Abstract
Some members of root-associated Bacillus species have been developed as biocontrol agents due to their contribution to plant protection by directly interfering with the growth of pathogens or by stimulating systemic resistance in their host. As rhizosphere-dwelling bacteria, these bacilli are surrounded and constantly interacting with other microbes via different types of communications. With this review, we provide an updated vision of the molecular and phenotypic responses of Bacillus upon sensing other rhizosphere microorganisms and/or their metabolites. We illustrate how Bacillus spp. may react by modulating the production of secondary metabolites, such as cyclic lipopeptides or polyketides. On the other hand, some developmental processes, such as biofilm formation, motility, and sporulation may also be modified upon interaction, reflecting the adaptation of Bacillus multicellular communities to microbial competitors for preserving their ecological persistence. This review also points out the limited data available and a global lack of knowledge indicating that more research is needed in order to, not only better understand the ecology of bacilli in their natural soil niche, but also to better assess and improve their promising biocontrol potential.
Keywords: Bacillus, rhizosphere, bioactive secondary metabolites, microbial interaction, biocontrol, molecular cross-talk, phenotype modulation
Introduction
Some Bacillus species of the B. subtilis complex are plant-associated and important members of the microbiome (Mendes et al., 2013; Müller et al., 2016; Fierer, 2017). During the last decades, their potential use as biocontrol agents with protective activity toward economically important plant pathogens has been highlighted thereby representing a promising alternative to chemical pesticides (Expósito et al., 2017; Fan et al., 2017; Finkel et al., 2017; Fira et al., 2018; Köhl et al., 2019). The efficacy of such bacilli in plant protection, as well as their constant presence in the strongly competitive rhizosphere niche, are due to their high potential to synthesize a wide range of volatile organic compounds (VOCs) and soluble bioactive secondary metabolites (BSMs). High structural diversity is observed in the patterns of VOCs formed by Bacillus (Caulier et al., 2019; Kai, 2020) but also in BSMs which can be either ribosomally synthesized and post-translationally modified like bacteriocins and lantibiotics or enzymatically formed via multi-modular mega-enzymes as in the case of polyketides (PKs), di-peptides or cyclic lipopeptides (CLPs) (Harwood et al., 2018; Kaspar et al., 2019; Rabbee et al., 2019). A prime role of some soluble BSMs and volatiles in plant protection is related to their strong antimicrobial activity leading to direct antagonism against plant pathogens (Raaijmakers and Mazzola, 2012; Borriss, 2015; Chowdhury et al., 2015a; Fan et al., 2018; Caulier et al., 2019; Rabbee et al., 2019; Kai, 2020). A second important biocontrol-related trait of those compounds is their ability to trigger an immune reaction in the host plants which leads to systemic resistance (Induced SR) rendering the plant less susceptible to pathogen infection (Pieterse et al., 2014; Chowdhury et al., 2015a; Fan et al., 2018; Caulier et al., 2019; Rabbee et al., 2019). An additional role of BSMs is also linked to an efficient plant root colonization ability of Bacillus which indirectly protects the plant by decreasing the space and nutrient availability for pathogens (Raaijmakers et al., 2010; Borriss, 2015; Nayak et al., 2020). Some BSMs also contribute to colonization since they are involved in the developmental processes of Bacillus social motility and biofilm formation (Raaijmakers and Mazzola, 2012; Borriss, 2015; Pandin et al., 2017).
As rhizosphere-dwelling bacteria, these plant-associated bacilli are influenced by various environmental factors, such as temperature, pH, moisture, light, and nutrient composition dictated by plant exudation (Santoyo et al., 2017). In this competitive niche, Bacillus species are also surrounded by and constantly interacting with a myriad of other (micro)organisms (Mendes et al., 2013; Traxler and Kolter, 2015; Fierer, 2017; Schmidt et al., 2019). In this review, we illustrate the diversity of BSMs produced by different Bacillus species and how this metabolome and phenotypic traits dictating ecological fitness can be impacted upon interaction with other fungal and bacterial microorganisms. The outcomes of volatile-based microbial interactions, in general, have been recently reviewed (Schmidt et al., 2015; Tyc et al., 2017). However, when dealing with interactions involving bacilli, information is scarce concerning possible changes in VOCs production upon cross-talk or perception of volatiles produced by other microorganisms (Chen et al., 2015; Tahir et al., 2017; Martínez-Cámara et al., 2019). Thus, we focus hereafter on interactions based on cross-talks mediated by the perception of soluble metabolites.
Diversity and Bioactivities of Bacillus BSMs
In the comparative genomic era, numerous adjustments have been done in the last years to clarify the phylogeny of the B. subtilis complex, which includes, among others, species, such as B. velezensis, B. amyloliquefaciens, B. atrophaeus, B. subtilis subspecies subtilis, B. licheniformis, B. pumilus, and B. siamensis with potential as biocontrol agents (Expósito et al., 2017; Fira et al., 2018; Maksimov et al., 2020), and which led to some confusion in species names but also to misassignments (Dunlap et al., 2016; Fan et al., 2017; Harwood et al., 2018; Du, 2019; Torres Manno et al., 2019). Many isolates, such as strains FZB42, QST713, or SQR9 formerly assigned to the B. subtilis or B. amyloliquefaciens species have been re-classified as B. velezensis representing the model species for plant-associated bacilli (Dunlap et al., 2016; Fan et al., 2017). A large part of the genome of these species is devoted to the production of antimicrobial compounds with up to 12% annotated as involved in the synthesis of bioactive secondary metabolites (Molinatto et al., 2016; Fan et al., 2017; Pandin et al., 2018).
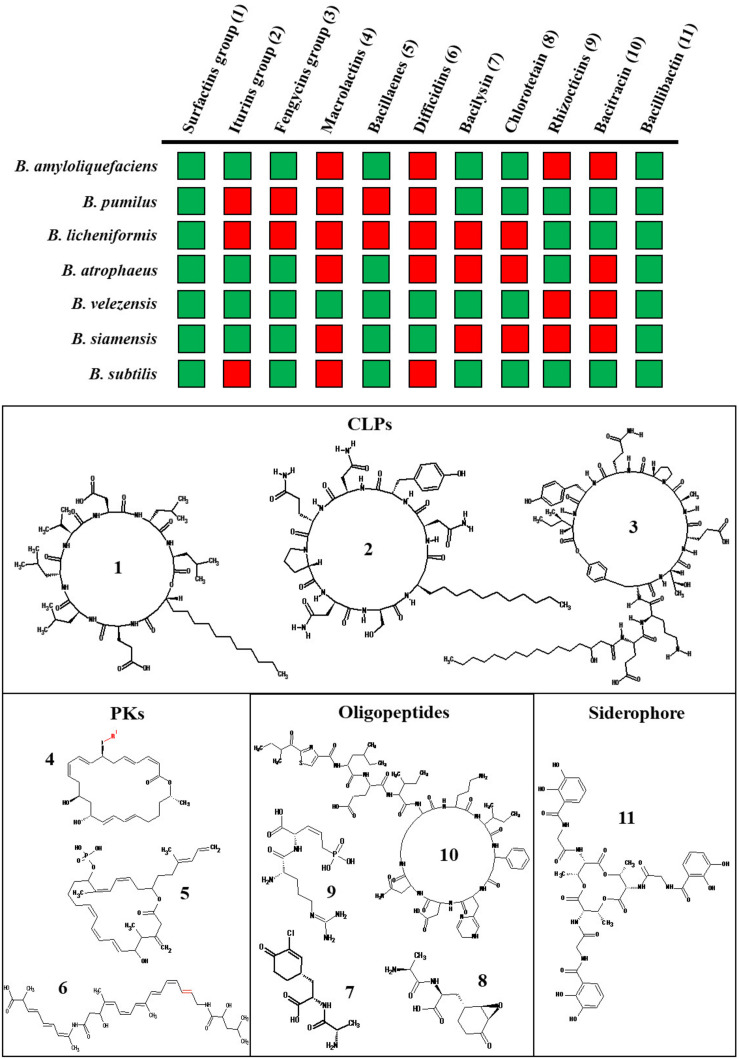
Non-ribosomal metabolites are synthesized either by polyketide synthases (PKS) or non-ribosomal peptide synthase (NRPS), both acting as assembly lines catalyzing different steps for the incorporation of amino acid residues (Dutta et al., 2014; Winn et al., 2016; Bozhüyük et al., 2019). The three main families of Bacillus CLPs are surfactins, fengycins, and iturins (Figure 1). According to this limited number of families identified so far, the structural diversity of Bacillus CLPs may appear quite limited compared to other bacterial genera, such as Pseudomonas, for which many more different groups have been discovered (Geudens and Martins, 2018; Götze and Stallforth, 2020). However, reduced specificity of adenylation domains allows substitutions at specific places in the peptide chain and the NRPS machinery can bind different fatty acids with various chain lengths in the initiation step leading to co-production of various homologs within the three families as illustrated in Figure 1 (Kraas et al., 2010; Bozhüyük et al., 2019). Interestingly, some CLP peptidic variants are synthesized through species-specific clusters, like pumilacidin and lichenysin which are only produced respectively by B. pumilus and B. licheniformis (Figure 1).
