Abstract
Purpose: Lymphocytes are central players in systemic anti-tumor immune responses. In this study, we aimed to identify the relationship between absolute lymphocyte count (ALC) nadir during definitive radiotherapy (RT) and survival outcomes in patients with esophageal squamous cell carcinoma (ESCC), as well as evaluate the effect of RT parameters on ALC during RT.
Materials and methods: We retrospectively reviewed 189 patients with stage I-IVA ESCC, who were treated with definitive RT at a single institution between 2012 and 2015. ALC values were assessed before, weekly during RT, and 1 month after the end of RT. Kaplan–Meier and Cox regression analyses were used to evaluate the relationship between ALC nadir during RT and patient outcomes. Predictors of low ALC nadir were assessed using univariate and multivariate logistic regression analyses.
Results: The median ALC before treatment was 1.73 × 103 cells/μL. Fifty-eight (58.2) percent of the patients exhibited low ALC nadir (≤ 0.38 × 103 cells/μL) during RT. A low ALC nadir during RT was significantly associated with poor OS, PFS, and LRFS. The planning target volume (PTV) was larger in patients with low ALC nadir compared with patients with high ALC nadir (418.5 vs. 347.7 cm3, P = 0.023). Multivariate logistic regression analysis revealed that tumor stage III-IVA (P = 0.002), low ALC before treatment (P = 0.028), large Log10(PTV) (P = 0.01), high heart V10 (P = 0.003), and high heart V20 (P = 0.028) were associated with low ALC nadir during RT.
Conclusions: In ESCC patients who received definitive RT, a low ALC nadir during RT was associated with large PTVs, and it was an independent prognostic factor of outcomes.
Keywords: radiotherapy, esophageal squamous cell carcinoma, radiation-induced lymphopenia, prognosis, immunosuppression
Introduction
Esophageal cancer (EC) is one of the most aggressive malignancies (1). Squamous cell carcinoma is the predominant histological type of EC in China, accounting for more than 90% of the total number of EC cases (2). Definitive chemoradiotherapy (dCRT) is the treatment of choice for unresectable or inoperable EC (3, 4). However, radiotherapy (RT) has various effects on the immune system of patients. Importantly, lymphocytes are the most radiosensitive cells of the hematopoietic system and are frequently depleted after RT, even at low radiation doses; hence, radiation-induced lymphopenia (RIL) is a common adverse effect of RT (5, 6).
Mounting evidence indicates that the immune system has multiple mechanisms for the identification and elimination of tumor cells, and lymphocytes are key players in these processes (7, 8). Several studies have demonstrated that RIL is associated with worse outcomes in a wide variety of malignancies, including EC (9–14). Moreover, it has been shown that in patients with stage I-III EC receiving chemoradiotherapy (CRT) with or without surgery, RIL is a significant prognostic factor associated with inferior survival (13, 14). Nevertheless, in the majority of previous studies, the most common pathological type (more than 80% of the cases) was esophageal adenocarcinoma (EAC). Moreover, the prognostic value of absolute lymphocyte count (ALC) in patients with esophageal squamous cell carcinoma (ESCC) treated with definitive RT remains elusive. In this study, we sought to identify the relationship between ALC during definitive RT and survival outcomes in ESCC patients, as well as to evaluate the effect of RT parameters on ALC.
Materials and Methods
Patient Selection
We retrospectively reviewed the records of ESCC patients who underwent definitive RT at a single institution from 2012 to 2015. Clinical TNM staging in all patients was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) guidelines. Study inclusion criteria were as follows: (1) Histologically or cytologically confirmed, treatment-naive esophageal squamous cell carcinoma; (2) stage I–IVA disease as determined by either contrast-enhanced computed tomography (CT), endoscopic ultrasonography (EUS) or positron emission tomography (PET)-CT; (3) Eastern Cooperative Oncology Group (ECOG) performance status 0–2; (4) patients with complete hematological data; (5) patients that completed the definitive RT plan. Patients with prior RT, second primary tumors or comorbidities that might have affected the lymphocyte count (e.g., autoimmune or inflammatory disorders) were excluded from the study.
Treatment Protocols
All the patients of this study received definitive RT alone or in combination with chemotherapy. All radiation treatments were delivered either as three-dimensional conformal radiotherapy (3D-CRT) or intensity modulated radiotherapy (IMRT) with a total dose of 50–68 Gy. Radiation was delivered by high-energy (6 or 15 MV) linear accelerators. According to the chemotherapy combination regimens, patients were divided into the following subgroups: RT alone, induced chemotherapy, concurrent CRT, and induced chemotherapy + concurrent CRT. In patients that underwent concurrent CRT, chemotherapy began on day 1 concurrent with the initial radiation treatments session; the concurrent CRT regimen was platinum and 5-fluorouracil (5-FU) doublet chemotherapy, a combination of platinum and taxane, or other commonly used chemotherapeutic agents.
Data Collection
ALC values were obtained within 1 week prior to treatment initiation, weekly during RT, and 1 month after RT. For patients with missing ALC data at a time point of interest, the closest value to the desired date was used. ALC nadir was defined as the minimum ALC value measured during RT. Lung dose-volume histogram (DVH) parameters, heart DVH parameters, and planning target volume (PTV) during RT were collected. Evaluation of the response of the primary tumor to treatment was performed using CT and esophagography 4–6 weeks after the completion of the treatment; response was defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.1 (15).
