Abstract
Background
Acute respiratory distress syndrome (ARDS) is a common and disabling disease with high rates of mortality and morbidity. The role of steroids in treating ARDS remains controversial. We aim to examine the evidence behind using glucocorticoids in the management of ARDS from the available studies.
Methods
We performed a literature review of major electronic databases for randomized controlled trials (RCTs) comparing glucocorticoids versus placebo in treating patients with ARDS. Our primary outcome was hospital mortality. Other outcomes included ICU mortality, number of ventilator-free days at day 28, incidence of nosocomial infections, and hyperglycemia. We performed a meta-analysis using a random effects model to calculate risk ratios (RR) and mean difference (MD) with their corresponding 95% confidence intervals (CI). A subsequent trial sequential analysis was performed to examine the strength of evidence and to guard against statistical type I and type II errors for our results.
Results
Eight RCTs were included in the final analysis totaling of 1091 patients, with a mean age of 57 ± 16, and 56.2% were male. In our pooled analysis, use of glucocorticoids was associated with a significant reduction in hospital mortality (RR 0.79; 95% CI 0.64–0.98; P = 0.03) and ICU mortality (RR 0.64; 95% CI 0.42–0.97; P = 0.04). Furthermore, glucocorticoid use was associated with an increased number of ventilator-free days at day 28 (MD 4.06 days; 95% CI 2.66–5.45; P < 0.01). Regarding adverse events, glucocorticoids use was not associated with an increased risk for nosocomial infections (RR 0.82; 95% CI 0.68–1.00; P = 0.05); however, it was associated with an increased risk of hyperglycemia (RR 1.11; 95% CI 1.01–1.24; P = 0.04). In our trial sequential analysis, the required diversity-adjusted information size (sample size = 2692 patients) was not reached, and the evidence was insufficient from the available RCTs.
Conclusion
Among patients with ARDS, use of glucocorticoids is associated with a significant reduction in mortality and duration of mechanical ventilation, without increased risk of hospital-acquired infections. However, based on a trial sequential analysis, these findings may be secondary to a false-positive (type I) error. Further studies are needed for a firm conclusion with guarding against possible statistical errors.
Keywords: Glucocorticoids, Corticosteroids, Acute respiratory distress syndrome, ARDS, Meta-analysis
Introduction
Acute respiratory distress syndrome (ARDS) is a common and disabling syndrome with high rates of mortality and morbidity. It affects 10% of patients admitted to intensive care units (ICUs) and almost 23% of mechanically ventilated patients. Additionally, ARDS has been found to have up to 35–45% mortality rate [1–3].
A recently published randomized controlled trial (RCT) showed a significant reduction in short-term and long-term mortality of ARDS patients who received dexamethasone within 24 h of ARDS onset [4]. In addition, an analysis involving individual patients’ data of four randomized controlled trials (RCTs) showed significant improvement in mortality and several other clinical outcomes with glucocorticoid use in ARDS patients [5]. However, their use in ARDS is still controversial, and the current society of critical care medicine guidelines have conditional recommendations for the use of glucocorticoids in patients with moderate-to-severe ARDS [6].
In this meta-analysis, we aim to examine the efficacy and safety of glucocorticoids in ARDS, as well as examine the strength of current evidence based on the available RCTs by performing a trial sequential analysis.
Methodology
Study design and study selection
Our study is a meta-analysis of RCTs performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) 2015 Statement [7]. Literature search utilizing major electronic databases including PubMed, Cochrane library, and Embase was conducted separately and independently by two reviewers (V.S.) and (M.S.) from inception to March 2020. Articles were first screened by abstracts and titles before exclusion. Review of full texts of eligible articles was performed before final inclusion or exclusion. Mesh term used: (“acute lung injury” OR “acute respiratory distress syndrome” OR “ARDS”) AND (“glucocorticoids” OR “corticosteroid” OR “steroids” OR “methylprednisolone” OR “dexamethasone” OR “hydrocortisone” OR “prednisolone”). In addition, references of relevant articles were reviewed for possible inclusion. Any discrepancy between the two reviewers was resolved by a third author (Y.Z.).
Inclusion and exclusion criteria
We included only RCTs that evaluated the role of glucocorticoids in the management of critically ill adult patients with established respiratory failure secondary to ARDS; ARDS was defined as acute hypoxemic respiratory failure with presence of bilateral infiltrates on chest imaging, PaO2/FiO2 < 300 without evidence of left ventricular failure or hydrostatic edema. Studies that examined prophylactic effects of glucocorticoids in patients at high risk for ARDS were excluded. In addition, we excluded studies with a high risk of bias as well as studies unavailable in English.
Two reviewers (E.I. and J.K.) extracted the data into predesigned tables independently and separately. Any discrepancy was resolved by a third reviewer (Y.Z.).
Quality assessment
Quality assessment of the included RCTs was performed using the Cochrane Collaboration’s tool for assessing risk of bias in randomized controlled trials [8]. Random sequence generation, allocation concealment, blindness of participants and health-care personnel, blindness of outcome assessment, incomplete outcome data, selective reporting, and other biases if any were present were assessed for each of the included RCTs based on authors’ judgement.
