Abstract
A seven-month-old cat was referred for evaluation of exercise intolerance and open-mouth breathing. Based on ultrasonographic examination, caudal vena cava (CVC) aneurysm associated with right congestive heart failure resulting from congenital heart disease was diagnosed. Conservative treatment for alleviating pulmonary hypertension mildly improved the clinical signs and decreased the heart size and CVC aneurysm diameter. However, the improvements were transient and four months after initiating therapy, the cat developed dyspnea and uncontrollable seizures and was euthanized.
Keywords: feline, polycythemia, pulmonary hypertension, right congestive heart failure, ventricular septal defect
A seven-month-old intact male Korean shorthair cat weighing 1.2 kg presented with a one-month history of exercise intolerance and open mouth breathing; it was referred to the Daegu Animal Medical Center owing to suspicions of diaphragmatic hernia. On physical examination, the cat was tachypneic (50 bpm) and a dilated jugular vein with pulsation and cyanotic mucous membrane was noted. Thoracic auscultation revealed a grade 3/6 systolic murmur from both the left and the right sides. A complete blood cell count showed polycythemia with a hematocrit of 57.7% (reference range: 24−45%). The serum chemistry profile also showed a slight elevation of alanine aminotransferase (124 U/l; reference range: 12−115 U/l). Thoracic radiographs revealed generalized cardiomegaly with a vertebral heart score of 11 and pruning of the peripheral pulmonary artery with increased radiolucency of the lung field (Fig. 1). Further, a large tubular, soft tissue structure was observed in the caudoventral mediastinal region, extending from the caudal margin of the heart to the diaphragm. Additionally, hepatomegaly was noted in the abdominal radiographs.
Fig. 1.
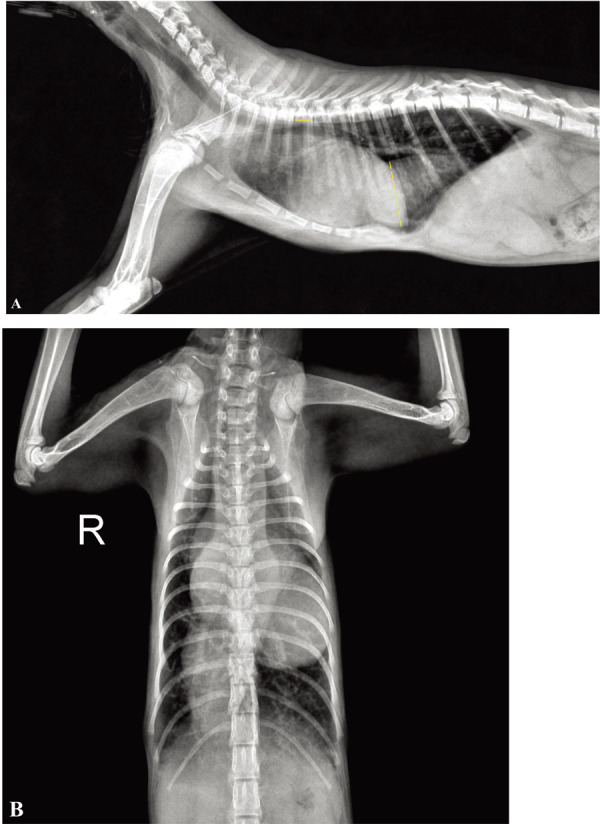
(A) Right lateral and (B) ventrodorsal radiograph of a seven-month-old Korean shorthair cat. Radiographs demonstrate severe cardiomegaly and pruning of the peripheral pulmonary artery with increased radiolucency of the lung field. A tubular soft-tissue density is visible on the caudoventral thorax extending from the heart to the diaphragm. To make a reasonable comparison before and after the medication treatment, the ratio of the diameter of the CVC to the length of 5th thoracic vertebral body (CVC:V) was measured. On this radiograph, the CVC:V ratio was 6.82. Dotted line, diameter of the CVC; solid line, length of 5th thoracic vertebral body.