FIGURE 1.
Main non-ribosomal BSMs produced by the various species in the B. subtilis complex. The BSMs production is indicated for the following species B. subtilis, B. siamensis, B. velezensis, B. atrophaeus, B. amyloliquefaciens, B. pumilus, and B. licheniformis by a green square whereas red square indicates an absence of production of the BSMs in this species (Zhao and Kuipers, 2016; Fan et al., 2017; Harwood et al., 2018; Du, 2019; Torres Manno et al., 2019). The surfactins, iturins, and fengycins groups include lichenycin (1;AA1:L-Gln) and pumilacidin (1;AA4: L-Leu, AA7 = I-Ile); mycosubtilin (2;AA6: D-Ser, AA7 = L-Thr) and bacillomycin (2; AA6: D-Ser, AA7 = L-Asn); maltacin (4;AA1: L-Ser), agrastatin (4;AA10: L-Val) and plipastatin (4;AA9: D-Tyr), respectively. The structure of the representative metabolite is indicated by a number and represented below. The possible variations in the PKs structure are highlighted in red. For the macrolactin family, the main variants are R = H; CO-CH2-COOH; CO-CH2-CH2-COOH or 6-O-succinyl-β-glucose (for review see Piel, 2010).
The three different types of CLPs retain specific but complementary functions considering biocontrol efficiency and, more generally, ecological fitness of the producing strains. By contributing to motility and biofilm formation, surfactins are involved in colonization of plant tissues which indirectly allow Bacillus to outcompete phytopathogens for space and nutrients. Surfactins are also involved in the molecular cross-talk with the host and it is well-characterized as an elicitor of plant immunity leading to ISR (Ongena and Jacques, 2008; Henry et al., 2011; García-Gutiérrez et al., 2013; Cawoy et al., 2015; Chowdhury et al., 2015a). Direct antibiotic activity of surfactins at biologically relevant concentrations toward soil-dwelling or plant-associated microbes has been only occasionally reported (Qi et al., 2010; Liu et al., 2014). By contrast, fengycins and iturins are best characterized for their antifungal activities against a wide range of plant pathogens (Caulier et al., 2019; Rabbee et al., 2019). This is mainly due to their ability to perturb fungal cell membrane integrity resulting in cytoplasm leakage and finally hyphae death and inhibition of spore germination (Chitarra et al., 2003; Romero et al., 2007; Deleu et al., 2008; Etchegaray et al., 2008; Gong et al., 2015; Gao et al., 2017; Zhang and Sun, 2018). The three CLPs retain some selectivity but may also act synergistically to inhibit fungal growth (Liu et al., 2014). The lipid composition of the plasma membrane could explain differences in the sensitivity of fungal targets to one or more CLPs (Wise et al., 2014; Fiedler and Heerklotz, 2015).
Besides lipopeptides, most species of the B. subtilis group also produce other non-ribosomal oligopeptide derivatives, such as bacilysin, chlorotetaine, bacitracins, and rhizocticins which are known to be efficient as antibacterial compounds targeting cell wall biosynthesis (Zhao and Kuipers, 2016). The siderophore bacillibactin is highly conserved in the B. subtilis group (Figure 1) and is induced in response to iron limitation in the environment. It allows Bacillus to efficiently acquire Fe3+ and other metals (Miethke et al., 2006, 2008; Li et al., 2014) thereby depriving phytopathogens of this essential element (Miethke et al., 2006; Niehus et al., 2017).
Polyketide biosynthesis is performed by successive condensation of small carboxylic acids mediated by core domains of the corresponding enzyme machinery but some PKs are synthesized via hybrid NRPS/PKS systems leading to the integration of amino acid residues (Piel, 2010; Olishevska et al., 2019). The three main PKs produced by Bacillus are difficidins, macrolactins, and bacillaenes, the latter being more widespread across species (Figure 1). The main PKs role is related to their antibacterial activity via the ability to inhibit protein biosynthesis in numerous phytopathogenic bacteria but certain antifungal activity has been reported for bacillaenes and macrolactins (Caulier et al., 2019; Olishevska et al., 2019).
Ribosomally synthetized BSMs encompass bacteriocins and lantibiotics including plantazolicin, subtilin, ericin, mersacidin, amylolysin, and amylocyclicin that are specifically produced by some species or strains (Brötz et al., 1998; van Kuijk et al., 2012; Arguelles Arias et al., 2013; Scholz et al., 2014; Torres Manno et al., 2019). These BSMs are responsible for growth inhibition of Gram-positive bacteria by acting via different modes of action (Abriouel et al., 2011; Acedo et al., 2018).
