Abstract
Extracellular protein interactions mediated by cell surface receptors are essential for intercellular communication in multicellular organisms. Assays to detect extracellular interactions must account for their often weak binding affinities and also the biochemical challenges in solubilising membrane-embedded receptors in an active form. Methods based on detecting direct binding of soluble recombinant receptor ectodomains have been successful, but genome-scale screening is limited by the usual requirement of producing sufficient amounts of each protein in two different forms, usually a “bait” and “prey”. Here, we show that oligomeric receptor ectodomains coupled to concatenated units of the light-generating Gaussia luciferase enzyme robustly detected low affinity interactions and reduced the amount of protein required by several orders of magnitude compared to other reporter enzymes. Importantly, we discovered that this flash-type luciferase exhibited a reaction-induced inhibition that permitted the use of a single protein preparation as both bait and prey thereby halving the number of expression plasmids and recombinant proteins required for screening. This approach was tested against a benchmarked set of quantified extracellular interactions and shown to detect extremely weak interactions (KDs ≥ μM). This method will facilitate large-scale receptor interaction screening and contribute to the goal of mapping networks of cellular communication.
Subject terms: Membrane proteins, Cell adhesion
Introduction
Proteins embedded in the cellular membrane are critically placed to transmit signals from the external environment to the interior of the cell to ensure coordinated and appropriate cellular behaviours from the earliest stages of development to the maintenance of adult tissues1. Importantly, these cell surface proteins are targets for therapeutic antibodies that are increasingly used to treat indications such as autoimmunity and cancer2. Despite their medical relevance, however, the specialised biochemical nature of membrane proteins has meant that they are often underrepresented in large-scale protein interaction screens3 which typically employ techniques such as biochemical purifications and transcription-based protein complementation assays4. Biochemically, membrane proteins are difficult to solubilise in their native conformation due to their amphipathic character, and the interactions between cell surface receptor proteins can be extremely transient—having dissociation half-lives of just fractions of a second—making protocols that include stringent wash steps unsuitable to detect them4,5.
Several approaches that address these biochemical challenges have been developed (reviewed recently in6) and include mass spectrometry-based techniques for association by proximity labelling7,8, genetic manipulation of cell surface protein repertoires in the context of a cell membrane9–11, and expression cloning12–14. A very successful approach has been the development of ELISA-style assays that detect direct binding events within large libraries of soluble recombinant receptor ectodomains15–17, including the AVEXIS (AVidity-based EXtracellular Interaction Screening) method developed by our laboratory18. This general approach relies on the ability to express the extracellular binding domain as a secreted recombinant protein that retains the extracellular binding activity, and has been particularly useful for common receptor architectural classes such as type I, type II, and GPI-anchored membrane proteins. It is important that structurally critical posttranslational modifications such as glycosylation and disulfide bonds are retained, and so eukaryotic protein expression systems such as mammalian cells are most commonly used. Usually, the receptor ectodomains are produced in two forms: a “bait” protein that can be immobilised on a solid substrate such as a microtitre plate, and a soluble “prey”. To circumvent the low binding affinities, the prey proteins are purposefully oligomerised to create highly avid binding probes4.
While these methods are highly specific and sensitive, a major barrier to increasing their scale as well as their wider adoption by other laboratories has been the large resource requirements to create large libraries containing hundreds of different proteins suitable for interaction screening. For each candidate receptor, two separate protein expression plasmids are required to produce both the bait and prey protein preparations. Also, in comparison to other methods, mammalian protein expression systems are expensive and often low-yielding, and because the number of interactions to be tested increases exponentially with the number of proteins screened, the large amount of protein required for screening is often a limitation. These constraining factors have meant that most extracellular interaction screens of this type have been limited to just a few hundred proteins15,16,18–20; however, mammalian genomes are known to contain up to 2000 receptors testable using this approach21–24. In principle, the required resources would be significantly reduced by developing an assay format where the receptor ectodomain can be used as both a bait and as a prey, halving the number of expression plasmids, transfections, and protein handling steps. In addition, significantly reducing the amount of protein required for each binding test would remove another limitation to increasing the scale of the screens.
One approach to reduce the amount of protein required could be to use a highly sensitive enzyme as the binding reporter. Previously, enzymes including alkaline phosphatase and beta-lactamase have been used, but enzymes with higher catalytic rates, such as horseradish peroxidase (HRP), and those that generate light such as luciferases are potentially more sensitive for an in vitro assay. Light-generating luciferase enzymes are very sensitive and have been developed for expression and secretion by mammalian cells25,26. These include aquatic luciferases that can produce blue light in an extremely bright but transient “flash” and have been further developed to produce long-lasting “glow” characteristics27. Conceptually, those enzymes that can be irreversibly inactivated with an inhibitor provide the option of using the same enzyme in a binding assay; for example, an enzyme-conjugated protein could be immobilised, its enzyme activity inhibited, and the interaction to another protein tested using the same enzyme as a binding reporter. The development of such an assay could result in the need for just a single protein expression plasmid and protein preparation thereby significantly reducing the amount of resources required for large scale extracellular protein interaction screening involving thousands of proteins.