On echocardiographic examination, a massive dilated right atrium with right ventricular concentric hypertrophy was revealed. Additionally, there was volume depletion on the left side of the heart, which mimicked left ventricular wall thickening and caused a decrease in the size of the left atrium. Tricuspid regurgitation was identified with a peak velocity of 6.07 m/sec. The pulmonary artery was dilated with a pulmonic velocity of 1.6 m/sec. No stenotic lesion was observed on B-mode echocardiogram. Peak pressure gradient was calculated between the right atrium and ventricle in the systolic phase (147 mmHg) and the value was consistent with severe pulmonary hypertension. Additionally, a 3-mm ventricular septal defect (VSD) was observed in the left ventricular outflow tract region just proximal to the aortic valve (Fig. 2). Color Doppler ultrasound revealed blood flow through the VSD. However, the exact shunt direction was difficult to determine based on the color Doppler method alone. Right to left shunting blood flow was confirmed via continuous wave Doppler imaging, with a peak velocity of 2.4 m/sec. Further, to optimally visualize the shunting flow characteristics, 1 ml of agitated saline microbubble was injected via the cephalic vein. Subsequently, injected bubble flow was seen in the left ventricle through the VSD, confirming right to left shunting (Fig. 3). No other structural anomaly was observed on the echocardiogram.
Fig. 2.
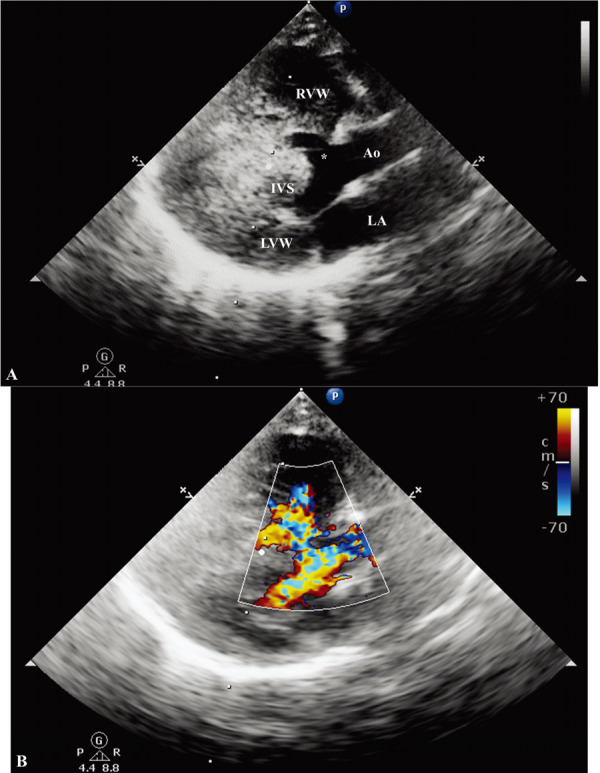
Right parasternal long axis, five-chamber view echocardiogram. (A) Two-dimensional echocardiography shows a 3-mm perimembranous ventricular septal defect. The right ventricular wall is markedly hypertrophied. (B) The color-Doppler demonstrates the turbulent flow across the left ventricular outflow tract and the right ventricle. Ao, aorta; LVW, left ventricular wall; RVW, right ventricular wall; IVS, interventricular septum; LA, left atrium. Asterisk (*), ventricular septal defect.
Fig. 3.
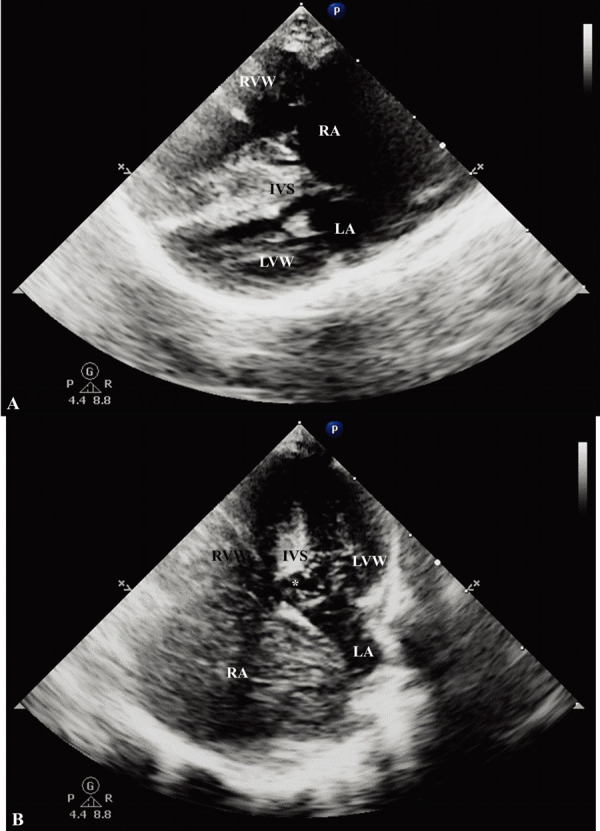
Right parasternal four-chamber and left apical four-chamber view echocardiogram. (A) Hypertrophied right ventricular wall and interventricular septum is noted. Additionally, there is a leftward displacement of the atrial septum which indicates elevated right atrial pressure. The left atrium is not well identified due to compression. (B) After microbubble injection, the bubbles cross the defect from the right ventricle to the left ventricle. LVW, left ventricular wall; RVW, right ventricular wall; RA, right atrium; LA, left atrium; IVS, interventricular septum. Asterisk (*), ventricular septal defect.
