Abstract
Non-neoplastic bone marrow disorders such as non-regenerative immune-mediated anemia, pure red cell aplasia, and myelodysplastic syndrome are major causes of non-regenerative anemia in dogs. However, there has been no study on the clinical and clinicopathological features of canine non-neoplastic bone marrow disorders in Japan. Hence, we first investigated the breed disposition of non-neoplastic bone marrow disorders that induce anemia as a retrospective study and found that Miniature Dachshund (MD) was a predisposed breed. Based on this finding, we investigated the clinical and clinicopathological features of non-neoplastic bone marrow disorders in MDs as a preliminary retrospective study, and we compared them between immunosuppressive treatment-responsive and -resistant MDs. We found that treatment-resistant MDs showed thrombocytosis and increased frequencies of dysplastic features in the peripheral blood. These results indicate that bone marrow disorders in treatment-resistant MDs might manifest distinct features compared with those in treatment-sensitive MDs, and sensitivity to immunosuppressive treatments could be predicted based on thrombocytosis and dysplastic features in the peripheral blood. Further studies that examine aberrations in the genome are needed to elucidate the pathophysiology of bone marrow disorders in MDs.
Keywords: canine, myelofibrosis, myelodysplastic syndrome, non-regenerative immune-mediated anemia, thrombocytosis
Bone marrow disorder is one of the major causes of non-regenerative anemia in dogs, and is categorized into neoplastic, hypoplastic, or dysplastic diseases [19]. Besides neoplastic diseases, bone marrow disorders that could cause non-regenerative anemia in dogs include aplastic anemia (AA), essential thrombocytosis (ET), myelofibrosis (MF), myelodysplastic syndrome (MDS), non-regenerative immune-mediated anemia (NRIMA), and pure red cell aplasia (PRCA) [5, 6, 14, 17, 21, 22]. Recently, a concept of precursor-targeted immune-mediated anemia (PIMA) was also proposed as an immune-mediated disease that causes non-regenerative anemia [12]. PIMA is characterized by phagocytosis of intact erythroid precursors by intact macrophages in bone marrow or spleen [12].
It has been previously reported in the United States that Labrador Retrievers and Miniature Dachshunds are predisposed to NRIMA and PIMA, respectively [2, 17]. However, no study on the breed predisposition of bone marrow disorders has been conducted in Japan. Hence, the primary objective of this study was to investigate the breed disposition of bone marrow disorders other than neoplastic diseases (non-neoplastic bone marrow disorders) that can induce anemia in dogs in Japan. In this retrospective study, we found that the breed Miniature Dachshund (MD) was significantly overrepresented when examining non-neoplastic bone marrow disorders that induce anemia. Thus, our secondary objective was to investigate the clinical and clinicopathological features of non-neoplastic bone marrow disorders observed in this breed.
MATERIALS AND METHODS
Retrospective investigation of predisposed breeds of bone marrow disorders that induce anemia
Medical records of dogs referred to Veterinary Medical Center of the University of Tokyo (UT-VMC) and diagnosed with non-neoplastic bone marrow disorders between April 2017 and March 2018 were retrospectively reviewed. Non-regenerative anemia was defined as moderate to severe anemia (Hct <30%) of minimum 5-day history with absolute reticulocyte count <60 × 103 /µl according to a previous study [17]. All dogs underwent a physical examination, complete blood count (CBC), blood biochemistry, radiographic and ultrasonographic examinations, and bone marrow aspiration. Dogs were excluded if they had apparent causes other than bone marrow disorders that could induce anemia based on the results of the blood examinations and diagnostic images described above. Diagnosis for each dog was made by three clinicians (A. Ohmi, H. Tomiyasu and H. Tsujimoto). Information on age, sex, and breed of the dogs were extracted from the medical records, and the predisposed breed was investigated.
Clinical and clinicopathological features of bone marrow disorders in MDs
In the first retrospective study, we found that MD was significantly overrepresented with non-neoplastic bone marrow disorders that induce anemia. Therefore, we decided to investigate the clinical and clinicopathological features of non-neoplastic bone marrow disorders observed in MDs. For this purpose, the medical records of dogs that were referred to UT-VMC and diagnosed with non-regenerative anemia due to non-neoplastic bone marrow disorders between April 2015 and March 2018 were retrospectively reviewed according to the same inclusion criteria as those in the first retrospective study.
The information on clinical history; signalments; the results of physical examination, CBC, blood biochemistry, autoagglutination test, direct Coombs test, radiographic and ultrasonographic examinations, and bone marrow aspiration; treatments, and outcomes were extracted from the medical records.