Perception of Fungi Triggers the Production of Appropriate BSMs
Several works have illustrated the impact of phytopathogenic fungi on BSMs production by soil bacilli. Some B. amyloliquefaciens, B. velezensis, and B. subtilis strains respond to the presence of antagonistic fungi by stimulating the production of the antifungal CLPs fengycins and/or iturins (Table 1). Not only the production of specific CLPs varies in a species-dependent manner but it is also highly dependent on the interacting fungal species. For example, much higher production of iturins and fengycins by B. subtilis 98S was observed in confrontation with Pythium aphanidermatum and Fusarium oxysporum but not with Botrytis cinerea (Cawoy et al., 2015). Further, upon interaction with fungi, some B. velezensis strains (SQR9, FZB42, and S499) overproduced either iturins or fencycins (Li et al., 2014; Chowdhury et al., 2015b; Kulimushi et al., 2017). For instance, Li et al. (2014) showed that when confronted with Sclerotinia sclerotiorum, B. velezensis SQR9 overproduces bacillomycin D (iturin family), but not fengycins. An overproduction of bacillomycin along with a reduced production of fengycins was also reported by Chowdhury et al. (2015b) upon B. velezensis FZB42 interaction with Rhizoctonia solani in the rhizosphere of lettuce plants. Differentially, Kulimushi et al. (2017), showed that strains S499 and FZB42 improved production of fengycin but not iturins upon interaction with Rhizomucor variabilis. Most of these studies also indicated that fengycins and iturins are the main BSMs responsible for antifungal activities (Table 1). Thus, Bacillus cells could specifically sense the presence of fungal competitors and accordingly overproduce appropriate antifungal BSMs to outcompete the interacting fungi. Moreover, besides modulating the production of fengycins and iturins, some strains of B. velezensis (SQR9, FZB42, and QST713) and B. subtilis (B9-5) may overproduce surfactins when sensing phytopathogenic fungi (Li et al., 2014; Chowdhury et al., 2015b; DeFilippi et al., 2018; Pandin et al., 2019). In support to this hypothesis, surfactin production of B. velezensis FZB42 was highly induced in the presence of fungal pathogen R. solani in the lettuce rhizosphere where it was found as the main produced compound (Chowdhury et al., 2015b). A similar response was recorded when B. velezensis SQR9 was confronted with S. sclerotiorum and Phytophthora parasitica (Li et al., 2014) or when B. subtilis B9-5 interacted in liquid medium with Rhizopus stolonifer (DeFilippi et al., 2018). In contrast to fengycins and iturins, surfactins are not strong direct antifungal metabolites in biologically relevant concentrations (Raaijmakers and Mazzola, 2012). Thus, it stays unclear why Bacillus responded by surfactin overproduction to the presence of antagonistic fungi. A possible explanation could be rooted in its global role promoting the rhizosphere and thereby, contributing to competition for nutrients and space with the interacting fungi (Ongena and Jacques, 2008; Rabbee et al., 2019).
TABLE 1.
Change in expression and bioactivity of BSMs produced by members of B. subtilis group, upon interaction with fungal species.
| BSMs | Change in expression | Involvement in antifungal activity | Bacillus species (strains) | Fungal species | References |
| Fengycins | 0 | Yes | B. subtilis (98S) | B. cinerea | Cawoy et al., 2015 |
| + | Yes | B. subtilis (98S) | F. oxysporum | Cawoy et al., 2015 | |
| + | No | B. subtilis (98S) | P. aphanidermatum | Cawoy et al., 2015 | |
| + | Yes | B. velezensis (S499) | R. variabilis | Kulimushi et al., 2017 | |
| + | Yes | B. velezensis (FZB42) | R. variabilis | Kulimushi et al., 2017 | |
| 0 | Yes | B. velezensis (QST713) | R. variabilis | Kulimushi et al., 2017 | |
| + | Yes | B. velezensis (SQR9) | Verticillium dahliae | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | F. oxysporum | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | Phytophthora parasitica var. nicotianae | Li et al., 2014 | |
| - | Mediating the plant defense expression | B. velezensis (FZB42) | R. solani | Chowdhury et al., 2015b | |
| + | ND | B. subtilis (B9-5) | R. stolonifer | DeFilippi et al., 2018 | |
| + | ND | B. subtilis (B9-5) | Fusarium sambucinum | DeFilippi et al., 2018 | |
| + | ND | B. subtilis (B9-5) | V. dahliae | DeFilippi et al., 2018 | |
| + | ND | B. velezensis (QST713) | Trichoderma aggressivum f. europaeum | Pandin et al., 2019 | |
| Iturins | 0 | Yes | B. subtilis (98S) | B. cinerea | Cawoy et al., 2015 |
| + | Yes | B. subtilis (98S) | F. oxysporum | Cawoy et al., 2015 | |
| + | No | B. subtilis (98S) | P. aphanidermatum | Cawoy et al., 2015 | |
| + | No | B. velezensis (SQR9) | V. dahliae | Li et al., 2014 | |
| + | No | B. velezensis (SQR9) | S. sclerotiorum | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | F. oxysporum | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | P. parasitica | Li et al., 2014 | |
| + | Mediating the plant defense expression | B. velezensis (FZB42) | R. solani | Chowdhury et al., 2015b | |
| Surfactins | + | Yes | B. velezensis (SQR9) | S. sclerotiorum | Li et al., 2014 |
| + | Yes | B. velezensis (SQR9) | R. solani | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | Fusarium solani | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | P. parasitica | Li et al., 2014 | |
| + | Mediating the plant defense expression | B. velezensis (FZB42) | R. solani | Chowdhury et al., 2015b | |
| + | ND | B. subtilis (B9-5) | R. solani | DeFilippi et al., 2018 | |
| + | ND | B. subtilis (B9-5) | F. sambucinum | DeFilippi et al., 2018 | |
| + | ND | B. subtilis (B9-5) | V. dahliae | DeFilippi et al., 2018 | |
| + | ND | B. velezensis (QST713) | T. aggressivum f. europaeum | Pandin et al., 2019 | |
| Bacillibactin | + | Yes | B. velezensis (SQR9) | V. dahliae | Li et al., 2014 |
| + | No | B. velezensis (SQR9) | S. sclerotiorum | Li et al., 2014 | |
| + | No | B. velezensis (SQR9) | F. oxysporum | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | R. solani | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | F. solani | Li et al., 2014 | |
| + | Yes | B. velezensis (SQR9) | P. parasitica | Li et al., 2014 |
“0” indicates no changes, “+” enhanced and, “–”decreased BSMs production by Bacillus upon interaction with fungi. “Yes” indicates fungitoxic activity, “No” no antifungal activity, “ND” indicates that BSMs with antifungal activity are not detected.
Even though the siderophore bacillibactin is produced by all members of the B. subtilis species complex (Figure 1), its possible overproduction upon microbial interactions has been poorly investigated. Interestingly, the work of Li et al. (2014) showed that B. velezensis SQR9 overproduces bacillibactin when grown in presence of a range of fungi including V. dahliae, S. sclerotiorum, F. oxysporum, R. solani, F. solani, and P. parasitica. This may be interpreted as a response of the bacterium to some iron-limitation in the medium caused by the fungi via the release of their own chelatants.
In B. subtilis, the expression of many BSMs biosynthesis genes is transcriptionally fine-tuned by compound-specific regulation but also by global regulators governing the transition to crucial developmental processes like motility, biofilm formation and sporulation (Inaoka et al., 2009; López et al., 2009; Vargas-Bautista et al., 2014). Fungal triggers may affect both types of regulatory systems involved in BSMs production. For instance, upon sensing F. verticillioides, the global stress-related regulator SigB is activated in B. subtilis NCIB3610 which in return enhances surfactin production (Bartolini et al., 2019). In interaction with F. culmorum under biofilm-conducive conditions, B. subtilis Bs12 down-regulates the expression of the sinR gene known as a repressor of biofilm formation which also negatively regulates surfactin production (Kearns et al., 2005; Khezri et al., 2016; Zhi et al., 2017). These observations strongly suggest that specific soluble signals, emitted by fungal pathogens, could be perceived by bacilli which in turn modulate BSMs synthesis. As observed by Bartolini et al. (2019), cells of the Bacillus colony, physically close to the fungal culture, responded to signals by over-expressing genes coding for transcription factors involved in CLPs synthesis regulation. In contrast, colony cells positioned on the opposite side of the fungi did not react to the fungus (Bartolini et al., 2019). This phenomenon indicates that the specific fungal metabolite diffuses on a short distance and has an influence on closely located Bacillus cells. Currently, no fungal compounds have been identified as triggers of BSM stimulation in Bacillus. Nonetheless, few commonly produced metabolites by Fusarium species were suggested to modify Bacillus behavior. It was shown that two cyclic depsipeptides (enniatins B1 and enniatins A1) and a pyrone (lateropyrone) had an antagonistic effect on B. subtilis growth (Ola et al., 2013). Fusaric acid also modified antibacterial activity of B. mojavensis but it was not related to a decrease in the production of specific BSMs (Bacon et al., 2004, 2006; Bani et al., 2014). These metabolites could also play a triggering role at sub-inhibitory concentration and could have an inducible effect on the range of Bacillus responses as has been shown for other signal metabolites (Bleich et al., 2015; Liu et al., 2018).
