Abstract
Levels of iron and iron-related proteins including ferritin are higher in the lung tissue and lavage fluid of individuals with chronic obstructive pulmonary disease (COPD), when compared to healthy controls. Whether more iron in the extracellular milieu of the lung associates with distinct clinical phenotypes of COPD, including increased exacerbation susceptibility, is unknown. We measured iron and ferritin levels in the bronchoalveolar lavage fluid (BALF) of participants enrolled in the SubPopulations and InteRmediate Outcome Measures In COPD (SPIROMICS) bronchoscopy sub-study (n = 195). BALF Iron parameters were compared to systemic markers of iron availability and tested for association with FEV1 % predicted and exacerbation frequency. Exacerbations were modelled using a zero-inflated negative binomial model using age, sex, smoking, and FEV1 % predicted as clinical covariates. BALF iron and ferritin were higher in participants with COPD and in smokers without COPD when compared to non-smoker control participants but did not correlate with systemic iron markers. BALF ferritin and iron were elevated in participants who had COPD exacerbations, with a 2-fold increase in BALF ferritin and iron conveying a 24% and 2-fold increase in exacerbation risk, respectively. Similar associations were not observed with plasma ferritin. Increased airway iron levels may be representative of a distinct pathobiological phenomenon that results in more frequent COPD exacerbation events, contributing to disease progression in these individuals.
Subject terms: Respiratory tract diseases, Iron
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of mortality and morbidity worldwide, resulting in almost 3 million deaths globally in 2016 and the loss of over 47 million potential life-years1. A significant portion of this burden of disease presents as exacerbation events, episodic surges of respiratory symptoms accompanied by a more rapid decline in lung function and higher mortality2,3. These exacerbations are largely attributed to recurrent acute infection, with host factors also playing a fundamental role4. Identifying and understanding host susceptibility risk factors that contribute to repeated exacerbations is imperative to understanding and treating COPD.
Although the overwhelming risk factor in the industrialized world for COPD development is tobacco smoking, genome wide association studies suggest a pathogenic role for abnormal iron homeostasis5. We recently demonstrated that a major iron metabolism protein, iron regulatory protein 2 (IRP2), drives lung inflammation and injury in a murine model of COPD5,6. In the lung, iron is found in both unbound and protein-bound forms, and several of the most abundant proteins in lung tissue and bronchoalveolar lavage fluid (BALF) bind to and regulate iron7–9. One such protein is ferritin, an octahedral polymeric shell composed of light chain (FTL) and heavy chain (FTH) subunits that stores ferric (Fe3+) iron atoms in a soluble, non-toxic form10. Previous studies have demonstrated release of ferritin from iron-loaded alveolar macrophages (AMs) in smokers, and AM ferritin mRNA levels are increased in active smokers and correlate with airflow limitation in COPD patients11,12. Furthermore, total levels of non-heme iron and of other iron-binding molecules including lipocalin-2 and lactoferrin, are increased in lung tissue, sputum, BALF, and AMs of COPD patients, relative to non-smokers9,11–21. Conversely, there is also ample evidence for iron deficiency in COPD, and anaemia in COPD is associated with worse patient outcomes, including mortality22,23. The biological relevance of such observations remains to be elucidated; however, these data strongly support a local iron overload signature in the extracellular milieu of the lung in COPD that is distinctive to systemic iron handling, which is intriguing as mainstream cigarette smoke contains little iron24,25.
Whether overloaded or deficient, abnormal iron stores may have important immunologic and microbiologic implications, and could provide insights into COPD pathogenesis and progression26. However, to our knowledge, iron parameters have not been examined for association with clinical phenotypes or outcomes (including exacerbation frequency) in a large, well-characterized COPD cohort. In this study, we measured BALF ferritin and iron levels in participants enrolled in the extensively characterized SubPopulations and InteRmediate Outcome Measures In COPD (SPIROMICS) cohort. We demonstrate that BALF ferritin and iron levels, but not systemic iron parameters, are associated with clinical measures of disease activity, most importantly exacerbation frequency. These findings support a hypothesis whereby higher BALF iron levels, reflective of iron overload in the lung microenvironment, may represent a novel pathobiological endotype in COPD.
Results
Bronchoscopy sub-study participants
SPIROMICS (ClinicalTrials.gov NCT01969344T4) is an on-going longitudinal multicentre observational study that recruited 2981 subjects, 40 to 80 years of age, including never-smokers (≤1 pack-year of tobacco-smoking history), current or former smokers (ever-smokers, ≥20 pack-years) without airflow obstruction, and ever-smokers with airflow obstruction27. A subgroup of subjects (n = 215) with post-bronchodilator FEV1 > 30% predicted and without an exacerbation in the prior six weeks were further enrolled in a bronchoscopy sub-study. The characteristics of participants of the bronchoscopy sub-study, in relation to the overall SPIROMICS cohort, are shown in Table 1. Compared to the entire SPIROMICS cohort, participants who underwent bronchoscopy were younger, had milder disease, and were less likely to have had a COPD exacerbation during study follow-up (Table 1). BALF ferritin and iron were measured in 195 out of the total 215 recruited participants in the bronchoscopy sub-study. Plasma ferritin was available for 119 of these 195 participants in the bronchoscopy sub-study, as well as for 1575 participants in the entire SPIROMICS cohort.
Table 1.
Participants enrolled in SPIROMICS and in the SPIROMICS bronchoscopy sub-study.
| SPIROMICS Cohort (n = 2974) |
Bronchoscopy Substudy (n = 195) |
P-value* | |
|---|---|---|---|
| Age (y) median [IQR] | 64 [56–70] | 59 [52–67] | <0.001 |
| Sex N (%) | 0.89 | ||
| Male | 1577 (53.0%) | 102 (52.3%) | |
|
Smoking Status at baseline N (%) |
0.91 | ||
| Current Non-Smoker | 1839 (62.7%) | 121 (63.4%) | |
| Current Smoker | 1093 (37.3%) | 70 (36.6%) | |
| GOLD Stage N (%) | <0.001 | ||
| 0 | 924 (31.1%) | 86 (44.1%) | |
| 1 | 404 (13.6%) | 32 (16.4%) | |
| 2 | 820 (27.6%) | 44 (22.6%) | |
| 3 | 433 (14.6%) | 8 (4.10%) | |
| 4 | 186 (6.3%) | 0 (0%) | |
| No COPD | 207 (7.0%) | 25 (12.8%) | |
| Haemoglobin (g/dL) median [IQR] | 14.3 [13.2–15.3] | 14.1 [13.2–14.9] | 0.10 |
|
Exacerbations during follow up N (%) |
969 (33.7%) | 39 (20.3%) | <0.001 |
*Kruskal-Wallis or Chi-square test comparing patients included in the Bronchoscopy substudy to remaining SPIROMICS patients. IQR: interquartile range.
