Abstract
A 3-years-old male golden retriever was presented for decreased activity (lethargy), anorexia, and titubation. Superficial lymph nodes were enlarged, and arrhythmia and tachycardia were auscultated. Fungal hyphae-like structures were detected in the biopsy samples from an enlarged lymph node and spleen. Nucleotide sequence of the internal transcribed spacer region of the fungi amplified by PCR was highly homologous to that of Inonotus pachyphloeus. The dog was treated with antifungal agents such as itraconazole, fluconazole, and voriconazole. Clinical signs resolved for 325 days but the dog died suddenly, possibly because of arrhythmia. Postmortem examination revealed the presence of a disseminated fungal infection. This report describes the case of canine systemic Inonotus sp. infection treated by an antifungal agent.
Keywords: antifungal agent, dog, Inonotus sp., systemic mycosis
Systemic fungal infection is rarely reported in dogs. It usually occurs in animals with immune-compromised conditions, such as diabetes, endocrine diseases, tumors, or viral infections [3]. Though Blastomyces, Histoplasma, Coccidioides, and Cryptococcus are well-known fungal pathogens that cause systemic fungal infections, other uncommon fungal pathogens have also been reported in dogs [17, 18]. Herein, we report a case of a canine with systemic mycosis caused by Inonotus sp.
A 3-years-old male golden retriever suffering from persistent lethargy, anorexia, and wobble for a month was referred to the Kagoshima University Veterinary Teaching Hospital. Upon physical examination, the dog was febrile (39.6°C) and lean, with a body condition score of 3 out of 5. Auscultation revealed arrhythmia and tachycardia (261 bpm). Superficial lymph nodes were slightly enlarged. The complete blood count (CBC) and serum chemistry indicated leukocytosis (21.5 × 109/l; reference range 5.05 to 16.8 × 109/l), mild neutrophilia (13.7 × 109/l; reference range 2.95 to 11.6 × 109/l), monocytosis (1.9 × 109/l; reference range 0.16 to 1.12 × 109/l), mild increased blood urea nitrogen level (13.7 mmol/l; reference range 3.28 to 10.4 mmol/l), hyperglobulinemia (67 g/l; reference range 27 to 44 g/l), and an elevation of the C-reactive protein concentration (105 mg/l; reference range <10mg/l). An abdominal radiography revealed splenomegaly and osteolysis of the L2 and L3 vertebrae (Fig. 1). Abdominal ultrasonography showed multiple hypoechoic nodules in the spleen (Fig. 2) and enlarged internal iliac lymph nodes. Echocardiography showed the left ventricular septum was hypoechoic and the left endocardium was hyperechoic, indicating heart muscle necrosis and endocarditis, respectively. A fine-needle aspiration (FNA) sample was obtained from a superficial cervical lymph node, the internal iliac lymph node, and spleen (Fig. 3A). Grocott stain-positive fungal hyphae-like structures were observed in the specimens (Fig. 3B). From these findings, we tentatively diagnosed this case as systemic mycosis. DNA was