Bacillus Phenotype Is Modulated Upon Perception of Bacterial Competitors
Some BSMs may also act as molecular determinants driving outcomes of interactions between B. subtilis and bacterial competitors as illustrated for the bacillaene polyketide displaying an essential protective role for survival in competition with Streptomyces soil isolates (Straight et al., 2007; Barger et al., 2012). However, there are few direct evidences for enhanced expression of BSMs upon interbacteria interactions. The only convincing examples involve the interaction of plant-associated bacilli with plant pathogens, such as Ralstonia solanacearum (Almoneafy et al., 2014) and Pseudomonas fuscovaginae (Kakar et al., 2014). In these two studies, improved expression of surfactin, bacilysin, and iturin biosynthesis genes were observed when Bacillus and pathogens were grown together in dual-cultures. Nevertheless, no clear indication about the enhanced production of the aforementioned BSMs based on their quantification nor improved antibacterial activities of Bacillus was presented as a result of this interaction.
Interestingly, at the phenotypical level, the development of soil bacilli is differentially altered upon sensing other bacteria from the same natural environment. Some of these phenotypical changes can be associated or due to a modulated production of specific BSMs. First, exogenous antibiotics or signals may stimulate biofilm formation which depends, to some extent, on surfactin production (López et al., 2009) and which may be viewed as a defensive response against exogenous toxic compounds and/or infiltration by competitors (Flemming et al., 2016; Townsley and Shank, 2017; Molina-Santiago et al., 2019). For instance, B. subtilis increased its relative subpopulation of biofilm matrix-producing cells in response to small molecules secreted by other bacterial species (López et al., 2009; Shank et al., 2011). The same phenomenon was illustrated for thiazolyl peptides emitted by closely related species, such as B. cereus and putatively formed by other soil microbes, such as Streptomyces isolates (Bleich et al., 2015). However, no change in surfactin production associated with the stimulation of biofilm was reported in these studies.
Besides biofilm formation, other mechanisms drive bacteria to initiate protective responses upon the detection of competitors. The flagellum-independent sliding motility is considered as an adaptive mechanism that allows bacterial cells to physically relocate in the context of a competitive interaction (Wadhams and Armitage, 2004; Jones et al., 2017; McCully et al., 2019). Upon sensing S. venezuelae, the B. subtilis ability to slide was increased (Liu et al., 2018). It depends in part on the production of surfactin (Grau et al., 2015; van Gestel et al., 2015) but a potential boost in lipopeptide synthesis upon the perception of the Streptomyces challenger was not demonstrated. Chloramphenicol and derivatives produced by S. venezuelae were identified as molecular triggers acting at subinhibitory concentrations for inducing Bacillus motility (Liu et al., 2018).
Multiple bacteria promote sporulation in B. subtilis which represents another example of alteration of the physiological development of this species. In a context of distant interactions, exogenous siderophores accelerate the differentiation of Bacillus cells into spores. It was notably shown for enterobactin from E. coli and for ferrioxamine E produced by Streptomycetes (Grandchamp et al., 2017). In iron-limited environments, B. subtilis cells would thus respond by taking up those “piratable” siderophores and start sporulating. This is not a general response to xenosiderophores since for instance, pyochelin from Pseudomonas does not affect Bacillus sporulation (Molina-Santiago et al., 2019). Nevertheless, the ability of siderophores to alter cellular differentiation in B. subtilis suggests that those molecules are likely to mediate complex microbial interactions in iron-depleted conditions, as often met in a soil environment. However, induction of B. subtilis sporulation by other bacteria may also occur in a cell-to-cell contact situation. Upon interaction with P. chlororaphis, its type VI secretion system acted as a trigger for sporulation, independently from its established role as cargo for delivering toxic effectors into the target Bacillus cells (García-Bayona and Comstock, 2018; Molina-Santiago et al., 2019).
That said, interspecies interactions may also result in inhibition rather than in stimulation of key developmental processes determining the fate of Bacillus multicellular communities. As an example, 2,4-diacetylphloroglucinol, a broad-spectrum antibiotic synthesized by fluorescent Pseudomonas, alters colony morphology, inhibits biofilm formation and sporulation in B. subtilis populations grown adjacent to P. protegens colonies (Powers et al., 2015). This antibiotic seems to act as an interspecific signaling molecule that inhibits bacterial differentiation at subinhibitory concentrations (Powers et al., 2015).
Conclusion
Here we provide an overview of the phenotypic and molecular responses of plant-beneficial soil bacilli upon sensing signals from other microorganisms that can be encountered in the rhizosphere niche. It is clear that BSMs production by Bacillus can be modulated upon interactions with other microbes and that key BSM-driven developmental processes may undergo unsuspected changes. It somehow illustrates the flexibility of these bacteria in re-directing their secondary metabolome to adapt environmental fitness upon sensing the presence of neighboring microorganisms. Nevertheless, the molecular mechanisms integrating the perception of exogenous triggers with a regulatory response leading to enhanced production of BSMs still remain unclear.
A significant boost in BSMs production by soil bacilli has been reported in most cases as an outcome from interactions with plant pathogenic fungi. This is of value in the context of biocontrol of fungal pathogens since direct antagonism is considered as the most powerful mode of action for suppression of plant diseases (Fravel, 2005; Frey-Klett et al., 2011; Köhl et al., 2019). By contrast, direct evidence for an impact of interbacteria interactions on the expression of the secondary metabolome in Bacillus is still globally missing. Nevertheless, interaction-mediated variations in colony morphology, motility, biofilm formation, or sporulation illustrate how soil bacilli can protect themselves from antimicrobials emitted by bacterial competitors. Such an impact on those key developmental processes should thus be coupled with significant modulation in the production of specific BSMs underpinning these phenotypes. Depending on the concentration, these BSMs would then act as antimicrobials in interference competition or as signals in cooperative interspecies communication processes not necessarily affecting the growth of the partners (Bleich et al., 2015; Liu et al., 2018). However, this has yet to be thoroughly demonstrated and future examination of developmental controls for BSMs biosynthesis will likely bring light upon the key principles driving environmental fitness of soil bacilli as intrinsically influenced by interspecies competition.
From an ecological viewpoint, further investigations would also help to better understand why soil amendment with selected bacilli, even at high doses, do not durably impact the composition of the rhizosphere microbiome despite their huge arsenal in antimicrobial weapons (Correa et al., 2009; Chowdhury et al., 2013; Kröber et al., 2014; Qiao et al., 2017) and by contrast with some other bacteria and fungi (Buddrus-Schiemann et al., 2010; Chowdhury et al., 2013; Erlacher et al., 2014; Thomas and Sekhar, 2016; Wu et al., 2016). Those bacilli may thus provide protection to their host plant toward microbial pathogen ingress but would avoid detrimental effect on its naturally selected beneficial microbiome which is of prime interest for future application as biocontrol agents.