BALF iron parameters in SPIROMICS
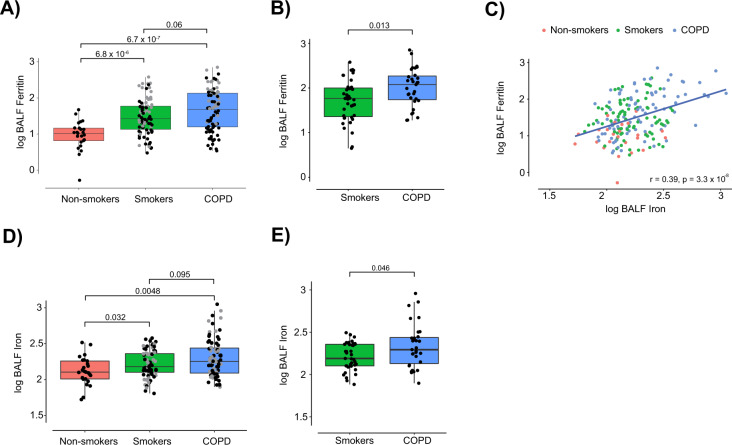
We first examined relationships between BALF iron parameters and COPD status. Relative to never-smokers (median 10.5 ng/mL), BALF ferritin levels were significantly increased in smokers without COPD (26.4 ng/mL; p < 0.0001) and in COPD participants (47.3 ng/mL; p < 0.0001) (Fig. 1A). Amongst current smokers, BALF ferritin was higher in participants with COPD than in those without COPD (median 119.4 vs. 58.2 ng/mL; p = 0.013) (Fig. 1B). To eliminate the possibility that increased BALF ferritin occurred as a result of alveolar epithelial damage and transepithelial protein leakage, a sensitivity analysis was performed by normalizing BALF ferritin to BALF total protein and revealed similar, if not stronger, associations with disease status relative to unnormalized levels (Supplemental Fig. 1A-B). Ferritin has a critical role in iron storage, both protecting the cell from the dangers of labile iron and making it available in times of increased demand10. In this study, BALF ferritin correlated (r = 0.34; p < 0.0001) with BALF iron, confirming the two are interrelated (Fig. 1C). Similar to ferritin, BALF total iron was also significantly increased in smokers without COPD (median 150.95 μg/L; p = 0.032) and in participants with COPD (179.1 μg/L; p = 0.0048) relative to never-smokers (107.9 μg/L; Fig. 1D). BALF iron in current smokers with COPD was also higher compared to those without COPD (196.9 vs. 154.7 μg/L; p = 0.046) (Fig. 1E).
Figure 1.
Bronchoalveolar lavage fluid (BALF) ferritin and iron levels are increased in smokers and participants with COPD. (A–E) Ferritin (ng/mL) and iron (mg/L) levels were measured in the BALF of SPIROMICS participants [never-smokers (n = 25), ever-smokers (including current and former smokers) without COPD (n = 86) and ever-smokers with COPD (n = 84, and n = 83 for ferritin and iron respectively)]. (A,D) Grey dots indicate current smokers at the time of baseline visit. (B,E) BALF ferritin and iron levels in current smokers without COPD (n = 39) and with COPD (n = 31) in SPIROMICS. (C) BALF ferritin association with BALF iron in never-smokers (n = 25, red), ever-smokers without COPD (n = 86, green) and ever-smokers with COPD (n = 84, and n = 83 for ferritin and iron respectively, blue) in SPIROMICS. Data (A,B,D,E) are presented as median with box indicating upper and lower quartiles, whiskers indicating extrema, and with P values calculated by non-parametric Kruskal-Wallis test. Linear associations (C) were tested with Pearson’s correlation coefficient.
Similar results were also observed in an independent validation cohort (n = 59 BALF samples, Supplemental Table 1). In that cohort, BALF ferritin was higher in smokers (median 57.9 ng/mL; p < 0.0001) and participants with COPD (77.6 ng/mL; p < 0.0001) compared to healthy non-smoker controls (5.44 ng/mL), and again higher in current smokers with COPD relative to those without (median 112.7 vs. 57.9 ng/mL; p = 0.045) (Supplemental Fig. 2A-B). BALF ferritin also correlated with BALF iron in this cohort (r = 0.43, p = 0.00064), and similarly BALF iron was higher in smokers (67.8 μg/L; p = 0.031) and participants with COPD (73.9 μg/L; p = 0.0011) compared to non-smokers (22.6 μg/L), but did not differ significantly between current smokers with and without COPD (p = 0.35) (Supplemental Fig. 2C–E). The above findings demonstrate that individuals with COPD have higher levels of ferritin and iron in the lavage fluid lining the lung, an observation that was strengthened by current smoking status.
BALF iron parameters and systemic iron status
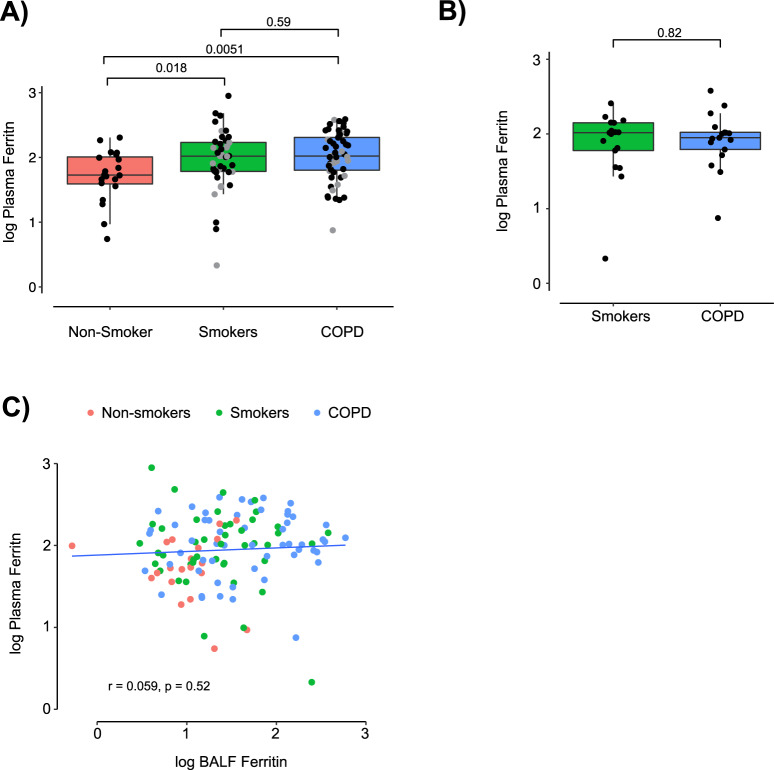
Circulating ferritin is widely used clinically as a surrogate of systemic and bone marrow iron stores, and is a component of routine anaemia work-up28. Additionally, ferritin is well-recognized as an acute phase reactant and is elevated in many chronic inflammatory diseases29–31. To evaluate whether increased BALF ferritin and iron were indicative of a global ferritin or iron increase, we next compared BALF and plasma ferritin levels in SPIROMICS bronchoscopy sub-study participants. As with BALF ferritin, plasma ferritin was significantly increased in smokers without COPD (median 104.5 ng/mL; p = 0.018) and in participants with COPD (105.0 ng/mL; p = 0.0051) relative to never-smokers (53.5 ng/mL) (Fig. 2A). However, unlike BALF ferritin, plasma ferritin was not higher in current smokers with COPD versus those without (104.0 vs. 89.0 ng/mL; p = 0.82) (Fig. 2B). Plasma ferritin did not associate with BALF ferritin, supporting the possible existence of two distinctive compartments for extracellular ferritin, and demonstrating that elevated BALF ferritin may not be merely a result of spillage from elevated plasma ferritin (Fig. 2C). The above plasma ferritin findings among bronchoscopy sub-study participants were also consistent with those of the entire SPIROMICS cohort (Supplemental Fig. 3A-B).
Figure 2.
Plasma ferritin increases in smokers and in COPD but is not associated with BALF ferritin. (A) Plasma ferritin (ng/mL) in never-smokers (n = 20), ever-smokers without COPD (n = 44) and ever-smokers with COPD (n = 55) in the SPIROMICS bronchoscopy sub-study were measured using a Luminex-based multiplex assay system as described62. Grey dots indicate current smokers at the time of baseline visit. (B) Plasma ferritin in current smokers without (n = 17) and with COPD (n = 17) in the SPIROMICS bronchoscopy sub-study. (C) Association between plasma ferritin and BALF ferritin. (A,B) median, 25th and 75th percentiles, and extrema; P values by non-parametric Kruskal-Wallis test. Linear associations (C) were tested with Pearson’s correlation coefficient.