Statistical Analysis
All statistical analyses were performed using SPSS version 22.0 (SPSS, Chicago, IL, USA) software package. Descriptive statistics were used to summarize patient characteristics at baseline, while lymphocyte nadir values over time were plotted to visualize the cell counts trends during treatment. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off value, with ALC nadir during RT and complete response (CR) rate as test and state variables, respectively. The relationship between ALC nadir (high vs. low) and the CR rate was assessed using Pearson's chi-squared test. The primary endpoints of the study were overall survival (OS), progression-free survival (PFS), and local recurrence-free survival (LRFS), all of which were determined from the day of therapy initiation until an event or censor. Survival curves were generated using the Kaplan-Meier method, and survival outcomes according to ALC nadir during RT were compared using the log-rank test. Univariate and multivariate analyses were conducted using the Cox proportional hazards model to determine risk factors for survival. Covariates identified by univariate analysis (UVA) with P-value < 0.10 were incorporated in the multivariate model, which was constructed with the forward stepwise method. Patient and treatment characteristics in each group (high ALC nadir vs. low ALC nadir) were tested for associations using Pearson's chi-squared test. Logistic regression analysis was used to identify RT-related factors that are associated with low ALC nadir during RT. Stepwise multivariate logistic regression analyses were used to assess the variables with P-value < 0.10 in the UVA. Pearson correlation coefficients (r) were used to determine the association between ALC nadirs and log10 (PTV). All statistical tests were two-sided, and P-value < 0.05 was used to indicate statistical significance.
Results
Patient Characteristics
A total of 189 ESCC patients were included in this study. The median age at diagnosis was 67 years (range, 44–92 years), and male patients accounted for 74.6% of the study population. The majority of patients (72%) had stage III disease. The median primary tumor length was 4.4 cm (range, 0.9–17 cm). Most patients (52.4%) received RT combined with chemotherapy, 39.2% of which underwent concurrent CRT, and 24.3% underwent induced chemotherapy. The majority of patients that underwent concurrent chemotherapy received platinum and 5-FU doublet chemotherapy (16.9%), followed by the combination of platinum and taxane (12.7%). A higher portion of patients underwent IMRT (65.1%) than 3D-CRT (34.9%). The median follow-up time was 46 months. Additional information about patient demographics, tumor characteristics, and treatment regimens are listed in Table 1.
Table 1.
Baseline patient, tumor, and treatment characteristics.
| Characteristic | All patients N = 189 | High ALC nadir N = 79 | Low ALC nadir N = 110 | p-value |
|---|---|---|---|---|
| Age, n (%) | 0.797 | |||
| ≤67 years | 96 (50.8) | 41 (42.7) | 55 (57.3) | |
| >67 years | 93 (49.2) | 38 (40.9) | 55 (59.1) | |
| Sex, n (%) | 0.044 | |||
| Male | 141 (74.6) | 53 (37.6) | 88 (62.4) | |
| Female | 48 (25.4) | 26 (54.2) | 22 (45.8) | |
| Smoking, n (%) | 0.206 | |||
| Ever | 94 (49.7) | 35 (37.2) | 59 (62.8) | |
| Never | 95(50.3) | 44 (46.3) | 51 (53.7) | |
| Drinking, n (%) | 0.339 | |||
| Ever | 77 (40.7) | 29 (37.7) | 48 (62.3) | |
| Never | 112 (59.3) | 50 (44.6) | 62 (55.4) | |
| Tumor location, n (%) | 0.147 | |||
| Cervical | 17 (9.0) | 4 (23.5) | 13 (76.5) | |
| Upper | 76 (40.2) | 37 (48.7) | 39 (51.3) | |
| Middle | 51 (27.0) | 23 (45.1) | 28 (54.9) | |
| Lower | 45 (23.8) | 15 (33.3) | 30 (66.7) | |
| Tumor length (cm) | 0.006 | |||
| ≤4.4 cm | 95 (50.3) | 49 (51.6) | 46 (48.4) | |
| >4.4 cm | 94 (49.7) | 30 (31.9) | 64 (68.1) | |
| Clinical stage, n (%) | 0.002 | |||
| I | 21 (11.1) | 13 (61.9) | 8 (38.1) | |
| II | 20 (10.6) | 14 (70.0) | 6 (30.0) | |
| III | 136 (72.0) | 50 (36.8) | 86 (63.2) | |
| IVA | 12 (6.3) | 2 (16.7) | 10 (83.3) | |
| Treatment regimen, n (%) | 0.910 | |||
| RT combined with chemo | 99 (52.4) | 41 (41.4) | 58 (58.6) | |
| RT alone | 90 (47.6) | 38 (42.2) | 52 (57.8) | |
| Concurrent chemo regimen, n (%) | 0.158 | |||
| 1. Platinum/taxane | 24 (12.7) | 10 (43.5) | 13 (56.5) | |
| 2. Platinum/5-FU | 32 (16.9) | 8 (25.0) | 24 (75.0) | |
| 3. Other | 18 (9.5) | 9 (50.0) | 9 (50.0) | |
| RT technology, n (%) | 0.172 | |||
| 3D-CRT | 66 (34.9) | 32 (48.5) | 34 (51.5) | |
| IMR | 123 (65.1) | 47 (38.2) | 76 (61.8) | |
| RT dose, n (%) | 0.476 | |||
| ≤60 Gy | 146 (77.2) | 59 (40.4) | 87 (59.6) | |
| >60 Gy | 43 (22.8) | 20 (46.5) | 23 (53.5) | |
| Pre-treatment ALC, n (%) | 0.180 | |||
| ≤1.73 × 103 cells/μL | 97 (51.3) | 36 (37.1) | 61 (68.9) | |
| >1.73 × 103 cells/μL | 92 (48.6) | 43 (46.7) | 49 (53.3) |
ALC, absolute lymphocyte count; RT, radiotherapy; chemo, chemotherapy; 5-FU, 5-fluorouracil; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy. Bold values indicate a statistically difference in statistical analysis (P < 0.05).