Here, we have explored the use of different enzyme reporters for low affinity extracellular protein interaction screening using an ELISA-style direct binding assay with the aim of reducing the amount of protein required, and investigate the possibility of using just a single recombinant protein construct for each protein tested. We report that highly avid constructs based on a flash luciferase—because the luciferase activity was inhibited by exposure to the substrate—enables the enzyme to be used as both bait and prey.
Results
Concatenated luciferases are highly expressed as part of an oligomeric binding probe
In the original AVEXIS method, the receptor ectodomains were expressed as soluble recombinant proteins containing a C-terminal antigen tag consisting of the rat Cd4 protein, a protein sequence from the cartilage oligomeric matrix protein (COMP) that produced highly-avid pentamers, and the enzyme beta-lactamase18,28 (Fig. 1a, b). With the aim of reducing the amount of protein required in this assay, we first tried to replace the beta-lactamase enzyme with more sensitive reporter enzymes including HRP29, and five concatenated units of either a “glow”-type nano-luciferase27 and “flash”-type Gaussia luciferase26,30 (Fig. 1a). To enable protein quantitation and purification, both a 6-histidine tag and a peptide sequence that is a substrate for the protein-biotin ligase which permits enzymatic biotinylation were included at the C-terminus (Fig. 1a). To determine the level at which these new prey constructs were produced, protein expression plasmids were transfected into HEK293 cells, and the supernatant containing the secreted protein harvested, purified, and quantified. We observed that both the Gaussia and nano-luciferase prey constructs were expressed at the same high levels as the beta-lactamase prey (Fig. 1c); by contrast, we repeatedly failed to detect expression of the prey protein encoding HRP (Fig. 1c). One possibility for this lack of expression is that the supply of the necessary heme co-factor was limiting31; however, supplementing the cell culture medium with hemin failed to improve expression, and so we did not pursue HRP as a reporter enzyme any further.
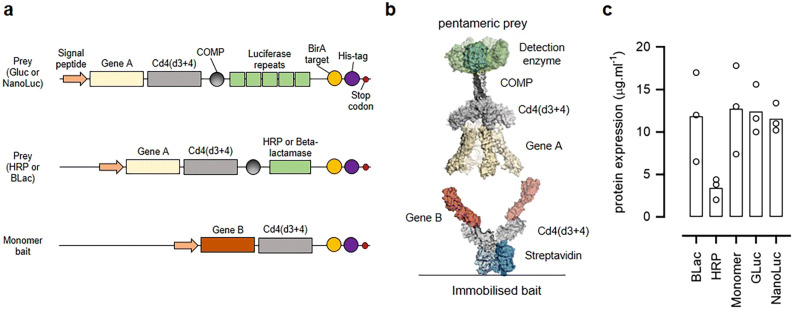
Figure 1.
Design and expression of prey constructs with different reporter enzymes. (a) Schematic diagrams of the protein constructs used in this study. Prey proteins consisting of the extracellular region of a protein of interest (Gene A) are fused at their C-terminus to the proteins tags: rat Cd4(d3 + 4) and the rat cartilage oligomeric matrix protein (COMP) followed by either five repeating units of luciferase, HRP, or beta-lactamase enzymes. Baits are expressed as monomers and consist of ectodomains of a protein of interest (Gene B) fused to the same rat Cd4(d3 + 4) tag but additionally containing a peptide sequence that is recognised by the enzyme BirA for the covalent addition of a single biotin molecule. Both baits and preys contain a terminal 6-his tag for purification. (b) Schematic representation of the AVEXIS assay involving a soluble highly avid pentameric enzyme-tagged prey protein and biotinylated bait protein immobilised on a streptavidin-coated microtitre plate. (c) Pentameric preys containing the HRP enzyme were expressed at low levels. Rat Cd200 prey constructs containing the named enzyme reporters were expressed by transient transfection of HEK293 cells and the secreted protein yield quantified after nickel affinity purification. Data points are values for three independent transfections and bars represent the means. BLac = beta-lactamase prey; GLuc = Gaussia luciferase prey; NanoLuc = Nano luciferase prey.
Low affinity extracellular interactions can be sensitively detected using luciferase reporter enzymes
To quantify the ability of each prey construct to detect low affinity interactions, we prepared a dilution series of each prey and probed them against a fixed quantity of monomeric enzymatically mono-biotinylated bait immobilised in a streptavidin-coated plate. We used a typical low affinity extracellular protein interaction (rat Cd200-Cd200R) which has an equilibrium dissociation constant of 2.5 μM and a dissociation half-life of 0.9 s32. We observed that both the Gaussia and nano-luciferases provided a more sensitive detection of the interaction compared to beta-lactamase whilst maintaining a low background signal (Fig. 2). In fact, even at the lowest concentration tested, there was still a robust signal that was clearly discriminated from the control, and especially using the Gaussia luciferase.