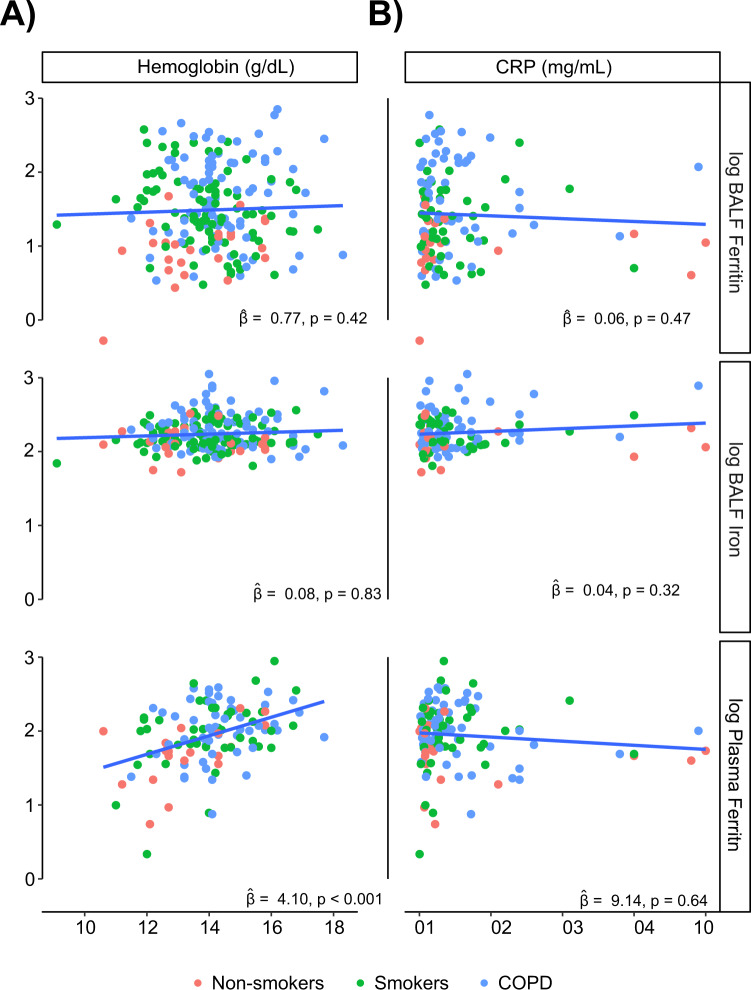
To test our hypothesis that increased BALF iron in COPD is indicative of a local pathogenic process independent of systemic iron levels, we investigated associations between BALF iron parameters and systemic iron (haemoglobin) and the systemic inflammatory marker C-reactive protein (CRP). BALF ferritin and iron did not associate with haemoglobin or CRP, whereas plasma ferritin strongly associated with haemoglobin and not CRP in bronchoscopy sub-study participants (Fig. 3A-B), and with both in the overall SPIROMICS cohort (Supplemental Fig. 4A-B).
Figure 3.
Local lung ferritin and iron levels do not correlate with systemic markers of iron storage or inflammation. (A) Haemoglobin (g/dL) and (B) CRP (μg/mL) levels in never-smokers (n = 25,20, red), ever-smokers without COPD (n = 85,44, blue) and ever-smokers with COPD (n = 83,55, green), as previously measured using a Luminex-based multiplex assay system62 and association with BALF ferritin (ng/mL), BALF iron (μg/L), and plasma ferritin (ng/mL) were tested with a linear model on the log-transformed markers and accounting for batch and site effects. denotes adjusted increase in log-10 ferritin associated with unit increase in log-10 marker.
BALF Iron Parameters, COPD Progression, and Exacerbation Risk
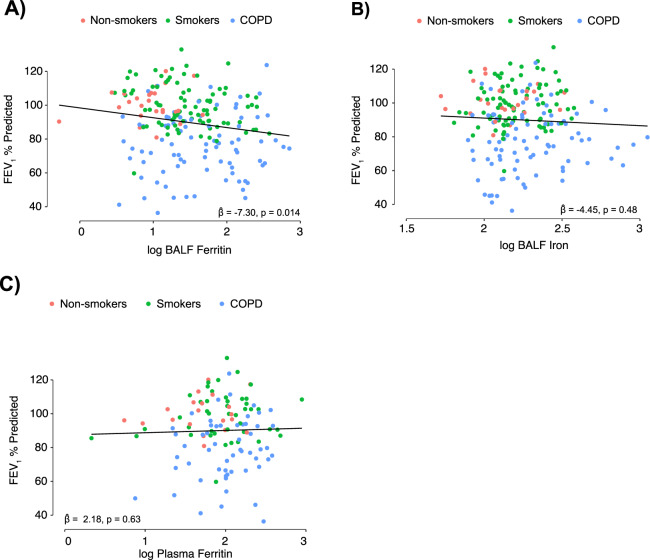
Cigarette smoking and COPD are associated with an accelerated decline in lung function, most commonly measured by spirometry and represented as FEV1 % predicted (forced expiratory volume in one second)32,33. In the SPIROMICS cohort, BALF ferritin negatively correlated with FEV1 % predicted in models adjusted for age, sex, smoking status and clinical site (10-fold increase in BALF ferritin = −7.3 points; confidence interval [CI] −13.10, −1.5; p = 0.01) (Fig. 4A). This was maintained upon removing never-smokers (adjusted = −7.53 points; CI −14.11, −0.94; p = 0.02) (Supplemental Table 2). BALF iron did not significantly correlate with FEV1% predicted (adjusted = −4.45 points; CI −16.87,7.97; p = 0.48) (Fig. 4B). Plasma ferritin also did not correlate with FEV1% predicted in adjusted models in the bronchoscopy sub-study ( = 2.18 points; CI −6.86,11.22; p = 0.63) (Fig. 4C) or in the overall SPIROMICS cohort (Supplemental Fig. 5). BALF ferritin, BALF iron or plasma ferritin were not associated with quantifiable radiographic measures of COPD such as small airway disease (PRMFSAD) or emphysema (PRMEMPH) (Supplemental Fig. 6A-B and Supplemental Table 2).
Figure 4.
Higher BALF ferritin levels are associated with lower lung function. (A–C) Correlation between BALF ferritin (ng/mL), BALF iron (mg/L), and plasma ferritin (ng/mL) in never-smokers (n = 25 for BALF ferritin and iron, 20 for plasma ferritin, red), ever-smokers without COPD (n = 86 for BALF ferritin and iron, 44 for plasma ferritin green) and ever-smokers with COPD (n = 84 for BALF ferritin, 83 for BALF iron, 55 for plasma ferritin, blue) and post-bronchodilator FEV1% predicted. Linear associations (A–C) were tested, adjusting for age, sex, smoking status and study site. denotes adjusted increase in log-10 ferritin associated with unit increase in log-10 marker.