Author Contributions
SA, TM, and MO conceived the idea, designed the outlines of the review, and wrote the manuscript. All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank A. Argüelles-Arias, G. Hoff, and A. Rigolet for reading the manuscript and for their helpful suggestions.
Footnotes
Funding. Research in the laboratory was supported by the Interreg FWVL V portfolio project SmartBiocontrol, and by the Excellence of Science Grant 30650620 (F.R.S. – FNRS Fonds National de la Recherche Scientifique).
References
- Abriouel H., Franz C. M. A. P., Ben Omar N., Gálvez A. (2011). Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 35 201–232. 10.1111/j.1574-6976.2010.00244.x [DOI] [PubMed] [Google Scholar]
- Acedo J. Z., Chiorean S., Vederas J. C., van Belkum M. J. (2018). The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiol. Rev. 42 805–828. 10.1093/femsre/fuy033 [DOI] [PubMed] [Google Scholar]
- Almoneafy A. A., Kakar K. U., Nawaz Z., Li B., Saand M. A., Chun-lan Y., et al. (2014). Tomato plant growth promotion and antibacterial related-mechanisms of four rhizobacterial Bacillus strains against Ralstonia solanacearum. Symbiosis 63 59–70. 10.1007/s13199-014-0288-9 [DOI] [Google Scholar]
- Arguelles Arias A., Ongena M., Devreese B., Terrak M., Joris B., Fickers P. (2013). Characterization of amylolysin, a novel lantibiotic from Bacillus amyloliquefaciens GA1. PLoS One 8:e83037. 10.1371/journal.pone.0083037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon C. W., Hinton D. M., Hinton A. (2006). Growth-inhibiting effects of concentrations of fusaric acid on the growth of Bacillus mojavensis and other biocontrol Bacillus species. J. Appl. Microbiol. 100 185–194. 10.1111/j.1365-2672.2005.02770.x [DOI] [PubMed] [Google Scholar]
- Bacon C. W., Hinton D. M., Porter J. K., Glenn A. E., Kuldau G. (2004). Fusaric acid, a Fusarium verticillioides metabolite, antagonistic to the endophytic biocontrol bacterium Bacillus mojavensis. Can. J. Bot. 82 878–885. 10.1139/B04-067 [DOI] [Google Scholar]
- Bani M., Rispail N., Evidente A., Rubiales D., Cimmino A. (2014). Identification of the main toxins isolated from Fusarium oxysporum f. sp. pisi race 2 and their relation with isolates’ pathogenicity. J. Agric. Food Chem. 62 2574–2580. 10.1021/jf405530g [DOI] [PubMed] [Google Scholar]
- Barger S. R., Hoefler B. C., Cubillos-Ruiz A., Russell W. K., Russell D. H., Straight P. D. (2012). Imaging secondary metabolism of Streptomyces sp. Mg1 during cellular lysis and colony degradation of competing Bacillus subtilis. Antonie Van Leeuwenhoek 102 435–445. 10.1007/s10482-012-9769-0 [DOI] [PubMed] [Google Scholar]
- Bartolini M., Cogliati S., Vileta D., Bauman C., Ramirez W., Grau R. (2019). Stress responsive alternative sigma factor SigB plays a positive role in the antifungal proficiency of Bacillus subtilis. Appl. Environ. Microbiol. 85:e00178-19. 10.1128/AEM.00178-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich R., Watrous J. D., Dorrestein P. C., Bowers A. A., Shank E. A. (2015). Thiopeptide antibiotics stimulate biofilm formation in Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 112 3086–3091. 10.1073/pnas.1414272112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borriss R. (2015). “Bacillus, a plant-beneficial bacterium,” in Principles of Plant-Microbe Interactions, ed. Lugtenberg B. (Cham: Springer; ), 379–391. 10.1007/978-3-319-08575-3_40 [DOI] [Google Scholar]
- Bozhüyük K. A., Micklefield J., Wilkinson B. (2019). Engineering enzymatic assembly lines to produce new antibiotics. Curr. Opin. Microbiol. 51 88–96. 10.1016/j.mib.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brötz H., Bierbaum G., Leopold K., Reynolds P. E., Sahl H. G. (1998). The lantibiotic mersacidin inhibits peptidoglycan synthesis by targeting lipid II. Antimicrob. Agents Chemother. 42 154–160. 10.1128/AAC.42.1.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buddrus-Schiemann K., Schmid M., Schreiner K., Welzl G., Hartmann A. (2010). Root colonization by Pseudomonas sp. DSMZ 13134 and impact on the indigenous rhizosphere bacterial community of barley. Microb. Ecol. 60 381–393. 10.1007/s00248-010-9720-8 [DOI] [PubMed] [Google Scholar]
- Caulier S., Nannan C., Gillis A., Licciardi F., Bragard C., Mahillon J. (2019). Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front. Microbiol. 10:302. 10.3389/fmicb.2019.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawoy H., Debois D., Franzil L., De Pauw E., Thonart P., Ongena M. (2015). Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 8 281–295. 10.1111/1751-7915.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Gozzi K., Yan F., Chai Y. (2015). Acetic acid acts as a volatile signal to stimulate bacterial biofilm formation. mBio 6:e00392-15. 10.1128/mBio.00392-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitarra G. S., Breeuwer P., Nout M. J. R., van Aelst A. C., Rombouts F. M., Abee T. (2003). An antifungal compound produced by Bacillus subtilis YM 10-20 inhibits germination of Penicillium roqueforti conidiospores. J. Appl. Microbiol. 94 159–166. 10.1046/j.1365-2672.2003.01819.x [DOI] [PubMed] [Google Scholar]
- Chowdhury S. P., Dietel K., Rändler M., Schmid M., Junge H., Borriss R., et al. (2013). Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS One 8:e68818. 10.1371/journal.pone.0068818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. P., Hartmann A., Gao X. W., Borriss R. (2015a). Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 - a review. Front. Microbiol. 6:780. 10.3389/fmicb.2015.00780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S. P., Uhl J., Grosch R., Alquéres S., Pittroff S., Dietel K., et al. (2015b). Cyclic lipopeptides of Bacillus amyloliquefaciens subsp. plantarum colonizing the lettuce rhizosphere enhance plant defense responses toward the bottom rot pathogen Rhizoctonia solani. Mol. Plant Microbe Interact. 28 984–995. 10.1094/mpmi-03-15-0066-r [DOI] [PubMed] [Google Scholar]
- Correa O. S., Montecchia M. S., Berti M. F., Ferrari M. C. F., Pucheu N. L., Kerber N. L., et al. (2009). Bacillus amyloliquefaciens BNM122, a potential microbial biocontrol agent applied on soybean seeds, causes a minor impact on rhizosphere and soil microbial communities. Appl. Soil Ecol. 41 185–194. 10.1016/j.apsoil.2008.10.007 [DOI] [Google Scholar]
- DeFilippi S., Groulx E., Megalla M., Mohamed R., Avis T. J. (2018). Fungal competitors affect production of antimicrobial lipopeptides in Bacillus subtilis Strain B9–5. J. Chem. Ecol. 44 374–383. 10.1007/s10886-018-0938-0 [DOI] [PubMed] [Google Scholar]
- Deleu M., Paquot M., Nylander T. (2008). Effect of fengycin, a lipopeptide produced by Bacillus subtilis, on model biomembranes. Biophys. J. 94 2667–2679. 10.1529/biophysj.107.114090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y., Ma J., Yin Z., Liu K., Yao G., Xu W., et al. (2019). Comparative genomic analysis of Bacillus paralicheniformis MDJK30 with its closely related species reveals an evolutionary relationship between B. paralicheniformis and B. licheniformis. BMC Genomics. 20:283. 10.1186/s12864-019-5646-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap C., Kim S. J., Kwon S. W., Rooney A. (2016). Bacillus velezensis is not a later heterotypic synonym of Bacillus amyloliquefaciens, Bacillus methylotrophicus, Bacillus amyloliquefaciens subsp. plantarum and ‘Bacillus oryzicola’ are later heterotypic synonyms of Bacillus velezensis based on phylogenomics. Int. J. Syst. Evol. Microbiol. 66 1212–1217. 10.1099/ijsem.0.000858 [DOI] [PubMed] [Google Scholar]
