Abstract
Purpose of review
Although the goal of preventive HIV vaccine design is primarily the induction of broadly neutralizing antibodies (bNAbs), recent evidence suggests that a protective response will also benefit from Fc effector functions. Here, we provide an update on the antibody response to HIV infection, including both Fab and Fc-mediated antibody responses. We also highlight recent studies showing the interplay between these functions, focusing primarily on studies published in the last year.
Recent findings
Identification and characterization of bNAb donors continues to provide insights into viral factors that are potentially translatable to vaccine design. Improved and more diverse measures of Fc effector function, and modulators thereof, are enabling a deeper understanding of their role in infection. New data providing mechanistic links between the innate and adaptive humoral immune responses are creating exciting opportunities for vaccine strategies, with the aim of eliciting a polyfunctional protective response.
Summary
New insights into the overall humoral response to HIV infection are defining diverse and synergistic mechanisms required for antibody protection from HIV through vaccination.
Keywords: antibody-dependent cellular cytotoxicity, broadly neutralizing antibodies, Fc effector function, HIV envelope, pediatric donors, superinfection
INTRODUCTION
Studies of HIV-infected donors over many years have provided extraordinary insights into the humoral immune response to the envelope glycoprotein (Env) of the virus. These antibody responses develop despite the fact that Env presents a significant immunological challenge, as it is heavily glycosylated, highly sequence variable and conformationally dynamic. As vaccines have traditionally sought to elicit neutralizing antibodies, which can provide sterilizing immunity, many studies have focused on these responses, particularly broadly neutralizing responses during infection. However, more recently, so-called ‘nonneutralizing’ antibodies with Fc effector function have gained increasing focus (see Zolla-Pazner et al. this issue). Here we review advances and gaps in our understanding of the antibody response to HIV Env, and the implications of such studies for HIV vaccine design.
STUDIES OF BROADLY NEUTRALIZING ANTIBODY DONORS CONTINUE TO PROVIDE CLUES FOR THEIR DEVELOPMENT
Broadly neutralizing antibodies (bNAbs), capable of recognizing diverse viral strains, are known to develop in a small proportion of HIV-infected donors [1]. The isolation of monoclonal antibodies (mAbs) from such donors has identified viral vulnerabilities spanning much of the HIV Env. The recent identification of bNAbs targeting the highly glycosylated ‘silent face’ extended these further, to cover all major exposed regions of the Env trimer [2].
Defining factors associated with the development of bNAbs continues to generate useful information, confirming the link of breadth with ethnicity, viral load, infection length, and viral diversity [3] (Table 1) (see Trkola et al. this issue). The latter three reflect the high levels of antigenic stimulation required to drive bNAb maturation from strain-specific precursors. However, all infected individuals mount neutralizing responses, and the magnitude of these ‘tier 2’ responses is not associated with breadth [4■]. Indeed, when normalized for viral load, overall Env diversity does not distinguish bNAb and non-bNAb donors [5]. In contrast, specific positively selected sites common to multiple bNAb donors targeting the same epitopes was temporally associated with the onset of breadth [5]. This is consistent with studies showing that bNAb maturation is associated with constrained viral escape and fitness costs [6,7]. Together, these factors suggest a need for prolonged exposure of maturing antibodies to viral variants [6,7], a finding supported by strategies employing slower administration of vaccines [8] (Table 1).
Table 1.
Contributors to broadly neutralizing antibody development and their potential impact on vaccine strategies
| Factors associated with bNAbs |
Impact on vaccine strategies |
|---|---|
| Ethnicity | Vaccination outcomes may vary by geographic location |
| Viral diversity/superinfection | Need for some variation in vaccines Not too much variation (shared determinants in prime boost scenarios to maintain primed responses) |
| Constrained viral escape | Prolonged administration of immunogens |
| Polyclonal bNAb responses | Renewed focus on vaccine strategies aimed at less genetically unusual bNAbs Use of multi-epitope vaccine platforms |
| RAB11FIP5 expression/altered NK function | Immune modulation as part of vaccine strategies |
| Association of Fc effector function with breadth | Adjuvanting to drive enhanced class switching Incorporation of immune complexes into vaccine strategies |
bNAbs, broadly neutralizing antibodies; NK, natural killer.
