Abstract
Lyme borreliosis is the most prevalent vector-borne disease in northern hemisphere. Borrelia burgdorferi sensu lato spirochetes are transmitted by Ixodes species ticks. During a blood meal, these spirochetes are inoculated into the skin where they multiply and often spread to various target organs: disseminated skin sites, the central nervous system, the heart and large joints. The usual diagnosis of this disease relies on serological tests. However, in patients presenting persistent clinical manifestations, this indirect diagnosis is not capable of detecting an active infection. If the serological tests are positive, it only proves that exposure of an individual to Lyme spirochetes had occurred. Although culture and quantitative PCR detect active infection, currently used tests are not sensitive enough for wide-ranging applications. Animal models have shown that B. burgdorferi persists in the skin. We present here our targeted proteomics results using infected mouse skin biopsies that facilitate detection of this pathogen. We have employed several novel approaches in this study. First, the effect of lidocaine, a local anesthetic used for human skin biopsy, on B. burgdorferi presence was measured. We further determined the impact of topical corticosteroids to reactivate Borrelia locally in the skin. This local immunosuppressive compound helps follow-up detection of spirochetes by proteomic analysis of Borrelia present in the skin. This approach could be developed as a novel diagnostic test for active Lyme borreliosis in patients presenting disseminated persistent infection. Although our results using topical corticosteroids in mice are highly promising for recovery of spirochetes, further optimization will be needed to translate this strategy for diagnosis of Lyme disease in patients.
Subject terms: Diseases, Infectious diseases, Microbiology, Bacteria, Infectious-disease diagnostics
Introduction
Lyme borreliosis is caused by Borrelia burgdorferi sensu lato (sl) group of spirochetal bacteria that is transmitted by a hard tick belonging to the Ixodes ricinus complex in North America and Eurasia. After inoculation of bacteria into the skin during a blood meal, the first clinical manifestation in the majority of patients is inflammatory response in skin at the site of infection known as erythema migrans (EM). Borreliae often disseminate to target organs: the central nervous system, joints, heart and the distant skin1. In the early local stage of infection, the clinical characteristics of EM are distinctive enough to allow diagnosis and treatment immediately. If EM is not detected, the clinical manifestations of disseminated infections are quite variable based upon the infecting strain and often are less specific. Thus, microbiological demonstration of the Borrelia infection is important. Several methods can be used, including culture but is not routinely used because it is time consuming, expensive, requires technical expertise and exhibits a low sensitivity for diagnosis of disseminated disease manifestations2. Quantitative PCR (qPCR) has also been performed by some laboratories, especially for synovial fluid, where it shows up to 80% sensitivity. Sensitivity of qPCR tends to be much lower for cerebrospinal fluid samples. Finally, indirect diagnosis using two-tier serological tests is routinely used in which the first tier is usually an ELISA, followed by immunoblotting only if ELISA results are positive or equivocal3,4. This method has several limitations: while its sensitivity is excellent in late stages of Lyme disease, positive predictive value is diminished by a high seroprevalence in healthy individuals from endemic areas, as well as by the fact that anti-Borrelia antibodies could persist for the entire life of a cured Lyme borreliosis patient. A positive serological result can thus never be interpreted as a reliable demonstration of the active presence of Borrelia in symptomatic patients, highlighting the need for alternative methods of diagnosis of an active infection. The disseminated disease can then be treated with different antibiotics such as amoxicillin, doxycycline or ceftriaxone5. In fact, treatment of patients early in infection usually completely resolves symptoms of Lyme disease. However, some symptoms, primarily manifested as persistent Lyme arthritis and chronic fatigue may persist in a number of patients after completion of antibiotic treatment regimen6.
Mouse model is often used to investigate physiopathology of human diseases when available. Lyme disease is well investigated particularly in susceptible C3H/HeN mice where B. burgdorferi infection results in inflammatory carditis and arthritic manifestations7. This mouse model has also allowed researchers to determine association of the specific Borrelia strains genotypes with either spirochetes dissemination or their lack of dissemination8,9. The skin has also been demonstrated as a site for B. burgdorferi strains multiplication and persistence using this animal model10,11.
As the role of the skin is extensively documented now for vector-borne diseases12–14, we developed a new approach for diagnosis of Lyme borreliosis by using proteomics analyses of the skin biopsies15,16. We set up the model in Lyme-infected mouse in early and late disseminated infections to identify protein markers for detection of B. burgdorferi sl infection. We identified protein markers of infection, mainly OspC, flagellin and DbpA in early infections15 and flagellin, GAPDH, different VlsE proteins late in infection16. Although we obtained promising results in the mouse model, translation of this approach to humans for diagnosis was not straightforward and presented several difficulties.
To overcome problems associated with adopting our approach for patients, we more carefully compared the protocols used in the mouse model. For early diagnosis of Lyme disease in patients, we suspected that the presence of lidocaine used as local anesthetic and the presence of host blood were critical for targeted proteomics. In addition, the low level of bacteria in the mouse skin during disseminated infection did not allow the detection of Borrelia proteins by proteomics. Therefore, we used a local immunosuppressive drug, clobetasol, to induce Borrelia proliferation in situ followed by PCR quantification and identification of protein markers of B. burgdorferi infection. We performed additional experiments in our mouse model to better define the effect of clobetasol in the infected skin and determine its direct effect on Borrelia protein expression. This has prepared us for an extensive clinical trial in patients demonstrating disseminated infections in the future.
Translational research and application of our protocols developed in the mouse model to humans will require further adjustments. In this paper, we focus on the effect of lidocaine and clobetasol on Borrelia present in the skin in C3H/HeN mouse model to optimize conditions for a novel diagnostic test development using targeted proteomics using skin of patients afflicted with Lyme borreliosis.
Results
Effect of lidocaine on Borrelia viability in vitro
In the past, lidocaine was suggested to exhibit antimicrobial activities17. In fact, a recent review described antimicrobial effects of local anesthetics when collecting biological fluids for microbial studies18. This local anesthetic significantly inhibits the growth of bacteria; such that it can possibly also affect Borrelia-infected skin when used before the human biopsy specimens are collected. To evaluate this possibility, we first tested the direct effect of lidocaine in vitro on B. afzelii. Lethal effects of lidocaine were determined by the loss of motility and disruption of the spiral shape of Borrelia. It indeed demonstrated lethal effects of lidocaine at the highest concentration we used, 5 mg/ml, at day 1 and 7 (Table 1) and even its lower, 2.5 mg/ml concentration. We obtained similar data with B. burgdorferi ss 297 strain (data not shown). The concentration generally used in patients for skin biopsy is 10 mg/ml (0.5 ml inoculation) suggesting our need to optimize its concentration for humans that will not affect Lyme spirochetes adversely.
Table 1.