Outcomes
Our primary outcome was in-hospital mortality defined as mortality before hospital discharge (if in-hospital mortality was not provided, we utilized the 60-day mortality or mortality at longest follow-up duration provided by each study in order of preference). Secondary outcomes included ICU mortality and number of ventilator free days at day 28. Safety outcomes included incidence of nosocomial infections and incidence of hyperglycemia.
Statistical analysis
Pooled risk ratios (RR) with their corresponding 95% confidence intervals (CI) for dichotomous data were calculated using the random Mantel-Haenszel method. We calculated weighted mean difference (MD) and their 95% corresponding confidence intervals for continuous variables using an inverse variance test. Heterogeneity was assessed using Cochrane Q and I2 tests. Sensitivity analysis was performed by sequential removal of trials for each outcome. In addition, we conducted a subgroup analysis based on timing of glucocorticoids administration, early (less than 7 days of ARDS onset) vs late (> 7 days of ARDS onset), severity of ARDS, and whether studies used lung protective ventilations or not. Further meta-regression analyses were performed based on the study-level covariates [age, duration of glucocorticoids treatment (days), daily dose of glucocorticoid equivalent to prednisone, age, mean positive end-expiratory pressure, partial pressure of arterial oxygen to fractional inspired oxygen (PaO2/FiO2)]. Revman v5.3 windows and the Comprehensive Meta-Analysis v3 software were used for the analysis.
Trial sequential analysis
To examine the strength of our results, we applied trial sequential analysis (TSA) boundaries to the meta-analysis to guard against the risk of false-positive (type I error) or false-negative (type II error) results [9]. We performed our analysis to maintain an overall two-sided type I error at 5% and to provide 80% power to calculate the diversity-adjusted information size in order to examine if the conclusion is sufficient or if further studies are needed to detect 20% relative risk reduction (RRR) of hospital and ICU mortality between the two groups. Further analyses were performed to calculate sample size required for 15% and 25% relative risk reduction of mortality. TSA software, Copenhagen Trial Unit, version 0.9.5.10 Beta was used to conduct the analysis.
Results
Summary of included studies
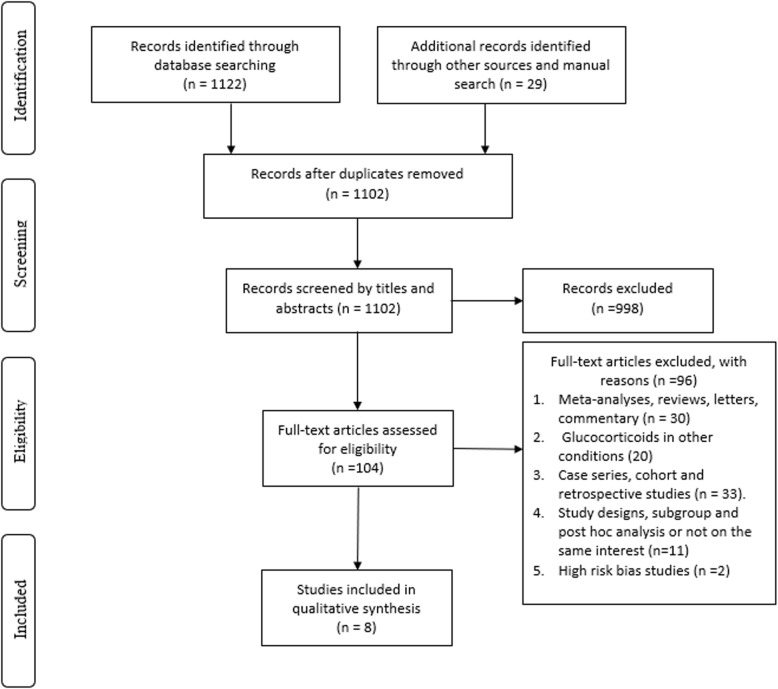
After review of electronic databases, we included 8 RCTs totaling 1091 patients with a mean age 57 ± 16 years, and 56.2% were male [4, 10–16]. Figure 1 illustrates the search process and study selection. Six trials initiated glucocorticoids treatment within 7 days of ARDS onset, while 2 trials initiated glucocorticoid treatment after 7 days of ARDS onset [11, 13]. Glucocorticoids were administered for a total of 7–14 days in five of the included trials. Two trials used extended duration of glucocorticoids (28–32 days) [12, 13]. In addition, one trial administered high-dose methylprednisolone (120 mg/Kg divided on 4 doses) for only 24 h [16]. Two studies were excluded from the final analysis due to high risk of bias concerning the blinding of participants and investigators [17, 18]. Table 1 explains the characteristics of included studies while Table 2 explains the demographic and clinical characteristics of the included patient populations in each study.
Fig. 1.
Flow chart of literature search and study selection
Table 1.