Changes in Lymphocyte Count Over Time
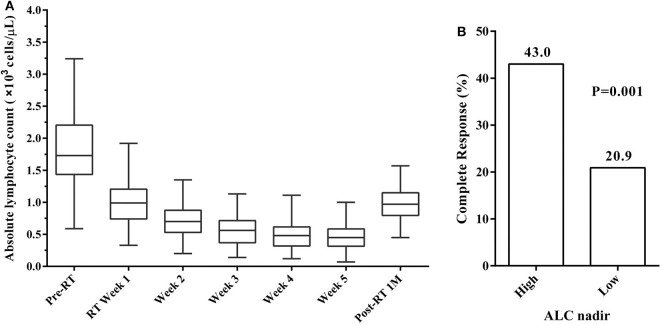
To get more insight into the changes in ALC during RT, ALC values were assessed over time (Figure 1A). The median ALC before treatment was 1.73 × 103 cells/μL. As expected, ALC decreased every week during RT, reaching a plateau by the end of RT in most patients. The ALC values during RT decreased to 0.99, 0.70, 0.56, 0.48, and 0.45 × 103 cells/μL from weeks 1 to 5, respectively. After the end of RT, ALC began to enter a slow recovery stage and returned to 0.97 × 103 cells/μL by 1 month post-RT.
Figure 1.
(A) Absolute lymphocyte count (ALC) trend from before radiotherapy (Pre-RT) through to 1 month after radiotherapy (Post-RT 1M). (B) Complete response (CR) rates by ALC nadir. CR rates was significantly higher in patients with high ALC nadir compared to those with low ALC nadir.
ROC curve analysis was performed to investigate the predictive value of ALC nadir during RT in CR rates. The optimal cut-off value of ALC nadir was determined as 0.38 × 103 cells/μL, and the sensitivity and specificity were 63.2 and 63.6%, respectively. We, therefore, defined ALC nadir >0.38 × 103 cells/μL as “high” and ≤0.38 × 103 cells/μL as “low” ALC nadir. High ALC nadir was significantly associated with higher CR rates (Pearson's chi-square test; P = 0.001; Figure 1B). In the 189 patients, the median ALC nadir during RT was 0.34 × 103 cells/μL (range, 0.07–1 × 103 cells/μL). 79 (41.8%) patients exhibited high ALC nadir levels, while the remaining 110 (58.2%) exhibited low ALC nadir levels.
ALC Nadir During RT Is Associated With Survival Outcomes
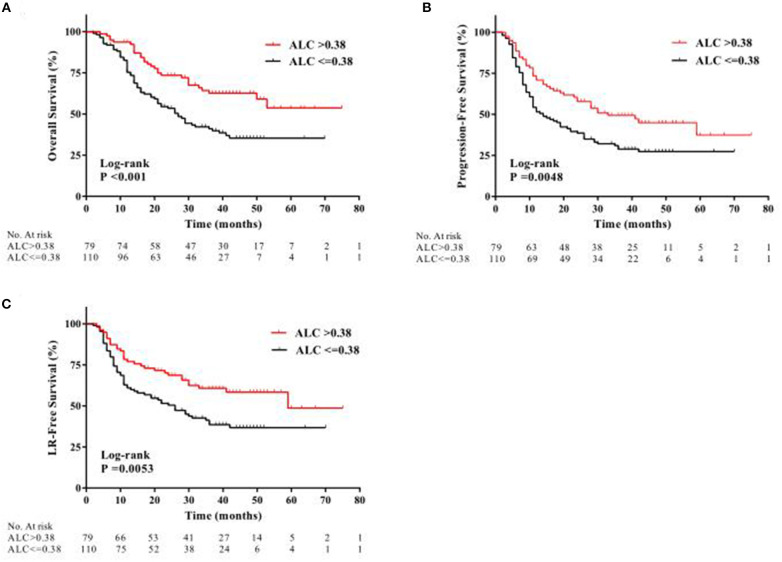
At the median follow-up time of 46 months, 95 patients (50.3%) had died. Compared with patients with high ALC nadir, patients with low ALC nadir had a significantly worse OS [hazard ratio [HR], 2.08; 95% confidence interval [CI], 1.37–3.05; P < 0.001; Figure 2A]. After adjusting for risk factors, multivariate analysis (MVA) revealed that tumor localization in the middle or lower third of the esophagus (HR, 2.94; 95% CI, 1.88–4.60; P < 0.001), stage III or IV tumor (HR, 2.34; 95% CI, 1.29–4.22; P = 0.005), treatment with RT alone (HR, 1.58; 95% CI, 1.03–2.43; P = 0.037), and low ALC nadir during RT (HR, 1.87; 95% CI, 1.20–2.94; P = 0.006) were independent factor predicting unfavorable OS (Table 2).
Figure 2.
Kaplan-Meier curves showing patient clinical outcomes: (A) overall survival, (B) progression-free survival, and (C) local recurrence-free (LR) survival between patients with high ALC nadir (red line) and with low ALC nadir (black line) during radiotherapy.
Table 2.