The effect of lidocaine on B. afzelii strain NE4049 in vitro.
| Compounds | Concentration | Day 1 | Day 7 |
|---|---|---|---|
| Control | ++++ | ++++ | |
| Lidocaine (mg/ml) | 5 | 0 | 0 |
| 2.5 | 0 | 0 | |
| 1.25 | ++ | ++ | |
| 0.62 | ++ | ++ | |
| 0.31 | ++++ | ++ | |
| 0.15 | ++++ | ++++ | |
| 0.078 | ++++ | ++++ | |
| 0.039 | ++++ | ++++ | |
| 0.019 | ++++ | ++++ | |
| 0.009 | ++++ | ++++ | |
| 0.0048 | ++++ | ++++ | |
| 0.002 | ++++ | ++++ | |
| 0.001 | ++++ | ++++ | |
| 0.0006 | ++++ | ++++ | |
| Gentamicin (µg/ml) | 100 | 0 | 0 |
| 50 | 0 | 0 | |
| 25 | 0 | 0 | |
| 12.5 | ++ | 0 | |
| 6.25 | ++ | + | |
| 3.12 | ++++ | ++ | |
| 1.56 | ++++ | ++++ | |
| 0.78 | ++++ | ++++ | |
| 0.39 | ++++ | ++++ | |
| 0.19 | ++++ | ++++ | |
| 0.09 | ++++ | ++++ | |
| 0.048 | ++++ | ++++ |
Borrelia motility and numbers were evaluated by observation and counting using Petroff-Hausser chamber under a dark-field microscope. Gentamicin was used as a positive control.
Key used: +: detectable viable B. afzelii in culture by microscopy; ++: at least one spirochete/field of view; +++: at least 10 spirochetes per field of view, ++++: an average of > 100 spirochetes per field of view).
Effect of lidocaine on Borrelia in vivo and its impact on proteomics data
In our mouse model, we also tested whether lidocaine can affect protein expression of Borrelia in the infected skin. Lidocaine affects the viability and vitality of B. afzelii as observed after 7 days of culture. The culture of infected skin injected with lidocaine exhibits less viable spirochetes than in control at day 7, as shown by dark field microscopy. B. afzelii survived in skin after lidocaine treatment because after 14 days of culture, spirochetes recovery appeared complete with spirochete numbers as numerous and motile as in the control (infected mouse without lidocaine—Table 2). Not surprisingly, no difference was observed between the two groups by qPCR since the technique detects DNA of both dead and live Borrelia. At day 14, the DNA detection was expressed as Ct since the skin was damaged and detection of gapdh and thus, relative quantification of spirochetes was not possible anymore.
Table 2.
Effect of lidocaine injection on day 7 (peak of Borrelia multiplication) in the skin of C3H/HeN mice infected with B. afzelii NE4049.
| Mouse no. | Protocol | Number and mobility of Borrelia in BSK-H complete medium | PCR on Borrelia-infected mouse skin | ||
|---|---|---|---|---|---|
| Day 7 After biopsy | Day 14 After biopsy | Day 0 (Quantification flaB/gapdh gene) | Day 7 After biopsy (Ct) | ||
| 1 | Infected skin at day 7 | ++++ | ++++ | 173 | 20 |
| 2 | ++++ | ++++ | 212 | 17 | |
| 3 | ++++ | ++++ | 252 | 16 | |
| 4 | ++++ | ++++ | 189 | 20 | |
| 5 | ++++ | ++++ | 169 | 19 | |
| 6 | ++++ | ++++ | 187 | 16 | |
| 7 | Infected skin at day 7 + lidocaine | ++ | ++++ | 173 | 17 |
| 8 | ++ | ++++ | 182 | 19 | |
| 9 | ++ | ++++ | 191 | 19 | |
| 10 | +++ | ++++ | 197 | 19 | |
| 11 | +++ | ++++ | 237 | 19 | |
| 12 | +++ | ++++ | 271 | 16 | |
Mouse skin biopsy samples were collected at day 7 post-inoculation, and cultured for 7–14 days. To determine the spirochetes burden in skin biopsies, qPCR was also performed at day 7 without culture. After 7 days of culture, quantification of B. afzelii in the skin relative to host DNA was not possible due to damage to the skin in BSK-H medium, which reduced quality of mouse DNA, inhibiting gapdh amplification and detection. Therefore, Ct is provided for flaB only after 7 days of in vitro culture. Samples 3 and 12 marked in bold indicate two skin samples selected for proteomic analysis.
We also checked the effect of lidocaine on the expression of B. afzelii proteins to confirm our viability assay results. Interestingly, when lidocaine is injected at day 7, i.e., at the peak of Borrelia multiplication in the skin and samples were processed immediately (Day 0—Table 3), difference in the number of Borrelia proteins identified in the control and the lidocaine-treated animal was not statistically significant. Thus, 64 versus 70 bacterial proteins and more than 4,600 mouse proteins were identified. One week of culture of the skin sample allowed B. afzelii multiplication that improved its proteins detection. In fact, a six–sevenfold increase in the number of Borrelia proteins was observed after culture (Day 7—Table 3).
Table 3.
Proteomic analysis of B. afzelii-infected skin.
| Treatment | fla/104 gapdh | fla/100 ng | Number of total identified proteins | Total number of identified peptides | Number of Borrelia proteins | Number of Borrelia peptides |
|---|---|---|---|---|---|---|
| Day 0 of culture | ||||||
| Without lidocaine | 252 | 836 | 4,274 | 31,100 | 70 | 206 |
| With lidocaine | 271 | 838 | 4,685 | 37,300 | 64 | 186 |
| Day 7 of culture | ||||||
| Without lidocaine | N.d | 15,200 | 3,140 | 21,733 | 474 | 2,736 |
| With lidocaine | N.d | 22,700 | 3,691 | 25,427 | 397 | 1992 |
At the peak of Borrelia multiplication in the infected skin at day 7, lidocaine was injected or not (control) before the skin biopsy. Skin was collected and directly analyzed (day 0) or cultivated for 7 days and then we selected the skin with the best qPCR count to perform proteomic analysis. For each biopsy sample, mouse and Borrelia proteins were identified using nanoLC-MS/MS, once for each biopsy.
Moreover, irrespective of the conditions used, we identified proteins previously detected in Borrelia-infected skin as infection markers in mice treated without lidocaine15,16, i.e. flagellin, OspC, enolase, GAPDH, lipoprotein gi|365823350, flagellar filament outer layer, elongation factor Tu, DbpA, GroEL and l-lactate dehydrogenase (Table 4).
Table 4.
Number of identified peptides per Borrelia identified proteins, with or without injection of lidocaine.
| Day 0 of culture | Day 7 of culture | |||
|---|---|---|---|---|
| Without lidocaine | With lidocaine | Without lidocaine | With lidocaine | |
| Flagellin | 11 | 13 | 18 | 16 |
| OspC | 8 | 7 | 10 | 9 |
| Enolase | 4 | 2 | 18 | 16 |
| GAPDH | 13 | 6 | 24 | 24 |
| Lipoprotein gi|365823350 | 10 | 8 | 13 | 11 |
| Flagellar filament outer layer | 7 | 7 | 18 | 18 |
| Elongation factor Tu | 5 | 5 | 23 | 24 |
| DbpA | 2 | 2 | 3 | 2 |
| GroEL | 8 | 8 | 33 | 33 |
| l-lactate dehydrogenase | 2 | 3 | 15 | 12 |
Skin biopsies samples were collected at the peak of multiplication, 7 days after Borrelia inoculation, and either directly frozen (day 0 of culture) or culture for 7 days.