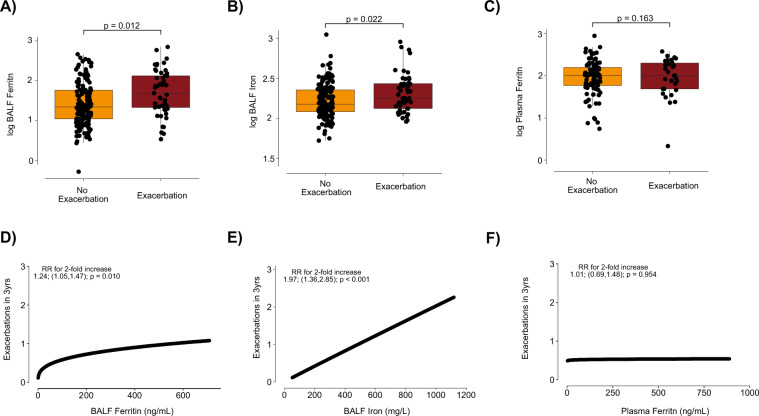
FEV1 is closely linked to COPD exacerbations. Patients with frequent exacerbations have steeper decline in FEV1, especially in those with early COPD, where each individual exacerbation results in a profound loss of lung function34–36. In SPIROMICS, participants in the bronchoscopy sub-study had less severe disease compared to the overall cohort, and therefore had fewer exacerbations (Table 1). Nevertheless, compared to participants without exacerbations during follow-up, those who did have exacerbations had higher levels of BALF ferritin in adjusted models (2-fold increase in ferritin = 1.39 points; CI 1.08, 1.80; p = 0.012). Similarly, compared to participants without exacerbations during follow-up, those who had exacerbations had higher levels of BALF iron (adjusted = 1.90 points; CI 1.11, 3.36; p = 0.022). Plasma ferritin did not associate with exacerbation events in adjusted models (= 0.95 points; CI 0.88, 1.02; p = 0.163) (Fig. 5A–C).
Figure 5.
Higher BALF ferritin and iron levels are associated with increased exacerbation risk. (A,B) BALF ferritin (ng/mL) and BALF iron (µg/mL) in participants who had had one or more acute COPD exacerbation (n = 49 for ferritin, n = 48 for iron) when compared to participants who did not (n = 146). (C) Plasma ferritin levels in participants with (n = 30) versus without (n = 89) exacerbations. (A–C) median, 25th and 75th percentiles, extrema; Adjusted P values (age, sex, smoking status and site) (D–F) Predicted exacerbation rate per SPIROMICS bronchoscopy sub-study participant over 3–5 years of follow up by BALF ferritin (ng/mL) (D), BALF iron (µg/mL) (E) or plasma ferritin (ng/mL) (F) were estimated with a zero-inflated negative binomial model for a participant with a median FEV1 % predicted for (D) BALF ferritin (n = 195), (E) BALF iron (n = 194), and (F) plasma ferritin (n = 119).
When analysed relative to the baseline visit in a zero-inflated negative binomial model, a 2-fold increase in BALF ferritin was associated with a 24% increase in the rate of yearly exacerbations (rate ratio [RR] 1.24; CI 1.05, 1.47; p = 0.010) (Fig. 5D, Supplemental Table 3). Comparable results were observed adjusting for participant age, sex and smoking status, with the point estimate not substantially changed (RR 1.23; CI 0.99, 1.53; p = 0.058) (Supplemental Table 3). Similar results were found for BALF iron, for which a 2-fold increase was associated with a 2-fold increase in the yearly rate of exacerbations (RR 1.97; CI 1.36, 2.85; p < 0.001 and adjusted RR 1.98; CI 1.33, 2.93; p < 0.001) (Fig. 5E, Supplemental Table 3). Notably, plasma ferritin was not associated with exacerbation rate in the bronchoscopy sub-study or in the overall cohort (Fig. 5F, Supplemental Table 3). After analysing only exacerbations following the bronchoscopy visit, many exacerbation events were lost and the association between exacerbation rate and BALF ferritin was no longer significant (adjusted RR 1.11; CI 0.88–1.40; p = 0.401), but a significant association was maintained for BALF iron (adjusted RR 1.81; CI 1.18–2.76; p = 0.006) (Supplemental Table 3).
Discussion
In this study, we found that airway iron parameters were significantly higher in the bronchoalveolar lavage fluid of individuals with COPD when compared to “healthy” smokers and controls, and that higher levels of BALF ferritin and iron associate with a greater risk for COPD exacerbation. These results, contrasting with the absence of similar associations with plasma ferritin, support the hypothesis that changes in “local” lung iron homeostasis contribute to COPD pathobiology.
The regulation of iron acquisition, utilization, and storage is fundamental to the correct function of lung epithelial, endothelial, and immune cells37. Iron is also crucial for the metabolism and growth of native and pathogenic microbes, including those associated with recurrent infections in COPD such as Haemophilus influenzae and Pseudomonas aeruginosa38,39. During infection, the host deploys a number of defence mechanisms in an attempt to sequester free iron away from bacteria, including promoting iron uptake, increasing intracellular iron storage capacity, and secreting proteins (e.g. ferritin, lactoferrin, lipocalin-2) to chelate remaining extracellular iron40–42. Saturation of these adaptive mechanisms directly (e.g. smoke) or indirectly (genetic susceptibility) may result in an excess of iron in the lung microenvironment, which in turn may influence the size and diversity of microbial populations that colonize and proliferate there. In support of this, we have also observed increased BALF levels of the siderophore lipocalin-2 in smokers and participants with COPD, when compared to control subjects in the SPIROMICS cohort (data not shown). Given the fact that infections are the dominant cause of COPD exacerbations, it follows that disrupting such a complex iron regulatory system and changing iron availability could potentially directly affect exacerbation risk in COPD patients. This is highlighted by the strong association between BALF iron levels and exacerbation frequency found in this study, without a concurrent association with FEV1, suggesting that this iron-mediated process may precede lung function decline in these patients.
In this study, the source of increased BALF iron and ferritin is unknown. Alveolar macrophages are iron- and ferritin-rich in smokers and COPD patients, but both lung epithelial and endothelial cells are also potential sources. As previously mentioned, mainstream cigarette smoke contains little iron24,25. We hypothesize that iron accumulates as a biological response to continuous smoke exposure. Specifically, smoke exposure leads to altered responses to hypoxia, higher erythropoietin (EPO) levels43 and expanded, but inefficient erythropoiesis in the bone marrow (unpublished data)44. This may in turn lead to changes in iron uptake and release in macrophages globally, including in the lung, to increase iron availability for hemoglobin synthesis45. Alternatively, alveolar macrophage iron metabolism is directly dysregulated upon smoke exposure. Alveolar macrophages from COPD patients have markedly depressed M1-responses46 and markers47,48 and lower phagocytic ability49. Such deactivation in the M1-polarization programme is accompanied by an increase in the expression of wound-healing M2 markers47,48. Iron regulatory responses also differ between macrophage phenotypes where M1-macrophages accumulate iron as part of a bacteriostatic stratagem, whilst the opposite is the case for M2-macrophages, in which iron release is favoured50. Changes in alveolar macrophage biology therefore may favor a phenotype of active iron release by the macrophage, leading to increased extracellular iron. Similarly, we believe that the increased level of BALF ferritin detected in this and other studies is a result of a biological response to smoke exposure. In the lung, intracellular ferritin is comprised of light and heavy chain subunits (FTL and FTH, respectively) in equal proportions, and has relatively low iron saturation compared to ferritin from other organs such as the liver or spleen51. Extracellular ferritin is also a combination of FTL and FTH, which in serum consists predominantly of FTL52,53. FTL may be more abundant than FTH in the BALF of healthy subjects54; however, we did not evaluate proportions of FTL and FTH in ferritin found in BALF, and most commercial ELISA kits, including those used in this study, are unable to discriminate between these subunits. The purpose of ferritin and iron release into the alveolar space is unclear and is currently the subject of further investigation. Ferritin can serve as an iron carrier; macrophages, in particular, use ferritin to deliver iron to hepatocytes and erythroid progenitors55,56.