More recently, binding of IgG1 to BG505 SOSIP trimer was shown to be a strong predictor of neutralization breadth [9]. In addition, a transcriptomic analysis of bNAb and non-bNAb donors implicated expression of RAB11FIP5, a Rab effector protein associated with recycling endosomes, and altered natural killer cell function in bNAb development [10■]. This suggests that both innate and adaptive components of humoral responses are important to support the development of bNAbs, a possibility explored in more detail below.
NOT ALL ANTIBODY MUTATIONS ARE CREATED EQUAL FOR BROADLY NEUTRALIZING ANTIBODY DEVELOPMENT
The association of bNAbs with high levels of somatic hypermutation (SHM) is well established. However, the availability of many bNAbs has enabled a deeper understanding of the specific mutational features associated with breadth. Mutations in the variable regions of immunoglobulins, which often interact directly with epitopes, accumulate in response to varying viral escape mutations within core epitopes, or to accommodate heterogeneous glycans [11,12] that form part of many bNAb epitopes. In addition, mutations in the framework regions are enriched in many bNAbs and likely to have functional relevance. For example, mutations in the elbow region of the framework regions have been shown to decrease thermostability, impact interdomain flexibility and paratope plasticity during bNAb development [13]. In silico simulation of SHM has shown that many functionally important bNAb mutations are highly improbable, occurring in activation-induced cytidine deaminase (AID) cold spots or requiring multiple nucleotide mutations [14■]. This poses a challenge for vaccine regimens, which will need to select these unusual mutations.
THE INFECTING VIRUS OF BROADLY NEUTRALIZING ANTIBODY DONORS AS A BASIS FOR IMMUNOGEN DESIGN
Intrinsic viral attributes also impact on the development of bNAbs during infection. A recent analysis of the influence of viral antigens in shaping antibody responses, including transmission pairs with similar antibody responses, indicated that the infecting virus has a significant impact [15■■]. Defining the features of bNAb-imprinting viruses, a concept inspired by influenza studies, thus has the potential to contribute to the identification of effective immunogens, should these features be identified. One such feature may be the level of glycosylation, with a more complete glycan shield associated with better bNAb responses [16■]. This suggests that breaches in this shield and exposure of underlying protein epitopes represent an immunological distraction from breadth [16■].
SUPERINFECTION AND BROADLY NEUTRALIZING ANTIBODIES: A NATURAL TEST OF PRIME-BOOST VACCINATION STRATEGIES
HIV superinfection (re-infection with a second strain following an established infection) has been associated with neutralization breadth, though the effect is moderate [17]. These data suggested the possibility that superinfection may recruit preexisting nAbs elicited during the initial infection, a situation analogous to a heterologous prime boost with distinct Envs. However, isolation of diverse mAbs from a superinfection case suggested that neutralization breadth was the result of multiple distinct B-cell lineages that arose in response to either the initial or the superinfecting virus [18]. Similarly, characterization of the plasma responses of four superinfected women in a separate cohort revealed that superinfection did not boost memory nAb responses primed by the first infection or promote nAbs to epitopes conserved in both infecting viruses, but elicited a de novo response to each virus [4■]. These studies, therefore, suggest that infection with two viruses results in an additive rather than synergistic effect, and that heterologous Envs may not be sufficient to focus the immune response onto conserved bNAb epitopes [4■].
HIV-INFECTED CHILDREN: A SPECIAL CASE FOR BROADLY NEUTRALIZING ANTIBODIES?