extracted from a superficial cervical lymph node using an FNA sample. We performed sequencing of internal transcribed spacer (ITS) regions in fungi amplified by polymerase chain reaction using ITS-1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) primers [22]. Intergenic spacer (IGS) regions were also amplified using LR12R (5′-GAACGCCTCTAAGTCAGAATCC-3′) and invSR1R: (5′-ACTGGCAGAATCAACCAGGTA-3′) primers [12]. Approximately 800 and 3,500 bp DNA fragments were amplified in the ITS and IGS regions, respectively. The nucleotide sequence of the fragments was determined at a commercial laboratory, Fasmac (Atsugi, Japan) (GenBank accession number LC342676.1, LC348387.1). The nucleotide sequence analysis using a basic local alignment search tool (BLAST) provided by the National Center for Biotechnology Information, showed 99% of homology with Inonotus pachyphloeus, a genus of fungi in the family Hymenochaetaceae. The nucleotide sequence of ITS of the colony was the same as that from the superficial cervical lymph node. Aspirate from lymph node was cultured on Sabouraud dextrose agar (SDA; 1% of peptone, 2% of glucose and 2% of agar) at 32°C. Within 7 days, one of the numerous velvety, cottony, white-brownish colonies that developed on SDA was sub-cultured on potato dextrose agar (PDA) (Oxoid Ltd., Hampshire, England), and incubated at 32°C. After 14 days, the isolate appeared as velvety, cottony, white-brownish on PDA. Microscopic examination of the isolate revealed the no-production of conidia and hyphae were hyaline. Because the colony did not form conidia, morphology classification was not possible. PCR amplification of the D1/D2 regions of the Large subunit (LSU) was performed with NL1 (5′-GCATATCAATAAGCGGAGGAAAA-3′) and NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) primers [14]. The nucleotide sequence of the fragments was determined at a commercial laboratory, Fasmac (Atsugi, Japan) (GenBank accession number LC523910). The phylogenetic tree was constructed with maximum likelihood algorithm [10]. The bootstrap method was applied to obtain statistical support for the phylogenetic tree branches, by using 1,000 round of resampling, using the Molecular Evolutionary Genetics Analysis (MEGA) computer software version X [13, 20]. The LSU sequence analysis comprised 14 LSU sequences of Inonotus sp., Tropicoporus sp., Sanghuangporus sp., Phellinus sp., and Fuscoporia sp. retrieved from Genbank (NCBI). The LSU sequences obtained from FNA sample showed 100% identity with I. pachyphloeus. The phylogenetic tree inferred from the analysis demonstrated that the isolate is identified as I. pachyphloeus (Fig. 4).
Fig. 1.
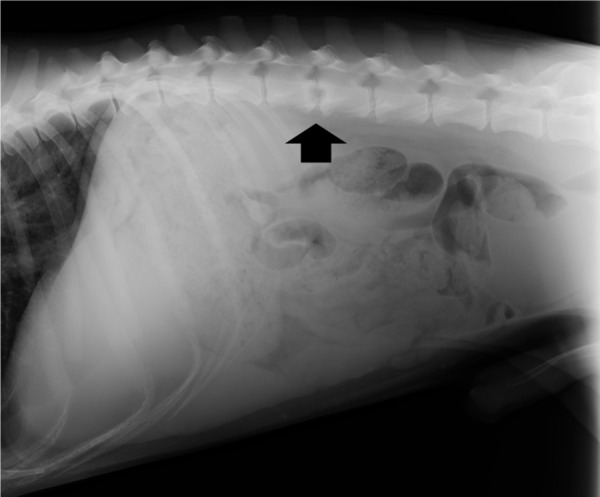
Right lateral decubitus position. Abdominal radiography revealed splenomegaly and osteolysis between L2 and L3 lumber vertebrae (arrow).
Fig. 2.
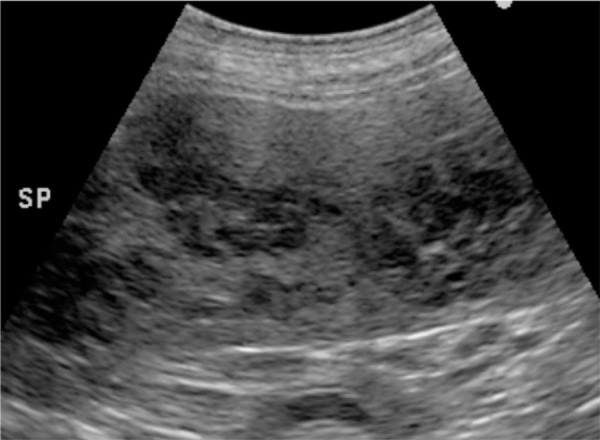
Abdominal ultrasonography showing hypoechoic multiple nodules in the spleen.
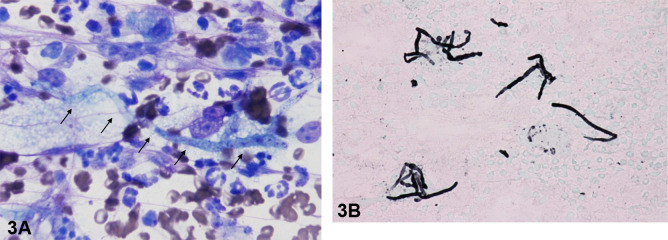
Fig. 3.