Our findings, in particular the discordance between BALF and plasma ferritin with clinical associations, extend on previous studies of serum ferritin levels in COPD patients. Serum ferritin is increased in smokers, and while some studies showed higher serum ferritin correlated with worse airflow obstruction, in others higher serum ferritin and iron correlated with spirometric protection against cigarette smoke57–59. Such findings do not inherently contradict our results, as blood and airway ferritin may be indicative of distinct pathologic mechanisms, and the phenomenon of local iron overload we demonstrated does not exclude systemic iron deficiency in some COPD patients. Distinguishing between local and systemic iron status is a crucial subject for future investigation, as treating one anatomic compartment with iron chelation or supplementation may exacerbate disease processes in another.
Our study has several important strengths. This is the largest study of iron metabolism in a prospective COPD cohort and exploits the fact that SPIROMICS is enriched for spirometrically mild-to-moderate disease, a deliberate design feature to discover factors that lead to disease progression. We included an independent validation cohort to corroborate some of our findings. To eliminate the possibility that increased BALF ferritin occurred as a result of alveolar epithelial damage and transepithelial protein leakage, we normalized BALF ferritin to BALF total protein, which strengthened BALF ferritin correlations with disease status.
Our study also has limitations. We determined smoking status by self-report, unverified in this analysis using objective measurements such as urine cotinine; we also limited current smoking status to the baseline visit, recognizing that study participants could have quit smoking between that visit and their bronchoscopy. Subjects who agreed to the bronchoscopy sub-study were self-selected and may not be representative of the general COPD population. Critically, the bronchoscopy sub-study population is overly represented by never-smokers and ever-smokers with preserved lung function and is constrained further by a safety check on adequate FEV1 on the date of bronchoscopy. This stringent selective process limits our statistical power, given the limited numbers of severe COPD cases and exacerbations. The timing of bronchoscopy, in some cases up to 1 year after the initial visit, further curtails the exacerbation event numbers. In addition, we measured BALF ferritin and iron at a single time point, and it is not known whether these levels are stable over time in a given patient, or fluctuate, especially around the time of an exacerbation or infection. We correlated BALF ferritin with many clinical variables and cannot exclude the possibility that iron metabolism is merely an intermediary between these variables and other more established predictors such as FEV1% predicted. Furthermore, our models assume equal follow-up time for study participants, whereas in reality that follow-up time varied substantially. Despite these limitations, we consider the over-representation of never-smokers and ever-smokers with preserved lung function a strength, in that this allowed us to test our hypothesis at an early disease stage, before significant lung function loss has taken place. Our study is also the first to associate BALF iron parameters with important COPD outcomes such as exacerbation risk, associations that do not exist with plasma ferritin. The lack of associations with radiographic measures of small airways disease and emphysema further support our hypothesis that higher iron may be associated with early disease and may be a factor in COPD pathogenesis. Given the impracticality of routine bronchoscopy outside the research setting, our goal is not to advocate for BALF ferritin or iron levels as COPD biomarkers, nor to suggest predictive or causal relationships. Instead, we highlight these associations to provide evidence for a role for abnormal airway iron metabolism in COPD pathogenesis and progression, to support exploration of the basic mechanisms behind this phenomenon as well as to draw attention to the potential of targeting lung iron overload in COPD.
In conclusion, BALF ferritin and iron levels are higher in subjects with COPD, an observation that correlates with heightened COPD exacerbation susceptibility. If replicated, these results suggest that iron overload might represent an under-recognized disease endotype. The pathobiologic mechanism behind these associations warrants further investigation.
Methods
Ethics statement
All clinical investigations are conducted according to the principles of the Declaration of Helsinki. The individual institutional review boards (IRBs) of all participating clinical centres approved all study protocols. All participants understood the purpose of the study and provided written informed consent before they underwent any research activities or procedures.
Study design and sample collection
SPIROMICS (ClinicalTrials.gov NCT01969344T4) is an ongoing longitudinal multicenter observational study funded by the National Heart, Lung, and Blood Institute27 that recruited 2981 subjects, 40 to 80 years of age, including some who had never smoked cigarettes (≤1 pack-year of tobacco-smoking history), current or former smokers (ever-smokers, greater than 20 pack-years) without airflow obstruction, and ever-smokers with airflow obstruction60. Data was collected at the initial study visit, including demographics, comorbidities, questionnaires, current cigarette smoke exposure and 6-minute walk distance (6MWD)27. The extent of airway-wall abnormality was characterized with HRCT scans of the lung, using Imbio (parametric response mapping, PRM) and VIDA Diagnostics software; small airway disease (PRMFSAD) was defined as areas of lung that are greater than −950 HU on inspiration but less than −856 HU on expiration, denoting air-trapping, while emphysema was defined as areas of lung that less than −950 HU on inspiration and less than −856 HU on expiration61.
Clinical data was collected at the baseline study visit and in follow-up visits as previously described27. Peripheral blood was collected as part of the baseline visit, and plasma biomarkers were measured using a Luminex-based multiplex assay; relevant to this study, plasma ferritin was shown to be equivalent to serum ferritin using this assay method62. Exacerbations were defined as health care utilization events (office visit, hospital admission, or emergency department visit for a respiratory flare-up) that were treated with antibiotics, systemic corticosteroids, or both. Exacerbation history was prospectively collected every 3 months for up to 5 years using a structured questionnaire2,60.
A subgroup of subjects (n = 215) with post-bronchodilator FEV1 > 30% predicted and without an exacerbation in the prior six weeks were further enrolled in the bronchoscopy sub-study, in which on the first of two visits, sputum induction was performed as previously described63. On the second visit, post-bronchodilator FEV1 was measured and only subjects with an FEV1 > 30% predicted were allowed to participate in the bronchoscopy portion of the study. BAL was performed in the right middle lobe and lingula by instilling two aliquots of 40 mL and one aliquot of 50 mL per lobe (260 mL total volume), after excluding an initial airway wash sample. Unfiltered BALF fluid was collected into a sterilized beaker or in multiple 50 mL conical tubes on ice, then centrifuged at 300 x g for 5 min and the supernatant aliquoted into 1 mL aliquots for storage at −80 °C, representing one BALF sample per patient as described previously63,64. Because no research plasma samples were obtained in the bronchoscopy sub-study, the plasma biomarkers, including ferritin, in this analysis were measured from the baseline visit samples. For participants in the bronchoscopy sub-study, exacerbation events were analysed both relative to the baseline visit and to the bronchoscopy visit (0-14 months after baseline visit).
To replicate the study findings in an independent cohort, never smokers (n = 20), healthy smokers (n = 21) with normal lung function and individuals with COPD (n = 18), recruited by the Department of Genetic Medicine, Weill Cornell Medical College underwent bronchoscopy with BALF isolated as described above (see Supplementary Information and Supplemental Table 1).
Ferritin measurement and normalization
BALF ferritin was quantified by ELISA using the Abcam Human Ferritin ELISA Kit (Cat#ab200018), which detects both ferritin heavy and light chain. BALF ferritin was normalized to total protein, measured using the Thermo Scientific Pierce BCA Protein Assay Kit (Cat#23225).