Characteristics of included studies
| Study name (first author and year) | Study design | Study groups and patients’ number | Inclusion criteria | Treatment regimen | Duration of treatment | Follow-up duration |
|---|---|---|---|---|---|---|
| Bernard 1987 | Multicenter randomized controlled trial |
Total patients, 99 Steroids, 50 Placebo, 49 |
Adult patients having ARDS withPaO2 ≤ 7 0mmhg (with FiO2 at least 40%) or PaO2/PAO2 ≤ 0.3; bilateral diffuse infiltrates on chest radiography, PAWP ≤ 18mmhg regardless of PEEP level. |
-Treatment started after ARDS onset. Methylprednisolone 30 mg every 6 h (4 doses only). |
24 h | Until death or for 45 days |
| Meduri 1998 |
Multicenter randomized controlled trial |
Total Patients, 24 Steroids, 16 Placebo, 8 |
Adult patients with hypoxemic respiratory failure diagnosed with ARDS, who were on mechanical ventilation for at least 7 days, with LIS of 2.5 or greater and less than 1-point reduction from day 1 of ARDS onset. |
-Treatment started after 7 days of ARDS onset. -Methylprednisolone 2 mg/kg per day (2 mg/kg from day 1 to day 14; 1 mg/kg from day 15 to 21; 0.5 mg/kg from day 22 to day 28; 0.25 mg/day from day 28 to day 32). |
32 days | Hospital length of stay. |
| Confalonieri 2005 | Multicenter randomized controlled trial |
Total patients, 46 Steroids, 22 Placebo, 23 |
Patients with clinical and radiographic evidence of pneumonia with bilateral or multi-lobar involvement and PaO2/FiO2 ratio less than 250. |
-Treatment started after diagnosis. -Hydrocortisone 200 mg bolus followed by an infusion of 10 mg/h. |
7 days | 60 days |
| Annane 2006 | Multicenter randomized controlled trial |
Total patients, 177 Steroids, 85 Placebo, 92 |
Patients with septic shock and ARDS; bilateral infiltrates on chest radiography; PaO2/FiO2 ≤ 200, PAWP ≤ 18 mmhg; no left atrial hypertension. |
-Treatment started after randomization which occurred within 8 h of disease onset. -Hydrocortisone 50 mg every 6 h and 9 alpha fludrocortisone 50 milligram orally once a day. |
7 days | 28 days |
| Steinberg 2006 | Multicenter randomized control trial |
Total patients, 180 Steroids, 89 Placebo, 91 |
Adult patients who had ARDS and mechanically ventilated for 7 to 28 days. PaO2/FiO2 ≤ 200 mmhg. |
-Treatment started after 7 to 28 days of ARDS onset. -Methylprednisolone: bolus 2 mg/kg followed by 0.5 mg/kg every 6 h for 14 days and then 0.5 mg/kg every 12 hours for 7 days and then tapering over 4days. |
21-25 days. | 60 days |
| Meduri 2007 | Multicenter randomized control trial |
Total patients, 91 Steroids, 63 Placebo, 28 |
Adult intubated patients with early ARDS (≤ 72 h) defined by the American-European Consensus definition. |
-Treatment started within 72 h of ARDS onset. -Methylprednisolone bolus dose of 1 mg/kg followed by an infusion of 1 mg/kg per day from day 1 to day 14; 0.5 mg/kg per day from day 15 to day 21; 0.25 mg/kg per day from day 22 to day 25; and 0.125 mg/kg per day from day 26 to day 28. |
Up to 28 days | Hospital length of stay. |
| Tongyoo 2016 | Single-center randomized controlled trial |
Total patients, 197 Steroids, 98 Placebo, 99 |
Adult patients with severe sepsis or septic shock, intubated with ARDS (according to criteria of ARDS by the American European Consensus or by the berlin criteria as moderate to severe ARDS) |
-Randomization within 12 h of ARDS onset. -Hydrocortisone 50 mg every 6 h for 7 days |
7 days | 28 days |
| Villar 2020 | Multicenter randomized control trial |
Total patients, 277 Steroids, 139 Placebo, 138 |
Adult intubated patients with acute onset of ARDS according to criteria of ARDS by the American European Consensus or by the berlin criteria as moderate to severe ARDS. | Dexamethasone 20 mg daily from day 1 to day 5, then 10 mg daily from day 6 to day 10. | 10 days | 60 days |
ARDS acute respiratory distress syndrome, PaO2 partial pressure pf arterial oxygen, PAWP pulmonary artery wedge pressure, PEEP positive end expiratory pressure, LIS lung injury score, FiO2 fraction of inhaled oxygen, APACHE acute physiologic and chronic health evaluation, MMHG millimeter of mercury, MG milligram, KG kilogram
Table 2.