Ultrasound of the caudal thorax revealed a fusiform-shaped dilated vessel, approximately 3 cm in diameter that communicated with the right atrium cranially and with the hepatic veins caudally, which is consistent with an intra-thoracic caudal vena cava (CVC) (Fig. 4). No obstructive lesions that may potentially cause a dilation of the CVC were identified. The hepatic veins were dilated, and the liver was subjectively enlarged, suggesting hepatic congestion. There was also a small amount of pleural effusion surrounding the dilated CVC. To summarize, the cat was diagnosed with right congestive heart failure that resulted from pulmonary hypertension. The underlying cause was Eisenmenger syndrome associated with VSD. Aneurysmal CVC was strongly suspected as a result of elevated right atrial pressure, although an additional congenital anomaly could not be ruled out. In addition, the right-to-left shunt led to polycythemia. The cat was treated with a diuretic (furosemide 2 mg/kg q12hr PO), angiotensin-converting-enzyme inhibitor (ramipril 0.125 mg/kg q24hr PO), vasodilator (sildenafil 2 mg/kg q12hr PO), and an inotrope (pimobendan 0.3 mg/kg q12hr PO). Additionally, antithrombic agent (clopidogrel 18.75 mg, q24hr PO) was prescribed for thrombotic risk as a complication of venous aneurysm.
Fig. 4.
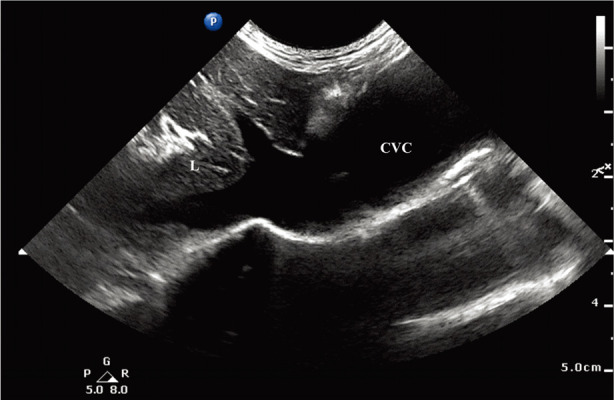
Ultrasound images obtained from right caudal thorax with the left-hand side of the image directed cranially. A 3 cm large tortuous vessel is seen on the caudal thorax that was identified as CVC since it is connected to the right atrium cranially and to the hepatic veins caudally. A small amount of pleural effusion is seen around the field. L, liver; CVC, caudal vena cava.
After one-week of medication, an echocardiogram revealed that the velocity of the tricuspid regurgitant jet decreased from 6.07 m/sec to 4.6 m/sec (pressure gradient: 145 to 85 mmHg). However, clinical signs did not improve and there was no difference in the size of the heart and the CVC aneurysm in the thoracic radiograph. Therefore, the dose of sildenafil was increased to 3 mg/kg q12hr (PO) along with a new prescription of a bronchodilator (theophylline 10 mg/kg q12hr PO). At the one-month post-presentation re-evaluation, the cat was reported to be more comfortable with fewer episodes of exercise intolerance that were shorter in duration than that before the treatment. The heart size (vertebral heart score: 9.6) and the diameter of the CVC decreased (Fig. 5). The ratio of the CVC diameter to the diameter of 5th thoracic vertebral body (CVC:V) was measured on a lateral thoracic radiograph, to make a reasonable comparison before and after the medication treatment. The measured CVC:V ratio before medication was 6.82 and 4.09 after medication, which confirmed that the CVC had reduced in size after the treatment.
Fig. 5.