The contribution of multiple different antibody specificities to breadth is likely also true of HIV-infected children. HIV-infected infants rapidly develop cross-reactive responses [19], and more recent studies of older children show this group to be enriched for elite neutralizers [20]. This highly selected group of non-progressing/slow-progressing children have unusually high viral loads over many years and immunological characteristics such as low levels of inflammation, well maintained CD4/CD8 responses and high levels of germinal center function [20,21], which may explain their unusually broad and potent responses [20]. Mapping studies suggest that breadth in children is mediated by a polyclonal response of multiple antibodies to known bNAb epitopes, which act additively to mediate breadth [22]. More detailed studies of one pediatric donor enabled the identification of a bNAb, BF520.1, that exhibits moderate breadth but lower levels of SHM and insertions/deletions than those that define many adult bNAbs [23]. The potentially easier pathways to breadth [23], also observed in some adults [24], raises the possibility that eliciting a diversity of moderately broad antibodies with less unusual genetic features may be a viable alternative to the current focus on elite neutralizers.
THE ROLE OF ISOTYPE IN HIV-DIRECTED ANTIBODY RESPONSES
Immunoglobulin isotype is a major modulator of Fc effector function, as described in more detail below. However, several studies over many decades have implicated this region in neutralization activity and antibody protection against HIV [25–27]. These data indicate that it is not only the Fab that dictates the affinity for antigen. However, this paradigm has not been adopted routinely into the many studies that are based on antibody isolation. Typically, in these studies the antibody variable regions are cloned into an IgG1 backbone, irrespective of the isotype of the naturally isolated antibody mAb (indeed, in many cases, this is not known). The most common subclass elicited in response to HIV in blood is IgG1; however, there are a number of bNAbs that have been isolated as other isotypes. In particular, membrane proximal bNAbs, such as 4E10, 10E8 and 2F5 were isolated as IgG3, suggesting a possible predisposition of certain classes of antibodies for particular isotypes [28]. In addition, new roles for isotype in HIV infection are being reported. This includes a previously unknown role for IgG3 in B-cell regulation, resulting in reduced sensitivity to B-cell stimulation in chronic HIV infection [29] (see Moir et al. this issue). Similarly, although previously thought to be antagonistic to IgG function, IgA has been shown to co-operate with IgG to enhance ADCC in cell types that express Fc alpha and gamma receptors [30]. The finding of new roles for isotypes including the modulation of neutralization, points to a need to characterize antibodies in their full natural context.
THE FC RESPONSE DURING INFECTION
The observation that antibody-dependent cellular cytotoxicity (ADCC) was associated with decreased risk of infection in the RV144 vaccine trial [31] lead to renewed energy in understanding Fc effector function in HIV infection. Not only is there extensive evidence to indicate that ADCC delays disease progression but a recent study suggested it may play a role in reducing transmission in serodiscordant couples [32]. Evidence from several studies shows an association between Fc effector function and HIV control, including functional IgG1 p24 antibodies [33] and higher more polyfunctional antibody responses [34,35]. Futhermore, the isolation from infected donors of several antibodies that exhibit potent Fc effector function, though not neutralization, suggests such antibodies are commonly induced [36,37]. This may allow for the development of new strategies that aim to ‘sensitize’ Env to ADCC attack by opening the trimer [38–40]. However, there are several aspects of Fc effector function that remain understudied in HIV infection.
WHAT DO CURRENT MEASURES OF ANTIBODY-DEPENDENT CELLULAR CYTOTOXICITY MEAN?
Unlike measures of neutralizing activity, which have been heavily standardized, many Fc effector function assays lack standardization and in-depth understanding of their mechanisms. Measurement of ADCC, in particular, employs several assays, which may measure slightly different outcomes [41,42]. Many ADCC assays include HIV envelope proteins coated onto target cells through binding to CD4, which exposes sites that would typically not be present on envelope trimer and are a major target for ADCC [43]. As a result of this, the field is moving towards using infected target cells, not without its own complications. During infection, the Nef and Vpu accessory proteins downregulate CD4 expression, limiting the exposure of CD4-induced HIV-1 Env epitopes [44]. Engineered infectious viruses may lack Nef expression resulting in incomplete CD4 downregulation, exposing CD4-induced sites and increasing ADCC [45■]. In addition, gp120 shed from infected cells binds to other uninfected CD4-expressing cells resulting in bystander killing [46]. As gp120 shedding likely occurs in vivo, the significance of this effect is unclear, complicating meaningful interpretations of such data. Furthermore, the absence of comprehensive studies defining the varying Env structures, and their proportion relative to the Env trimer on the surface of infected cells, makes it difficult to define whether CD4i responses are biologically important. These assay limitations impact studies of HIV infection and vaccination that make use of ‘systems serology’, the simultaneous measurement of diverse Fc functions and their modulators (including glycosylation, isotype and Fc receptor binding) (see Ackerman et al. this issue) [47]. These assays allow for a comprehensive and important overview of humoral activity, but because of their high-throughput nature often rely on methods based on coating cells assays.