- Dutta S., Whicher J. R., Hansen D. A., Hale W. A., Chemler J. A., Congdon G. R., et al. (2014). Structure of a modular polyketide synthase. Nature 510 512–517. 10.1038/nature13423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher A., Cardinale C., Grosch R., Grube M., Berg G. (2014). The impact of the pathogen Rhizoctonia solani and its beneficial counterpart Bacillus amyloliquefaciens on the indigenous lettuce microbiome. Front. Microbiol. 5:175. 10.3389/fmicb.2014.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray A., de Castro Bueno C., de Melo I. S., Tsai S. M., de Fátima Fiore M., Silva-Stenico M. E., et al. (2008). Effect of a highly concentrated lipopeptide extract of Bacillus subtilis on fungal and bacterial cells. Arch. Microbiol. 190 611–622. 10.1007/s00203-008-0409-z [DOI] [PubMed] [Google Scholar]
- Expósito R. G., de Bruijn I., Postma J., Raaijmakers J. M. (2017). Current insights into the role of rhizosphere bacteria in disease suppressive soils. Front. Microbiol. 8:2529. 10.3389/fmicb.2017.02529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B., Blom J., Klenk H. P., Borriss R. (2017). Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “Operational Group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 8:22. 10.3389/fmicb.2017.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B., Wang C., Song X., Ding X., Wu L., Wu H., et al. (2018). Bacillus velezensis FZB42 in 2018: the gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 9:2491. 10.3389/fmicb.2018.02491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler S., Heerklotz H. (2015). Vesicle leakage reflects the target selectivity of antimicrobial lipopeptides from Bacillus subtilis. Biophys. J. 109 2079–2089. 10.1016/j.bpj.2015.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer N. (2017). Embracing the unknown: disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 15 579–590. 10.1038/nrmicro.2017.87 [DOI] [PubMed] [Google Scholar]
- Finkel O. M., Castrillo G., Herrera Paredes S., Salas González I., Dangl J. L. (2017). Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 38 155–163. 10.1016/j.pbi.2017.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fira D., Dimkić I., Berić T., Lozo J., Stanković S. (2018). Biological control of plant pathogens by Bacillus species. J. Biotechnol. 285 44–55. 10.1016/j.jbiotec.2018.07.044 [DOI] [PubMed] [Google Scholar]
- Flemming H. C., Wingender J., Szewzyk U., Steinberg P., Rice S. A., Kjelleberg S. (2016). Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14 563–575. 10.1038/nrmicro.2016.94 [DOI] [PubMed] [Google Scholar]
- Fravel D. R. (2005). Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 43 337–359. 10.1146/annurev.phyto.43.032904.092924 [DOI] [PubMed] [Google Scholar]
- Frey-Klett P., Burlinson P., Deveau A., Barret M., Tarkka M., Sarniguet A. (2011). Bacterial-Fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 75 583–609. 10.1128/mmbr.00020-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Han J., Liu H., Qu X., Lu Z., Bie X. (2017). Plipastatin and surfactin coproduction by Bacillus subtilis pB2-L and their effects on microorganisms. Antonie Van Leeuwenhoek 110 1007–1018. 10.1007/s10482-017-0874-y [DOI] [PubMed] [Google Scholar]
- García-Bayona L., Comstock L. E. (2018). Bacterial antagonism in host-associated microbial communities. Science 361:eaat2456. 10.1126/science.aat2456 [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez L., Zeriouh H., Romero D., Cubero J., de Vicente A., Pérez-García A. (2013). The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate- and salicylic acid-dependent defence responses. Microb. Biotechnol. 6 264–274. 10.1111/1751-7915.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geudens N., Martins J. C. (2018). Cyclic lipodepsipeptides from Pseudomonas spp. – Biological swiss-army knives. Front. Microbiol. 9:1867. 10.3389/fmicb.2018.01867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong A.-D., Li H.-P., Yuan Q.-S., Song X.-S., Yao W., He W.-J., et al. (2015). Antagonistic mechanism of iturin A and plipastatin A from Bacillus amyloliquefaciens S76-3 from wheat spikes against Fusarium graminearum. PLoS One 10:e0116871. 10.1371/journal.pone.0116871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götze S., Stallforth P. (2020). Structure, properties, and biological functions of nonribosomal lipopeptides from pseudomonads. Nat. Prod. Rep. 37 29–54. 10.1039/C9NP00022D [DOI] [PubMed] [Google Scholar]
- Grandchamp G. M., Caro L., Shank E. A. (2017). Pirated siderophores promote sporulation in Bacillus subtilis. Appl. Environ. Microbiol. 83:e03293-16. 10.1128/AEM.03293-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau R. R., De Oña P., Kunert M., Leñini C., Gallegos-Monterrosa R., Mhatre E., et al. (2015). A duo of potassium-responsive histidine kinases govern the multicellular destiny of Bacillus subtilis. mBio 6:e00581-15. 10.1128/mBio.00581-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. R., Mouillon J.-M., Pohl S., Arnau J. (2018). Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol. Rev. 42 721–738. 10.1093/femsre/fuy028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G., Deleu M., Jourdan E., Thonart P., Ongena M. (2011). The bacterial lipopeptide surfactin targets the lipid fraction of the plant plasma membrane to trigger immune-related defence responses. Cell. Microbiol. 13 1824–1837. 10.1111/j.1462-5822.2011.01664.x [DOI] [PubMed] [Google Scholar]
- Inaoka T., Wang G., Ochi K. (2009). ScoC regulates bacilysin production at the transcription level in Bacillus subtilis. J. Bacteriol. 191 7367–7371. 10.1128/JB.01081-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. E., Ho L., Rees C. A., Hill J. E., Nodwell J. R., Elliot M. A. (2017). Streptomyces exploration is triggered by fungal interactions and volatile signals. eLife 6:e21738. 10.7554/eLife.21738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M. (2020). Diversity and distribution of volatile secondary metabolites throughout Bacillus subtilis isolates. Front. Microbiol. 11:559. 10.3389/fmicb.2020.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar K. U., Duan Y. P., Nawaz Z., Sun G., Almoneafy A. A., Hassan M. A., et al. (2014). A novel rhizobacterium Bk7 for biological control of brown sheath rot of rice caused by Pseudomonas fuscovaginae and its mode of action. Eur. J. Plant Pathol. 138 819–834. 10.1007/s10658-013-0356-7 [DOI] [Google Scholar]
- Kaspar F., Neubauer P., Gimpel M. (2019). Bioactive secondary metabolites from Bacillus subtilis: a comprehensive review. J. Nat. Prod. 82 2038–2053. 10.1021/acs.jnatprod.9b00110 [DOI] [PubMed] [Google Scholar]