Although we conducted only a single proteomic analysis for one sample each from lidocaine treated and untreated control mice, the lidocaine seems to adversely affect protein detection, since more Borrelia proteins were detected without lidocaine treatment after 7 days of infection in the skin biopsies (Table 3). The in vitro culture improved the number of identified peptides per Borrelia, in agreement with Borrelia multiplication observed in culture, despite lidocaine-treatment of mice (Table 4). Thus, the culture of skin-biopsy improves the health of bacteria increasing protein expression such that the efficiency of detection of Borrelia proteins and peptides improved more (474 and 2,736) in the skin of mice without lidocaine treatment than in the lidocaine-treated mice (397 and 1992). It is likely that culture also washes out the lidocaine reducing its impact on B. afzelii multiplication to prevent further exacerbation of these differences between treated and untreated samples (Boulanger-unpublished data).
We observed a similar phenomenon in the skin of patients with erythema migrans (data not shown). In vitro culture of human infected skin for several weeks allowed the detection of flagellin and OspC, which was otherwise not possible.
Topical corticosteroid application increases localized growth of different Borreliae in the mouse skin 40–50 days after inoculation
We first compared the reactivation of different Borrelia species and strains after application of clobetasol twice a day for two days at the site of inoculation (lower back of mouse). After dissemination, i.e., 40–50 days after the inoculation, Borreliae multiplied very well irrespective of the strain used when compared with the controls (infected but non reactivated by clobetasol) as shown by PCR quantification. We found that B. afzelii NE4049 (tick isolated strain) and IBS106 (human isolate) were the best Borrelia species strains to respond to the application of clobetasol (Fig. 1). We also tested whether local cutaneous application of clobetasol would enhance Borrelia multiplication at a distant anatomical site. After clobetasol application, cultures of the skin at the site of inoculation, the heart, the joint and the blood of infected mice were carried out. While a much higher quantity of Borrelia were recovered from the skin samples compared to the non-reactivated infected-skin, cultures of all other organs of clobetasol-treated mice remained at the same level as the untreated infected mice. Interestingly, the blood remained negative after clobetasol application. It strongly argues against any systemic effect of topical corticosteroid treatment (data not shown).
Figure 1.
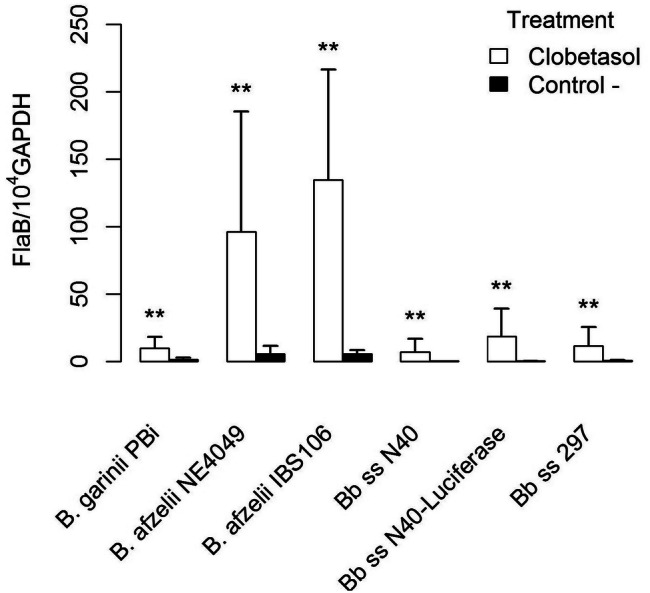
Comparison of reactivation of several Borrelia burdgdorferi sensu lato strains as detected by qPCR in the skin at the site of inoculation (lower back) after occurrence of disseminated infection (40–50 days post inoculation). Different groups were compared by a Mann–Whitney test and p values for each strain from left to right are: 0.003, 0.002, 0.008, 0.006, 0.002, and 0.001.
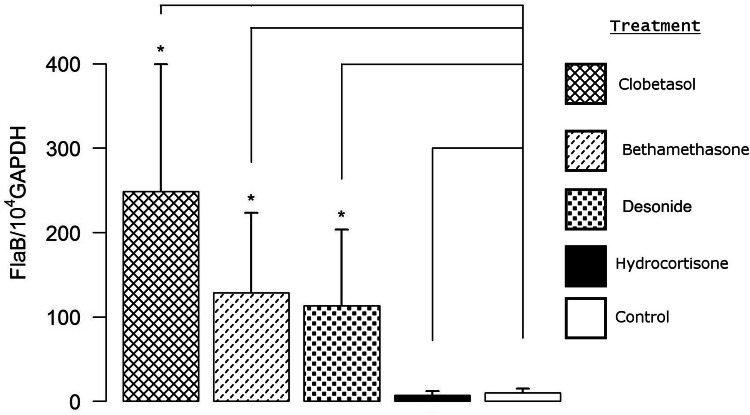
Since B. afzelii multiplies very well in mouse skin, we used it to study the effect of topical corticosteroids that exhibit distinct anti-inflammatory activities19. Clobetasol propionate cream is considered as a corticosteroid with a very high anti-inflammatory activity, while bethametasone dipropionate and desonide cream have a high and, hydrocortisone a low anti-inflammatory activity. As shown in (Fig. 2), clobetasol was found to be most effective for bacterial reactivation. Therefore, we used it for all further experiments.
Figure 2.
Determination of B. afzelii NE4049 reactivation at day 50 by qPCR, at the site of inoculation after application of topical corticoids with different levels of anti-inflammatory activities: clobetasol propionate 0.05% (very high); bethamethasone valerate 0.1% (high); desonide 0.1% (high); hydrocortisone 0.5% (low); control (non-reactivated infected skin). Different groups were compared by a Mann–Whitney test and p values relative to control from left to right are: 0.02, 0.02, 0.02, and 0.4.
Comparison of reactivation of the two strains of B. afzelii at different sites
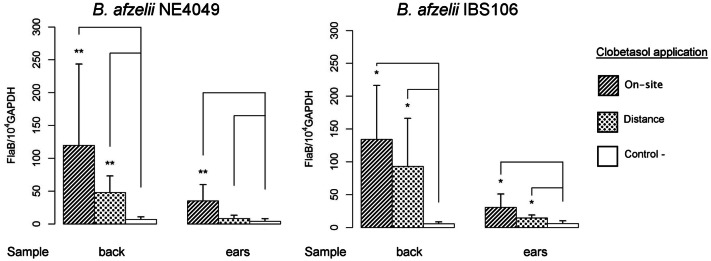
We examined the mechanism of B. afzelii reactivation more thoroughly using two strains, one isolated from a tick and another from a patient. We tested the reactivation levels and determined whether we can reactivate Borreliae at a site distant from the site of inoculation. When the topical corticosteroid was applied at the site of Borrelia inoculation (back of the mouse), Borrelia multiplication was detected efficiently by qPCR, primarily at the local site (Fig. 3). In the mouse model, all skin samples harbored live Borreliae; however, a reactivation at distance (ear) location from the site of inoculation also induced Borrelia multiplication in the back of mice. When the corticosteroid was applied on the ears, distant from the site of inoculation, we also detected Borrelia multiplication, albeit less intense than when applied at the site of inoculation. We did not observe a significant difference in the susceptibility to reactivation between the two strains of B. afzelii, NE4049 and IBS106 (Fig. 3).
Figure 3.
