 82.0 (18.9) | 75.4 (20.1) | 77.9 (21.2) | 77.0 (17.4) | 70.3 (22.2) | 76.9 (21.0) | 84.2 (21.3) | 74.5 (27.9) | 87.2 (21.7) | 78.8 (20.5) |
| eGFR < 60 mL/min/1.73 m2, n (%) | 35 (6.9) | 31 (15.8) | 14 (13.6) | 15 (12.9) | 22 (25.3) | 74 (13.2) | 16 (7.2) | 1 (16.7) | 2 (15.4) | 210 (11.6) |
α-GI alpha-glucosidase inhibitors, BG biguanides, BMI body mass index, CVD cardiovascular disease, DPP4i dipeptidyl peptidase 4 inhibitors, eGFR estimated glomerular filtration rate, GLP-1RA glucagon-like peptide 1 receptor agonists, HbA1c glycated hemoglobin, SGLT2i sodium–glucose cotransporter 2 inhibitors, SU sulfonylureas, TZD thiazolidinediones
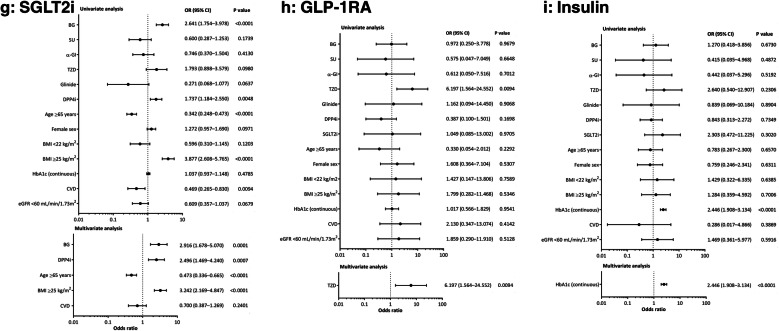
The clinical factors associated with the choice of each second-line treatment are shown in Fig. 3 and summarized in Table S5. Patients who were prescribed second-line biguanides, sulfonylureas, α-GIs, thiazolidinediones, SGLT2is, or glinides therapy were likely to have been prescribed a DPP4i as first-line monotherapy. Patients on second-line biguanides were also likely to be younger, and to have better renal function (Fig. 3a). Those receiving second-line sulfonylureas commonly had worse glycemic control and poorer renal function (Fig. 3b), while patients prescribed second-line α-GIs were more likely to have a BMI of < 22 kg/m2 (Fig. 3c). Patients given second-line thiazolidinediones were likely to be older and to have lower HbA1c (Fig. 3d), and those who received second-line glinides were more likely to have been prescribed an SGLT2i as first-line monotherapy, to be men, and to have poorer renal function, and less likely to have a BMI of ≥ 25 kg/m2 (Fig. 3e). Participants receiving second-line DPP4is were more likely to be ≥ 65 years old and less likely to have a BMI of ≥ 25 kg/m2 (Fig. 3f). In contrast, those prescribed second-line SGLT2is were more likely to be overweight, less likely to be ≥ 65 years old, and were likely to have been prescribed biguanides or DPP4is as first-line monotherapy (Fig. 3g). Patients on second-line GLP-1RAs were likely to have received thiazolidinediones as first-line monotherapy (Fig. 3h). Finally, those prescribed second-line insulin generally had worse glycemic control (Fig. 3i). Sex and cardiovascular history did not have a significant effect on second-line treatment choice.
Fig. 3.