Fungal hyphae-like structures were observed in the spleen and lymph node. (A) Fine-needle biopsy on the spleen. Thin, poorly septate fungal hyphae (arrows) with neutrofills, Wright–Giemsa, ×100 objective. (B) Fine-needle biopsy on internal iliac lymph node. Septate fungal hyphae were Grocott-stain positive. Grocott, ×100 objective.
Fig. 4.
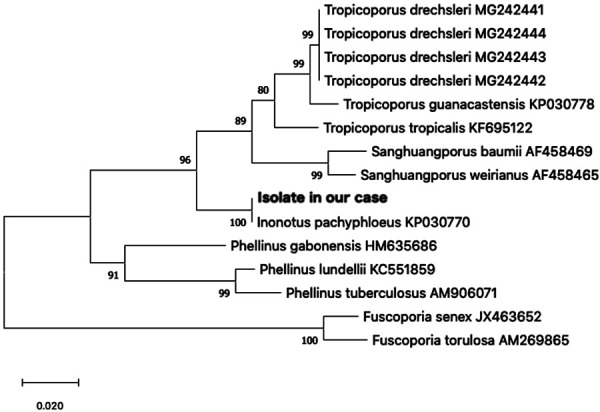
Maximum likelihood phylogenetic tree. Derived from the alignment of Large Subunit (LSU) partial sequences of the superficial cervical lymph node (FNA sample), with representative species obtained from GenBank (NCBI). Maximum likelihood bootstrap support values higher than 70% are indicated at the nodes. The tree was rooted to Fuscoporia senex and F. torulosa.
Treatment was started empirically with 4.6 mg/kg/twice/day itraconazole (Itraconazole; Kobayashi Kako Co., Ltd., Fukui, Japan) and 6.8 mg/kg enrofloxacin (Baytril; BAYER Pharmaceutical Co., Ltd., Berlin, Germany). One point two mg/kg diltiazem hydrochloride (Herbesser; Mitubishi Tanabe Pharma Co., Ltd., Osaka, Japan) was also prescribed for arrhythmia. Two weeks later, we changed antiarrhythmic agent to 1 mg/kg/twice/day sotalol hydrochloride (Sotacor; Bristol-Myers Squibb Pharmaceutical Co., Ltd., New York, NY, USA) because arrhythmia did not improve. Lymphadenopathy of the body surface, arrhythmia, and tachycardia improved, and plasma C-reactive protein concentration decreased to the normal range (7 mg/l) within 4 weeks after starting the treatment. However, the dog presented with a head tilt 5 weeks later. We suspected central nervous system involvement of the fungal infection and changed the antifungal agent to 10 mg/kg fluconazole (Diflucan; Pfizer Inc., New York, NY, USA), but the clinical symptom was not improved, and superficial lymph nodes became enlarged again. We restored the medication to itraconazole (11.3 mg/kg/twice/day) with dose escalation. Three months later, the dog presented with worsened (relapsed) head tilt, horizontal nystagmus, and titubation with marked elevation of C-reactive protein concentration (70 mg/l). The macro-dilution assays testing the susceptibility of filamentous fungi to the antifungal drug fluconazole (FLZ), itraconazole (ITZ) and voriconazole (VRZ) were performed according to the CLSI M38-A2 guidelines [5]. Because of the isolate did not produce conidia on the PDA, we pick up amount (approximately 5 cubic millimeter) of a fresh colony and inoculated into 2 ml of RPMI1640 medium containing various concentration of antifungals. Minimal inhibitory concentrations (MIC) were determined after incubation at 35°C for 5 days [2]. For the MICs were defined as the lowest concentration that prevents any discernible growth (clear tubes) [4, 5]. The MICs for the clinical isolate were 32 mg/l for FLZ, 8 mg/l for ITZ and 0.0635 mg/l for VRZ. From these results, 5 mg/kg/twice/day voriconazole (Voriconazole; Nihon Generic Co., Ltd., Tokyo, Japan) was prescribed, which lead to resolution of symptoms for 7 months except the mild head tilt. Renal function deteriorated gradually, and the dog died suddenly 10.5 months from the first admission, possibly because of arrhythmia. Gross findings of necropsy revealed multiple yellowish white nodular lesions in the spleen (Fig. 5), liver, endocardium of right ventricle, lung, and surface of right femur. Moreover, foci of discoloration and hemorrhage were in the left ventricular myocardium. Macroscopically, there were no remarkable changes in the brain. On histopathological examination, acute myocardial necrosis, suggestive of myocardial infarction, were observed without any organism. In the nodular lesions, multiple granulomas with hyphae were formed in the spleen, liver, endocardium of right ventricle, surface of right femur, and pulmonary hilar lymph nodes. Several mycelial aggregates were observed in the renal pelvic cavity. The pons had a localized perivascular cuffing, mild edema, and acute swelling of oligodendrocytes.