- Kearns D. B., Chu F., Branda S. S., Kolter R., Losick R. (2005). A master regulator for biofilm formation by Bacillus subtilis. Mol. Microbiol. 55 739–749. 10.1111/j.1365-2958.2004.04440.x [DOI] [PubMed] [Google Scholar]
- Khezri M., Jouzani G. S., Ahmadzadeh M. (2016). Fusarium culmorum affects expression of biofilm formation key genes in Bacillus subtilis. Braz. J. Microbiol. 47 47–54. 10.1016/j.bjm.2015.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhl J., Kolnaar R., Ravensberg W. J. (2019). Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front. Plant Sci. 10:1–19. 10.3389/fpls.2019.00845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraas F. I., Helmetag V., Wittmann M., Strieker M., Marahiel M. A. (2010). Functional dissection of surfactin synthetase initiation module reveals insights into the mechanism of lipoinitiation. Chem. Biol. 17 872–880. 10.1016/j.chembiol.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Kröber M., Wibberg D., Grosch R., Eikmeyer F., Verwaaijen B., Chowdhury S. P., et al. (2014). Effect of the strain Bacillus amyloliquefaciens FZB42 on the microbial community in the rhizosphere of lettuce under field conditions analyzed by whole metagenome sequencing. Front. Microbiol. 5:252. 10.3389/fmicb.2014.00252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulimushi P. Z., Arias A. A., Franzil L., Steels S., Ongena M. (2017). Stimulation of fengycin-type antifungal lipopeptides in Bacillus amyloliquefaciens in the presence of the maize fungal pathogen Rhizomucor variabilis. Front. Microbiol. 8:850. 10.3389/fmicb.2017.00850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B., Li Q., Xu Z., Zhang N., Shen Q., Zhang R. (2014). Responses of beneficial Bacillus amyloliquefaciens SQR9 to different soilborne fungal pathogens through the alteration of antifungal compounds production. Front. Microbiol. 5:636. 10.3389/fmicb.2014.00636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Kyle S., Straight P. D. (2018). Antibiotic stimulation of a Bacillus subtilis migratory response. mSphere 3:e00586-17. 10.1128/mSphere.00586-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang N., Qiu M., Feng H., Vivanco J. M., Shen Q., et al. (2014). Enhanced rhizosphere colonization of beneficial Bacillus amyloliquefaciens SQR9 by pathogen infection. FEMS Microbiol. Lett. 353 49–56. 10.1111/1574-6968.12406 [DOI] [PubMed] [Google Scholar]
- López D., Vlamakis H., Losick R., Kolter R. (2009). Cannibalism enhances biofilm development in Bacillus subtilis. Mol. Microbiol. 74 609–618. 10.1111/j.1365-2958.2009.06882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimov I. V., Singh B. P., Cherepanova E. A., Burkhanova G. F., Khairullin R. M. (2020). Prospects and applications of lipopeptide-producing bacteria for plant protection (Review). Appl. Biochem. Microbiol. 56 15–28. 10.1134/S0003683820010135 [DOI] [Google Scholar]
- Martínez-Cámara R., Montejano-Ramírez V., Moreno-Hagelsieb G., Gustavo S., Valencia-Cantero E. (2019). The volatile organic compound dimethylhexadecylamine affects bacterial growth and swarming motility of bacteria. Folia Microbiol. 65 523–532. 10.1007/s12223-019-00756-6 [DOI] [PubMed] [Google Scholar]
- McCully L. M., Bitzer A. S., Seaton S. C., Smith L. M., Silby M. W. (2019). Interspecies Social Spreading: interaction between two sessile soil bacteria leads to emergence of surface motility. mSphere 4:e00696-18. 10.1128/msphere.00696-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes R., Garbeva P., Raaijmakers J. M. (2013). The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 37 634–663. 10.1111/1574-6976.12028 [DOI] [PubMed] [Google Scholar]
- Miethke M., Klotz O., Linne U., May J. J., Beckering C. L., Marahiel M. A. (2006). Ferri-bacillibactin uptake and hydrolysis in Bacillus subtilis. Mol. Microbiol. 61 1413–1427. 10.1111/j.1365-2958.2006.05321.x [DOI] [PubMed] [Google Scholar]
- Miethke M., Schmidt S., Marahiel M. A. (2008). The major facilitator superfamily-type transporter YmfE and the multidrug-efflux activator Mta mediate bacillibactin secretion in Bacillus subtilis. J. Bacteriol. 190 5143–5152. 10.1128/JB.00464-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Santiago C., Pearson J. R., Navarro Y., Berlanga-Clavero M. V., Caraballo-Rodriguez A. M., Petras D., et al. (2019). The extracellular matrix protects Bacillus subtilis colonies from Pseudomonas invasion and modulates plant co-colonization. Nat. Commun. 10:1919. 10.1038/s41467-019-09944-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinatto G., Puopolo G., Sonego P., Moretto M., Engelen K., Viti C., et al. (2016). Complete genome sequence of Bacillus amyloliquefaciens subsp. plantarum S499, a rhizobacterium that triggers plant defences and inhibits fungal phytopathogens. J. Biotechnol. 238 56–59. 10.1016/j.jbiotec.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Müller D. B., Vogel C., Bai Y., Vorholt J. A. (2016). The plant microbiota: systems-level insights and perspectives. Annu. Rev. Genet. 50 211–234. 10.1146/annurev-genet-120215-034952 [DOI] [PubMed] [Google Scholar]
- Nayak S. K., Nayak S., Patra J. K. (2020). “Rhizobacteria and its biofilm for sustainable agriculture: a concise review” in New and Future Developments in Microbial Biotechnology and Bioengineering, eds Singh J. S., Singh D. P. (Amsterdam: Elsevier; ), 165–175. 10.1016/B978-0-444-64279-0.00013-X [DOI] [Google Scholar]
- Niehus R., Picot A., Oliveira N. M., Mitri S., Foster K. R. (2017). The evolution of siderophore production as a competitive trait. Evolution 71 1443–1455. 10.1111/evo.13230 [DOI] [PubMed] [Google Scholar]
- Ola A. R. B., Thomy D., Lai D., Brötz-Oesterhelt H., Proksch P. (2013). Inducing secondary metabolite production by the endophytic fungus Fusarium tricinctum through coculture with Bacillus subtilis. J. Nat. Prod. 76 2094–2099. 10.1021/np400589h [DOI] [PubMed] [Google Scholar]
- Olishevska S., Nickzad A., Déziel E. (2019). Bacillus and Paenibacillus secreted polyketides and peptides involved in controlling human and plant pathogens. Appl. Microbiol. Biotechnol. 103 1189–1215. 10.1007/s00253-018-9541-0 [DOI] [PubMed] [Google Scholar]
- Ongena M., Jacques P. (2008). Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 16 115–125. 10.1016/j.tim.2007.12.009 [DOI] [PubMed] [Google Scholar]
- Pandin C., Darsonval M., Mayeur C., Le Coq D., Aymerich S., Briandet R. (2019). Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl. Environ. Microbiol. 85:e00327-19. 10.1128/AEM.00327-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandin C., Le Coq D., Canette A., Aymerich S., Briandet R. (2017). Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb. Biotechnol. 10 719–734. 10.1111/1751-7915.12693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandin C., Le Coq D., Deschamps J., Védie R., Rousseau T., Aymerich S., Briandet R. (2018). Complete genome sequence of Bacillus velezensis QST713: a biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J. Biotechnol. 278 10–19. 10.1016/j.jbiotec.2018.04.014 [DOI] [PubMed] [Google Scholar]