Univariate and multivariate Cox regression analysis of factors associated with clinical outcomes.
| Characteristics | OS | PFS | LRFS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age | |||||||||||||||
| ≤67 | 1 | 1 | 1 | ||||||||||||
| >67 | 1.62(1.08–2.44) | 0.020 | NS | 1.13 (0.79–1.62) | 0.493 | 1.33 (0.89–1.99) | 0.169 | ||||||||
| Gender | |||||||||||||||
| Male | 1 | 1 | |||||||||||||
| Female | 0.77 (0.47–1.25) | 0.292 | 0.81 (0.53–1.23) | 0.321 | 0.75 (0.46–1.22) | 0.250 | |||||||||
| Smoking | |||||||||||||||
| Ever | 1 | 1 | 1 | 1 | |||||||||||
| Never | 0.75 (0.50–1.13) | 0.164 | 0.78 (0.55–1.12) | 0.184 | 1.77 (1.17–2.67) | 0.007 | 1.66 (1.09–2.50) | 0.017 | |||||||
| Drinking | |||||||||||||||
| Ever | 1 | 1 | 1 | ||||||||||||
| Never | 0.63 (0.42–0.95) | 0.027 | NS | 0.64 (0.45–0.92) | 0.015 | NS | 1.73 (1.16–2.60) | 0.008 | NS | ||||||
| Tumor location | |||||||||||||||
| Cervical or Upper | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||
| Mid or Lower | 3.02 (1.96–4.64) | <0.001 | 2.94 (1.88–4.60) | <0.001 | 2.33 (1.61–3.37) | <0.001 | 2.59 (1.78–3.76) | <0.001 | 2.61 (1.70–3.99) | <0.001 | 2.64 (1.72–4.04) | <0.001 | |||
| Tumor length | |||||||||||||||
| ≤4.4 cm | 1 | 1 | 1 | ||||||||||||
| >4.4 cm | 1.86 (1.23–2.80) | 0.003 | NS | 1.70 (1.18–2.45) | 0.004 | NS | 1.51 (1.01–2.28) | 0.047 | NS | ||||||
| Clinical stage | |||||||||||||||
| I–II | 1 | 1 | 1 | 1 | 1 | ||||||||||
| III–IVA | 2.07 (1.17–3.66) | 0.012 | 2.34 (1.29–4.22) | 0.005 | 1.76 (1.09–2.85) | 0.021 | 1.84 (1.11–3.04) | 0.018 | 1.45 (0.87–2.43) | 0.156 | |||||
| Treatment regimen | |||||||||||||||
| RT combined with chemo | 1 | 1 | 1 | 1 | |||||||||||
| RT alone | 1.73 (1.15–2.59) | 0.009 | 1.58 (1.03–2.43) | 0.037 | 1.24 (0.87–1.78) | 0.233 | 1.55 (1.03–2.32) | 0.035 | NS | ||||||
| Chemo regimen | |||||||||||||||
| Platinum/taxane | 1 | 1 | 1 | ||||||||||||
| Platinum/5-FU | 1.36 (0.52–3.56) | 0.531 | 1.13 (0.57–2.26) | 0.723 | 1.93 (0.80–4.65) | 0.145 | |||||||||
| Other | 1.58 (0.98–2.54) | 0.058 | 1.16 (0.79–1.71) | 0.441 | 1.33 (0.80–2.21) | 0.276 | |||||||||
| RT technology | |||||||||||||||
| 3D-CRT | 1 | 1 | 1 | ||||||||||||
| IMRT | 0.76 (0.50–1.14) | 0.184 | 0.67 (0.46–0.96) | 0.030 | NS | 0.71 (0.47–1.08) | 0.106 | ||||||||
| RT dose | |||||||||||||||
| ≤60 Gy | 1 | 1 | 1 | ||||||||||||
| >60 Gy | 0.73 (0.44–1.22) | 0.233 | 0.77 (0.49–1.21) | 0.257 | 0.73 (0.44–1.22) | 0.231 | |||||||||
| Pre-treatment ALC | |||||||||||||||
| ≤1.73 × 103 cells/μL | 1 | 1 | 1 | ||||||||||||
| >1.73 × 103 cells/μL | 0.87 (0.58–1.31) | 0.507 | 0.78 (0.55–1.12) | 0.180 | 0.66 (0.44–1.00) | 0.050 | |||||||||
| ALC nadir during RT | |||||||||||||||
| >0.38 × 103 cells/μL | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||
| ≤0.38 × 103 cells/μL | 2.10 (1.35–3.26) | 0.001 | 1.87 (1.20–2.94) | 0.006 | 1.69 (1.16–2.47) | 0.006 | 1.55 (1.05–2.29) | 0.028 | 1.82 (1.18–2.80) | 0.007 | 1.91 (1.23–2.94) | 0.004 | |||
OS, overall survival; PFS, progression-free survival; LRFS, local recurrence-free survival;HR, hazard ratio; CI, confidence interval; RT, radiotherapy; chemo, chemotherapy; 5-FU, 5-fluorouracil; IMRT, intensity modulated radiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; ALC, absolute lymphocyte count; NS, non-significant. Bold values indicate a statistically difference in statistical analysis (P < 0.05).
During follow-up, a total of 120 patients (63.5%) had locoregional or distant progression, with a median PFS of 19 months. Kaplan-Meier analysis indicated a significantly worse PFS in patients with low ALC nadir compared with patients with high ALC nadir (HR, 1.69; 95% CI, 1.18–2.43; P = 0.0048; Figure 2B). According to UVA, drinking history, tumor localization, tumor size, clinical stage, RT method, and ALC nadir, were all significantly associated with PFS (all P < 0.05; Table 2). MVA revealed that tumor localization in the middle or lower third of the esophagus (HR, 2.58; 95% CI, 1.78–3.76; P < 0.001), stage III or IV tumor (HR, 1.84; 95% CI, 1.11–3.04; P = 0.018), and low ALC nadir (HR, 1.55; 95% CI, 1.05–2.29; P = 0.028) during RT were significantly associated with inferior PFS (Table 2).
Locoregional progression was observed in 94 patients (49.7%) during follow-up. Similarly, low ALC nadir during RT predicted for worse LRFS (HR, 1.81; 95% CI, 1.20–2.70; P = 0.0053; Figure 2C). Furthermore, MVA analysis showed that smoking history (HR, 1.66; 95% CI, 1.09–2.50; P = 0.017), tumor localization in the middle or lower third of the esophagus (HR, 2.64; 95% CI, 1.72–4.04; P < 0.001), and low ALC nadir during RT (HR, 1.91; 95% CI, 1.23–2.94; P = 0.004) were significantly associated with worse LRFS (Table 2).