Patient baseline factors associated with the choice of second-line treatment. Logistic regression models were used to identify factors that were significantly associated with treatment choice. Second-line agents were a biguanides, b sulfonylureas, c alpha-glucosidase inhibitors, d thiazolidinediones, e glinides, f dipeptidyl peptidase 4 inhibitors, g sodium–glucose cotransporter 2 inhibitors, h glucagon-like peptide 1 receptor agonists, or i insulin. α-GI alpha-glucosidase inhibitors, BG biguanides, BMI body mass index, CVD cardiovascular disease, DPP4i dipeptidyl peptidase 4 inhibitors, eGFR estimated glomerular filtration rate, GLP-1RA glucagon-like peptide 1 receptor agonists, HbA1c glycated hemoglobin, SGLT2i sodium–glucose cotransporter 2 inhibitors, SU sulfonylureas, TZD thiazolidinediones
Discussion
This paper reports the baseline characteristics of the patients enrolled in J-DISCOVER, i.e., Japanese patients with T2DM who were being initiated on second-line treatment in 2014–2015, and analyzes patient factors associated with selection of specific second-line treatment. Data were analyzed for 1806 patients with a mean age, HbA1c, and BMI of 61.7 years, 7.7%, and 25.5 kg/m2, respectively; 49.3% of patients had a BMI ≥ 25 kg/m2, while 70.9% and 28.7% of patients had HbA1c ≥ 7% and ≥ 8%, respectively. Compared with previous observational studies [8, 9], HbA1c in the J-DISCOVER population was higher, but age and BMI were similar. Mean HbA1c was probably higher in this study because the study was limited to uncontrolled patients who were starting second-line treatment.
Commonly prescribed first-line monotherapy in this study were DPP4is (53.7% of patients), biguanides (21.4%), sulfonylureas (7.2%), α-GIs (6.8%), glinides (3.5%), and thiazolidinediones (3.4%). These results reflect the absence of specific treatment recommendation for first-line treatment in Japanese guidelines compared with US and European guidelines that explicitly specify metformin as first-line treatment [2]. Indeed, metformin was the most frequently prescribed non-insulin monotherapy in the USA in 2012 (49.9% of approx. 121 million scripts), followed by sulfonylureas (26.7%) and DPP4is (8.0%) [15]. Our findings also differ from an analysis of claims data (2008–2013) from 103 Japanese hospitals, in which the most common first-line prescriptions in adult patients (aged 40–70 years, N > 7000) with T2DM were biguanides (26.5%), DPP4is (25.2%), sulfonylureas (18.4%), α-GIs (15.2%), and thiazolidinediones (8.3%) [16]. It is difficult to compare our findings with these two studies, as the methods and patient populations were different, and data were collected over different periods; however, the proportion of first-line biguanides prescriptions in our analysis was similar to that in the earlier Japanese claims analysis, and DPP4is usage was approximately twofold higher in our study than the earlier one, while prescriptions for other agents were lower. Therefore, we assume that many patients who would have been prescribed agents other than biguanides or DPP4is in 2008–2013 were prescribed DPP4is in 2014–2015. DPP4is were the most common choice for second-line treatment in both studies, particularly in patients who received a biguanide first line; both studies also showed that patients prescribed first-line DPP4is were most likely to be given biguanides second line. Combining biguanides and DPP4is is expected to have a synergistic effect, as DPP4is enhance insulin secretion and inhibit glucagon secretion by reducing endogenous GLP-1 degradation [17–19], while metformin increases the number of GLP-1 receptors, enhances the insulin secretion activity of pancreatic β-cells, and may stimulate GLP-1 secretion from L cells [16, 20]. In the current study, the majority of patients received second-line treatment with a mode of action different from the first as recommended by Japanese treatment guidelines [4]. The higher use of DPP4is as the first- or the second-line agent in the current study (84.7%) may reflect various favorable characteristics of this class of agent in the clinical practice of diabetes care, including low risk of hypoglycemia and weight gain [2, 19], safety (at reduced doses) in patients with renal failure [21], cytoprotective effects on pancreatic β-cells [22], and improved glycemic stability [23]. In addition, compared with Caucasians, Japanese patients with T2DM appear to be more predisposed to impaired insulin secretion rather than increased insulin resistance [6, 24], Therefore, Japanese patients may receive greater benefit from DPP4is, which increase the endogenous incretin levels and promote insulin secretion in a glucose-dependent manner. Notably, the rationale for using DPP4is in Japanese patients is supported by a systematic review and meta-analysis, which showed that DPP4is exhibit better glucose-lowering efficacy in Asians than in other ethnic groups [25].