Quantification of B. afzelii NE4049 and IBS 106 by real-time qPCR after application of clobetasol on the back or on the ears of mice 40–50 days after Borrelia inoculation in the lower back of mice. Control (−) depicts infected skin collected from the back without reactivation. Different groups were compared by a Mann–Whitney test and p values relative to control from left to right for NE4049 are: 0.002, 0.002, 0.002, and 0.08, and for IBS 106 are: 0.01, 0.01, 0.02, and 0.01.
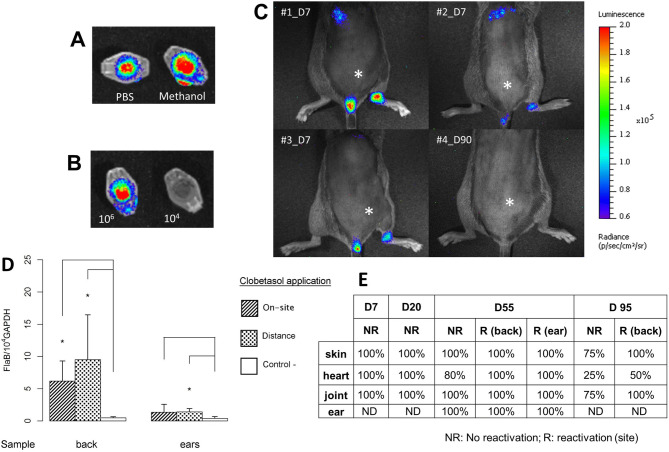
Reactivation of B. burgdorferi ss detected by determining luciferase activity in the skin
To better appreciate the extent of Borrelia in the skin and whether the reactivation was strictly cutaneous, we used a luciferase-expressing bioluminescent B. burgdorferi N40/D10E9 strain20. We first determined the best solvent, PBS or methanol, to dissolve luciferin and defined the minimal concentration of bacteria (104 and 106) needed to visualize by live-imaging (Fig. 4A, B). Methanol was found to be better than PBS when used for the in vivo experiments. After injection of 106 luciferase-expressing Borrelia, we made a kinetic analysis at 24 h, and 7, 20, 55 and 95 days post-infection. B. burgdorferi only reactivated with the topical corticosteroid at 55 and 95 days. Interestingly, spirochetes could only be detected by bioluminescence imaging at day 7, the usual peak of multiplication in this model11. The right joint, which was close to the inoculation site, was positive as well as the bladder and the heart (Fig. 4C: three representative mice are shown). Since we did not detect bioluminescence at day 20, 55 and 95 post-infection with or without reactivation, we detected B. burgdorferi by culture and qPCR in parallel to ensure that the spirochetes were present in the skin. As for B. afzelii, we confirmed the reactivation by qPCR at day 55 (Fig. 4D—other points of the kinetics not shown) and the presence of N40 in the skin and its dissemination in deep organs (heart and joint) by culture (Fig. 4E). Irrespective of the technique used and the time point selected, spirochetes were detected in the skin. However, the level of reactivation was lower for B. burgdorferi ss compared to B. afzelii that is shown in Fig. 1.
Figure 4.
Live-imaging of luciferase-expressing B. burgdorferi strain N40D10/E9. In vitro assay was first performed to select the most suitable solvent to be used. (A) d-Luciferin substrate was diluted either in PBS or in methanol in Eppendorf tube to select the best solvent to detect bioluminescence. (B) Examination of two luciferase-expressing N40 concentrations added to the Eppendorf tubes, 104 and 106 spirochetes, with the substrate. In vivo assay: (C) Measurement of luciferase-expressing B. burgdorferi ss N40 strain injected in C3H/HeN mice at 7 days post-infection (natural peak of Borrelia multiplication-3 mice were selected) or 90 days (clobetasol reactivation—mouse#4) post-infection. The intradermal injection site of luciferase-expressing Borrelia is marked by an asterisk. (D) Quantification of luciferase-expressing N40 strain in the skin of C3H/HeN mice at day 55: the site of Borrelia inoculation was always at the lower back of mice, but the clobetasol reactivation was either “on site” of inoculation or “at distance”. Infected but non-reactivated mice were included as controls. The skin was collected either from the back (site of inoculation) or from the ear (distant skin from the inoculation site). Different groups were compared by a Mann–Whitney test and p values relative to control mice from left to right for back are: 0.01 and 0.01, and for ear are: 0.2 and 0.01. (E) The study was completed by culture of other tissues at different time points: heart, right joint, skin at the site of inoculation (back) and skin at a distant site (ear). The clobetasol application site is marked by an ‘R’ for reactivated mice (Day 55 and 95) or ‘NR’ for infected skin but ‘not reactivated’. “%” positive in this table was calculated using the number of mouse organ from which Borrelia could be recovered by culture with respect to the total number of samples cultured and examined.
Effect of corticosteroids on Borrelia in vitro
To test whether corticosteroids could have a direct effect on the growth of Borreliae in vitro and whether we need to avoid its topical application, we isolated infected skin at day 40 after B. afzelii inoculation, added prednisolone in vitro and measured the quantities of Borrelia as compared with samples of skin treated in situ with clobetasol. Mouse skins infected with B. afzelii were cultured for 11 and 19 days and spirochetes counted at each time point. Data in Fig. 5 shows that while skin treatment with topical corticosteroid had a strong influence on the quantities of spirochetes retrieved by culture, adding prednisolone to the culture medium had insignificant effect on bacterial multiplication. These results rule out a direct action of corticosteroids on the growth of Borrelia, but rather increase in spirochete numbers in the immunosuppressed skin tissue due to either increased spirochete infiltration, prevention of Borreliae killing by innate immune response of mice, or both. Of note, relative spirochetes quantification by qPCR was not possible using skin immersed in BSK-H complete medium since the skin was too damaged after 3 weeks to quantify mouse gapdh gene, but it was possible to count live spirochetes by dark field microscopy.
Figure 5.
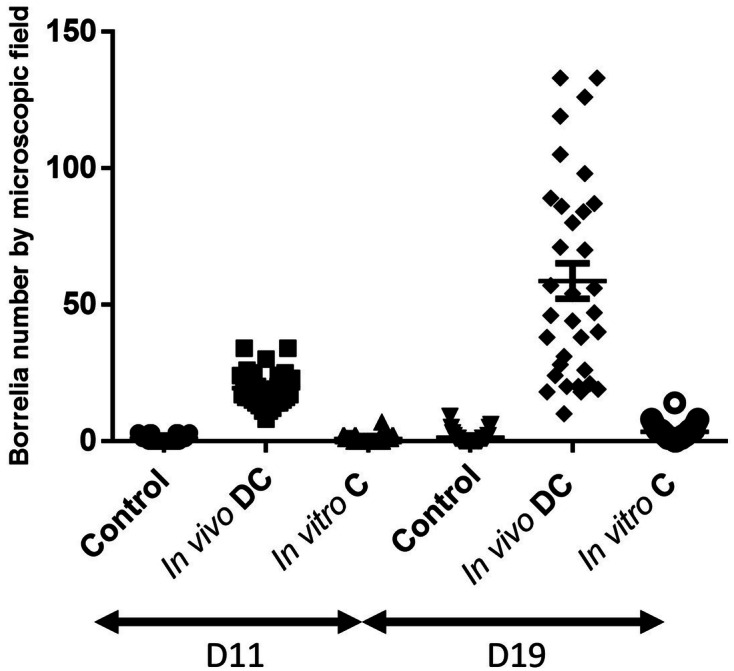
The effect of clobetasol (DC) application in vivo or incubation of B. afzelii NE4049-infected skin with cortisone (C) in vitro at day 40 of inoculation on the lower back of mice. Infected skins (3 biopsy punches per mouse) were incubated in BSK-H complete medium at 33 °C for 11 and 19 days and: cultured directly for control (infected but non-reactivated mouse), reactivated in vivo by skin application of clobetasol (DC) and cultured, or cultured directly with cortisone included in culture medium. Borreliae were counted by dark-field microscopy using a Petroff-Hausser chamber at two time points.