DISCOVERING NEW FC EFFECTOR FUNCTIONS AND EXPANDING MECHANISMS
Although ADCC is the most widely studied Fc effector function, it is not the only function with a role to play in HIV infection and vaccination. Antibody-dependent cellular phagocytosis was recently found to be a correlate of reduced infection risk in rhesus macaques immunized with a DNA prime-Ad5 SIV-mac239 Env-based vaccine regimen [48■], illustrating its importance in the potential prevention of HIV infection. Interestingly, depending on the route of immunization, two different kinds of phagocytosis were important, mediated either by monocytes or neutrophils. Neutrophil-mediated phagocytosis has only recently explored in HIV infection using HIV-specific antibodies [49,50], and has shown distinct phagocytic outcomes to monocytes. This indicates the importance of exploring Fc effector functions in a wider variety of cell types than those routinely used.
Another Fc effector function that is largely unexplored in HIV infection is trogocytosis, though it has been extensively described in cancer. In the presence of antibody, THP-1 cells (a monocytic cell line) nibbles membrane and accompanying proteins off target cells coated with gp120 [51]. Membrane nibbling results in rapid cell death of target cells, suggesting that repeated trogocytic attack may be an important tool in vaccination. However, the impact of trogocytosis on HIV disease progression is not known. Furthermore, several groups have also found a potential role for trogocytosis in various ADCC assays, making it clear that further study of this function is needed [41,42]
An unaddressed gap in Fc effector studies during HIV infection is their impact on viral escape, which could strengthen studies of their relevance in HIV infection. In an elegant humanized mouse study, Horwitz et al. showed that infusion of a nonneutralizing gp41 mAb resulted in selection of a viral escape mutation in a Fc-dependent manner [52]. Although one study has shown that ADCC exerts pressure on the virus in chronic infection [53], expansion of these studies to acute infection is an area that needs further work.
GENETICS OF ANTIBODIES AND THEIR RECEPTORS
A growing area of research in antibody responses to HIV infection is immunoglobulin genetic diversity, and its contribution to neutralization and Fc effector function. In HIV-infected individuals, several HIV-reactive antibody clonotypes that are common to multiple individuals, known as ‘public antibodies’, have been described [54]. This is promising for vaccine efforts, suggesting that there are common modalities for the induction of a protective response across individuals [54]. There have also been exciting developments in understanding repertoires common to HIV infection and vaccination. For example, V2-directed antibodies associated with reduced risk of infection from the RV144 vaccine trial have been reported to have restricted light chain gene usage [55]. However, the isolation of similar antibodies from HIV-infected individuals revealed an additional light chain gene able to mediate such interactions, increasing the repertoire able to produce such antibodies [56].
Although exciting advances are being made in the variable region, large gaps in the constant region repertoires remain, especially in African populations [57]. An added complication is the historical definition of allotypes based on SNPs that affect serology, but do not capture all allelic variation. Although several allotypes have been associated with susceptibility or resistance to a variety of diseases [58] and HIV vaccine efficacy [59], little is known about allelic variation and its impact on HIV-directed and other antibody responses in different populations.