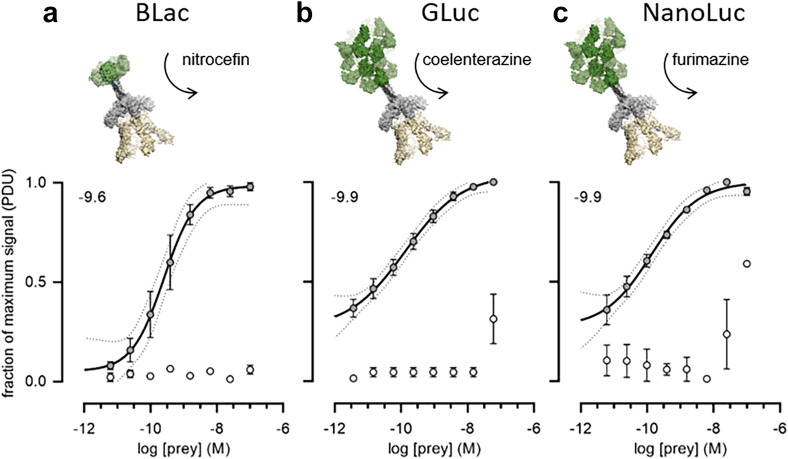
Figure 2.
Luciferase reporters provide highly sensitive detection of the Cd200-Cd200R interaction. Highly avid rat Cd200 prey proteins containing the reporter enzymes (a) beta-lactamase (BLac), (b) Gaussia luciferase (GLuc) or (c) nano-luciferase (NanoLuc) were probed for interactions with a biotinylated rat Cd200R bait protein immobilised in streptavidin-coated microtitre plates. Prey capture was quantified using absorbance at 485 nm for hydrolysis products of the beta-lactamase colourimetric substrate nitrocefin, and luminescence using the substrates coelenterazine (Gaussia luciferase) and furimazine (nano-luciferase). Cd200 was used as a negative control bait (empty circles). The normalised signal for each data point was calculated as described in the Methods such that background signal would be 0 and the maximum signal 1. The log IC50 for each interpolated curve is displayed on each graph. Representative experiments shown with n = 3 for each concentration of prey with s.e.m. for each data point and the 95% CI for the interpolated curves indicated as dotted lines.
Reaction-induced inhibition of Gaussia ‘flash’ luciferase permits single construct use as both bait and prey
With the ultimate aim of using the same ectodomain-containing protein expression construct as both a bait and a prey, we first asked whether a highly avid prey protein could substitute as a bait in our assay. We immobilised biotinylated Cd200R Gaussia luciferase pentamers on streptavidin-coated microtitre plates and probed them for interactions with a Cd200 beta-lactamase tagged prey. We observed that the interaction could be robustly detected and the sensitivity of the assay increased when compared to immobilised monomer baits (Fig. 3a).
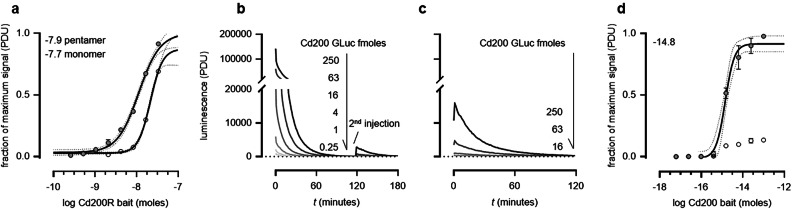
Figure 3.
Reaction-induced inactivation of Gaussia luciferase enables the use of a single construct as both a bait and a prey. (a) Pentameric prey proteins can be used as a bait for sensitive interaction detection. Biotinylated Cd200R Gaussia luciferase-tagged pentamer (filled circles) or monomers (empty circles) were serially diluted and immobilised on a streptavidin-coated microtitre plate as bait proteins and probed for interactions with a beta-lactamase-tagged Cd200 pentamer. (b) Coelenterazine was added to a dilution series of purified Cd200 Gaussia luciferase pentamer which elicited a strong luminescence signal that was gradually lost after around an hour. A second administration of substrate did not generate the same bright luminescence signal. (c) Gaussia enzymatic activity is not restored after a nickel-affinity purification of the inactivated luciferase. Reaction inhibited Gaussia luciferase was purified using Ni–NTA agarose beads with several wash steps before addition of fresh coelenterazine (100 pmol). (d) Pentameric (non-biotinylated) Cd200 Gaussia luciferase prey was probed against biotinylated and inactivated pentameric Cd200R Gaussia luciferase bait (filled circles). The Cd200 prey was also probed against a reaction-inhibited pentameric Gaussia luciferase Cd200 protein used as a non-interacting control bait (empty circles). The inflection point for each interpolated curve is displayed as the log baits (moles) value on each graph. Representative experiments shown with n = 3 for each quantity of prey with SEM for each data point and the 95% CI for the interpolated curves.