Discussion
We plan to develop a novel diagnostic assay for active Lyme borreliosis employing targeted proteomics on skin biopsy specimens from patients15. Indeed, we suspect persistence of Borrelia in the skin of patients with disseminated infections as shown in mouse and dog models7, 21. To prepare our clinical studies, we plan to use lidocaine, a local anesthetic for local biopsy and a topical corticosteroid to reactivate Borrelia in the skin of patients with disseminated Lyme disease. In the present study, we show that lidocaine can affect protein detection of B. burgdorferi sl if the skin biopsy sample was analyzed immediately. The culture of infected skin biopsy further improved the pathogen detection. We have previously shown that topical application of corticosteroid triggers the local multiplication of Borrelia as detected by qPCR and culture16. To generate additional data on the mechanisms of corticosteroid reactivation in the skin, we examined different parameters such as the site and the level of reactivation according the class of corticosteroids used and whether the reactivation was also possible in vitro using infected skin biopsies to avoid the application of corticosteroid on the skin of patient.
The C3H/HeN mouse model represents a good system to study Lyme borreliosis and it has been studied for a long time to decipher pathology of this disease22. The skin was described as a site of persistence7 and we confirmed by different experiments that the skin could constitute a promising biological sample to develop a new diagnostic test for direct detection of Borrelia11,16. Translational projects involving proteomics have also been considered for the diagnosis of Lyme disease in patients15.
Diagnosis of Lyme disease in patients relies on observation of the specific clinical manifestations together with the positive serological test results. The direct detection of Borrelia by culture and qPCR is rarely employed and are reserved for specialized laboratories where relevant technical expertise is available4,23. However, serology is often unsatisfactory since in endemic areas, seroprevalence is quite high in the healthy/recovered population, so a positive result cannot be interpreted as demonstration of an active infection. In addition, some patients with late disseminated disease still suffer from persistent symptoms after completion of antibiotic treatment regimen. Several patients continue to display persistent inflammatory symptoms in absence of live bacteria21. Therefore, it is important to prove the absence of live Borreliae to ascertain the lack of active infection reducing unnecessary use of antibiotics for treatment.
We and others have previously showed that B. burgdorferi sl can persist in the mouse skin for several weeks, albeit at lower levels7,16 We have shown that a short course of clobetasol cream application can be an efficient way to stimulate the number of Lyme spirochete in the skin in mouse model, allowing increase to a level sufficient for efficient detection of Borrelia proteins by targeted proteomics in a reproducible manner. We conducted studies presented here using topical corticosteroids to improve recovery of spirochetes in skin biopsies and obtained data using mouse model of infection with different Lyme spirochetes. Outcomes of this study will be very useful for us to develop a diagnostic test, and after full optimization, employ it for spirochetes detection in Lyme disease patients in the future.
When a skin biopsy is part of a diagnosis in human medicine, a localized anesthetic such as lidocaine is injected before the skin biopsy. Lidocaine has been reported to exhibit antibacterial activities17,18. Therefore, we first investigated the effect of this anesthetic on Borrelia, both in vitro and in vivo. In our experiments, we demonstrated the lethal effect of lidocaine on Borrelia species in vitro when used at high concentrations. The injection of lidocaine at the site of spirochetes inoculation adversely affected the viability of Borrelia as shown by culture, and also adversely affected the proteomic sensitivity. One week of culture helped to recover live Borreliae allowing us to detect 7 times more proteins. Culture also likely helped in diluting the effect of lidocaine on spirochete viability. Our pilot studies with patient samples confirmed these observations (Boulanger—unpublished data).
We have previously shown that the topical corticosteroid, clobetasol can reactivate Borrelia in the skin in mouse model of Lyme borreliosis16. To better prepare the protocol for use in human patients, we further evaluated the effect of topical corticosteroid on spirochetes reactivation. First, we tested whether the three Borrelia species that are most pathogenic in humans reactivate similarly after clobetasol treatment. Interestingly, among all three Borrelia species tested, reactivation of B. afzelii was found to be the best after this treatment. It could be due to the fact that in the natural environment, rodents are the main reservoirs for this species24 and thus, is likely more adapted to mouse model than the other Borrelia species. In addition, B. afzelii is known to have a peculiar affinity for the human skin, as it is the only agent responsible for acrodermatitis chronica atrophicans, the late cutaneous manifestation of disseminated Lyme borreliosis, in which the bacteria may persist in the skin for an extended period25. This species is also the most frequently isolated from early cutaneous manifestations such as erythema migrans or borrelial lymphocytoma in patients from Europe26, but this may merely reflect the high prevalence of B. afzelii in ticks from this geographical area. The mechanism underlying Borrelia growth in murine skin following application of topical steroids is not clear. We tested different topical steroids that demonstrate differential anti-inflammatory activities. We observed a clear link between the magnitude of observed effect and the activity of the molecule tested with clobetasol proprionate being the most efficient to increase Borrelia recovery from mouse skin. One possible interpretation is that in mouse skin, the persistence of Lyme spirochetes is under the control of the host skin immunity. Indeed, it has been shown that B. burgdorferi can persist for months in the skin in absence of inflammation (M. Wooten-personal communication). Topical corticosteroids, with their local immunosuppressive effects, can likely induce a break in immune-tolerance and reactivate the persistent bacteria. Alternatively, these chemicals also create a strong vasoconstriction, which may lead to a decrease in local skin temperature. As the optimal temperature to grow Lyme spirochetes in vitro is around 33 °C and the mouse skin is approximately 37 °C, the effect of temperature may be an alternative explanation for this observation. To detect any direct effect of corticosteroids on the growth of Borrelia, infected skin biopsies were cultured in the presence of prednisolone. We did not observe any effect of prednisolone, likely demonstrating that corticosteroids break the tolerance of the immune system to B. burgdorferi sl in the skin in vivo but does not affect growth during in vitro cultivation.
We then investigated the effect of topical corticosteroids on Borreliae proliferation in different organ systems of mice. It has been shown that in mice7, like in dogs21, B. burgdorferi can be recovered following disseminated infection from every part of the skin. The application of topical steroids on the back of infected mice had a clear, although mild effect on the quantity of Borrelia recovered from a distant site, ears of the same animal. This finding can be attributed to the diffusion of the steroid in the skin to some extent. Importantly, this short course of treatment had no impact on spirochetes presence in other distant organs, such as the joints or the heart, and no Borrelia DNA was amplified from the blood, thus favoring a purely a cutaneous effect of topical corticosteroids. These data are clearly reassuring for expansion of this protocol to humans.