Total iron measurements
After centrifugation (1000 × g for 5 mins), 60 μL of BALF was digested with 40 μL of 50% Nitric Acid (in distilled H2O) containing a final concentration of 0.1% digitonin for 2 hours at 60 °C. Total iron, including both bound and unbound forms, was measured in triplicate in 20 μL of digested fractions using a graphite furnace atomic absorption spectrophotometer (GFAAS, Perkin Elmer PinAAcle 900z), comparing unknown values to a standard curve of known concentrations of iron (1000 PPM in 2% Nitric Acid).
Statistical analysis
Clinical characteristics of SPIROMICS participants enrolled in the bronchoscopy sub-study were compared to those of all SPIROMICS participants, summarized using means and standard deviations or counts and percentages as appropriate. BALF Ferritin and iron were analysed on the log 10 scale. Plasma ferritin, haemoglobin and CRP were measured as previously described62, and analysed on the log scale, accounting for site and batch effects. Associations between BALF ferritin and baseline characteristics were performed using Kruskal-Wallis tests for categorical variables, and Pearson correlations for continuous variables, and were unadjusted unless otherwise specified. Sensitivity analyses including all SPIROMICS participants were also performed to study associations in the overall cohort. COPD exacerbations were analyzed in three ways. First, participants were dichotomized into those with any exacerbations between their baseline visit and the end of study follow-up versus those without exacerbations in this timeframe, and ferritin levels were compared across groups. Second, participants were dichotomized into those with any exacerbations between the bronchoscopy visit and the end of the study follow-up, versus those without, and ferritin levels were compared across groups. Third, the rate of exacerbations per participant per year was estimated using a negative binomial zero-inflated model, with % FEV1 predicted as the predictor in the binomial model. Ferritin associations were studied in models unadjusted as well as adjusted for age, sex, and smoking status at baseline or at the time of the bronchoscopy, as appropriate. Adjustment for study site prevented model convergence and was thus removed. Yearly exacerbation rate ratios, as well as 95% confidence intervals (CI) were estimated, and the unadjusted model was then used to plot predicted exacerbation rates in three years in a participant with a median FEV1% predicted. BALF ferritin levels in a validation cohort (see Supplemental Table 1) were similarly analysed on a log 10 scale, and compared across non-smokers, smokers without COPD, and participants with COPD.
Supplementary information
Acknowledgements
We thank Dr. Augustine M.K. Choi for critical discussion and insight. This work is supported by the US National Institute of Health–National Heart, Lung and Blood Institute R00-HL125899 (S.M.C.), U01 HL128964, U01 HL137880, P01 HL114501, R01 HL122438, R01 HL136682 (F.J.M.), T32-HL134629, a COPD Research Grant from the CHEST foundation in partnership with AstraZeneca LP and Sunovion Pharmaceuticals Inc and the Stony-Wold Herbert Fund (W.Z.Z). The authors thank the SPIROMICS participants and participating physicians, investigators and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E Alexis, MD; Wayne H Anderson, PhD; Mehrdad Arjomandi, MD; Igor Barjaktarevic, MD, PhD; R Graham Barr, MD, DrPH; Lori A Bateman, MSc; Surya P Bhatt, MD; Eugene R Bleecker, MD; Richard C Boucher, MD; Russell P Bowler, MD, PhD; Stephanie A Christenson, MD; Alejandro P Comellas, MD; Christopher B Cooper, MD, PhD; David J Couper, PhD; Gerard J Criner, MD; Ronald G Crystal, MD; Jeffrey L Curtis, MD; Claire M Doerschuk, MD; Mark T Dransfield, MD; Brad Drummond, MD; Christine M Freeman, PhD; Craig Galban, PhD; MeiLan K Han, MD, MS; Nadia N Hansel, MD, MPH; Annette T Hastie, PhD; Eric A Hoffman, PhD; Yvonne Huang, MD; Robert J Kaner, MD; Richard E Kanner, MD; Eric C Kleerup, MD; Jerry A Krishnan, MD, PhD; Lisa M LaVange, PhD; Stephen C Lazarus, MD; Fernando J Martinez, MD, MS; Deborah A Meyers, PhD; Wendy C Moore, MD; John D Newell Jr, MD; Robert Paine, III, MD; Laura Paulin, MD, MHS; Stephen P Peters, MD, PhD; Cheryl Pirozzi, MD; Nirupama Putcha, MD, MHS; Elizabeth C Oelsner, MD, MPH; Wanda K O’Neal, PhD; Victor E Ortega, MD, PhD; Sanjeev Raman, MBBS, MD; Stephen I. Rennard, MD; Donald P Tashkin, MD; J Michael Wells, MD; Robert A Wise, MD; and Prescott G Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Lisa Viviano, BSN; SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), grants from the NIH/NHLBI (U01 HL137880 and U24 HL141762), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Novartis Pharmaceuticals Corporation; Nycomed GmbH; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; and Theravance Biopharma and Mylan.
Author contributions
S.M.C conceived the hypothesis and designed the study. W.Z.Z. and S.M.C. interpreted the data and drafted the initial manuscript. S.A.K., J.J.B., and K.K. performed the measurements in the study. S.O. and R.G.C. provided samples for the validation cohort. W.K.O, C.M.F., S.A.C., S.P.P., J.M.W., C.D., N.P., I.B., P.G.W., C.C., R.P.B., A.P.C., G.J.C., R.P., N.H., M.K.H., R.J.K., J.L.C., F.J.M., were involved in data collection and processing. C.O. and K.V.B. provided the statistical analysis. All authors participated in manuscript editing for critical intellectual content and approved the final manuscript.
Competing interests
Dr. Bowler served on the advisory boards (GlaxoSmithKline, Boehringer Ingelheim, and Mylan Pharmaceuticals) and received research grants from GlaxoSmithKline and Boehringer Ingelheim not related to this manuscript and these activities have not influenced my work on this manuscript. Dr. Christenson reports personal fees from AstraZeneca, personal fees from GlaxoSmithKline, personal fees from Amgen, personal fees from Glenmark, personal fees from Sunovion, non-financial support from Genentech, non-financial support from Medimmune, outside the submitted work. Dr. Comellas reports grants from NIH, non-financial support from VIDA, personal fees from GSK, outside the submitted work. Dr. Cooper reports grants from Equinox Health Clubs, personal fees from Equinox Health Clubs, grants from Amgen, personal fees from PulmonX, other from GlaxoSmithKline, outside the submitted work; and work part-time on scientific engagement for the GlaxoSmithKline Global Respiratory Franchise. Dr. Criner reports grants from Boehringer- Ingelheim, grants from Novartis, grants from Astra Zeneca, grants from Respironics, grants from MedImmune, grants from Actelion, grants from Forest, grants from Pearl, grants from Ikaria, grants from Aeris, grants from PneumRx, grants from Pulmonx, other from HGE Health Care Solutions, Inc, other from Amirall, other from Boehringer- Ingelheim, other from Holaira, outside the submitted work. Dr. Han reports personal fees from GSK, personal fees from BI, personal fees from AZ, other from Novartis, other from Sunovion, outside the submitted work. Dr. Hansel reports grants and personal fees from AstraZeneca, grants from Boehringer Ingelheim, grants from NIH, grants from COPD Foundation, personal fees from Mylan, outside the submitted work. Dr. Barjaktarevic reports personal fees from Astra Zeneca, personal fees from Boehringer Ingelheim, grants from AMGEN, grants and personal fees from GE Healthcare, personal fees from Grifols, personal fees from Verona Pharma, personal fees from GSK, personal fees from CSL Behring, personal fees from Mylan/Theravance, during the conduct of the study. Dr. Kaner reports personal fees from Boehringer Ingelheim, grants and personal fees from Genentech, outside the submitted work. Dr. Martinez reports personal fees and non-financial support from American College of Chest Physicians, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Boehringer Ingelheim, non-financial support from ProterrixBio, personal fees from Columbia University, personal fees and non-financial support from ConCert, personal