To use the same protein as both a bait and a prey, it will be necessary to inhibit the enzymatic activity of the immobilised protein, and so we asked whether the “flash” activity of the Gaussia luciferase led to the irreversible inhibition of the enzyme. For the pentameric prey constructs, this would require inactivation of the luciferase enzyme reporter when arrayed as a bait such that the luminescent activity is reduced by > 90% for the remaining duration of the assay (~ 2 h) and is not dependent upon a labile or soluble inhibitor that would be removed during wash steps. We observed that once the coelenterazine substrate is added to the Gaussia luciferase enzyme immobilised on the microtitre plate, after an initial very bright burst of light, there is a gradual decline in luminescence that becomes undetectable after an hour (Fig. 3b). Subsequent additions of coelenterazine substrate did not result in further increases in luminescence demonstrating a reaction-induced inhibition of the enzyme (Fig. 3b). To determine if this inactivation was due to a labile reaction-induced inhibitor that would be removed during assay washing steps, the coelenterazine-treated luciferase-containing prey proteins were re-purified using their 6-his tags by Ni–NTA affinity chromatography. By adding coelenterazine substrate a second time to these purified enzymes, we observed that the inactivation of the Gaussia luciferase enzyme was not reversed (Fig. 3c). Together, these results demonstrate that the inhibition of the Gaussia luciferase enzyme cannot be easily reversed by wash steps and so will not interfere with the subsequent application of the prey reporter in an interaction assay.
The substrate-dependent inactivation of Gaussia luciferase therefore provided an opportunity to use the same protein expression plasmids to produce both the biotinylated bait and soluble prey. To determine if reaction-inhibited Gaussia luciferase Cd200R pentamer could be used as a bait, it was expressed as a biotinylated protein and a dilution series was immobilised in wells of a streptavidin-coated plate. Coelenterazine was added to inactivate the luciferase enzyme in the immobilised protein, and then each well was probed with the non-biotinylated Cd200 Gaussia luciferase fusion pentamer before coelenterazine was again added to detect interactions. The Cd200-Cd200R interaction was robustly detected over a wide range of bait quantities (1–100 fmole per well in a 96 well plate) (Fig. 3d). Together, these data demonstrate that the pentameric Gaussia luciferase reporter enzyme can be used as both a bait and a prey for low affinity extracellular protein interaction screening.
The Gaussia luciferase single protein construct system detects very low affinity interactions in a benchmarked screening assay
While the rat Cd200-Cd200R interaction represents a typical extracellular receptor-ligand pair, there are other extracellular receptor interactions that have a significantly lower affinity4. To determine the limit of sensitivity of this assay, we used a set of quantified interactions within a family of six paralogous receptors that belong to the zebrafish junctional cell adhesion molecule (Jam) family that have previously been used to benchmark extracellular interaction screening33. It is known that the interaction between JamB1 and JamC1 is essential for myoblast fusion in vivo34, and the interactions within these receptors have been systematically determined and quantified using surface plasmon resonance. These interactions therefore represent a useful set of both homophilic and heterophilic interactions with a range of affinities from the relatively strong JamB1–JamC2 interaction (t1/2 > 2 s) to the very weak JamA1–JamC2 interaction (t1/2 < 0.4 s), as well as several known negatives35; the expected interactions are depicted graphically in Fig. 4a. Each of the six Jam proteins were expressed as monomeric biotinylated baits, a beta-lactamase pentamer prey, a Gaussia luciferase (non-biotinylated) pentamer prey, and a Gaussia luciferase pentamer biotinylated bait. The proteins were normalised according to the parameters previously established for optimal interaction detection and all 36 possible interactions tested in a pairwise fashion with each Jam bait arrayed in a streptavidin-coated plate, and all six Jam preys probed against them. The original beta-lactamase prey version of the assay performed as previously described35, easily detecting the highest affinity interactions. It also detected all the weaker (t1/2 < 0.4 s) interactions, although two false positives (JamA1 prey – JamC1 bait, and JamA2-JamC2) were identified at this assay stringency (Fig. 4b). We found that using the Gaussia luciferase prey with the monomer bait performed exceptionally well, clearly detecting all interactions, including the very weakest ones with no false positives (Fig. 4c). Using the single plasmid system where the Gaussia luciferase pentamer is used as both a biotinylated bait and non-biotinylated prey, the assay performed better than the original beta-lactamase prey detecting all the interactions with only the signal for the weakest JamC1 homophilic interaction overlapping with the distribution of background signals (Fig. 4d). Given the ability to use the Gaussia luciferase pentamers as baits, we next asked whether the same biotinylated protein preparations could be used as both a bait and a prey. After the immobilisation of the Gaussia luciferase pentamer and inhibition of the enzymatic activity by coelenterazine addition, an additional wash step with a buffer containing free biotin to block all remaining biotin binding sites was performed before again adding the Gaussia luciferase pentamer to detect interactions. We observed that the assay performed well for the higher affinity JamB1-JamC2 and JamB1-JamC1 interactions, but did not detect the weaker JamB2– JamC2 interaction in either bait-prey orientation (Fig. 4e). Setting a threshold to detect the weaker interactions led to an increase in false positives suggesting this format of the assay would be best used to detect interactions with interaction half-lives of a second or longer.