Fig. 5.
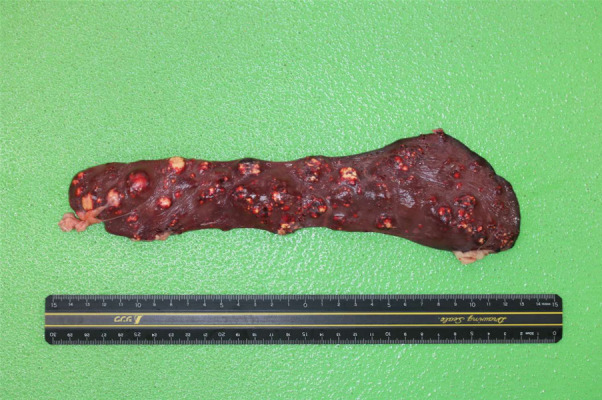
Autopsy photograph showing the spleen with yellowish white nodular lesions.
This report describes a canine case of systemic fungal infection caused by Inonotus pachyphloeus. Because it is a non-pigmented mold, infection caused by Inonotus spp. was recognized as a hyalohyphomycosis. Inonotus sp. is placed in the family Hymenochaetaceae. The family Hypmenochaetaceae contains also species belonging Tropicoporus and Phellinus genus. Infection caused by the family Hymenochaetaceae is rare and only three canine cases and nine human cases could be found in eleven reports [6,7,8,9, 15,16,17,18,19, 21, 23]. In one report, a 3.5-years-old Irish wolfhound was described with an intra-thoracic mass and suspected diskospondylitis. Tropicoporus tropicalis (=Inonotus tropicalis) was isolated the thoracic mass. The dog was euthanized during thoracotomy and necropsy was not performed [18]. The other case report described a 9-years-old French bulldog with fungal myocarditis with pericardial effusion. T. tropicalis was isolated from the pericardial effusion. The dog had been treated with cyclosporine and prednisone for atopic dermatitis at the time of diagnosis [17]. The dog was euthanized without any treatment for T. tropicalis. The other case report described a 9-years-old female mongrel dog with a painful mass over the right prescapular area and T. tropicalis. was isolated from the mass [11]. The dog was treated with itraconazole, but it was euthanized because of the unfavorable clinical outcome. Seven human cases were in immunocompromised status because of X-linked chronic granulomatous disease, a genetic disorder in which neutrophils are unable to phagocytize bacteria and fungi. Inonotus sp. and Tropicoporus sp. were isolated from subcutaneous abscesses [6, 7, 15, 16, 19, 21], lung, and brain [9]. One human patient was immunocompromised because of chronic myeloid leukemia [8]. Tropicoporus tropicalis was isolated from the lung and skin. The other one human patient was highly susceptible to infection because of type 2 diabetic mellitus [23]. Inonotus undulates was isolated from subcutaneous tissue. In this case, the fungal infection was invasive and the infection had spread to systemic organs, including the spleen, lung, heart, bone, and liver.