Predictors of Low ALC Nadir During RT
One hundred ten patients (58.2%) exhibited low ALC nadir during RT. We found no significant differences in age, smoking history, drinking history, tumor localization, treatment regimen, RT method, RT dose, and pre-treatment ALC values between patients with low and high ALC nadir. Pearson's chi-squared tests indicated that male patients (P = 0.044), patients with larger tumors (>4.4 cm; P = 0.006), and advanced clinical stage patients (P < 0.001) were more likely to have a low ALC nadir during RT (Table 1).
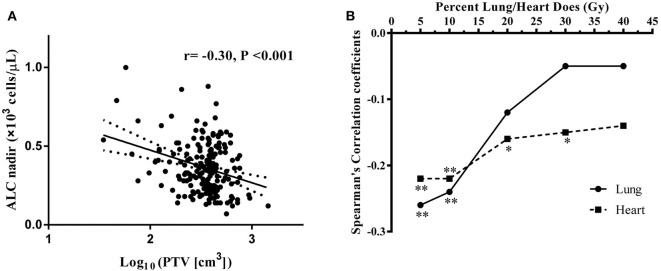
The median PTV was significantly higher in patients with low ALC nadir (418.5 ± 204.7 cm3) compared with patients with high ALC nadir (347.7 ± 157.4 cm3; P = 0.023). Consistently, a significant negative correlation was observed between ALC nadir during RT and log10(PTV) (r = −0.30; P < 0.001; Figure 3A). To examine this further, we evaluated the correlation of low ALC nadir with lung or heart dosimetry parameters. To this end, we assessed the Spearman correlation between low ALC nadir and the percentage of lung and heart receiving 5 to 40 Gy (lung V5–V40, heart V5–V40; Figure 3B). We found that high lung V5 (r = −0.26; P < 0.001), lung V10 (r = −0.24; P = 0.001), heart V5 (r = −0.22; P = 0.002), and heart V10 (r = −0.22; P = 0.002) strongly correlated with lower ALC nadir (P < 0.01). This correlation subsequently lessened at heart V20 (r = −0.16; P = 0.029) and heart V30 (r = −0.15; P = 0.037). Lung V20–V40, as well as heart V40, showed no significant correlation with low ALC nadir (P ≥ 0.05). Stepwise multivariate logistic regression demonstrated that patients with tumor stage III-IVA [odds ratio [OR], 3.46; 95% CI, 1.56–7.68; P = 0.002], lower ALC value before treatment (OR, 0.50; 95% CI, 0.27–0.93; P = 0.028), larger Log10(PTV) (OR, 8.31; 95% CI, 1.65–41.78; P = 0.01), higher heart V10 (OR, 1.05; 95% CI, 1.02–1.09; P = 0.003), and higher heart V20 (OR, 0.95; 95% CI, 0.91–1.00; P = 0.028) had a lower ALC nadir (Table 3). Of note, although patients were treated with different therapeutic schedules, there were no significant effects of therapeutic scheduling on the ALC nadir level.
Figure 3.
(A) Correlation between log10(PTV) and absolute lymphocyte count (ALC) nadir during RT. (B) Spearman correlation coefficients between the percentages of lung/heart dose (Gy) and lymphocyte nadirs at varying percentages of lung (solid line)/heart doses (dashed line) for patient. Significance indicated at **P < 0.01, and *P < 0.05.
Table 3.
Univariate and multivariate logistic regression of factors associated with low ALC nadir.
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (years)* | 0.99 (0.96–1.02) | 0.586 | ||
| Tumor location | ||||
| Cervical or Upper | 1 | |||
| Mid or Lower | 1.20 (0.68–2.15) | 0.531 | ||
| Tumor length (cm)* | 1.28 (1.09–1.49) | 0.003 | NS | |
| Clinical stage | ||||
| I–II | 1 | 1 | ||
| III–IVA | 3.56 (1.72–7.38) | 0.001 | 3.46 (1.56–7.68) | 0.002 |
| Treatment regimen | ||||
| RT combined with chemo | 1 | |||
| RT alone | 0.97 (0.54–1.73) | 0.910 | ||
| Chemo regimen | ||||
| Platinum/taxane | 1 | |||
| Platinum/5-FU | 2.31 (0.73–7.28) | 0.154 | ||
| Other | 0.88 (0.47–1.63) | 0.678 | ||
| RT technology | ||||
| 3D-CRT | 1 | |||
| IMRT | 1.52 (0.83–2.79) | 0.173 | ||
| RT dose (Gy)* | 1.00 (0.93–1.09) | 0.844 | ||
| Pre-treatment ALC (×103 cells/μL)* | 0.56 (0.32–0.97) | 0.039 | 0.50 (0.27–0.93) | 0.028 |
| Log10 [PTV [cm3]]* | 5.34 (1.41–20.16) | 0.013 | 8.31 (1.65–41.78) | 0.010 |
| Lung DVH* | ||||
| V5 (%) | 1.03 (1.01–1.06) | 0.001 | NS | |
| V10 (%) | 1.04 (1.01–1.07) | 0.004 | NS | |
| V20 (%) | 1.05 (1.00–1.10) | 0.034 | NS | |
| V30 (%) | 1.06 (0.98–1.14) | 0.186 | NS | |
| V40 (%) | 1.08 (0.96–1.23) | 0.213 | NS | |
| Mean does (Gy) | 1.17 (1.05–1.30) | 0.004 | NS | |
| Heart DVH* | ||||
| V5 (%) | 1.01 (1.00–1.02) | 0.006 | NS | |
| V10 (%) | 1.01 (1.00–1.02) | 0.005 | 1.05 (1.01–1.09) | 0.003 |
| V20 (%) | 1.01 (1.00–1.03) | 0.073 | 0.95 (0.91–1.00) | 0.028 |
| V30 (%) | 1.01 (0.99–1.03) | 0.251 | NS | |
| V40 (%) | 1.01 (0.98–1.05) | 0.461 | NS | |
| Mean does (Gy) | 1.05 (1.01–1.09) | 0.007 | NS | |
ALC, absolute lymphocyte count; OR, odds ratio; CI, confidence interval; RT, radiotherapy; chemo, chemotherapy; 5-FU, 5-fluorouracil; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity modulated radiotherapy; PTV, planning target volume; DVH, dose-volume histogram; Lung V5/10/20/30/40, the percentage of lung receiving 5/10/20/30/40 Gy; Heart V5/10/20/30/40, the percentage of heart receiving 5/10/20/30/40 Gy; NS, non-significant.