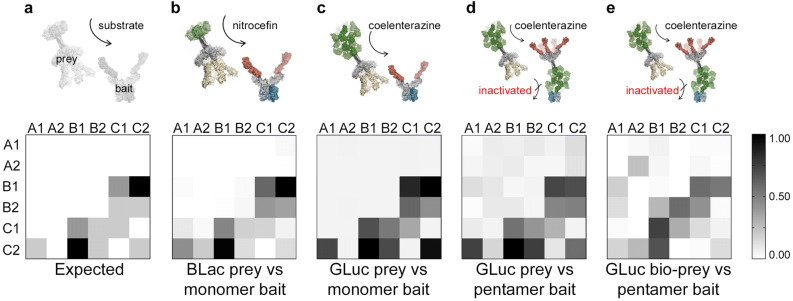
Figure 4.
Benchmarking the Gaussia luciferase single protein construct system within the zebrafish Jam family protein interactions shows it can detect low affinity interactions with a low false positive rate. The bait and prey versions of the six paralogues of the zebrafish Jam family were expressed and used in different implementations of the receptor interaction assay including both monomer and pentameric Gaussia luciferase bait formats. The indicated baits were arrayed on streptavidin-coated plates and systematically probed with the preys for a total of 36 binary interaction tests in each prey-bait combination. (a) Graphical representation of expected and observed interactions of different affinities within the zebrafish Jam family. The expected interactions within the zebrafish Jam family are shaded according to their measured dissociation half lives (black = t1/2 > 2 s; dark grey 0.4 < t1/2 < 2 s; light grey = t1/2 < 0.4 s); the scale in (b) to (e) represents the normalised signal output from the assay. (b) Assay read out using the original AVEXIS assay with the beta-lactamase reporter prey against monomeric baits, and (c) using the new Gaussia luciferase reporter prey against the same array of monomeric baits. In (d), the Gaussia luciferase pentameric protein is used as a bait, inactivated, and then a same protein construct expressed in a non-biotinylated form as the prey. In (e), the same biotinylated Gaussia luciferase pentameric protein preparation is used as both a bait and a prey. Representative experiments are shown with n = 3 for each prey-bait binary interaction test and summary data are shown in Figure S1.
Discussion
Despite their fundamental importance in many biological processes and attractiveness as drug targets, identifying extracellular protein interactions between membrane-embedded receptor proteins still poses technical challenges. A great deal of progress has been made, and especially by using ELISA-style assays that detect direct binary binding events within libraries of receptor ectodomains expressed as soluble recombinant proteins in eukaryotic cells15–18. While these assays were designed to systematically screen for interactions between hundreds of different proteins, they are not generally suitable for thousands of proteins, a scale that would be necessary to test the majority of tractable receptor proteins encoded by a mammalian genome. Here, we have made progress on tackling two of the main barriers that have prevented this increase in scale: reducing the amount of protein required per binding test by increasing assay sensitivity, and, importantly, designing the assay so that just one—rather than the usual two—plasmid expression constructs and sample preparations per protein are needed.
The use of the “flash” Gaussia luciferase presented the opportunity of not only increasing the sensitivity of the prey reporter, and thereby reducing the amount of protein required, but also, because of the reaction-induced inhibition, using the same protein preparation as both a bait and a prey. Previously, we used monomeric biotinylated bait proteins that were clustered around immobilised streptavidin18; however, by taking this approach, we were unable to control the local clustering. By using “pre-clustered” pentameric proteins as the bait, we observed that we could also reduce the amount of immobilised bait protein needed. We established that the inhibition of the luciferase was not easily reversible: re-purifying the protein with extensive washing steps, for example, did not result in any enzyme reactivation. Because the experimental properties of the enzyme inhibition were sufficient for our purposes, we did not investigate the molecular basis of inhibition in any further detail. It is known that Cu2+ ions are potent inhibitors of Gaussia luciferase36 and post-reaction structures of the photoprotein obelin37 which also uses coelenterazine as a substrate suggest that the enzyme inhibitor could be a product of coelenterazine catalysis, possibly coelenteramide. Alternatively, coelenterazine oxidation may lead to permanent inactivation of the enzyme due to the formation of inhibitory adducts. Our initial idea was to use HRP as a reporter enzyme that could be inactivated because it is a sensitive enzyme that can simply and irreversibly inhibited by azide38, and there are a large number of commercially-available substrates. However, we found that the inclusion of HRP in a pentamer expression construct failed due to poor protein expression that was enzymatically inactive, possibly due to protein misfolding or the lack of an essential co-factor in our HEK293 culture.