In our case, there were no notable clinical history before the episode. The dog and the owner had been living a standard Japanese home and gone for a walk around a suburban area. The dog did not have a medical history associated with an immunocompromised condition, nor received immunosuppressive agents. Hypergammaglobulinemia of the dog indicated the ability to produce antibodies. We evaluated oxygen radical production ability of white blood cells by measuring luminol-dependent chemiluminescence induced by opsonized zymosan using whole peripheral blood [1, 2]. Oxygen radical production level was not different from that in control healthy dogs (data not shown). The etiology and the route of infection in the dog are still unknown. Antifungal treatment with itraconazole was not effective for one dog in the previous report [11]. In humans, 6 were breakthrough infections in patients receiving prophylactic itraconazole or posaconazole [6, 7, 9, 16, 19, 21] and voriconazole was effective for 6 cases in controlling this infection [6,7,8, 15, 16, 19]. In our case, itraconasole was effective as an early stage of infection but could not completely control the infection. From the results of the sensitivity test, we treated the dog with voriconazole to control the infection for several months. However, the dog eventually died from widespread infection.
This is a case report documenting systemic Inonotus sp. infection in a dog without any noticeable disease. Although the clinical symptoms were improved temporarily by antifungal agents, this infection carried a poor prognosis and the lesions were observed in the spleen, liver, heart, lung, bone, and other organs. It is necessary to examine more cases to determine the appropriate therapy.
REFERENCES
- 1.Allen R. C., Stjernholm R. L., Steele R. H.1972. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem. Biophys. Res. Commun. 47: 679–684. doi: 10.1016/0006-291X(72)90545-1 [DOI] [PubMed] [Google Scholar]
- 2.Andersen B. R., Brendzel A. M., Lint T. F.1977. Chemiluminescence spectra of human myeloperoxidase and polymorphonuclear leukocytes. Infect. Immun. 17: 62–66. doi: 10.1128/IAI.17.1.62-66.1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biegańska M., Dardzińska W., Dworecka-Kaszak B.2014. Fungal colonization-an additional risk factor for diseased dogs and cats? Ann. Parasitol. 60: 139–146. [PubMed] [Google Scholar]
- 4.Cantón E., Espinel-Ingroff A., Pemán J.2009. Trends in antifungal susceptibility testing using CLSI reference and commercial methods. Expert Rev. Anti Infect. Ther. 7: 107–119. doi: 10.1586/14787210.7.1.107 [DOI] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A2, Clinical and Laboratory Standards Institute, Philadelphia. [Google Scholar]
- 6.Davis C. M., Noroski L. M., Dishop M. K., Sutton D. A., Braverman R. M., Paul M. E., Rosenblatt H. M.2007. Basidiomycetous fungal Inonotus tropicalis sacral osteomyelitis in X-linked chronic granulomatous disease. Pediatr. Infect. Dis. J. 26: 655–656. doi: 10.1097/INF.0b013e3180616cd0 [DOI] [PubMed] [Google Scholar]
- 7.De Ravin S. S., Parta M., Sutton D. A., Wickes B. L., Thompson E. H., Wiederhold N. P., Nakasone K. K., Alimchandani M., OConnell A., Notarangelo L., Kang E., Malech H. L., Zelazny A. M.2014. Paravertebral mushroom: identification of a novel species of Phellinus as a human pathogen in chronic granulomatous disease. J. Clin. Microbiol. 52: 2726–2729. doi: 10.1128/JCM.00667-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Cruz A., Kwon M., Guinea J., Escribano P., Jiménez M. D. C. M., Pulido A., Parra V., Serrano D., Gayoso J., Martín J. L. D., Bouza E.2018. Inonotosis in patient with hematologic malignancy. Emerg. Infect. Dis. 24: 180–182. doi: 10.3201/eid2401.171265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haidar G., Zerbe C. S., Cheng M., Zelazny A. M., Holland S. M., Sheridan K. R.2017. Phellinus species: An emerging cause of refractory fungal infections in patients with X-linked chronic granulomatous disease. Mycoses 60: 155–160. doi: 10.1111/myc.12573 [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa M., Kishino H., Yano T.1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 22: 160–174. doi: 10.1007/BF02101694 [DOI] [PubMed] [Google Scholar]