Indicates a continuous variable with units indicated in parenthesis. Bold values indicate a statistically difference in statistical analysis (P < 0.05).
Discussion
In this study, we observed a significant lymphocyte depletion following RT in ESCC patients, followed by a gradual recovery in ALC after the end of RT. We also found that a lower ALC nadir during RT was significantly associated with poor OS, PFS, and LRFS. The prognostic value of ALC nadir during RT remained significant after adjustment for confounding risk factors. Finally, we found that low ALC nadir was associated with the clinical stage, ALC before treatment, PTV, heart V10, and heart V20. These results are very clinically relevant, because with the development of modern RT techniques, RIL has emerged as a strong and potentially modifiable risk factor. Adjusting RT parameters to minimize immunosuppression caused by RIL may help optimize the therapeutic effect of RT in ESCC and other malignancies.
Our study indicated that ALC value was a prognostic factor in patients with ESCC who received definitive RT. Lymphocytes play critical roles in promoting systemic anti-tumor immune responses. Lymphopenia is a main manifestation of immunosuppression, and several retrospective studies have demonstrated a strong link between treatment-induced lymphopenia and inferior outcomes in a wide variety of cancers, including glioblastoma, lung cancer, pancreatic cancer, cervical cancer, and EC (9–14). Furthermore, a prospective study has also confirmed the correlation between lymphocyte count and outcomes in patients with high-grade glioma (16). However, the definition of treatment-related lymphopenia varied among these studies. Previous studies have also reported that maintaining a high ALC during treatment was associated with higher pathologic complete response (pCR) rates in patients with rectal cancer, gastric cancer, and EC (17–19). In our study, we used clinical CR rates as state variables to determine the optimal cut-off value of ALC nadir during RT. Consistent with previously reported findings, our data indicated that a high ALC nadir during RT was associated with higher CR rates and improved survival outcomes. Interestingly, some retrospective studies have reported that low ALC values before treatment are associated with poor outcomes in cancer patients (20, 21). Our results showed that low ALC nadir during RT was associated with low ALC value before treatment. Given the exquisite radiosensitivity of lymphocytes, a change in lymphocyte count may also be a prognostic factor worthy of attention.
By MVA, we identified several radiation treatment parameters that could significantly predict low ALC nadir during RT. Lymphocytes are the most radiosensitive hematopoietic cells and are frequently depleted by RT using a 50% lethal dose of 1 to 2 Gy (5, 6). Nevertheless, the mechanisms responsible for RIL remain unclear. Lymphoid organ radiation, including bone marrow, thymus, spleen, and lymph nodes, could likely contribute to RIL (22–24). Radiation can directly damage hematopoietic stem cells in bone marrow or mature lymphocytes in lymphoid tissues, as well as stimulate the secretion of immunosuppressive cytokines, promoting immunosuppression (25). Additionally, as EC develops in close proximity to the heart and lungs, lymphocytes receive a significant dose of radiation through the large blood vessels that are in the radiation field (14). Tang et al. (12) analyzed the relationship between DVH parameters and lymphocyte nadir in 771 non-small cell lung cancer (NSCLC) patients and observed that lymphocyte nadir was associated with gross tumor volume (GTV). Rudra et al. (26) assessed the effects of different RT volumes on lymphopenia risk in glioblastoma patients and found that the reduction in the irradiated brain volume might mitigate treatment-induced lymphopenia. Furthermore, the results of RTOG 0617 showed that higher cardiac doses and larger PTV were significant predictors of poor OS (27). Due to the esophagus's anatomical position near the heart, lungs, and several large blood vessels, we can reasonably extrapolate that these effects would be enhanced in EC treated with RT. Our study supported that PTV, heart V10, and heart V20 are significant prognostic factors of low ALC nadir. Our findings also suggested that larger volumes of radiation result in more severe damage in lymphocytes. Analysis of DVH parameters showed that lung V5–V10 and heart V5–V10 had the highest correlation coefficients, suggesting that RIL occurs primarily as a result of frequent low-dose radiation damage in circulating lymphocytes. In addition, we found that the administration of chemotherapy, in combination with RT, had no significant effect on lymphocytes.