Both Gaussia and nano-luciferases had several advantages as their use as reporter enzymes in these assays. Both were highly expressed as pentameric oligomers using our mammalian expression system, and were highly sensitive, reducing the protein quantity required for screening. In addition, once expressed, the recombinant protein can be normalised directly in tissue culture medium without the need for further processing. There is scope for optimising this approach further because we did observe some reduction in the signal:noise ratio for the weakest interactions when using the Gaussia luciferase pentamers as the bait. This could be due to incomplete luciferase enzyme inhibition or possibly some low level monomer exchange due to the COMP-mediated oligomerisation between the immobilised bait and prey; even with these caveats, this assay format out-performed the original beta-lactamase assay when using a non-biotinylated prey. The performance of the assay was affected when using the same biotinylated protein preparation for both bait and prey suggesting that this assay format would be best deployed by increasing assay stringency and detecting relatively high affinity interactions (t1/2 > 1.0 s). This increase in background may be due to the exchange of biotinylated prey and baits during the prey incubation step or bait protein stripping with the unconjugated biotin wash used to block all remaining biotin binding sites on the streptavidin-coated plate. Although it doesn’t take advantage of using the one-construct system, we did observe that replacing the beta-lactamase enzyme with Gaussia luciferase resulted in very high signal:noise ratios across all the known interactions, including the very weak ones leading to a significant improvement in assay performance. Similarly, while it cannot be inhibited in the same way as the Gaussia luciferase, and can therefore only be used as a prey reporter construct, we found that the “glow” properties of the nano luciferase was a significant improvement on the use of beta-lactamase as a prey reporter enzyme. The increased sensitivity coupled with the long-lasting luminescence made screening a large number of assay plates very practical. Indeed, we have recently used this assay format to investigate the receptor-ligand interactions involved in the recognition of hepatocyte host cells by the liver stage (sporozoite) of the malaria-causing parasite P. falciparum. Using this implementation of the assay, it was possible to produce sufficient protein for a very large receptor interaction screen (88 parasite ligands versus 182 human receptors) by conveniently transfecting cells in 24-well plates rather than larger (250 mL volume) Erlenmeyer flasks39.
Systematically screening for direct interactions using ELISA-style binary binding assays against all ~ 2,000 tractable receptor proteins in a mammalian genome necessitates an assay that has a throughput of ~ 107 interaction tests. Importantly, this assay should be able to detect weak interactions (KDs ≤ 50 µM) that are a feature of many extracellular interactions4. We found that the use of Gaussia luciferase as a reporter enzyme in a highly avid pentamer construct both reduced the amount protein required and enabled binary interaction screening using a single protein preparation. These refinements have therefore significantly reduced the amount of resources required and will make large-scale extracellular interaction screening less reliant on access to large-scale protein expression infrastructures.
Methods
Recombinant protein expression and purification
All proteins were expressed as secreted proteins by transient transfection of the human HEK293E cell line grown in suspension as described28. Enzyme and tag sequences were synthesized by gene synthesis (Geneart) essentially as described40. The bait proteins were co-transfected with a plasmid for expression of the E. coli enzyme BirA, which monobiotinylates a specific lysine residue in a C-terminal peptide sequence. The transfected cells were incubated for 5 days (in the presence of D-biotin or hemin where specified at 50 μM). The supernatants were harvested by centrifugation at 3000g for 20 min and either stored at 4 °C until use or purified. Except for HRP constructs, 2 mM sodium azide was used as a preservative for stored supernatants. Where required, proteins were purified from spent tissue culture media using Ni2+-NTA resin using an AKTA pure instrument or HisTrap96 well plates (GE Healthcare) as described previously41 or according to manufacturer’s instructions. Briefly, plates were spun to remove storage solutions and washed with binding buffer (20 mM phosphate, 0.5 M NaCl, 20 mM imidazole, pH7.4). Supernatants were loaded, spun, and washed three times with binding buffer before being eluted in 500 µl of elution buffer (20 mM phosphate, 0.5 M NaCl, 200 mM imidazole, pH 7.4).
Enzyme sequences
The following enzyme sequences were used: Gaussia luciferase, accession number: AAG54095 residues K18 to D185; nano-luciferase, AHH41346 residues V2 to T171; beta-lactamase WP_125075679 residues H19 to W282; horseradish peroxidase P00433 residues Q31 to S338.
Avidity-based extracellular interaction detection
Binding assays were performed as previously described28. Briefly, both bait and prey protein preparations were normalised to activities that have been previously shown to detect transient interactions (monomeric dissociation half-lives of less than 0.1 s) with a low false positive rate18. Biotinylated baits that had been purified were immobilised in the wells of a streptavidin-coated 96-well microtitre plate (NUNC). Normalised preys were added, incubated for 1 h at room temperature, washed three times in PBS / 0.1% Tween-20, and once in PBS, after which (for the beta-lactamase constructs) 125 µg/mL of nitrocefin was added and absorbance values measured at 485 nm on a Fluostar Optima (BMG laboratories). This method was modified for the alternative prey constructs to suit their enzyme requirements: for Gaussia luciferase, coelenterazine was added at 1 μM (or as indicated); to nano-luciferase, fumerazine was added at 1 μM. Luminescence was measured on a Fluostar Omega (BMG) with no filter and 1 s integration.