Baseline clinical characteristics of patients
| Study | Study groups | Total number | Age | Male No. (%). | APACHE III score | Respiratory rate | PaO2/FiO2 | PEEP |
|---|---|---|---|---|---|---|---|---|
| Bernard 1987 | Steroid | 50 | 55 ± 2 | NA | NA | NA | NA | 8 ± 1 |
| Control | 49 | 56 ± 2 | NA | NA | NA | NA | 7 ± 1 | |
| Meduri 1998 | steroid | 16 | 47 ± 3.9 | 5 (31%) | 58(14) | NA | 161(14) | 12(1.2) |
| control | 8 | 51 ± 6.6 | 4 (50%) | 55(16) | NA | 141(19) | 14(1.7) | |
| Confalonieri 2005 | steroid | 23 | 60.4 ± 17.3 | 17 (74%) | 17.2 ± 4.1 | NA | 141 ± 49 | NA |
| control | 23 | 66.6 ± 14.7 | 15 (65%) | 18.2 ± 4 | NA | 178 ± 58 | NA | |
| Annane 2006 | steroid | 85 | 61 ± 16 | 56 (66 %) | NA | 18.5 ± 3 | 104 ± 42 | 6.8 ± 2.7 |
| control | 92 | 59 ± 18 | 65 (70%) | NA | 17.9 ± 3.1 | 108 ± 45 | 7.4 ± 3 | |
| Steinberg 2006 | steroid | 89 | 49 ± 19 | 40 (45%) | 87.6 ± 27.5 | NA | 126 ± 42 | 12.9 ± 5.6 |
| control | 91 | 49.2 ± 16.5 | 58 (64%) | 84.6 ± 29.4 | NA | 126 ± 40 | 12.3 ± 4.7 | |
| Meduri 2007 | steroid | 63 | 50.1 ± 15.3 | 34 (54%) | 60.2 ± 20.2 | NA | 118.4 ± 51.2 | 13 ± 5 |
| control | 28 | 53.2 ± 15.3 | 13 (46%) | 57.9 ± 21 | NA | 125.9 ± 38.6 | 11.2 ± 4 | |
| Tongyoo 2016 | steroid | 98 | 64.5 ± 17.3 | 50 (51%) | 21.7 ± 5.7 | NA | 175.4 ± 6.9 | 7.3 ± 3 |
| control | 99 | 64.3 ± 16 | 51 (52%) | 21.9 ± 5.7 | NA | 172.4 ± 6.7 | 6.8 ± 2.5 | |
| Villar 2020 | steroid | 139 | 56 ± 14 | 96 (69%) | NA | 23(5) | 142.4 ± 37.3) | 12.6 ± 2.7 |
| control | 138 | 58 ± 15 | 95 (69%) | NA | 23(5) | 143.5 ± 33.4 | 12.5 ± 2.6 |
Data are provided number and percent (%) or mean ± SD. PaO2 partial pressure pf arterial oxygen, PEEP positive end expiratory pressure, FiO2 fraction of inhaled oxygen, APACHE acute physiologic and chronic health evaluation
Figure 2 explains the results of the quality assessment based on authors’ judgment.
Fig. 2.
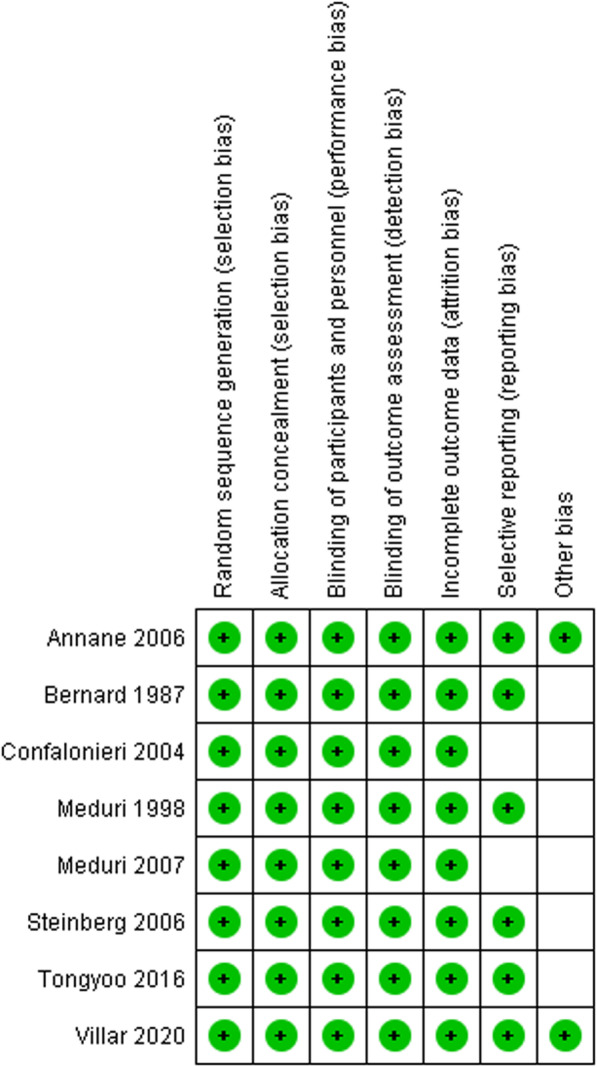
Risk of bias assessment based on authors’ judgment for each of the included RCTs. Blank items indicate an unclear risk of bias
Clinical outcomes
Hospital and ICU-mortality
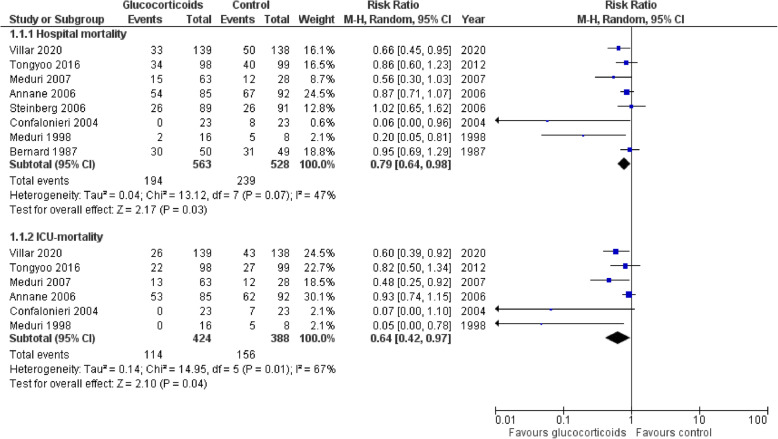
Use of glucocorticoids was associated with a significant reduction of hospital mortality (RR 0.79; 95% CI 0.64–0.98; P = 0.03; I2 = 47%) and ICU mortality (RR 0.64; 95% CI 0.42–0.97; P = 0.04, I2 67%) (Fig. 3). Sensitivity analysis with sequential trial removal revealed consistent results.