CONCLUSION
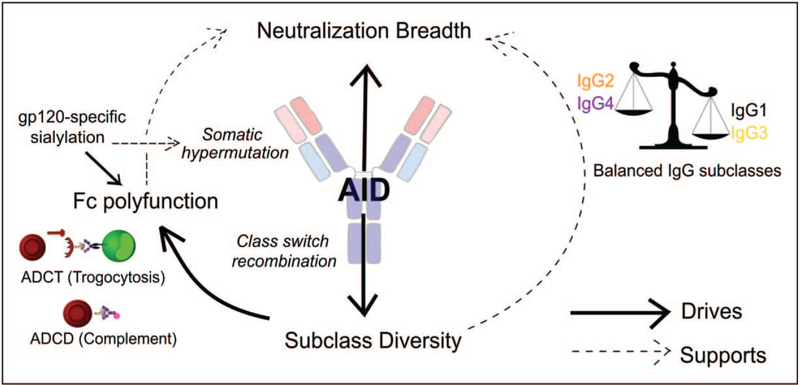
Although many studies have characterized the contribution of neutralizing antibodies and Fc effector function during infection, the link between the two is less well characterized. Two studies have described an association between Fc effector function and the development of neutralization breadth. We showed that individuals who developed bNAbs also had highly polyfunctional Fc effector function early in infection [60■]. Increased IgG subclass diversity was associated with germinal center activity measured both by CXCL13 and activation-induced cytidine deaminase levels in B cells from these bNAb individuals [60■]. Subsequently, Lofano et al. [61■■] confirmed in a second cohort that antibody-dependent complement deposition was higher in bNAb individuals. Furthermore, sialylated gp120 specific mAbs were also found to be elevated in bNAb individuals [61■■]. The translational value of these findings was confirmed following the infusion of sialylated immune complexes into mice, where enhanced antigen deposition in germinal centers resulted in improved antibody responses in a complement-dependent manner [61■■]. These studies provide a mechanistic link between the Fab and the Fc, and provide evidence for a supporting role of Fc effector function in the elicitation of bNAbs (Fig. 1). These common features could be used to support the development of both functional and potent antibodies in response to vaccination.
FIGURE 1.
Co-operation between Fab-mediated and Fc-mediated functions. Neutralization breadth is driven in part through the action of activation-induced cytidine deaminase (AID) and resulting somatic hypermutation. IgG subclass diversity of the Fc is driven by AID and class switch recombination, which in turn drives Fc polyfunction. The balance of antiinflammatory and pro-inflammatory subclasses, sialylation of antibodies and Fc effector function, such as antibody-dependent complement deposition (ADCD) and antibody-dependent cellular phagocytosis (ADCP) may have a supportive role in the development of breadth through the enhancement of antigen presentation and increase in somatic hypermutation.
KEY POINTS.
Studies of bNAb donors suggest that viral determinants of immunogenicity may be valuable for immunogen design.
Superinfected and pediatric bNAb donors provide unique insights into bNAb development.
Expanded understanding of existing and new Fc functions during HIV infection and how to measure them meaningfully.
Neutralizing and Fc effector responses are mechanistically linked, presenting opportunities for generating polyfunctional vaccine responses.
Acknowledgements
We thank colleagues, students and collaborators for useful discussions.
Financial support and sponsorship
P.L.M. is supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa. S.I.R. is supported by a H3 Africa grant (U01A136677). We acknowledge funding from a U01 grant (AI116086–01), the Centre for the AIDS Programme of Research (CAPRISA) and the SA Medical Research Council SHIP program.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Landais E, Moore PL. Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers. Retrovirology 2018; 15:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou T, Zheng A, Baxa U, et al. A neutralizing antibody recognizing primarily N-linked glycan targets the silent face of the HIV envelope. Immunity 2018; 48:500.e6–513.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rusert P, Kouyos RD, Kadelka C, et al. , Swiss HIV Cohort Study. Determinants of HIV-1 broadly neutralizing antibody induction. Nat Med 2016; 22:1260–1267. [DOI] [PubMed] [Google Scholar]
- 4.Sheward DJ, Marais J, Bekker V, et al. HIV superinfection drives de novo antibody responses and not neutralization breadth. Cell Host Microbe 2018; 24:593.e3–599.e3.■ Detailed mapping of four superinfected donors showed that superinfection did not boost memory nAb responses primed by the first infection, but elicited a de novo response to two viruses.