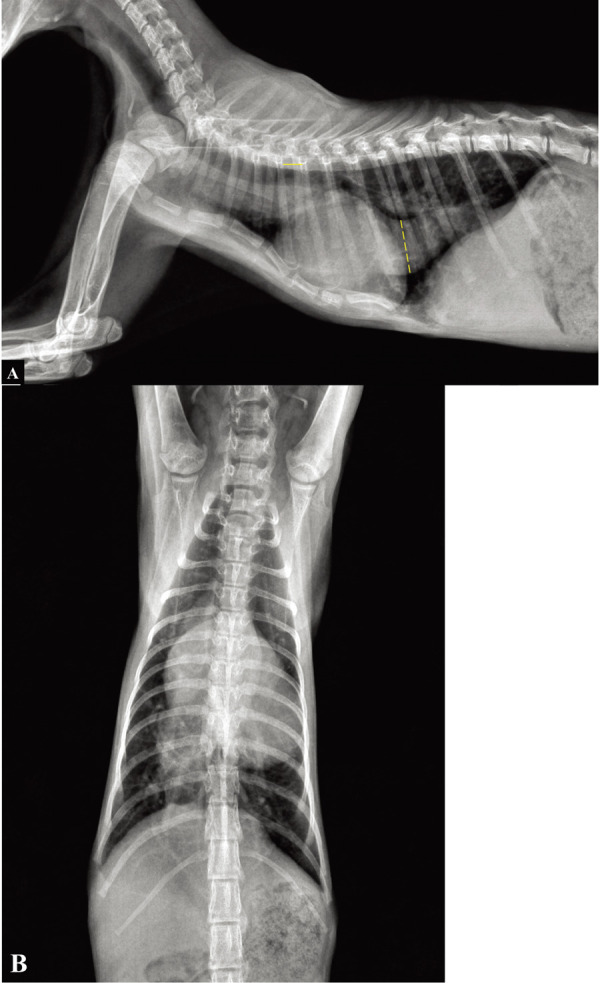
Right lateral (A) and ventrodorsal (B) thoracic radiographs following 1 month after starting medication. Reduction of the CVC was noted, as compared to a previous thoracic radiograph. On this radiograph, the CVC:V ratio was 4.09. Dotted line, diameter of the CVC; solid line, length of 5th thoracic vertebral body.
The condition of the cat was well maintained for the next three months with the same treatment. However, four months after the start of the treatment, the cat showed symptoms of dyspnea and seizures at the local hospital where it was first examined before being referred to our hospital. The cause of the seizures was presumed to be due to hyperviscosity secondary to hypoxic polycythemia; however, other causes like electrolyte imbalance, hypoglycemia, or an additional anomaly like a portosystemic shunt were not ruled-out. The owner refused further diagnostic testing as well as a therapeutic phlebotomy. Therefore, the seizures were symptomatically treated with phenobarbital (3 mg/kg q12hr); after an additional 2 weeks, the cat was euthanized due to uncontrollable seizures.
An aneurysm is a focal dilation of the vessel that occurs mostly in the arterial system [9]. However, an aneurysm in the venous system is rarely reported in both human and veterinary medicine. Venous aneurysm has been described in the linguofacial, maxillary, jugular vein, cranial vena cava, and CVC in dogs, and in the maxillary and jugular vein of a horse [3, 5, 6, 10]. To the best of our knowledge, CVC aneurysm has not been reported in a cat before.
In human medicine, aneurysm of the inferior vena cava (IVC) is usually asymptomatic and is diagnosed incidentally in most cases. Some patients present with pain and limb edema [1]. However, it can be lethal if complications occur, such as thrombosis, pulmonary embolism, or bleeding result from the rupture of the aneurysm [4, 9]. The etiology of IVC aneurysm is classified into a congenital or acquired form. Previous studies have reported various causes of IVC aneurysm such as congenital weakness of the vessel wall, trauma, inflammation, long-standing systemic venous hypertension, and neoplasms [8].
According to Gradman and Steinberg (1993), IVC aneurysm can be classified into four types based on the anatomical and embryological characteristics: type 1 aneurysm involves the suprahepatic IVC without venous obstruction, type 2 aneurysm associated with interruption of the IVC above or below the hepatic veins, type 3 aneurysm involves the infrarenal IVC with no venous anomaly, and type 4 aneurysm is miscellaneous [2]. Montero-Baker (2015) proposed a therapeutic algorithm using the Gradman and Steinberg classification [7]. A conservative approach with annual surveillance is recommended for type 1, as this aneurysm is usually asymptomatic and found in patients with right-sided heart failure. Medical management focused on the optimization of cardiac function tends to decrease the risk of rupture by improving venous overdistention. For type 2−4 aneurysms, operative intervention is recommended due to the high risk of thromboembolism and rupture. According to reported cases, open resection, ligation, and embolization of the aneurysm have been successfully performed in type 2−4 aneurysms. The present case was diagnosed as an intrathoracic CVC aneurysm with right congestive heart failure resulting from a congenital cardiac anomaly. There was no evidence of obstruction or interruption at the confluence of the hepatic vein and CVC. Therefore, this CVC aneurysm can be classified as type 1 based on the Gradman and Steinberg classification.