Fig. 3.
Forest plot for hospital and ICU mortality
Subgroup analysis showed that there was no hospital mortality benefit with late administration (more than 7 days of ARDS onset) of glucocorticoids (RR 0.52; 95% CI 0.11–2.52; P = 0.42; 2 studies, 204 patients) while mortality benefit remained significant with early glucocorticoids administration (RR 0.80; 95% CI 0.65–0.98; P = 0.03; 6 studies, 887 patients). Further subgroup analysis revealed that there was no significant difference in hospital mortality in studies that used glucocorticoids without lung protective ventilation (RR 0.79; 95% CI 0.58–1.07; P = 0.12; 6 studies, 667 patients), while the mortality reduction remained significant in studies incorporating a lung protective ventilation strategy (RR 0.75; 95% CI 0.58–98; P = 0.04; 2 studies, 474 patients). Meta-regression analysis did not suggest any effects of the study-level covariates on hospital mortality; however, prolonged duration of glucocorticoid treatment and higher PEEP were associated with decreased ICU mortality (P < 0.05) (Supplementary Figure 1 & 2).
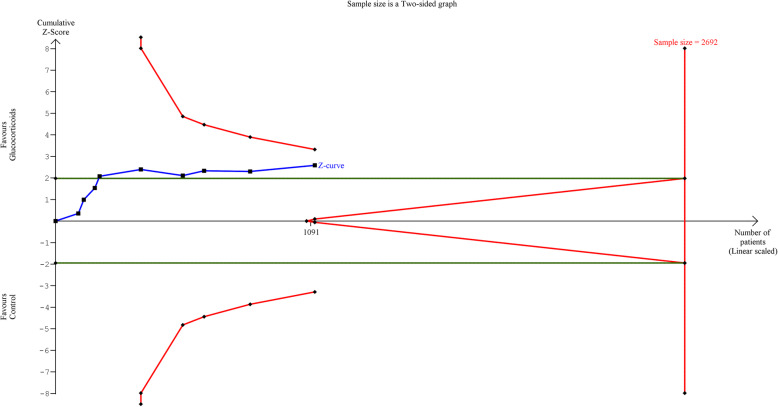
In a trial sequential analysis for hospital mortality, the cumulative Z-curve crossed the Alpha boundary of significance, indicating sufficient statistical significance favoring glucocorticoids over the control group. However, since the cumulative Z-curve failed to cross the TSA boundary and the diversity-adjusted information size (sample size) calculated (2692 patients) was not reached, the conclusion is insufficient, and further studies are needed (Fig. 4). Similarly, regarding ICU mortality, the conclusion was insufficient, and further studies are needed (Supplementary Figure 3). Similar conclusions were obtained upon performing sensitivity analyses with 15% and 25% RRR in mortality.
Fig. 4.
Trial sequential analysis for hospital mortality. The diversity-adjusted information size (sample size) is 2692 patients. The cumulative Z-line (blue line with small black squares representing each trial) crosses the alpha monitoring boundary (horizontal green line) indicating statistical significance for the efficacy of glucocorticoids. However, The Z-line failed to cross the TSA boundary (concave red line), and since the required sample size was not reached, there is lack of firm evidence supporting improved hospital mortality in the glucocorticoids group
Number ventilator-free days at day 28
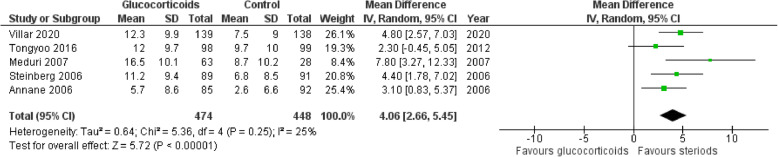
There was a significant increase in the number of ventilator-free days at day 28 in patients treated with glucocorticoids in comparison to the control group (MD 4.06 days; 95% CI 2.66–5.45; P < 0.01; I2 = 25%) (Fig. 5).