Significant associations were found between the choice of second-line treatment and several patient characteristics, including first-line treatment, age, BMI, HbA1c levels, and renal function. Sex and cardiovascular history did not have a significant effect on second-line treatment choice. Patients who were prescribed second-line biguanides were more likely to have been prescribed a DPP4i as first-line monotherapy, to be younger, and to have better renal function. Since combining biguanides and DPP4is is expected to have a synergistic effect, it is reasonable that biguanides were selected as second-line prescriptions for subjects treated with DPP4is first line. Avoiding using biguanides in the elderly patients and/or individuals with poor renal function is in accordance with the general recommendations for appropriate use of biguanides. As may be expected, patients prescribed second-line insulin or sulfonylureas were more likely to have poor glycemic control at baseline, whereas those receiving thiazolidinediones generally had lower baseline HbA1c. This may indicate a belief that sulfonylureas will reduce HbA1c levels more rapidly than other agents. In addition, patients in this study who received a sulfonylurea second line were older (mean 62.9 years) and had poorer renal function, which may pose a risk for hypoglycemia [26]; however, these data were collected before the Japanese Diabetes Society issued guidelines for glycemic control in elderly patients, which emphasizes the importance of avoiding hypoglycemia in elderly patients [27], and thus may not reflect current practice. Participants receiving second-line DPP4is were commonly older and less likely to be overweight. Patients who were prescribed an SGLT2i second-line were more likely to be overweight.
The number of patients receiving an SGLT2i second line was relatively low (12.2%). However, there is a possibility that prescribing practice has since changed, as these data were collected only a short time after SGLT2is became available in Japan; recommendations on the correct use of this class were released in Japan in June 2014 [28]. Several cardiovascular outcomes trials and large international observational studies have shown that SGLT2is reduce cardiovascular events, heart failure hospitalizations, and renal events [29–36]. Furthermore, the consensus report and guideline in the USA and Europe have recommended SGLT2is or GLP1-RAs for patients with T2DM and CVD or at high CV risk. These may influence the prescribing pattern of Japanese physicians [3, 37].
In this study, the percentage of patients treated with insulin as a second-line treatment is very small. Although it may implicate clinical inertia [38], the Japanese guidelines specify that one of the indications for insulin therapy is when good glycemic control is not achieved even after treatment with oral medication for diabetes [4]. As HbA1c was relatively low at baseline in this study (7.7%) and all the patients were enrolled at the initiation of a second-line treatment, the low insulin treatment rate could indicate that physicians are trying to control HbA1c with oral diabetes medications before insulin therapy.
There are some limitations to the current study. First, the information of the treatment pattern is restricted because of the limited number of patients. Second, though this study successfully enrolled patients from 142 sites of clinics and hospitals across Japan, including primary care physicians who are not specialized in diabetes, these data may not be a true representative of Japanese daily practice owing to the limited number of sites as well as the limited number of patients from each site.
Conclusion
This analysis of J-DISCOVER baseline data has shown patient characteristics associated with choice of second-line treatment as well as the treatment reality for Japanese patients with T2DM who are initiating second-line treatment. Whereas metformin is the preferred first-line treatment for T2DM in US and European guidelines, in this Japanese study DPP4is were the most frequently prescribed agents both first- and second-line treatment. Biguanides were also commonly used, while SGLT2is were prescribed in a relative minority of patients. The results of this study indicate that physicians chose second-line treatment by considering the mode of action of first-line treatment, as well as patients’ background characteristics and clinical conditions (age, BMI, HbA1c, and renal function).
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the investigators and patients participating in the J-DISCOVER study.
Funding
The J-DISCOVER study was funded by AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd., Osaka Japan. The journal’s Rapid Service Fee was funded by AstraZeneca K.K.
Editorial Assistance
Editorial assistance in the preparation of this article was provided by Toni Dando, of inScience Communications, Springer Healthcare. Support for this assistance was funded by AstraZeneca K.K. and Ono Pharmaceutical Co., Ltd., Osaka Japan.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
List of Investigators
For a full list of the study investigators please see the Electronic Supplementary Material.