The vena cava is a highly compliant vessel with a low-pressure capacity [11]. The diameter of CVC is known to be closely related to right-sided heart pressure. A study reported that cats with right-sided heart failure had a CVC that was on average 1.5 times the diameter of CVCs in normal healthy cats [11].
In the present case, medical treatment for alleviating pulmonary hypertension and right congestive heart failure reduced the diameter of the aneurysm, indicating a possible correlation between the CVC aneurysm and elevated right-sided heart pressure (Figs. 1 and 5). This type of CVC aneurysm can be palliatively managed by medical treatments focused on reducing the right atrial pressure. However, it cannot be cured without removing the essential cause of the elevation of right atrial pressure, which was not possible in this patient. Additionally, an antithrombic agent should be prescribed for preventing complications of the aneurysm such as pulmonary embolism.
This report has a limitation. Although pulmonary hypertension can resulted from VSD alone, an additional pathology could be present in the pulmonary vasculature. This could have been revealed through additional tests, including computed tomography or necropsy, which were not performed due to refusal by the owner.
In conclusion, this is the first reported case of a CVC aneurysm in a cat. The intrathoracic CVC aneurysm identified in this case was the result of elevated right atrial pressure secondary to pulmonary hypertension, which was a consequence of VSD, although an additional congenital anomaly cannot be ruled out. The patient was managed for a short period by reducing the elevated right atrial pressure; however, the treatment was only palliative.
CONFLICT OF INTEREST
The researcher claims no conflicts of interest.
REFERENCES
- 1.Atalar M. H.2016. Aneurysm of the inferior vena cava: imaging findings. Austin J Radiol 3: 1053. [Google Scholar]
- 2.Gradman W. S., Steinberg F.1993. Aneurysm of the inferior vena cava: case report and review of the literature. Ann. Vasc. Surg. 7: 347–353. doi: 10.1007/BF02002888 [DOI] [PubMed] [Google Scholar]
- 3.Hilbert B. J., Rendano V. T.1975. Venous aneurysm in a horse. J. Am. Vet. Med. Assoc. 167: 394–396. [PubMed] [Google Scholar]
- 4.Inoue M., Sudo T., Yamaguchi M., Seo S., Miyamoto T., Misumi T., Shimizu W., Irei T., Suzuki T., Onoe T., Shimizu Y., Hinoi T., Tashiro H.2018. Aneurysm of the inferior vena cava with thrombosis. Clin. Case Rep. 6: 402–406. doi: 10.1002/ccr3.1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee N. D., Danoff K., Etue S., Rush J. E.2007. Cranial vena cava aneurysm in a dog. J. Vet. Cardiol. 9: 47–51. doi: 10.1016/j.jvc.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 6.Lockwood A. J., Sinnott-Stutzman V. B., Mouser P. J., Tsai S. L.2018. Azygos continuation of the caudal vena cava with segmental aneurysm, lung lobe torsion and pulmonary thromboembolism in a dog. Clin. Case Rep. 6: 363–369. doi: 10.1002/ccr3.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montero-Baker M. F., Branco B. C., Leon L. L., Jr, Labropoulos N., Echeverria A., Mills J. L., Sr.2015. Management of inferior vena cava aneurysm. J. Cardiovasc. Surg. (Torino) 56: 769–774. [PubMed] [Google Scholar]
- 8.Mookadam F., Rowley V. B., Emani U. R., Al-Harthi M. S., Baxter C. M., Wilansky S., Tajik J. A., Raslan S. F.2011. Aneurysmal dilatation of the inferior vena cava. Echocardiography 28: 833–842. doi: 10.1111/j.1540-8175.2011.01457.x [DOI] [PubMed] [Google Scholar]
- 9.Qaradaghi L., Imren V. Y., Ereren E., Tumer N. B.2010. Inferior vena cava aneurysm with hemothorax: rare presentation. Ann. Vasc. Surg. 24: 823.e11–14. doi: 10.1016/j.avsg.2010.02.019 [DOI] [PubMed] [Google Scholar]
- 10.Salmeri K. R., Bellah J. R., Ackerman N., Homer B.1991. Unilateral congenital aneurysm of the jugular, linguofacial, and maxillary veins in a dog. J. Am. Vet. Med. Assoc. 198: 651–654. [PubMed] [Google Scholar]
- 11.Vosugh D., Nazem M. N.2019. Radiological evaluation of caudal vena cava in domestic shorthair cats with regard to right heart failure diagnosis. Bulg. J. Vet. Med. 22: 220–226. doi: 10.15547/bjvm.2055 [DOI] [Google Scholar]