Interaction detection analysis for comparison of different reporter outputs
Each of the enzymes compared in this study has a different output whether colorimetric or luminescent with a different signal intensity. To compare them using a normalised signal output that accounted for the background signal, we used the equation S = (Sobs−Smin) / (Smax−Smin); where ‘S’ is the ‘fraction of maximum signal’ displayed on data plots, ‘Sobs’ is the raw signal in a test well, ‘Smin’ is the average background signal taken from Cd4 negative control bait wells, and ‘Smax’ is the highest signal recorded for the standard interaction at the highest prey concentration. Using this equation, background signal would be 0, and the maximum signal 1.
Benchmark assay using the zebrafish JAM family proteins
Each of the six Jam proteins35 were expressed as the monomeric biotinylated bait, the beta-lactamase pentamer prey, the Gaussia luciferase pentamer prey and the Gaussia luciferase pentamer biotinylated bait. Each protein was purified by nickel affinity purification as described41 and normalised according to the parameters established in the standard assay for optimal interaction detection: the prey protein of interest at 100 pM; 1 pmol of mono-biotinylated bait; 10 fmoles of biotinylated luciferase pentameric bait. These constructs were then tested for binding in a pairwise fashion with each Jam bait arrayed in a streptavidin-coated plate, and each set of six Jam preys tested for binding, making a total of 36 binary interaction tests for each prey-bait combination. In the case of the pentamer bait an additional inactivation step using an excess of coelenterazine was used followed by a wash before the addition of prey protein. The beta-lactamase prey was assessed using accumulated hydrolysis of the colorimetric substrate nitrocefin, while the Gaussia luciferase preys were assessed using luminescence shortly after substrate addition.
Supplementary information
Acknowledgements
This work was funded by the Wellcome Trust Grant 206194.
Author contributions
F.G. performed the experiments. F.G. and G.J.W. wrote the manuscript.
Data availability
All data generated or analysed during this study are included in this published article, or available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67468-7.
References
- 1.Ben-Shlomo, I., Yu Hsu, S., Rauch, R., Kowalski, H. W. & Hsueh, A. J. Signaling receptome: a genomic and evolutionary perspective of plasma membrane receptors involved in signal transduction. Sci. STKE2003, RE9, doi:10.1126/stke.2003.187.re9 (2003). [DOI] [PubMed]
- 2.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer. 2015;15:361–370. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun P, et al. An experimentally derived confidence score for binary protein–protein interactions. Nat. Methods. 2009;6:91–97. doi: 10.1038/nmeth.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright GJ. Signal initiation in biological systems: the properties and detection of transient extracellular protein interactions. Mol. Biosyst. 2009;5:1405–1412. doi: 10.1039/B903580J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Merwe PA, Barclay AN. Transient intercellular adhesion: the importance of weak protein–protein interactions. Trends Biochem. Sci. 1994;19:354–358. doi: 10.1016/0968-0004(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 6.Wood L, Wright GJ. Approaches to identify extracellular receptor–ligand interactions. Curr. Opin. Struct. Biol. 2019;56:28–36. doi: 10.1016/j.sbi.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Frei AP, et al. Direct identification of ligand–receptor interactions on living cells and tissues. Nat. Biotechnol. 2012;30:997–1001. doi: 10.1038/nbt.2354. [DOI] [PubMed] [Google Scholar]
- 8.Sobotzki N, et al. HATRIC-based identification of receptors for orphan ligands. Nat. Commun. 2018;9:1519. doi: 10.1038/s41467-018-03936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong ZS, Ohnishi S, Yusa K, Wright GJ. Pooled extracellular receptor–ligand interaction screening using CRISPR activation. Genome Biol. 2018;19:205. doi: 10.1186/s13059-018-1581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S, Bartholdson SJ, Couch ACM, Yusa K, Wright GJ. Genome-scale identification of cellular pathways required for cell surface recognition. Genome Res. 2018;28:1372–1382. doi: 10.1101/gr.231183.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood L, Wright GJ. High-content imaging for large-scale detection of low-affinity extracellular protein interactions. SLAS Discov. 2019;24:987–999. doi: 10.1177/2472555219879053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin HY, Wang XF, Ng-Eaton E, Weinberg RA, Lodish HF. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- 14.Mathews LS, Vale WW. Expression cloning of an activin receptor, a predicted transmembrane serine kinase. Cell. 1991;65:973–982. doi: 10.1016/0092-8674(91)90549-e. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Martin N, et al. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell. 2018;174:1158–1171. doi: 10.1016/j.cell.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Ozkan E, et al. An extracellular interactome of immunoglobulin and LRR proteins reveals receptor-ligand networks. Cell. 2013;154:228–239. doi: 10.1016/j.cell.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser JJ, et al. An extracellular biochemical screen reveals that FLRTs and Unc5s mediate neuronal subtype recognition in the retina. Elife. 