fees and non-financial support from Genentech, personal fees and non-financial support from GlaxoSmithKline, personal fees and non-financial support from Inova Fairfax Health System, personal fees from Integritas, personal fees from MD Magazine, personal fees from Methodist Hospital Brooklyn, personal fees and non-financial support from Miller Communicatinos, personal fees and non-financial support from National Association for Continuing Education, personal fees and non-financial support from Novartis, personal fees from New York University, personal fees and non-financial support from Pearl Pharmaceuticals, personal fees and non-financial support from PeerView Communications, personal fees and non-financial support from Prime Communications, personal fees and non-financial support from Puerto Rican Respiratory Society, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Sunovion, personal fees and non-financial support from Theravance, personal fees from UpToDate, personal fees from WebMD/MedScape, personal fees from Western Connecticut Health Network, other from Afferent/Merck, non-financial support from Gilead, non-financial support from Nitto, personal fees from Patara/Respivant, personal fees from PlatformIQ, personal fees and non-financial support from Potomac, other from Biogen, personal fees and non-financial support from University of Alabama Birmingham, other from Veracyte, non-financial support from Zambon, personal fees from American Thoracic Society, grants from NIH, personal fees and non-financial support from Physicians Education Resource, personal fees from Rockpointe, other from Prometic, personal fees from Rare Disease Healthcare Communications, other from Bayer, other from Bridge Biotherapeutics, personal fees and non-financial support from Canadian Respiratory Network, other from ProMedior, personal fees and non-financial support from Teva, personal fees from France Foundation, personal fees and non-financial support from Dartmouth, outside the submitted work. Dr. Woodruff reports personal fees from Glaxosmithkline, personal fees from NGM biopharmaceuticals, personal fees from Amgen, personal fees from Glenmark Pharmaceuticals, personal fees from Theravance, personal fees from Clarus Ventures, personal fees from Astra Zeneca, personal fees from 23andMe, personal fees from Sanofi, personal fees from Regeneron, personal fees from Genentech, outside the submitted work. Dr. Wells reports grants from NIH/NHLBI, during the conduct of the study; grants from NIH/NCATS, grants from Bayer, grants and other from GSK, other from Boehringer Ingelheim, grants and other from Mereo BioPharma, other from Quintiles, other from PRA, outside the submitted work. All other authors have no conflicts to disclose.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-67047-w.
References
- 1.Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet390, 1151–1210, 10.1016/s0140-6736(17)32152-9 (2017). [DOI] [PMC free article] [PubMed]
- 2.Han MK, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet. Respir Med. 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soler-Cataluna JJ, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qureshi H, Sharafkhaneh A, Hanania NA. Chronic obstructive pulmonary disease exacerbations: latest evidence and clinical implications. Ther Adv Chronic Dis. 2014;5:212–227. doi: 10.1177/2040622314532862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeMeo DL, et al. Integration of genomic and genetic approaches implicates IREB2 as a COPD susceptibility gene. American journal of human genetics. 2009;85:493–502. doi: 10.1016/j.ajhg.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloonan SM, et al. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nat Med. 2016;22:163–174. doi: 10.1038/nm.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilig EA, et al. Manganese and iron transport across pulmonary epithelium. American journal of physiology. Lung cellular and molecular physiology. 2006;290:L1247–1259. doi: 10.1152/ajplung.00450.2005. [DOI] [PubMed] [Google Scholar]
- 8.Wu L, Ma L, Nicholson LF, Black PN. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respiratory medicine. 2011;105:329–336. doi: 10.1016/j.rmed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Fracchia, A. et al. A comparative study on ferritin concentration in serum and bilateral bronchoalveolar lavage fluid of patients with peripheral lung cancer versus control subjects. Oncology 56, 181–188, doi:11962 (1999). [DOI] [PubMed]
- 10.Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. A possible role for secreted ferritin in tissue iron distribution. Journal of neural transmission (Vienna, Austria: 1996) 2011;118:337–347. doi: 10.1007/s00702-011-0582-0. [DOI] [PubMed] [Google Scholar]
- 11.Wesselius LJ, Nelson ME, Skikne BS. Increased release of ferritin and iron by iron-loaded alveolar macrophages in cigarette smokers. American journal of respiratory and critical care medicine. 1994;150:690–695. doi: 10.1164/ajrccm.150.3.8087339. [DOI] [PubMed] [Google Scholar]
- 12.Philippot Q, et al. Increased iron sequestration in alveolar macrophages in chronic obstructive pulmonary disease. PloS one. 2014;9:e96285. doi: 10.1371/journal.pone.0096285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takemoto K, Kawai H, Kuwahara T, Nishina M, Adachi S. Metal concentrations in human lung tissue, with special reference to age, sex, cause of death, emphysema and contamination of lung tissue. International archives of occupational and environmental health. 1991;62:579–586. doi: 10.1007/BF00381111. [DOI] [PubMed] [Google Scholar]
- 14.Corhay JL, et al. Iron content in human alveolar macrophages. The European respiratory journal. 1992;5:804–809. [PubMed] [Google Scholar]
- 15.Ghio AJ, et al. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. American journal of respiratory and critical care medicine. 2008;178:1130–1138. doi: 10.1164/rccm.200802-334OC. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto H, et al. Differences in plasma and sputum biomarkers between COPD and COPD-asthma overlap. The. European respiratory journal. 2014;43:421–429. doi: 10.1183/09031936.00024313. [DOI] [PubMed] [Google Scholar]
- 17.Mateos F, Brock JH, Perez-Arellano JL. Iron metabolism in the lower respiratory tract. Thorax. 1998;53:594–600. doi: 10.1136/thx.53.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson AB, Bohling T, Heires A, Linder J, Rennard SI. Lower respiratory tract iron burden is increased in association with cigarette smoking. The Journal of laboratory and clinical medicine. 1991;117:493–499. [PubMed] [Google Scholar]
- 19.McGowan SE, Henley SA. Iron and ferritin contents and distribution in human alveolar macrophages. The Journal of laboratory and clinical medicine. 1988;111:611–617. [PubMed] [Google Scholar]
- 20.Wesselius LJ, Flowers CH, Skikne BS. Alveolar macrophage content of isoferritins and transferrin. Comparison of nonsmokers and smokers with and without chronic airflow obstruction. The American review of respiratory disease. 1992;145:311–316. doi: 10.1164/ajrccm/145.2_Pt_1.311. [DOI] [PubMed] [Google Scholar]
- 21.Nelson ME, O’Brien-Ladner AR, Wesselius LJ. Regional variation in iron and iron-binding proteins within the lungs of smokers. American journal of respiratory and critical care medicine. 1996;153:1353–1358. doi: 10.1164/ajrccm.153.4.8616566. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Rivera C, et al. Anemia is a mortality predictor in hospitalized patients for COPD exacerbation. COPD. 2012;9:243–250. doi: 10.3109/15412555.2011.647131. [DOI] [PubMed] [Google Scholar]
- 23.Putcha N, et al. Anemia and Adverse Outcomes in a Chronic Obstructive Pulmonary Disease Population with a High Burden of Comorbidities. An Analysis from SPIROMICS. Ann Am Thorac Soc. 2018;15:710–717. doi: 10.1513/AnnalsATS.201708-687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mussalo-Rauhamaa H, Leppanen A, Salmela SS, Pyysalo H. Cigarettes as a source of some trace and heavy metals and pesticides in man. Archives of environmental health. 1986;41:49–55. doi: 10.1080/00039896.1986.9935765. [DOI] [PubMed] [Google Scholar]