Using luciferase expressing B. burgdorferi, we only detected bacteria at day 7 post-infection. From previous experiments, we already know that this time point corresponds to an intense local multiplication of Lyme spirochetes in the mouse skin, confirming previously published data20. Unfortunately, the technique lacks sufficient sensitivity at later stages of infection. Therefore, we cannot as yet conclude on the extent of the reactivation after several weeks of infection. Culture and qPCR were found to be reliable methods to demonstrate the viability, persistence and amplification of Borrelia in the skin in all our assays.
We provide evidence that two weeks of in vitro culture can significantly improve the pathogen recovery from the skin. Up to now, most studies have focused on plasma/serum biomarkers while skin proteomics has rarely been used for diagnostics. This approach is challenging due to the putatively low recovery of pathogen components amongst a large pool of host proteins. To address this problem, the high specificity and sensitivity of targeted proteomics makes it a promising strategy. By this technique, Mycobacterium tuberculosis proteins were detected in human serum of infected patients27, and the combination with immuno-capture enabled the detection of Yersinia pestis proteins in dried blood spots28. Using skin probes as starting material, protein profiling of cutaneous leishmaniasis lesions was compared with normal skin, however, no Leishmania protein was identified amongst human proteins, possibly because of rather scarcity of this parasite in the skin29.
In the vector-borne diseases, skin has been documented as an essential interface for pathogen transmission12,30. More precisely, proteomic analysis could help identify new markers for persistent infection that can be used for direct diagnosis. We have successfully developed mass spectrometry-based proteomics using murine Borrelia-infected skin biopsies, then in human skin samples naturally infected with Borrelia, with quantification possible at low femtomolar range15. This proteomic approach is currently being evaluated for the early diagnosis of Lyme disease on a large cohort of patients (manuscript in preparation). It could then be tested for late stage of infection diagnosis since in mouse and dog models, the persistence of B. burgdorferi sl in the skin has been clearly demonstrated. To improve B. burgdorferi detection, their reactivation by topical corticosteroid can be followed by quantification using qPCR, allowing identification of the specific proteins expressed at the later stages of infection. If similar phenomenon of skin persistence exists for Borreliae in humans, it might constitute a new approach to identify active markers of infection in patients with disseminated infections16. Interestingly, persistence of cultivable Borrelia at the site of untreated, spontaneously cured erythema migrans has been repeatedly documented in patients with symptoms of disseminated disease, providing a strong rational to test this hypothesis31–33. As most patients with symptoms of late disseminated Lyme disease do not remember occurrence of a previous erythema migrans rash, a short course of topical corticosteroids on randomly chosen asymptomatic skin may be helpful to increase the sensitivity of the detection of either protein or DNA from Borrelia bystanders. Detection of spirochetes particularly in individuals exhibiting persistent Lyme disease manifestations will pave this new avenue for a straightforward confirmatory bacteriological diagnosis in often complex clinical situations observed in such patients.
In this paper, we emphasize the importance of filling the gap between mouse model outcomes and clinical research. Thus, we propose that protocol adjustments are necessary when a translational project is performed on humans. We clearly show that anesthetics of the lidocaine family can hamper the detection of microorganism proteins, confirming previous report18. We plan to develop a test for diagnosis of active Lyme borreliosis using mass spectrometry-based detection of spirochetal proteins. This new technique represents an interesting approach for the diagnosis of this disease, especially if Lyme spirochetes persist for months in the skin of humans.
Methods
Animals and Borrelia species
C3H/HeN mice (male or female) were raised at the Institute of bacteriology. Three different species of Borrelia were tested: B. burgdorferi ss, B. garinii and B. afzelii. Different strains belonging to these 3 main species were used for the infection experiments: B. burgdorferi ss strain N40D10/E9-expressing luciferase20, B. burgdorferi ss strain N40 (isolated from tick) and strain 297 (isolated from patient with neuroborreliosis), B. afzelii strain NE4049 (isolated from tick) and IBS 106 (isolated from a patient with erythema migrans), and B. garinii strain PBi (isolated from patient with neuroborreliosis). These strains were cultured in BSK-H complete medium (Sigma) at 33 °C and passaged ≤ 7 times before using them for mouse infections. Animals were infected intradermally in the lower part of the back as previously described34.
Lidocaine effect in vitro and in vivo on B. afzelii NE4049
In vitro
We first tested the effect of different concentrations of lidocaine chlorhydrate without preservative (Aguettant 10 mg/ml stock solution; 5 mg/ml was the highest concentration, then twofold dilution) in vitro on a culture of B. afzelii NE4049. Gentamicin (100 µg/ml as highest concentration, then twofold dilution) was included as a positive control. The spirochetes were co-incubated with the different drugs in 96 well-microtiter plate for 24 h, then transferred into Eppendorf tube with 1 ml of BSK-H complete medium and incubated at 33 °C for 7 days. Observation by dark field microscopy was performed to evaluate the shape, the mobility and the density of Borreliae in each well at days 1 and 7.
In vivo
We also assessed the effect of lidocaine injection in Borrelia-infected skin, 7 days after Borrelia inoculation. Lidocaine (50 µl of a 10 mg/ml solution) was injected at the site of bacteria inoculation; allowed the anesthetic to diffuse for 30 min and then collected the skin biopsy specimens. One part was directly frozen at − 80 °C for qPCR and proteomic studies (Day 0), while the other part was cultured for 7 (Day 7) and 14 days. At day 7, qPCR and proteomics analyses were also conducted to measure the effect of culture on the expression of Borrelia proteins, and their detection.
Proteomics
Mouse skin biopsies (15 mg) were manually extracted by 600 µL of Laemmli sample buffer in a 1 ml potter tissue grinder (Berktree, Cary, NC). Proteins (50 µg) were resolved by 12% SDS-PAGE15. Gel bands (10 ± 1 bands) of 2 mm were excised manually. Pre-digestion, digestion and nano-Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (nanoLC-MS/MS) analyses were carried out as described previously16, once for each sample. Mass data collected during nano-LC–MS/MS were converted into “.mgf” files with MSConvert software (Proteowizard, version 3.0.6090), and interpreted using Mascot (Matrix Science, version 2.5.1). Searches were performed against an in house generated protein database composed of Borrelia and mouse protein sequences extracted from NCBInr and UniProtKB-SwissProt, respectively. For bacteria, four different reference databases were used depending on the strains analyzed: B. burgorferi ss B31 (1,758 entries at May 07, 2013), B. burgdorferi ss N40 (1,480 entries at January 30, 2015), B. afzelii PKo (2,186 entries at October 16, 2014), and B. garinii (B. bavariensis) PBi (1,720 entries at August 30, 2013). The Mascot results were independently loaded into Proline software (Proline Studio Release, version 1.6). All spectra leading to an identification exceeding a minimum set threshold (Mascot Ion Score > 25, peptide length > 7 amino acids, pretty rank = 1) were considered. Resulting spectra were then filtered to obtain a protein false discovery rate of less than 1%.