- 5.Mabvakure BM, Scheepers C, Garrett N, et al. Positive selection at key residues in the HIV envelope distinguishes broad and strain-specific plasma neutralizing antibodies. J Virol 2019; 93:; pii: e01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anthony C, York T, Bekker V, et al. Co-operation between strain-specific and broadly neutralizing responses limited viral escape, and prolonged exposure of the broadly neutralizing epitope. J Virol 2017; 91:e00828–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reh L, Magnus C, Kadelka C, et al. Phenotypic deficits in the HIV-1 envelope are associated with the maturation of a V2-directed broadly neutralizing antibody lineage. PLoS Pathog 2018; 14:e1006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tam HH, Melo MB, Kang M, et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc Natl Acad Sci U S A 2016; 113:E6639–E6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadelka C, Liechti T, Ebner H, et al. , Swiss HIV Cohort Study. Distinct, IgG1-driven antibody response landscapes demarcate individuals with broadly HIV-1 neutralizing activity. J Exp Med 2018; 215:1589–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley T, Peppa D, Pedroza-Pacheco I, et al. RAB11FIP5 expression and altered natural killer cell function are associated with induction of HIV broadly neutralizing antibody responses. Cell 2018; 175:387.e17–399.e17.■ A transcriptomic analysis of bNAb and non-bNAb donors implicated natural killer cells and Rab11 recycling endosomal transport in regulation of HIV-1 bnAb development.
- 11.Andrabi R, Su CY, Liang CH, et al. Glycans function as anchors for antibodies and help drive HIV broadly neutralizing antibody development. Immunity 2017; 47:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rantalainen K, Berndsen ZT, Murrell S, et al. Co-evolution of HIV envelope and apex-targeting neutralizing antibody lineage provides benchmarks for vaccine design. Cell Rep 2018; 23:3249–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson R, Watts BE, Ergin HN, et al. Selection of immunoglobulin elbow region mutations impacts interdomain conformational flexibility in HIV-1 broadly neutralizing antibodies. Nat Commun 2019; 10:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiehe K, Bradley T, Meyerhoff RR, et al. Functional relevance of improbable antibody mutations for HIV broadly neutralizing antibody development. Cell Host Microbe 2018; 23:759.e6–765.e6.■ An elegant study showing that bnAbs are enriched for improbable mutations, and demonstrating their functional relevance. This has importance for vaccine strategies, which will need to select these unusual mutations.
- 15.Kouyos RD, Rusert P, Kadelka C, et al. Tracing HIV-1 strains that imprint broadly neutralizing antibody responses. Nature 2018; 561:406–410.■■ Analysis of the role of viral antigens in shaping antibody responses, including transmission pairs with similar antibody responses, indicated that the infecting virus has a moderate but significant impact. The identification of viruses with ‘bNAb-imprinting capacities’ may provide the basis of effective immunogens.
- 16.Wagh K, Kreider EF, Li Y, et al. Completeness of HIV-1 envelope glycan shield at transmission determines neutralization breadth. Cell Rep 2018; 25:893.e7–908.e7.■ A second study determining the importance of features of the transmitted virus in determining the quality of subsequent neutralization responses during infection.