Fig. 5.
Forest plot for number of ventilator-free days at day 28
Adverse events
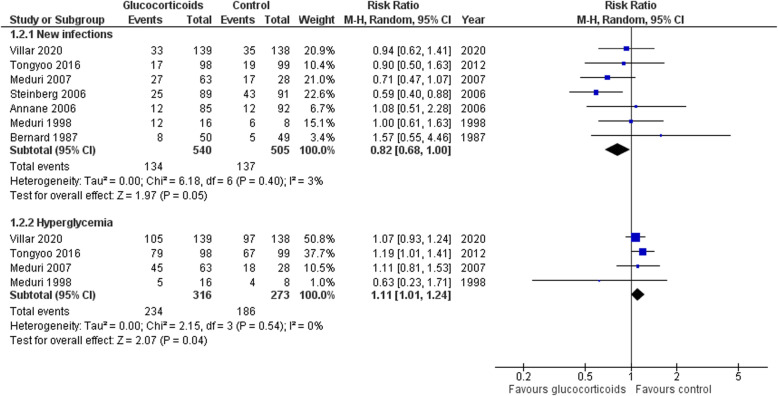
Use of glucocorticoids was not associated with an increased risk of hospital-acquired infections (RR 0.82; 95% CI 0.68–1.00; P = 0.05; I2 = 3%) but was associated with an increased risk of hyperglycemia (RR 1.11; 95% CI 1.01–1.24; P = 0.04; I2 = 0%) (Fig. 6). Meta-regression analysis did not suggest any covariates effects on the adverse events.
Fig. 6.
Forest plot for adverse events, infection, and hyperglycemia
Discussion
In our meta-analysis, use of glucocorticoids in patients with ARDS was associated with a significant reduction in hospital and ICU mortality and duration of mechanical ventilation. While there was no increased risk of hospital-acquired infections with glucocorticoid use, there was an increased risk of hyperglycemia. In trial sequential analysis, these findings could be secondary to a false-positive (type I) error, and further studies are needed for sufficient evidence as the required sample size was not reached by the available RCTs.
Current guidelines of American Thoracic Society/European Society of intensive care medicine/Society of Critical Care Medicine have strong recommendations for the use of low tidal volume (4–8 ml/kg of ideal body weight), limiting inspiratory pressure (plateau pressure < 30 cm H2O), and prone positioning in moderate-to-severe ARDS. Furthermore, the use of recruitment maneuvers and higher PEEP strategies have conditional recommendations in patients with moderate-to-severe ARDS. In addition, glucocorticoids have a conditional recommendation in early moderate-to-severe ARDS, and their use is still controversial [6, 19].
There are three distinct phases in the development of ARDS including exudative, proliferative, and fibrotic phases [1]. As lung fibrosis is associated with increased duration of mechanical ventilation and increased rates of mortality, steroids are considered a potent anti-inflammatory agent that can attenuate the inflammatory process and subsequently decrease further lung injury and fibrosis [1].
A recently published randomized controlled trial revealed that early use of dexamethasone in patients with moderate-to-severe ARDS was associated with a significant reduction in mortality and duration of mechanical ventilation [4]. Similar results were noticed in patients with sepsis or septic shock with moderate-to-severe ARDS treated with methylprednisolone in comparison to placebo [10]. In these two recent trials, glucocorticoid use was evaluated with lung protective mechanical ventilation and low tidal volume, as opposed to the other trials conducted before 2005 where low tidal volumes were not implemented in the trial protocols. This strategy which limits tidal volume to 4–8 ml/kg of ideal body weight and alveolar pressure to less than 30 cm H2O showed a significant reduction in mortality and increased number of ventilator-free days at day 28 [20].
In our subgroup analysis, we found that there was no mortality benefit in studies that evaluated glucocorticoids without a lung-protective ventilation strategy likely secondary to worsening lung injury. High tidal volumes delivered to an already injured lung may worsen lung injury leading to alveolar rupture, air leaks, and barotrauma with worse clinical outcomes [20–22]. Furthermore, we found that late administration of glucocorticoids (after 7 days of ARDS onset) was not associated with improved outcomes despite lower risk ratio (0.52) but with a high p value, a finding that is limited by the low number of patients and studies in this subgroup (2 studies, 204 patients). However, this supports the concept that steroids exert their action through downregulation of the inflammatory response and decrease alveolar capillary permeability which occurs early in the exudative phase and is linked to lung injury [1]. In exploratory meta-regression, we found that patients who were treated with prolonged duration of glucocorticoid administration and received higher PEEP had lower ICU but not hospital mortality; a finding that is limited by the low number of studies that reported ICU mortality and needs to be examined in further trials.