- Piel J. (2010). Biosynthesis of polyketides by trans-AT polyketide synthases. Nat. Prod. Rep. 27 996–1047. 10.1039/b816430b [DOI] [PubMed] [Google Scholar]
- Pieterse C. M. J., Zamioudis C., Berendsen R. L., Weller D. M., Van Wees S. C. M., Bakker P. A. H. M. (2014). Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52 347–375. [DOI] [PubMed] [Google Scholar]
- Powers M. J., Sanabria-Valentín E., Bowers A. A., Shank E. A. (2015). Inhibition of cell differentiation in Bacillus subtilis by Pseudomonas protegens. J. Bacteriol. 197 2129–2138. 10.1128/JB.02535-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi G., Zhu F., Du P., Yang X., Qiu D., Yu Z., et al. (2010). Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 31 1978–1986. 10.1016/J.PEPTIDES.2010.08.003 [DOI] [PubMed] [Google Scholar]
- Qiao J. Q., Yu X., Liang X. J., Liu Y. F., Borriss R., Liu Y. Z. (2017). Addition of plant-growth-promoting Bacillus subtilis PTS-394 on tomato rhizosphere has no durable impact on composition of root microbiome. BMC Microbiol. 17:131. 10.1186/s12866-017-1039-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers J. M., de Bruijn I., Nybroe O., Ongena M. (2010). Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol. Rev. 34 1037–1062. 10.1111/j.1574-6976.2010.00221.x [DOI] [PubMed] [Google Scholar]
- Raaijmakers J. M., Mazzola M. (2012). Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 50 403–424. 10.1146/annurev-phyto-081211-172908 [DOI] [PubMed] [Google Scholar]
- Rabbee M., Ali M., Choi J., Hwang B., Jeong S., Baek K., et al. (2019). Bacillus velezensis: a valuable member of bioactive molecules within plant microbiomes. Molecules 24:1046. 10.3390/molecules24061046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D., de Vicente A., Rakotoaly R. H., Dufour S. E., Veening J.-W., Arrebola E., et al. (2007). The Iturin and Fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant Microbe Interact. 20 430–440. 10.1094/MPMI-20-4-0430 [DOI] [PubMed] [Google Scholar]
- Santoyo G., Hernández-Pacheco C., Hernández-Salmerón J., Hernández-León R. (2017). The role of abiotic factors modulating the plant-microbe-soil interactions: toward sustainable agriculture. A review. Span. J. Agric. Res. 15 1–15. [Google Scholar]
- Schmidt R., Cordovez V., De Boer W., Raaijmakers J., Garbeva P. (2015). Volatile affairs in microbial interactions. ISME J. 9 2329–2335. 10.1038/ismej.2015.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Ulanova D., Wick L., Bode H., Garbeva P. (2019). Microbe-driven chemical ecology: past, present and future. ISME J. 13 2656–2663. 10.1038/s41396-019-0469-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R., Vater J., Budiharjo A., Wang Z., He Y., Dietel K., et al. (2014). Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 196 1842–1852. 10.1128/JB.01474-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank E. A., Klepac-Ceraj V., Collado-Torres L., Powers G. E., Losick R., Kolter R. (2011). Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proc. Natl. Acad. Sci. U.S.A. 108 E1236– E1243. 10.1073/pnas.1103630108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight P. D., Fischbach M. A., Walsh C. T., Rudner D. Z., Kolter R. (2007). A singular enzymatic megacomplex from Bacillus subtilis. Proc. Natl. Acad. Sci. U.S.A. 104 305–310. 10.1073/pnas.0609073103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir H. A. S., Gu Q., Wu H., Raza W., Safdar A., Huang Z., et al. (2017). Effect of volatile compounds produced by Ralstonia solanacearum on plant growth promoting and systemic resistance inducing potential of Bacillus volatiles. BMC Plant. Biol. 17:133. 10.1186/s12870-017-1083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Sekhar A. C. (2016). Effects due to rhizospheric soil application of an antagonistic bacterial endophyte on native bacterial community and its survival in soil: a case study with Pseudomonas aeruginosa from banana. Front. Microbiol. 7:493. 10.3389/fmicb.2016.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres Manno M. A., Pizarro M. D., Prunello M., Magni C., Daurelio L. D., Espariz M. (2019). GeM-Pro: a tool for genome functional mining and microbial profiling. Appl. Microbiol. Biotechnol. 103 3123–3134. 10.1007/s00253-019-09648-8 [DOI] [PubMed] [Google Scholar]
- Townsley L., Shank E. A. (2017). Natural-product antibiotics: cues for modulating bacterial biofilm formation. Trends Microbiol. 25 1016–1026. 10.1016/j.tim.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler M. F., Kolter R. (2015). Natural products in soil microbe interactions and evolution. Nat. Prod. Rep. 32 956–970. 10.1039/c5np00013k [DOI] [PubMed] [Google Scholar]
- Tyc O., Song C., Dickschat J. S., Vos M., Garbeva P. (2017). The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 25 280–292. 10.1016/j.tim.2016.12.002 [DOI] [PubMed] [Google Scholar]
- van Gestel J., Vlamakis H., Kolter R. (2015). From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate. PLoS Biol. 13:e1002141. 10.1371/journal.pbio.1002141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kuijk S., Noll K. S., Chikindas M. L. (2012). The species-specific mode of action of the antimicrobial peptide subtilosin against Listeria monocytogenes Scott A. Lett. Appl. Microbiol. 54 52–58. 10.1111/j.1472-765X.2011.03170.x [DOI] [PubMed] [Google Scholar]
- Vargas-Bautista C., Rahlwes K., Straight P. (2014). Bacterial competition reveals differential regulation of the pks genes by Bacillus subtilis. J. Bacteriol. 196 717–728. 10.1128/JB.01022-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhams G. H., Armitage J. P. (2004). Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5 1024–1037. 10.1038/nrm1524 [DOI] [PubMed] [Google Scholar]
- Winn M., Fyans J. K., Zhuo Y., Micklefield J. (2016). Recent advances in engineering nonribosomal peptide assembly lines. Nat. Prod. Rep. 33 317–347. 10.1039/c5np00099h [DOI] [PubMed] [Google Scholar]
- Wise C., Falardeau J., Hagberg I., Avis T. J. (2014). Cellular lipid composition affects sensitivity of plant pathogens to Fengycin, an antifungal compound produced by Bacillus subtilis strain CU12. Phytopathology 104 1036–1041. 10.1094/PHYTO-12-13-0336-R [DOI] [PubMed] [Google Scholar]
- Wu B., Wang X., Yang L., Yang H., Zeng H., Qiu Y. M., et al. (2016). Effects of Bacillus amyloliquefaciens ZM9 on bacterial wilt and rhizosphere microbial communities of tobacco. Appl. Soil Ecol. 103 1–12. 10.1016/j.apsoil.2016.03.002 [DOI] [Google Scholar]
- Zhang L., Sun C. (2018). Fengycins, cyclic lipopeptides from marine Bacillus subtilis Strains, kill the plant-pathogenic fungus Magnaporthe grisea by inducing reactive oxygen species production and chromatin condensation. Appl. Environ. Microbiol. 84:e00445-18. 10.1128/AEM.00445-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Kuipers O. P. (2016). Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genomics 17:882. 10.1186/s12864-016-3224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi Y., Wu Q., Xu Y. (2017). Genome and transcriptome analysis of surfactin biosynthesis in Bacillus amyloliquefaciens MT45. Sci. Rep. 7:40976. 10.1038/srep40976 [DOI] [PMC free article] [PubMed] [Google Scholar]