In addition to immunosuppressive effects, immunostimulating roles have also been described for RT. RT exerts strong anti-tumor effects by increasing the immunogenicity of cancer cells (28). Furthermore, the RT-induced modulation of the tumor microenvironment can promote the recruitment of immune cells in the tumor, as well as enhance the tumor cell recognition and elimination by immune cells (29). Preclinical studies demonstrated that PD-L1 expression was upregulated in the tumor microenvironment after RT (30, 31). Since the approval of anti-CTLA4 therapy (ipilimumab) for unresectable or metastatic melanoma in 2011 (32), the development of anti-cancer immunotherapy agents has progressed rapidly. As the number of preclinical and clinical studies assessing the anti-tumor effects of RT combined with immunotherapy, it is vital to eliminate the immunosuppressive effects of RT to achieve optimal anti-cancer effects (33). In clinical practice, however, we have traditionally ignored the effect of radiation parameters on lymphocytes in the development of RT plans. Our findings suggested that reducing radiation field size could minimize the risk of RIL during RT. The radiation target volume of EC has remained a topic of persistent controversy among radiation oncologists. One of the most controversial points is whether to opt for elective nodal irradiation (ENI) or involved-field irradiation (IFI). ENI involves delivering RT to the primary tumor, as well as the irradiation of clinically uninvolved regional lymph nodes at risk of micrometastases of the treated disease (34). However, previous studies confirmed that ENI did not change failure patterns and survival outcomes of local advanced stage EC after definitive chemoradiotheraty (35–38). Compared with ENI, IFI has a smaller radiation target volume. Combined with the results of our study, IFI may be a better choice for reducing RIL. Further research is required to test this hypothesis. Additionally, the ability of proton beam therapy (PBT) to conform high radiation dose to the tumor volume while reducing the unintentional radiation dose to adjacent healthy tissues has the potential to decrease RIL (39, 40). RT could quite possibly be an immunologic adjuvant if the RT plan is right.
Our study has several limitations. Importantly, our study is a single institution retrospective study; hence, there was a significant risk of selection bias and information bias. Although in our institution, we perform routine blood tests before RT and weekly during RT, there is considerable variation in this procedure. For patients with missing blood data at a time point of interest, the closest value to the desired date was used. Moreover, there were several potential confounding variables in our study, such as auxiliary medications or infections that might have affected the lymphocyte count. Although all the patients of our study received definitive RT, there might have been variation in the therapeutic scheduling due to the wide time span of treatment. Finally, even though using ROC curve analysis, we identified the cut-off ALC nadir value that most accurately predicts response, the optimal threshold needs to be validated in larger cohorts from multi-institutional studies.
Despite these limitations, our study suggests a strong link between RIL and prognosis in patients with ESCC. It also identifies several parameters that could modulate the lymphocyte count during RT, providing further insight into the mechanisms behind RIL. Additionally, our findings further demonstrate the importance of maintaining an intact immune system during anti-tumor therapy. Adjusting potential risk factors of RIL to enhance host immunity may help optimize the therapeutic benefit of RT in patients with ESCC and other malignancies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
ML and XW designed the study. XW drafted the manuscript. ZZ and PW participated in data collection. XG and LZ coordinated, edited, and finalized the drafting of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would such as to express their great thanks to the Natural Science Foundation of China.
Glossary
Abbreviations
- ALC
absolute lymphocyte count
- AJCC
American Joint Committee on Cancer
- CI
confidence interval
- CR
complete response
- CRT
chemoradiation therapy
- CT
computed tomography
- dCRT
definitive chemoradiotherapy
- DVH
dose-volume histogram
- EAC
esophageal adenocarcinoma
- ECOG
Eastern Cooperative Oncology Group
- ESCC
esophageal squamous cell carcinoma
- ENI
elective nodal irradiation
- EUS
endoscopic ultrasonography
- GTV
gross tumor volume
- HR
hazard ratio
- IFI
involved-field irradiation
- IMRT
intensity modulated radiotherapy
- LRFS
local recurrence-free survival
- MVA
multivariate analysis
- NSCLC
non-small cell lung cancer
- OARs
organs-at-risk
- OR
odds ratio
- OS
overall survival
- PBT
proton beam therapy
- pCR
pathologic complete response
- PET
positron emission tomography
- PFS
progression-free survival
- PTV
Planning target volume
- RECIST
Response Evaluation Criteria in Solid Tumors
- RIL
radiation-induced lymphopenia
- ROC
receiver operating characteristic
- RT
radiotherapy
- RTOG
Radiation Therapy Oncology Group
- UVA
univariate analysis
- 3D-CRT
three-dimensional conformal radiation therapy
- 5-FU
5-fluorouracil.
Footnotes
Funding. This study was funded by the Natural Science Foundation of China (NSFC 81672995).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. (2018) 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. (2017) 14:33–41. 10.20892/j.issn.2095-3941.2016.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group. JAMA. (1999) 281:1623–7. 10.1001/jama.281.17.1623 [DOI] [PubMed] [Google Scholar]
- 4.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. (2002) 20:1167–74. 10.1200/JCO.2002.20.5.1167 [DOI] [PubMed] [Google Scholar]
- 5.Trowell OA. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. (1952) 64:687–704. 10.1002/path.1700640403 [DOI] [PubMed] [Google Scholar]
- 6.Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res. (1990) 123:224–7. 10.2307/3577549 [DOI] [PubMed] [Google Scholar]
- 7.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. (1998) 188:2357–68. 10.1084/jem.188.12.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi H, Yuan J, Keshavarz-Fathi M, Murphy JF, Rezaei N. Immunotherapy of cancers comes of age. Exp Rev Clin Immunol. (2017) 13:1001–15. 10.1080/1744666X.2017.1366315 [DOI] [PubMed] [Google Scholar]