Our analysis revealed a 21% risk reduction in hospital mortality among ARDS patients treated with glucocorticoids with a number needed to treat of nine patients to prevent one death. In addition, there was a 4-day increase in the number of ventilator-free days at day 28. Despite these favorable outcomes, there was no increased risk of hospital acquired infections; in contrast, our analysis showed a tendency toward reduction of acquired infections, a finding that could be explained by the decreased duration of mechanical ventilation and subsequently ICU length of stay. However, these findings should be interpreted cautiously until confirmed in further larger studies.
In order to examine the strength of the evidence and whether more randomized controlled trials are needed for sufficient conclusion regarding mortality benefit, we performed a trial sequential analysis to guard against false positive (type I) or false negative (type II) errors. While the mortality benefit reached statistical significance, based on our analysis, the mortality benefit could be secondary to a false positive (type I) error, and the evidence is insufficient as the sample size required for detection of 20% RRR in mortality between the two groups while avoiding statistical errors is 2692 patients that was not reached by the available data (1091) patients were included in our analysis). Further, well-controlled randomized clinical trials are required for a strong conclusion about the efficacy of steroids in managing ARDS patients. Additionally, the focus should be on the type, dose, and duration of glucocorticoids therapy as we included studies that evaluated different glucocorticoids with variable dosages and durations. However, until further studies are performed, the significant risk reduction and the low number needed to treat may justify the use of glucocorticoids in patients with ARDS, especially those with an underlying etiology similar to patients enrolled in the included RCTs (sepsis, septic shock, and pneumonia).
Our results are consistent with previously published meta-analyses. However, we included the recently published trial and only included patients with established ARDS, and we excluded studies with high risk of bias as well as retrospective studies which were included in previous reviews [23, 24]. Furthermore, we were able to perform subgroup and meta-regression analyses based on study-level covariates. In addition, we conducted trial sequential analysis to examine the strength of the evidence and concluded that further studies are needed for a strong and firm evidence of glucocorticoids efficacy in ARDS patients with paying special attention of the duration, dose, and timing of glucocorticoids administration.
Our results are non-generalizable to patients with ARDS secondary to coronavirus disease-2019 (COVID-19) and other viral pneumonias such as H1N1 influenza. To date, there is only one retrospective study that examined outcomes of COVID-19 patients treated with steroids. The study by Wu et al. found lower risk of death (hazard ratio 0.38; 95% CI 0.20–0.72; p = 0.003) in patients with ARDS treated with methylprednisolone [25]. In addition, a non-peer reviewed article was published recently and reported reduction in the duration of supplemental oxygen and improved radiographic findings in 26 patients with severe COVID-19 but it is unknown how many patients had ARDS in this cohort [26]. Since we lack patient-level data and the information are missing in the published literature, we were unable to perform a subgroup analysis for patients with viral etiology of ARDS. However, it is known that glucocorticoids are associated with worse outcomes in patients with ARDS secondary to H1N1 influenza virus as demonstrated by previous cohort studies and meta-analyses [27–29]. Based on the current available evidence for the management of COVID-19 patients, Society of Critical Care Medicine and European Society of intensive care medicine guidelines have recommended against the use of corticosteroids in mechanically ventilated patients without ARDS and issued a weak recommendation for the use of low dose steroids (hydrocortisone 200 mg per day) in those with ARDS and/or refractory septic shock [30].
Limitations
Our study has several limitations. There is significant advancement in critical care management between older and modern studies as most of the studies did not adopt lung-protective ventilation. Second, we included RCTs that investigated different types and dosages of glucocorticoids with various durations. Third, as we lack patient-level data, we could not perform analyses based on the severity and underlying etiology of ARDS.
Conclusion
Among patients with ARDS, use of glucocorticoids is associated with significant reduction in mortality and duration of mechanical ventilation without an increased risk of infection but with an increased incidence of hyperglycemia. In our trial sequential analysis, we revealed that the evidence is insufficient from the available RCTs, and further studies are required for a firm conclusion with guarding against possible statistical errors.
Supplementary information
Additional file 1: Supplementary Figure 1. Regression of duration of glucocorticoid treatment on ICU mortality. Longer duration was associated with lower rates of ICU mortality (P < 0.05).
Additional file 2: Supplementary Figure 2. Regression of PEEP on ICU mortality. Higher PEEP was associated with lower rates of ICU mortality (P < 0.05).
Additional file 3: Supplementary Figure 3. Trial sequential analysis for ICU-mortality. The diversity-adjusted information size (sample size) is 3,244 patients. The cumulative Z-line (blue line with small black squares representing each trial) crosses the alpha monitoring boundary (horizontal green line) indicating statistical significance for the efficacy of glucocorticoids. However, The Z-line failed to cross the TSA boundary (concave red line), and since the required sample size was not reached, there is lack of firm evidence supporting improved hospital mortality in the glucocorticoids group.