Disclosures
Naoto Katakami holds an endowed chair (Department of Metabolism and Atherosclerosis) established by funds from Kowa, and has received research funds from MSD and lecture fees from Arkray, Astellas, Astra Zeneca, Nippon Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Mitsubishi Tanabe Pharma, MSD, Novartis, Novo Nordisk, Ono Pharmaceutical, Otsuka, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, Sanofi-Aventis, and Shionogi. Tomoya Mita has received research funds from MSD, Takeda Pharmaceutical, Kowa, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma and Ono Pharmaceutical, and lecture fees from Astra Zeneca, Nippon Boehringer Ingelheim, Eli Lilly, Kowa, Mitsubishi Tanabe Pharma, MSD, Ono Pharmaceutical, and Takeda Pharmaceutical. Mitsuyoshi Takahara has received subsidy from The Japan Diabetes Society, and has endowed departments by commercial entitles from Astra Zeneca, Ono Pharmaceutical, MSD, Keiseikai Hospital, Taisho Toyama Pharmaceutical, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Novo Nordisk. Iichiro Shimomura has received Research funding from Mochida Pharmaceutical, Japan Agency for Medical Research and Development, Rohto Pharmaceutical, and Kowa, and has received subsidies or donations from Takeda Pharmaceutical, Novo Nordisk, Midori Health Care Foundation, Kyowa Hakko Kirin, Dainippon Sumitomo Pharma, Daiichi Sankyo, Mochida Pharmaceutical, Ono Pharmaceutical, Teijin Pharma, Mitsubishi Tanabe Pharma, Suzuken Memorial Foundation, Shionogi, Japan Diabetes Foundation, MSD, Sanofi, Astra Zeneca, Novartis, Kissei Pharma, Japan Foundation for Applied Enzymology, Astellas, Gokeikai Osaka Kaisei Hospital, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Eli Lilly, The Japan Diabetes Society, MSD Life Science Foundation and Kowa Life Science Foundation. Hirotaka Watada has acted as an advisory board member for Astellas, Astra Zeneca, Nippon Boehringer Ingelheim, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, and Takeda Pharmaceutical and as a speaker for Astellas, Astra Zeneca, Nippon Boehringer Ingelheim, Dainippon Sumitomo Pharma, Eli Lilly, Kowa, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, and Takeda Pharmaceutical, and has received research support from Astellas, Nippon Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Kissei Pharma, Kowa, Kyowa Hakko Kirin, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novartis, Novo Nordisk, Ono Pharmaceutical, Pfizer, Sanofi, Sanwa Kagaku Kenkyusho, Taisho Toyama Pharmaceutical, Takeda Pharmaceutical, Teijin Pharma, and Terumo. Toshitaka Yajima and Fumitaka Wada are employees of Astra Zeneca. Masaru Kawashima is an employee of Ono Pharmaceutical.
Compliance with Ethics Guidelines
All procedures followed will be in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients included in the study. The full list of institutional review boards/ethics committees that approved this study can be found in the Supplementary Material.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12311861.
References
- 1.International Diabetes Foundation. IDF diabetes atlas eighth edition. 2017. https://diabetesatlas.org/resources/2017-atlas.html. Accessed 14 Jan 2019.
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. doi: 10.2337/dc14-2441. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;2018(41):2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Japanese Diabetes Society . Treatment guide for diabetes 2018–2019. Tokyo: Bunkodo; 2018. [Google Scholar]
- 5.Haneda M, Noda M, Origasa H, et al. Japanese clinical practice guideline for diabetes 2016. Diabetol Int. 2018;9:1–45. doi: 10.1007/s13340-018-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujihara K, Hanyu O, Heianza Y, et al. Comparison of clinical characteristics in patients with type 2 diabetes among whom different antihyperglycemic agents were prescribed as monotherapy or combination therapy by diabetes specialists. J Diabetes Investig. 2016;7:260–269. doi: 10.1111/jdi.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueki K, Sasako T, Okazaki Y, et al. Effect of an intensified multifactorial intervention on cardiovascular outcomes and mortality in type 2 diabetes (J-DOIT3): an open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:951–964. doi: 10.1016/S2213-8587(17)30327-3. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama H, Araki SI, Kawai K, et al. Declining trends of diabetic nephropathy, retinopathy and neuropathy with improving diabetes care indicators in Japanese patients with type 2 and type 1 diabetes (JDDM 46) BMJ Open Diabetes Res Care. 2018;6:e000521. doi: 10.1136/bmjdrc-2018-000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashino Y, Izumi K, Okamura S, et al. Duration of diabetes and types of diabetes therapy in Japanese patients with type 2 diabetes: The Japan Diabetes Complication and its Prevention prospective study 3 (JDCP study 3) J Diabetes Investig. 2017;8:243–249. doi: 10.1111/jdi.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complicat. 2017;31:1188–1196. doi: 10.1016/j.jdiacomp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Nicolucci A, Charbonnel B, Gomes MB, et al. Treatment patterns and associated factors in 14 668 people with type 2 diabetes initiating a second-line therapy: results from the global DISCOVER study programme. Diabetes Obes Metab. 2019;21:2474–85. [DOI] [PMC free article] [PubMed]