- 9.Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Canc Netw. (2015) 13:1225–31. 10.6004/jnccn.2015.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol hematol. (2018) 123:42–51. 10.1016/j.critrevonc.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 11.Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. (2015) 38:259–65. 10.1097/COC.0b013e3182940ff9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. (2014) 89:1084–91. 10.1016/j.ijrobp.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 13.Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. (2017) 99:128–35. 10.1016/j.ijrobp.2017.05.037 [DOI] [PubMed] [Google Scholar]
- 14.Deng W, Xu C, Liu A, van Rossum PSN, Deng W, Liao Z, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. (2019) 133:9–15. 10.1016/j.radonc.2018.12.002 [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 16.Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. (2011) 17:5473–80. 10.1158/1078-0432.CCR-11-0774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heo J, Chun M, Noh OK, Oh YT, Suh KW, Park JE, et al. Sustaining blood lymphocyte count during preoperative chemoradiotherapy as a predictive marker for pathologic complete response in locally advanced rectal cancer. Cancer Res Treat. (2016) 48:232–9. 10.4143/crt.2014.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang D, Yang Y, Zhang S, Su Z, Peng T, Wang X, et al. Regulatory T-cell density and cytotoxic T lymphocyte density are associated with complete response to neoadjuvant paclitaxel and carboplatin chemoradiotherapy in gastric cancer. Exp Ther Med. (2018) 16:3813–20. 10.3892/etm.2018.6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang P, Jiang W, Davuluri R, Xu C, Krishnan S, Mohan R, et al. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol. (2018) 128:584–90. 10.1016/j.radonc.2018.02.025 [DOI] [PubMed] [Google Scholar]
- 20.Mohsen A, Taalab M, Abousamra N, Mabed M. Prognostic significance of absolute lymphocyte count, absolute monocyte count, and absolute lymphocyte count to absolute monocyte count ratio in follicular non-hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. (2020). 10.1016/j.clml.2020.03.007. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 21.Ueno A, Maeda R, Kin T, Ito M, Kawasaki K, Ohtani S. Utility of the absolute lymphocyte count and neutrophil/lymphocyte ratio for predicting survival in patients with metastatic breast cancer on eribulin: a real-world observational study. Chemotherapy. (2020). 10.1159/000507043. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Toya R, Yoshida N, Shono T, Matsuyama T, Ninomura S, et al. Spleen dose-volume parameters as a predictor of treatment-related lymphopenia during definitive chemoradiotherapy for esophageal cancer. In Vivo. (2018) 32:1519–25. 10.21873/invivo.11409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman NB, Sherry AD, Anderson J, Osmundson EC. Dose-dependent association of vertebral body irradiation and lymphopenia during chemoradiation for esophageal cancer. Int J Radiat Oncol Biol Phys. (2018) 102:e37–8. 10.1016/j.ijrobp.2018.07.535 [DOI] [Google Scholar]
- 24.Sini C, Fiorino C, Perna L, Noris Chiorda B, Deantoni CL, Bianchi M, et al. Dose-volume effects for pelvic bone marrow in predicting hematological toxicity in prostate cancer radiotherapy with pelvic node irradiation. Radiother Oncol. (2016) 118:79–84. 10.1016/j.radonc.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 25.Ladbury CJ, Rusthoven CG, Camidge DR, Kavanagh BD, Nath SK. Impact of radiation dose to the host immune system on tumor control and survival for stage iii non-small cell lung cancer treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys. (2019) 105:346–55. 10.1016/j.ijrobp.2019.05.064 [DOI] [PubMed] [Google Scholar]
- 26.Rudra S, Hui C, Rao YJ, Samson P, Lin AJ, Chang X, et al. Effect of radiation treatment volume reduction on lymphopenia in patients receiving chemoradiotherapy for glioblastoma. Int J Radiat Oncol Biol Phys. (2018) 101:217–25. 10.1016/j.ijrobp.2018.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. (2020) 38:706–14. 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. (2018) 9:185. 10.3389/fphar.2018.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao XY, Liu CY, He JF, Wang LS, Zhang T. Combination of checkpoint inhibitors with radiotherapy in esophageal squamous cell carcinoma treatment: a novel strategy. Oncol Lett. (2019) 18:5011–21. 10.3892/ol.2019.10893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. (2014) 124:687–95. 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MF, Chen PT, Chen WC, Lu MS, Lin PY, Lee KD. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. (2016) 7:7913–24. 10.18632/oncotarget.6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Culver ME, Gatesman ML, Mancl EE, Lowe DK. Ipilimumab: a novel treatment for metastatic melanoma. Ann Pharmacother. (2011) 45:510–9. 10.1345/aph.1P651 [DOI] [PubMed] [Google Scholar]
- 33.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. (2012) 83:1306–10. 10.1016/j.ijrobp.2011.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res. (2018) 24:5058–71. 10.1158/1078-0432.CCR-17-3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamashita H, Okuma K, Wakui R, Kobayashi-Shibata S, Ohtomo K, Nakagawa K. Details of recurrence sites after elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) combined with chemotherapy for thoracic esophageal squamous cell carcinoma–a retrospective analysis. Radiother Oncol. (2011) 98:255–60. 10.1016/j.radonc.2010.10.021 [DOI] [PubMed] [Google Scholar]
- 36.Cheng YJ, Jing SW, Zhu LL, Wang J, Wang L, Liu Q, et al. Comparison of elective nodal irradiation and involved-field irradiation in esophageal squamous cell carcinoma: a meta-analysis. J Radiat Res. (2018) 59:604–15. 10.1093/jrr/rry055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita H, Takenaka R, Omori M, Imae T, Okuma K, Ohtomo K, et al. Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study. Radiat Oncol. (2015) 10:171. 10.1186/s13014-015-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M, Zhang X, Zhao F, Luo Y, Kong L, Yu J. Involved-field radiotherapy for esophageal squamous cell carcinoma: theory and practice. Radiat Oncol. (2016) 11:18. 10.1186/s13014-016-0589-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiraishi Y, Fang P, Xu C, Song J, Krishnan S, Koay EJ, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. (2018) 128:154–60. 10.1016/j.radonc.2017.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang JI, Jr, Slater JD, Yang GY. Proton therapy for esophageal cancer. Thorac Cancer. (2012) 3:91–3. 10.1111/j.1759-7714.2011.00084.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.