Reactivation of Borrelia after dissemination by topical corticosteroid application
In situ reactivation
C3H/HeN mice (3–4 weeks old) were infected with different Borrelia species and strains via needle inoculation. Mice were inoculated intradermally in the dorsal thoracic area with 103 spirochetes suspended in 100 μL of BSK-H. Two to 3 weeks after bacterial inoculation, the presence of Borrelia-specific IgG antibodies in the mice sera was determined by ELISA (data not shown). Mice were sacrificed by cervical dislocation and different organs and the blood were collected for Borrelia culture and/or qPCR. After bacterial dissemination, spirochetes were reactivated in mouse skin between days 40 to 90 post-infection by applying topical corticosteroid at the site of inoculation (back) or at distant site (ear), twice a day for 2 days. Non-reactivated infected mice were used as negative controls (absence of Borrelia reactivation).
Comparison of Borrelia reactivation after application of different dermocorticoids
Topical corticosteroids with different anti-inflammatory activities19 have been tested in vivo: ~ 10 mg of 0.05% clobetasol propionate (very high) or 0.1% desonide (high) or 0.1% bethamethasone valerate (high) or 0.5% hydrocortisone cream were applied for one minute by massage to the dorsal thoracic area of the B. afzelii NE4049-infected mice, twice a day for 2 days. Quantification and Borrelia culture by qPCR were performed on skin biopsies collected at the site of cream application.
Comparison of B. afzelii reactivation in situ or distant site
In B. afzelii-infected mice, two sites of clobetasol-reactivation were compared: the site of inoculation in the back and a distant site, the ear. After euthanasia, different organs were collected to detect Borrelia presence by culture and/or by qPCR.
Reactivation of Borrelia burgdorferi ss N40-luciferase in the skin of infected mice
In order to better measure the extent of the reactivation of Borrelia in the skin, we carried out experiments of imaging using Borrelia-luciferase using codon-optimized luciferase expressing N40 D10/E9 strain20.
In vitro imaging
To validate the expression of luciferase in B. burgdorferi ss for in vivo imaging, first 104 and 106 spirochetes were treated with 50 µL of d-Luciferin sodium salt diluted in PBS or in methanol (10 or 50 mg/mL, Sigma-Aldricht) in Eppendorf tubes. Luminescence was estimated using IVIS Lumina X5 imaging system (Perkin Elmer, Massachusetts, USA) with 5 min exposures. These were the same settings used for in vivo imaging experiments (see below).
In vivo imaging
C3H/HeN mice were observed at different days after inoculation of luc-expressing B. burgdorferi N40 (106 spirochetes injected: 3 mice observed at day 7 after infection (day 7) and 2 mice at day 90 (day 90) after infection. Before imaging on day 90, topical corticosteroid was applied on mouse skin for 2 days. Each animal was anesthetized in oxygen-rich induction chamber with 2% isoflurane and then, injected intraperitoneally with 100 µL of d-luciferin in methanol (XenoLight d-Luciferin, Perkin Elmer). Mouse was installed in ventral position in the chamber of the IVIS Lumina X5 system (Perkin Elmer). Anesthesia was maintained during the entire imaging process and animal body temperature was regulated using a digitally thermostatic bed integrated within the IVIS system. The series of luminescence images was acquired during first 40 min after d-Luciferin injection. Data acquisition and analysis were performed by using the Living Image 4.5.5 software (Perkin Elmer). Luminescence was expressed in photons/s/cm2/sr and normalized with respect to imaging time, area imaged, and the distance between the light source (i.e., the mouse) and the charge-coupled device camera.
After each imaging session, mice were euthanized and B. burgdorferi infected-skin (site of inoculation on the back), heart, distant skin (ear) and joint were cultured to recover live spirochetes and quantified by PCR.
In vitro versus in vivo reactivation with cortisone
B. afzelii-infected skin was collected at around day 40, after either two days of in vivo topical application with clobetasol or by adding in vitro dissolved prednisolone (20 mg) to the culture of infected skin35. Culturing of infected skin without reactivation served as control. The culture of infected skin biopsy punch (6 mm) was followed for 11 and 19 days observation by dark field microscopy and spirochetes counted, and quantified by qPCR.
Culture and qPCR of B. burgdorferi sensu lato from mouse organs or blood
Mice were sacrificed by cervical dislocation at day 3 following topical corticosteroid treatment. One-cm2 area of dorsal thoracic skin, and an ear, heart and joint were removed for qPCR and culture. Blood was also collected for in vitro culture. For detection of B. burgdorferi sl by culture, different mouse organs were dissected aseptically. Collected organs and blood (3 drops) were placed in 6 ml of BSK-H complete medium containing 30 μg of rifampicin (BioRad). Culture tubes were incubated at 33 °C and examined weekly for the presence of spirochetes by dark-field microscopy as described previously34. For each organ and blood sample, tissue materials were divided into two parts: the first part was tested for live spirochetes presence following in vitro culture. If the culture remained negative after incubation, the second part was tested for DNA using qPCR. For all skin samples, culture and/or qPCR were performed.
Estimation of Borrelia load in mouse skin by qPCR
Mouse tissue samples were tested for the presence of B. burgdorferi sl using qPCR that targeted the flaB gene as described previously36. DNA was extracted from the skin of individual mice on a MagNA Pure equipment. Quantification of the B. burgdorferi-specific flaB gene was performed on a LightCycler system (Roche Diagnostics). Quantification of the mouse-specific gapdh gene was performed on an ABI Prism 7,500 instrument (Applied Biosystem), using a commercial kit (TaqMan rodent GADPH control reagent; Applied Biosystem). The number of B. burgdorferi sl spirochetes in tissue samples was standardized per 104 mouse gapdh gene copies11.
Statistical analyses
For different kinetic analyses, between 5 and 20 mice were used for each time point of the protocol. For proteomic analyses, for different infection protocols, between 10 and 22 mice were inoculated. We selected the mouse skin samples with the highest PCR quantification to maximize protein detection. Data were analyzed with R core team version 3.6.3 (https://www.r-project.org/index.html) to compute the mean with the standard deviation (SD) and generate bar plots. Groups were compared by a Mann–Whitney test with a Benjamini Hochberg correction when multiple tests were performed. Significant p value is reported as follow: “*” for p < 0.05 and “**” for p < 0.01.
Ethics statement
The protocols carried out in this study were approved by the Comité Régional d’Ethique en Matière d’Expérimentation Animale de Strasbourg (CREMEAS—Committee on the Ethics of Animal Experiments of the University of Strasbourg). Name of the ethics statement is /No. CREMEAS 2015062210282757 and APAFIS # 9317. The protocols performed on animals follow the European guidelines: “directive 2010/63/EU” under the animal facilities #: d67-482-34.
Acknowledgements
We thank the French Proteomics Infrastructure (ProFI; ANR-10-INSB-08-03), the PHRC (Programme Hospitalier de Recherche Clinique) HUS 6084 and the French Ministry of Research, Agence Nationale de la Recherche No. ANR-16-CE17-0003-01 for their financial support. We thank the Centre National Reference Center Borrelia for its technical support.
Author contributions
BL, PC, AG, CP, CB and NB performed the experiments and analyzed results. LS, NB, BJ supervised the experiments. NB and LES got financial support. NB, CL, NP and LES wrote the paper and NP provided bioluminescent N40 strain and edited the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Bastien Lefeuvre and Paola Cantero.