- 12.Katakami N, Mita T, Takahara M, et al. Rationale and design for the J-DISCOVER Study: DISCOVERing the treatment reality of type 2 diabetes in a real-world setting in Japan—a protocol. Diabetes Ther. 2018;9:165–175. doi: 10.1007/s13300-017-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. doi: 10.1093/biomet/80.1.27. [DOI] [Google Scholar]
- 14.Sweeney RE, Ulveling EF. A transformation for simplifying the interpretation of coefficients of binary variables in regression analysis. Am Stat. 1972;26:30–32. [Google Scholar]
- 15.Hampp C, Borders-Hemphill V, Moeny DG, et al. Use of antidiabetic drugs in the US, 2003–2012. Diabetes Care. 2014;37:1367–1374. doi: 10.2337/dc13-2289. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe M, Motonaga R, Terawaki Y, et al. Prescription of oral hypoglycemic agents for patients with type 2 diabetes mellitus: a retrospective cohort study using a Japanese hospital database. J Diabetes Investig. 2017;8:227–234. doi: 10.1111/jdi.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahren B. DPP-4 inhibitors. Best Pract Res Clin Endocrinol Metab. 2007;21:517–533. doi: 10.1016/j.beem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003;26:2929–2940. doi: 10.2337/diacare.26.10.2929. [DOI] [PubMed] [Google Scholar]
- 19.Xourgia EP, Karampousli AK, Melidonis EA. DPP-4 Inhibitors vs. SGLT-2 inhibitors; cons and pros. J Ren Med. 2017;1:7. [Google Scholar]
- 20.Cho YM, Kieffer TJ. New aspects of an old drug: metformin as a glucagon-like peptide 1 (GLP-1) enhancer and sensitiser. Diabetologia. 2011;54:219–222. doi: 10.1007/s00125-010-1986-3. [DOI] [PubMed] [Google Scholar]
- 21.Dicker D. DPP-4 inhibitors: impact on glycemic control and cardiovascular risk factors. Diabetes Care. 2011;34(Suppl 2):S276–278. doi: 10.2337/dc11-s229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita A, Mukai E, Hiratsuka A, et al. Distinct effects of dipeptidyl peptidase-4 inhibitor and glucagon-like peptide-1 receptor agonist on islet morphology and function. Endocrine. 2016;51:429–439. doi: 10.1007/s12020-015-0733-4. [DOI] [PubMed] [Google Scholar]
- 23.Leibowitz G, Cahn A, Bhatt DL, et al. Impact of treatment with saxagliptin on glycaemic stability and beta-cell function in the SAVOR-TIMI 53 study. Diabetes Obes Metab. 2015;17:487–494. doi: 10.1111/dom.12445. [DOI] [PubMed] [Google Scholar]
- 24.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 26.Piątkiewicz P. Hypoglycemia in elderly type 2 diabetes patients (editorial) Diabetes Manag. 2016;6:071–075. [Google Scholar]
- 27.Haneda M, Ito H. Glycemic targets for elderly patients with diabetes. Diabetol Int. 2016;7:331–333. doi: 10.1007/s13340-016-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recommendations on the proper use of SGLT2 inhibitors. Diabetol Int 2020;11:1–5. 10.1007/s13340-019-00415-8 [DOI] [PMC free article] [PubMed]
- 29.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 30.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 31.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 32.Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors) Circulation. 2017;136:249–259. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosiborod M, Lam CSP, Kohsaka S, et al. Cardiovascular events associated with SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL 2 study. J Am Coll Cardiol. 2018;71:2628–2639. doi: 10.1016/j.jacc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 35.Zeiniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 36.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019 doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 37.Consentino F, Grant PJ, Aboyans V, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2019 doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 38.Gentile S, Ceriello A, Strollo F, et al. A multicenter Italian survey on diabetes care units reveals a somewhat slow attitude in treatment guideline implementation: are we dealing with therapeutic inertia? Diabetes Res Open J. 2016;2:33–44. doi: 10.17140/DROJ-2-127. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.