2015;4:e08149. doi: 10.7554/eLife.08149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bushell KM, Sollner C, Schuster-Boeckler B, Bateman A, Wright GJ. Large-scale screening for novel low-affinity extracellular protein interactions. Genome Res. 2008;18:622–630. doi: 10.1101/gr.7187808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sollner C, Wright GJ. A cell surface interaction network of neural leucine-rich repeat receptors. Genome Biol. 2009;10:R99. doi: 10.1186/gb-2009-10-9-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin S, et al. Construction of a large extracellular protein interaction network and its resolution by spatiotemporal expression profiling. Mol. Cell Proteomics. 2010;9:2654–2665. doi: 10.1074/mcp.M110.004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Almen MS, Nordstrom KJ, Fredriksson R, Schioth HB. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009;7:50. doi: 10.1186/1741-7007-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bausch-Fluck D, et al. The in silico human surfaceome. Proc. Natl. Acad. Sci. USA. 2018;115:E10988–E10997. doi: 10.1073/pnas.1808790115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Cunha JP, et al. Bioinformatics construction of the human cell surfaceome. Proc. Natl. Acad. Sci. USA. 2009;106:16752–16757. doi: 10.1073/pnas.0907939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diehn M, Bhattacharya R, Botstein D, Brown PO. Genome-scale identification of membrane-associated human mRNAs. PLoS Genet. 2006;2:e11. doi: 10.1371/journal.pgen.0020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat. Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maguire CA, et al. Gaussia luciferase variant for high-throughput functional screening applications. Anal Chem. 2009;81:7102–7106. doi: 10.1021/ac901234r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall MP, et al. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 2012;7:1848–1857. doi: 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerr, J. S. & Wright, G. J. Avidity-based extracellular interaction screening (AVEXIS) for the scalable detection of low-affinity extracellular receptor-ligand interactions. J. Vis. Exp., e3881. 10.3791/3881 (2012). [DOI] [PMC free article] [PubMed]
- 29.Martell JD, et al. A split horseradish peroxidase for the detection of intercellular protein–protein interactions and sensitive visualization of synapses. Nat. Biotechnol. 2016;34:774–780. doi: 10.1038/nbt.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remy I, Michnick SW. A highly sensitive protein–protein interaction assay based on Gaussia luciferase. Nat. Methods. 2006;3:977–979. doi: 10.1038/nmeth979. [DOI] [PubMed] [Google Scholar]
- 31.Grigorenko V, Andreeva I, Borchers T, Spener F, Egorov A. A genetically engineered fusion protein with horseradish peroxidase as a marker enzyme for use in competitive immunoassays. Anal. Chem. 2001;73:1134–1139. doi: 10.1021/ac000684t. [DOI] [PubMed] [Google Scholar]
- 32.Wright GJ, et al. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity. 2000;13:233–242. doi: 10.1016/s1074-7613(00)00023-6. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Gallagher-Jones M, Barker C, Wright GJ. A benchmarked protein microarray-based platform for the identification of novel low-affinity extracellular protein interactions. Anal. Biochem. 2012;424:45–53. doi: 10.1016/j.ab.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell GT, Wright GJ. Jamb and jamc are essential for vertebrate myocyte fusion. PLoS Biol. 2011;9:e1001216. doi: 10.1371/journal.pbio.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell GT, Wright GJ. Genomic organisation, embryonic expression and biochemical interactions of the zebrafish junctional adhesion molecule family of receptors. PLoS ONE. 2012;7:e40810. doi: 10.1371/journal.pone.0040810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inouye S, Sahara Y. Identification of two catalytic domains in a luciferase secreted by the copepod Gaussia princeps. Biochem. Biophys. Res. Commun. 2008;365:96–101. doi: 10.1016/j.bbrc.2007.10.152. [DOI] [PubMed] [Google Scholar]
- 37.Liu ZJ, et al. Crystal structure of obelin after Ca2+-triggered bioluminescence suggests neutral coelenteramide as the primary excited state. Proc. Natl. Acad. Sci. USA. 2006;103:2570–2575. doi: 10.1073/pnas.0511142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortiz de Montellano PR, David SK, Ator MA, Tew D. Mechanism-based inactivation of horseradish peroxidase by sodium azide. Formation of meso-azidoprotoporphyrin IX. Biochemistry. 1988;27:5470–5476. doi: 10.1021/bi00415a013. [DOI] [PubMed] [Google Scholar]
- 39.Segireddy, R. R. et al. A screen for Plasmodium falciparum sporozoite surface protein binding to human hepatocyte surface receptors identifies novel host-pathogen interactions. bioRxiv 2020.02.02.929190. doi:10.1101/2020.02.02.929190 (2020). [DOI] [PMC free article] [PubMed]
- 40.Crosnier C, et al. A library of functional recombinant cell-surface and secreted P. falciparum merozoite proteins. Mol. Cell Proteomics. 2013;12:3976–3986. doi: 10.1074/mcp.O113.028357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartholdson SJ, et al. Semaphorin-7A is an erythrocyte receptor for P. falciparum merozoite-specific TRAP homolog MTRAP. PLoS Pathog. 2012;8:e1003031. doi: 10.1371/journal.ppat.1003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article, or available from the corresponding author upon reasonable request.