- 17.Cortez V, Odem-Davis K, McClelland RS, et al. HIV-1 superinfection in women broadens and strengthens the neutralizing antibody response. PLoS Pathog 2012; 8:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams KL, Wang B, Arenz D, et al. Superinfection drives HIV neutralizing antibody responses from several B cell lineages that contribute to a polyclonal repertoire. Cell Rep 2018; 23:682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goo L, Chohan V, Nduati R, Overbaugh J. Early development of broadly neutralizing antibodies in HIV-1 -infected infants. Nat Med 2014; 20:655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muenchhoff M, Adland E, Karimanzira O, et al. Nonprogressing HIV-infected children share fundamental immunological features of nonpathogenic SIV infection. Sci Transl Med 2016; 8:358ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roider J, Maehara T, Ngoepe A, et al. High-frequency, functional HIV-specific T-follicular helper and regulatory cells are present within germinal centers in children but not adults. Front Immunol 2018; 9:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ditse Z, Muenchhoff M, Adland E, et al. HIV-1 subtype Cinfected children with exceptional neutralization breadth exhibit polyclonal responses targeting known epitopes. J Virol 2018; 92:; pii: e00878–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonich CA, Williams KL, Verkerke HP, et al. HIV-1 neutralizing antibodies with limited hypermutation from an infant. Cell 2016; 166:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cale EM, Gorman J, Radakovich NA, et al. Virus-like particles identify an HIV V1V2 apex-binding neutralizing antibody that lacks a protruding loop. Immunity 2017; 46:777.e10–791.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tudor D, Yu H, Maupetit J, et al. Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc Natl Acad Sci U S A 2012; 109:12680–12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharf O, Golding H, King LR, et al. Immunoglobulin G3 from polyclonal human immunodeficiency virus (HIV) immune globulin is more potent than other subclasses in neutralizing HIV type 1. J Virol 2001; 75:6558–6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheeseman HM, Olejniczak NJ, Rogers PM, et al. Broadly neutralizing antibodies display potential for prevention of HIV-1 infection of mucosal tissue superior to that of nonneutralizing antibodies. J Virol 2017; 91:; pii: e01762–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molinos-Albert LM, Clotet B, Blanco J, Carrillo J. Immunologic insights on the membrane proximal external region: a major human immunodeficiency virus type-1 vaccine target. Front Immunol 2017; 8:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kardava L, Sohn H, Youn C, et al. IgG3 regulates tissue-like memory B cells in HIV-infected individuals. Nat Immunol 2018; 19:1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duchemin M, Khamassi M, Xu L, et al. IgA targeting human immunodeficiency virus-1 envelope gp41 triggers antibody-dependent cellular cytotoxicity cross-clade and cooperates with gp41-specific IgG to increase cell lysis. Front Immunol 2018; 9:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. , MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–2220. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz MJ, Salido J, Abusamra L, et al. Evaluation of different parameters of humoral and cellular immune responses in HIV Serodiscordant heterosexual couples: humoral response potentially implicated in modulating transmission rates. EBioMedicine 2017; 26:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung AW, Mabuka JM, Ndlovu B, et al. Viral control in chronic HIV-1 subtype C infection is associated with enrichmentofp24 IgG1 with Fc effector activity. AIDS 2018; 32:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadanand S, Das J, Chung AW, et al. Temporal variation in HIV-specific IgG subclass antibodies during acute infection differentiates spontaneous controllers from chronic progressors. AIDS 2018; 32:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ackerman ME, Mikhailova A, Brown EP, et al. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog 2016; 12:e1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams KL, Stumpf M, Naiman NE, et al. Identification of HIV gp41-specific antibodies that mediate killing of infected cells. PLoS Pathog 2019; 15:e1007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith SA, Burton SL, Kilembe W, et al. VH1–69 utilizing antibodies are capable of mediating nonneutralizing Fc-mediated effector functions against the transmitted/founder gp120. Front Immunol 2018; 9:3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anand SP, Prevost J, Baril S, et al. Two families of Env antibodies efficiently engage Fc-gamma receptors and eliminate HIV-1-infected cells. J Virol 2019; 93:; pii: e01823–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richard J, Veillette M, Ding S, et al. Small CD4 mimetics prevent HIV-1 uninfected bystander CD4 + T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine 2016; 3:122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen X, Bogers WM, Yates NL, et al. Cross-linking of a CD4-mimetic miniprotein with HIV-1 Env gp140 alters kinetics and specificities of antibody responses against HIV-1 Env in macaques. J Virol 2017; 91:; pii: e00401–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kramski M, Schorcht A, Johnston AP, et al. Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity. J Immunol Methods 2012; 384:51–61. [DOI] [PubMed] [Google Scholar]
- 42.Pollara J, Orlandi C, Beck C, et al. Application of area scaling analysis to identify natural killer cell and monocyte involvement in the GranToxiLux antibody dependent cell-mediated cytotoxicity assay. Cytometry A 2018; 93:436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veillette M, Desormeaux A, Medjahed H, et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 2014; 88:2633–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veillette M, Coutu M, Richard J, et al. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 2015; 89:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prevost J, Richard J, Medjahed H, et al. Incomplete downregulation of CD4 expression affects HIV-1 Env conformation and antibody-dependent cellular cytotoxicity responses. J Virol 2018; 92:; pii: e00484–18.■ A study comparing different viral constructs in ADCC assays, showing that decreased Nef expression, as a result of reporter gene insertion, results in downregulated CD4 and enhanced ADCC activity.