- 25.Behera SN, Xian H, Balasubramanian R. Human health risk associated with exposure to toxic elements in mainstream and sidestream cigarette smoke. Sci Total Environ. 2014;472:947–956. doi: 10.1016/j.scitotenv.2013.11.063. [DOI] [PubMed] [Google Scholar]
- 26.Agoro R, Taleb M, Quesniaux VFJ, Mura C. Cell iron status influences macrophage polarization. PloS one. 2018;13:e0196921. doi: 10.1371/journal.pone.0196921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couper D, et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hallberg L, et al. Screening for iron deficiency: an analysis based on bone-marrow examinations and serum ferritin determinations in a population sample of women. Br J Haematol. 1993;85:787–798. doi: 10.1111/j.1365-2141.1993.tb03225.x. [DOI] [PubMed] [Google Scholar]
- 29.Evensen KJ, Swaak TJ, Nossent JC. Increased ferritin response in adult Still’s disease: specificity and relationship to outcome. Scand J Rheumatol. 2007;36:107–110. doi: 10.1080/03009740600958504. [DOI] [PubMed] [Google Scholar]
- 30.Zandman-Goddard G, Shoenfeld Y. Ferritin in autoimmune diseases. Autoimmun Rev. 2007;6:457–463. doi: 10.1016/j.autrev.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29:401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogelmeier CF, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. American journal of respiratory and critical care medicine. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 34.Seemungal TA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 35.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dransfield MT, et al. Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;195:324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, W. Z., Butler, J. J. & Cloonan, S. M. Smoking-induced iron dysregulation in the lung. Free radical biology & medicine, 10.1016/j.freeradbiomed.2018.07.024 (2018). [DOI] [PMC free article] [PubMed]
- 38.Whitby PW, Vanwagoner TM, Seale TW, Morton DJ, Stull TL. Transcriptional profile of Haemophilus influenzae: effects of iron and heme. J Bacteriol. 2006;188:5640–5645. doi: 10.1128/JB.00417-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minandri F, et al. Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection. Infect Immun. 2016;84:2324–2335. doi: 10.1128/IAI.00098-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carraway MS, Ghio AJ, Taylor JL, Piantadosi CA. Induction of ferritin and heme oxygenase-1 by endotoxin in the lung. Am J Physiol. 1998;275:L583–592. doi: 10.1152/ajplung.1998.275.3.L583. [DOI] [PubMed] [Google Scholar]
- 41.Damron FH, Oglesby-Sherrouse AG, Wilks A, Barbier M. Dual-seq transcriptomics reveals the battle for iron during Pseudomonas aeruginosa acute murine pneumonia. Sci Rep. 2016;6:39172. doi: 10.1038/srep39172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seifert M, et al. Effects of the Aspergillus fumigatus siderophore systems on the regulation of macrophage immune effector pathways and iron homeostasis. Immunobiology. 2008;213:767–778. doi: 10.1016/j.imbio.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Sharma RK, Chakrabarti S. Anaemia secondary to erythropoietin resistance: important predictor of adverse outcomes in chronic obstructive pulmonary disease. Postgraduate medical journal. 2016 doi: 10.1136/postgradmedj-2015-133814. [DOI] [PubMed] [Google Scholar]
- 44.Terashima T, Wiggs B, English D, Hogg JC, van Eeden SF. The effect of cigarette smoking on the bone marrow. American journal of respiratory and critical care medicine. 1997;155:1021–1026. doi: 10.1164/ajrccm.155.3.9116981. [DOI] [PubMed] [Google Scholar]
- 45.Rybinska I, Cairo G. Mutual Cross Talk Between Iron Homeostasis and Erythropoiesis. Vitamins and hormones. 2017;105:143–160. doi: 10.1016/bs.vh.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 46.McCrea KA, Ensor JE, Nall K, Bleecker ER, Hasday JD. Altered cytokine regulation in the lungs of cigarette smokers. Am J Respir Crit Care Med. 1994;150:696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 47.Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Frontiers in immunology. 2014;5:435. doi: 10.3389/fimmu.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaykhiev R, et al. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. Journal of immunology (Baltimore, Md.: 1950) 2009;183:2867–2883. doi: 10.4049/jimmunol.0900473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hodge S, et al. Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. American journal of respiratory cell and molecular biology. 2007;37:748–755. doi: 10.1165/rcmb.2007-0025OC. [DOI] [PubMed] [Google Scholar]
- 50.Recalcati S, Locati M, Gammella E, Invernizzi P, Cairo G. Iron levels in polarized macrophages: regulation of immunity and autoimmunity. Autoimmunity reviews. 2012;11:883–889. doi: 10.1016/j.autrev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Ryan TP, et al. Pulmonary ferritin: differential effects of hyperoxic lung injury on subunit mRNA levels. Free radical biology &. medicine. 1997;22:901–908. doi: 10.1016/s0891-5849(96)00483-2. [DOI] [PubMed] [Google Scholar]
- 52.Worwood M, Dawkins S, Wagstaff M, Jacobs A. The purification and properties of ferritin from human serum. Biochem J. 1976;157:97–103. doi: 10.1042/bj1570097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S, Hevi S, Chuck SL. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood. 2004;103:2369–2376. doi: 10.1182/blood-2003-09-3050. [DOI] [PubMed] [Google Scholar]
- 54.Stites SW, Plautz MW, Bailey K, O’Brien-Ladner AR, Wesselius LJ. Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. American journal of respiratory and critical care medicine. 1999;160:796–801. doi: 10.1164/ajrccm.160.3.9811018. [DOI] [PubMed] [Google Scholar]
- 55.Sibille JC, Kondo H, Aisen P. Interactions between isolated hepatocytes and Kupffer cells in iron metabolism: a possible role for ferritin as an iron carrier protein. Hepatology. 1988;8:296–301. doi: 10.1002/hep.1840080218. [DOI] [PubMed] [Google Scholar]
- 56.Leimberg MJ, Prus E, Konijn AM, Fibach E. Macrophages function as a ferritin iron source for cultured human erythroid precursors. J Cell Biochem. 2008;103:1211–1218. doi: 10.1002/jcb.21499. [DOI] [PubMed] [Google Scholar]
- 57.Ghio AJ, Hilborn ED. Indices of iron homeostasis correlate with airway obstruction in an NHANES III cohort. International journal of chronic obstructive pulmonary disease. 2017;12:2075–2084. doi: 10.2147/copd.S138457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee CH, et al. Association of serum ferritin levels with smoking and lung function in the Korean adult population: analysis of the fourth and fifth Korean National Health and Nutrition Examination Survey. International journal of chronic obstructive pulmonary disease. 2016;11:3001–3006. doi: 10.2147/copd.s116982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shibata Y, et al. Elevated serum iron is a potent biomarker for spirometric resistance to cigarette smoke among Japanese males: the Takahata study. PloS one. 2013;8:e74020. doi: 10.1371/journal.pone.0074020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woodruff PG, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galban CJ, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Neal WK, et al. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. Journal of translational medicine. 2014;12:9. doi: 10.1186/1479-5876-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Freeman CM, et al. Design of a multi-center immunophenotyping analysis of peripheral blood, sputum and bronchoalveolar lavage fluid in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) Journal of translational medicine. 2015;13:19. doi: 10.1186/s12967-014-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wells, J. M. et al. Safety and Tolerability of Comprehensive Research Bronchoscopy in COPD: Results from the SPIROMICS Bronchoscopy Sub-Study. Ann Am Thorac Soc, 10.1513/AnnalsATS.201807-441OC (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.