- 11.Hevia A., Iachini R., Fernández J., Lazzari J., Suárez-Alvarez R., Abrantes R., Toranzo A., Refojo N., Canteros C.2019. Mycosis Due to Tropicoporus tropicalis (= Inonotus tropicalis) in a domestic dog. Mycopathologia 184: 701–706. doi: 10.1007/s11046-019-00368-1 [DOI] [PubMed] [Google Scholar]
- 12.Kumar M., Shukla P. K.2005. Use of PCR targeting of internal transcribed spacer regions and single-stranded conformation polymorphism analysis of sequence variation in different regions of rrna genes in fungi for rapid diagnosis of mycotic keratitis. J. Clin. Microbiol. 43: 662–668. doi: 10.1128/JCM.43.2.662-668.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S., Stecher G., Li M., Knyaz C., Tamura K.2018. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 35: 1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzman C. P., Robnett C. J.1998. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 73: 331–371. doi: 10.1023/A:1001761008817 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen D. K., Davis C. M., Chinen J., Vallejo J. G., Noroski L. M.2009. Basidiomycetous Inonotus (Phellinus) tropicalis osteomyelitis in pediatric and adult X-linked chronic granulomatous disease. J. Allergy Clin. Immunol. 123: S13. doi: 10.1016/j.jaci.2008.12.060 [DOI] [Google Scholar]
- 16.Ramesh M., Resnick E., Hui Y., Maglione P. J., Mehta H., Kattan J., Bouvier N. M., LaBombardi V., Victor T. R., Chaturvedi S., Cunningham-Rundles C.2014. Phellinus tropicalis abscesses in a patient with chronic granulomatous disease. J. Clin. Immunol. 34: 130–133. doi: 10.1007/s10875-013-9967-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas T., Pipe-Martin H., Kim K. S., Leissinger M. K., Bauer R. W., Grasperge B. J., Grooters A. M., Sutton D. A., Pariaut R.2015. Fungal myocarditis and pericardial effusion secondary to Inonotus tropicalis (phylum Basidiomycota) in a dog. J. Vet. Cardiol. 17: 142–148. doi: 10.1016/j.jvc.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 18.Sheppard B. J., McGrath E., Giuffrida M., Craft S. L. M., Kung C. Y., Smith M. E.2013. Report of wood decay fungus Inonotus tropicalis (phylum Basidiomycota) from a dog with a granulomatous mediastinal mass. J. Vet. Diagn. Invest. 25: 566–572. doi: 10.1177/1040638713499341 [DOI] [PubMed] [Google Scholar]
- 19.Shigemura T., Nakazawa Y., Amano Y., Sudo A., Watanabe M., Kobayashi M., Kobayashi N., Koike K., Agematsu K., Nishimura K.2015. Subcutaneous abscess due to the basidiomycete Phellinus mori in a patient with chronic granulomatous disease. Infection 43: 371–375. doi: 10.1007/s15010-015-0724-7 [DOI] [PubMed] [Google Scholar]
- 20.Stecher G., Tamura K., Kumar S.2020. Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 37: 1237–1239. doi: 10.1093/molbev/msz312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutton D. A., Thompson E. H., Rinaldi M. G., Iwen P. C., Nakasone K. K., Jung H. S., Rosenblatt H. M., Paul M. E.2005. Identification and first report of Inonotus (Phellinus) tropicalis as an etiologic agent in a patient with chronic granulomatous disease. J. Clin. Microbiol. 43: 982–987. doi: 10.1128/JCM.43.2.982-987.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White T. J., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, pp. 315–322. In: PCR Protocols: A Guide to Methods and Applications. Academic Press, Inc., New York. [Google Scholar]
- 23.Williamson D., Pandey S., Taylor S., Rogers K., Storey L., Marshall M. R., Holland D.2011. A case of infection caused by the basidiomycete Phellinus undulatus. J. Med. Microbiol. 60: 256–258. doi: 10.1099/jmm.0.025569-0 [DOI] [PubMed] [Google Scholar]