- 46.Richard J, Prevost J, Baxter AE, et al. Uninfected bystander cells impact the measurement of HIV-specific antibody-dependent cellular cytotoxicity responses. MBio 2018; 9:; pii: e00358–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alter G, Dowell KG, Brown EP, et al. High-resolution definition of humoral immune response correlates of effective immunity against HIV. Mol Syst Biol 2018; 14:e7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackerman ME, Das J, Pittala S, et al. Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med 2018; 24:1590–1598.■ An important study in nonhuman primates showing that reduced risk of infection following vaccination was associated with different isotype and cell-driven phagocytosis, determined by either intramuscular or aerosol immunization.
- 49.Worley MJ, Fei K, Lopez-Denman AJ, et al. Neutrophils mediate HIV-specific antibody-dependent phagocytosis and ADCC. J Immunol Methods 2018; 457:41–52. [DOI] [PubMed] [Google Scholar]
- 50.Powell RLR, Fox A, Itri V, Zolla-Pazner S. Primary human neutrophils exhibit a unique HIV-directed antibody-dependent phagocytosis profile. J Innate Immun 2019; 11:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson SI, Crowther C, Mkhize NN, Morris L. Measuring the ability of HIV-specific antibodies to mediate trogocytosis. J Immunol Methods 2018; 463:71–83. [DOI] [PubMed] [Google Scholar]
- 52.Horwitz JA, Bar-On Y, Lu CL, et al. Nonneutralizing antibodies alter the course of HIV-1 infection in vivo. Cell 2017; 170:637.e10–648.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung AW, Isitman G, Navis M, et al. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A 2011; 108:7505–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Setiff I, McDonnell WJ, Raju N, et al. Multi-donor longitudinal antibody repertoire sequencing reveals the existence of public antibody clonotypes in HIV-1 infection. Cell Host Microbe 2018; 23:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liao HX, Bonsignori M, Alam SM, et al. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 2013; 5:453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Eeden C, Wibmer CK, Scheepers C, et al. V2-directed vaccine-like antibodies from HIV-1 infection identify an additional K169-binding light chain motif with broad ADCC activity. Cell Rep 2018; 25:3123.e6–3135.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson CT, Glanville J, Marasco WA. The individual and population genetics of antibody immunity. Trends Immunol 2017; 38:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oxelius VA, Pandey JP. Human immunoglobulin constant heavy G chain (IGHG) (Fcgamma) (GM) genes, defining innate variants of IgG molecules and B cells, have impact on disease and therapy. Clin Immunol 2013; 149:475–486. [DOI] [PubMed] [Google Scholar]
- 59.Kratochvil S, McKay PF, Chung AW, et al. Immunoglobulin G1 allotype influences antibody subclass distribution in response to HIV gp140 vaccination. Front Immunol 2017; 8:1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson SI, Chung AW, Natarajan H, et al. HIV-specific Fc effector function early in infection predicts the development of broadly neutralizing antibodies. PLoS Pathog 2018; 14:e1006987.■ First study showing an association between Fc effector function and the development of broadly neutralizing antibodies during infection.
- 61.Lofano G, Gorman MJ, Yousif AS, et al. Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci Immunol 2018; 3:; pii: eaat7796.■■ A mechanistic study relevant to vaccine design that provides insight into how Fc effect or function may drive and support broadly neutralizing responses in vivo.