References
- 1.Steere A, et al. Lyme borreliosis. Nat Rev Dis Prim. 2016;2:1–13. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanek G, Wormser G, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 3.Stanek G, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17:69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- 4.Borchers A, Keen C, Huntley A, Gershwin M. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82–115. doi: 10.1016/j.jaut.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Wormser GP, et al. Practice guidelines for the treatment of lyme disease. Clin Infect Dis. 2000;31:S1–S14. doi: 10.1086/314053. [DOI] [PubMed] [Google Scholar]
- 6.Turk SP, et al. Post-treatment Lyme disease symptoms score: developing a new tool for research. PLoS ONE. 2019;14:e0225012. doi: 10.1371/journal.pone.0225012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S, de Souza M, Janotka J, Smith A, Persing D. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol. 1993;143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 8.Hodzic E, Feng S, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun. 2003;71:5042–5055. doi: 10.1128/IAI.71.9.5042-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodzic E, Feng S, Freet KJ, Borjesson DL, Barthold SW. Borrelia burgdorferi population kinetics and selected gene expression at the host–vector interface. Infect Immun. 2002;70:3382–3388. doi: 10.1128/IAI.70.7.3382-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bockenstedt L, et al. What ticks do under your skin: two-photon intravital imaging of Ixodes scapularis feeding in the presence of the lyme disease spirochete. Yale J Biol Med. 2014;87:3–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Kern A, et al. Heterogeneity of Borrelia burgdorferi sensu stricto population and its involvement in Borrelia pathogenicity: study on murine model with specific emphasis on the skin interface. PLoS ONE. 2015;10:e0133195. doi: 10.1371/journal.pone.0133195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard Q, Jaulhac B, Boulanger N. Smuggling across the border: How arthropod-borne pathogens evade and exploit the host defense system of the skin. J Invest Dermatol. 2014;134:1211–1219. doi: 10.1038/jid.2014.36. [DOI] [PubMed] [Google Scholar]
- 13.Caljon G, et al. The dermis as a delivery site of Trypanosoma brucei for tsetse flies. PLoS Pathog. 2016;12:e1005. doi: 10.1371/journal.ppat.1005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ménard R, et al. Looking under the skin: the first steps in malarial infection and immunity. Nat Rev Microbiol. 2013;11:701–712. doi: 10.1038/nrmicro3111. [DOI] [PubMed] [Google Scholar]
- 15.Schnell G, et al. Discovery and targeted proteomics on cutaneous biopsies: a promising work toward an early diagnosis of Lyme disease. Mol Cell Proteom. 2015;14:1254–1264. doi: 10.1074/mcp.M114.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grillon A, et al. Identification of Borrelia protein candidates in mouse skin for potential diagnosis of disseminated Lyme borreliosis. Sci Rep. 2017;7:16719. doi: 10.1038/s41598-017-16749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson S, Saint John B, Dine A. Local anesthetics as antimicrobial agents: a review. Surg Infect (Larchmt). 2008;9:205–213. doi: 10.1089/sur.2007.036. [DOI] [PubMed] [Google Scholar]
- 18.Razavi B, Fazly Bazzaz B. A review and new insights to antimicrobial action of local anesthetics. Eur J Clin Microbiol Infect Dis. 2019;38:991–1002. doi: 10.1007/s10096-018-03460-4. [DOI] [PubMed] [Google Scholar]
- 19.Lebrun-Vignes B, Chosidow O. Dermocorticoïdes. EMC AKOS (Traité Médecine) 2011;7:1–6. [Google Scholar]
- 20.Chan K, Alter L, Barthold SW, Parveen N. Disruption of bbe02 by insertion of a luciferase gene increases transformation efficiency of Borrelia burgdorferi and allows live imaging in lyme disease susceptible C3H mice. PLoS ONE. 2015;10:e0129532. doi: 10.1371/journal.pone.0129532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straubinger R, Summers B, Chang Y, Appel M. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J Microbiol. 1997;35:111–116. doi: 10.1128/jcm.35.1.111-116.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barthold S, Beck D, Hansen G, Terwilliger G, Moody K. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 23.Aguero-Rosenfeld M, Wang G, Schwartz I, Wormser G. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Duijvendijk G, Sprong H, Takken W. Multi-trophic interactions driving the transmission cycle of Borrelia afzelii between Ixodes ricinus and rodents: a review. Parasit Vectors. 2015;8:643. doi: 10.1186/s13071-015-1257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenormand C, et al. Expanding the clinicopathological spectrum of late cutaneous Lyme borreliosis (acrodermatitis chronica atrophicans [ACA]): a prospective study of 20 culture- and/or polymerase chain reaction (PCR)-documented cases. J Am Acad Dermatol. 2016;74:685–692. doi: 10.1016/j.jaad.2015.10.046. [DOI] [PubMed] [Google Scholar]
- 26.Müllegger R, Glatz M. Skin manifestations of Lyme borreliosis: diagnosis and management. Am J Clin Dermatol. 2008;9:355–368. doi: 10.2165/0128071-200809060-00002. [DOI] [PubMed] [Google Scholar]
- 27.Mehaffy C, Dobos KM, Nahid P, Kruh-Garcia NA. Second generation multiple reaction monitoring assays for enhanced detection of ultra-low abundance Mycobacterium tuberculosis peptides in human serum. Clin Proteom. 2017;14:21. doi: 10.1186/s12014-017-9156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rifflet A, et al. Quantification of low abundance Yersinia pestis markers in dried blood spots by immuno-capture and quantitative high-resolution targeted mass spectrometry. Eur J Mass Spectrom. 2019;25:268–277. doi: 10.1177/1469066718795978. [DOI] [PubMed] [Google Scholar]
- 29.da Silva Santos C, et al. Proteome profiling of human cutaneous leishmaniasis lesion. J Invest Dermatol. 2015;135:400–410. doi: 10.1038/jid.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernard Q, Grillon A, Lenormand C, Ehret-Sabatier L, Boulanger N. Skin interface, a key player for Borrelia multiplication and persistence in Lyme borreliosis. Trends Parasitol. 2020;36:304–314. doi: 10.1016/j.pt.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 31.van Dam AP, et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect Dis. 1993;17:708–717. doi: 10.1093/clinids/17.4.708. [DOI] [PubMed] [Google Scholar]
- 32.Kuiper H, et al. Isolation of Borrelia burgdorferi from biopsy specimens taken from healthy-looking skin of patients with Lyme borreliosis. J Clin Microbiol. 1994;32:715–720. doi: 10.1128/JCM.32.3.715-720.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strle F, et al. Persistence of Borrelia burgdorferi sensu lato in resolved erythema migrans lesions. Clin Infect Dis. 1995;21:380–389. doi: 10.1093/clinids/21.2.380. [DOI] [PubMed] [Google Scholar]
- 34.Kern A, et al. Tick saliva represses innate immunity and cutaneous inflammation in a Murine model of lyme disease. Vector-Borne Zoonotic Dis. 2011;11:1343–1350. doi: 10.1089/vbz.2010.0197. [DOI] [PubMed] [Google Scholar]
- 35.Straubinger R, Straubinger A, Summers B, Jacobson R. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J Infect Dis. 2000;181:1069–1081. doi: 10.1086/315340. [DOI] [PubMed] [Google Scholar]
- 36.Hidri N, et al. Lyme endocarditis. Clin Microbiol Infect. 2012;18:E531–E532. doi: 10.1111/1469-0691.12016. [DOI] [PubMed] [Google Scholar]