Acknowledgements
NA
Authors’ contributions
YZ: study design, literature search, data analysis, data extraction, drafting the manuscript, and final approval of the manuscript. M.B.: study design, data analysis, drafting the manuscript, and final approval of the manuscript. E.I.: literature search, data extraction, drafting the manuscript, and final approval of the manuscript. V.S.: literature search, drafting the manuscript, and final approval of the manuscript. J.K..: data extraction, drafting the manuscript, and final approval of the manuscript. F.R.: drafting the manuscript and final approval of the manuscript. M.S.: literature search, drafting the manuscript, and final approval of the manuscript. A.B.: data analysis, literature search, drafting the manuscript, and final approval of the manuscript. M.O: literature search, drafting the manuscript, and final approval of the manuscript. S. Demian: data extraction, drafting the manuscript, and final approval of the manuscript. S. Deliwala: drafting the manuscript and final approval of the manuscript. I.A.: drafting the manuscript and final approval of the manuscript. R.R.: study design, data analysis, literature search, drafting the manuscript, and final approval of the manuscript.
Funding
There was no funding for this work.
Availability of data and materials
Data and materials are available and can be presented upon request.
Ethics approval and consent to participate
NA
Consent for publication
The authors give Journal of Intensive Care the consent for publishing this manuscript. There are no personal information that required consents.
Competing interests
The authors have no competing interests to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40560-020-00464-1.
References
- 1.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med [Internet] 2017;377:562–572. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA [Internet] 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med [Internet] 2012;38:1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 4.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med [Internet] 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 5.Meduri GU, Bridges L, Shih M-C, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med [Internet] 2016;42:829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit Care Med [Internet] 2017;45:2078–2088. doi: 10.1097/CCM.0000000000002737. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev [Internet] 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ [Internet] 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol [Internet] 2017;17:39. doi: 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care [Internet] 2016;20:329. doi: 10.1186/s13054-016-1511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg KP, Hudson LD, Goodman RB, Hough CL, Lanken PN, Hyzy R, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med [Internet] 2006;354:1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 12.Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest [Internet], Available from. 2007;131:954–63 http://www.ncbi.nlm.nih.gov/pubmed/17426195. [DOI] [PubMed]
- 13.Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA [Internet] 1998;280:159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 14.Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, et al. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med [Internet] 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 15.Annane D, Sébille V, Bellissant E, Ger-Inf-05 Study Group Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med [Internet] 2006;34:22–30. doi: 10.1097/01.CCM.0000194723.78632.62. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med [Internet] 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 17.Sabry N, Omar E. Corticosteroids and ICU Course of Community Acquired Pneumonia in Egyptian Settings. Pharmacology & Pharmacy. 2011;2:73–81. doi: 10.4236/pp.2011.22009. [DOI] [Google Scholar]
- 18.Rezk N, Ibrahim A. Effects of methylprednisolone in early ARDS. Egypt J Chest Dis Tuberc. 2013;62(1):167–72. doi: 10.1016/j.ejcdt.2013.02.013. [DOI] [Google Scholar]
- 19.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med [Internet] 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 20.Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med [Internet] 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 21.Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med [Internet] 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 22.Slutsky AS, Ranieri VM, et al. N Engl J Med [Internet] 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q, Shi J-X, Hu R, Li Q, Zhang C-Y, Li J-S, et al. Exp Ther Med [Internet] 2019;18:4913–4920. doi: 10.3892/etm.2019.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S, Liu D, Zhang H, Zhang X, Wan B. Effect of different doses and time-courses of corticosteroid treatment in patients with acute respiratory distress syndrome: a meta-analysis. Exp Ther Med [Internet] 2019;18:4637–4644. doi: 10.3892/etm.2019.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med [Internet]. 2020; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32167524. [DOI] [PMC free article] [PubMed]
- 26.Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, Dong N TQ. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. medRxiv. 2020;2003.2006.
- 27.Lansbury L, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. Cochrane Database Syst Rev [Internet] 2019;2:CD010406. doi: 10.1002/14651858.CD010406.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brun-Buisson C, Richard J-CM, Mercat A, Thiébaut ACM, Brochard L. REVA-SRLF a/H1N1v 2009 registry group. Early corticosteroids in severe influenza a/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med [Internet] 2011;183:1200–1206. doi: 10.1164/rccm.201101-0135OC. [DOI] [PubMed] [Google Scholar]
- 29.Ruan S-Y, Lin H-H, Huang C-T, Kuo P-H, Wu H-D, Yu C-J. Exploring the heterogeneity of effects of corticosteroids on acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care [Internet] 2014;18:R63. doi: 10.1186/cc13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med [Internet]. 2020; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32222812. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure 1. Regression of duration of glucocorticoid treatment on ICU mortality. Longer duration was associated with lower rates of ICU mortality (P < 0.05).
Additional file 2: Supplementary Figure 2. Regression of PEEP on ICU mortality. Higher PEEP was associated with lower rates of ICU mortality (P < 0.05).
Additional file 3: Supplementary Figure 3. Trial sequential analysis for ICU-mortality. The diversity-adjusted information size (sample size) is 3,244 patients. The cumulative Z-line (blue line with small black squares representing each trial) crosses the alpha monitoring boundary (horizontal green line) indicating statistical significance for the efficacy of glucocorticoids. However, The Z-line failed to cross the TSA boundary (concave red line), and since the required sample size was not reached, there is lack of firm evidence supporting improved hospital mortality in the glucocorticoids group.
Data Availability Statement
Data and materials are available and can be presented upon request.