In the examinations of bone marrow smears, 1,000 cells were evaluated on each slide, and the cellularity, myeloid-to-erythroid (M-E) ratios, and blast cell to all nucleated cell (ANC) ratios were evaluated as previously described [18]. In addition, granulocyte maturation ratio and erythroid maturation ratio were investigated as previously reported [20]. Granulocyte maturation ratio was calculated using the following formula: (myeloblasts + promyelocytes + myelocytes)/ (metamyelocytes + band neutrophils + segmented neutrophils). Erythroid maturation ratio was calculated using the following formula: (rubriblasts + prorubricytes)/ (rubricytes + metarubricytes). The reference ranges of granulocyte maturation and erythroid maturation ratios were set as 0.04–0.15 and 0.02–0.12, respectively, according to a previous study [20]. The following dysplastic features in each cell lineage were investigated: megaloblastic appearances, fragmented nuclei, and binucleate cells in erythroid cells; ring neutrophils, giant metamyelocytes, giant band neutrophils, and pseudo-Pelger-Huet cell morphology in myeloid cells; dysmorphic megakaryocytes with multiple nuclei, mononucleate megakaryocytes, and micro-megakaryocytes in megakaryocytes [10, 21], and the frequency of each dysplastic feature was calculated by dividing the number of cells with dysplastic features by total number of cells of the same cell lineage in each case. Regarding megakaryocytes, 25 cells of this lineage were examined in each slide to calculate the frequency of dysplastic features, according to a previous study [4]. These evaluations of bone marrow smear were conducted by one clinician (A. Tani). In addition, the bone marrow core biopsy specimens were evaluated using hematoxylin-eosin staining to examine fibrosis in the bone marrow.
Drugs used for the treatment and responsiveness to the treatments were also investigated. Since dogs with NRIMA or PRCA have been shown to respond to treatment within a maximum of 10 weeks after treatment initiation [17], dogs that could be followed for more than 2 months after treatment initiation were included for the evaluation of treatment responsiveness. The hematological improvement due to treatments was defined as the absence of blood transfusion for more than 2 months after last blood transfusion according to the response criteria in human medicine [7].
Comparison of clinical and clinicopathological features
The distributions of age and sex, the results of CBC and blood biochemistry at diagnosis, and findings of peripheral blood and bone marrow smears were compared among MDs based on treatment responsiveness. The dogs were divided into treatment-responsive and -resistant groups based on the existence of hematological improvement with treatments, and the clinical and clinicopathological features described above were compared between the two groups. Since the number of platelets was significantly different between the two groups, receiver operator characteristic (ROC) analysis was performed to calculate sensitivity, specificity and optimal cutoff value of the number of platelets to predict the resistance to treatment.
Statistical analysis
The Fisher’s exact test was used to determine the predisposed breed and sex, and to compare the frequencies of morphological findings in peripheral blood and bone marrow smears. The Mann-Whitney U test was used to compare age and results of CBC and blood biochemistry. The Kaplan-Meier method and log-rank test were used to compare overall survival. ROC curves were prepared to evaluate the accuracy of the number of platelets in predicting the resistance to treatment. A P-value <0.05 was considered statistically significant. All statistical analyses were performed using the statistical program R 3.5.0 (http://cran.r-project.org).
RESULTS
Investigation of the predisposed breed of bone marrow disorders that induce non-regenerative anemia
Thirty-three dogs were included in the first retrospective study. The median age was 10.9 years (range: 1–15.8 years). Sixteen dogs were male (10 were castrated) and 17 were female (13 were neutered). There were 22 MDs, 3 Toy Poodles, 2 mix-breed dogs, and 1 each of Cavalier King Charles Spaniel, French Bulldog, Shetland Sheepdog, Shiba Inu, Pug, and Yorkshire Terrier. The odds ratios (ORs) of these dogs against all dogs admitted to UT-VMC for the same period are shown in Table 1. Among these breeds, the OR of MD was 12.20 (95% confidence interval: 5.61–28.12), and this breed was significantly overrepresented (P<0.001).
Table 1. The prevalence of each breed diagnosed as non-neoplastic bone marrow disorders with non-regenerative anemia.
| Breed | Number of cases | Number of dogs admitted to UT-VMC |
Odd’s ratio | P-value |
|---|---|---|---|---|
| Miniature Dachshund | 22 | 402 | 12.20 | <0.001 |
| Toy Poodle | 3 | 377 | 0.62 | 0.612 |
| Mix | 2 | 222 | 0.73 | 1 |
| Cavalier King Charles Spaniel | 1 | 34 | 2.52 | 0.340 |
| French Bulldog | 1 | 94 | 0.88 | 1 |
| Shetland Sheepdog | 1 | 52 | 1.62 | 0.471 |
| Shiba Inu | 1 | 104 | 0.79 | 1 |
| Pug | 1 | 41 | 2.08 | 0.394 |
| Yorkshire Terrier | 1 | 109 | 0.75 | 1 |
UT-VMC, Veterinary Medical Center of The University of Tokyo.
Clinical and clinicopathological features of bone marrow disorders in MDs
Based on the results of the first retrospective study, we decided to investigate the clinical and clinicopathological features of bone marrow disorders that induce non-regenerative anemia in MDs. Twenty-one MDs were included in the second retrospective study. The median age was 12.5 years (range: 4.3–15.8 years). Seven dogs were male (5 were castrated) and 14 were female (12 were spayed). During the same period, 311 male, 510 castrated male, 201 female, and 535 spayed female dogs were referred to UT-VMC, and it was revealed that spayed female MDs were significantly overrepresented (P=0.035). MDs were treated at the primary hospital using corticosteroids (17 dogs), antibiotics (14 dogs), blood transfusion (7 dogs), cyclosporine (3 dogs), mycophenolate mofetil (2 dogs), and leflunomide (1 dog). On the first admission to UT-VMC, physical examination showed mild to severe pale mucous membranes in 21 dogs, cardiac murmur in 5 dogs, tachycardia in 1 dog, and tachypnea in 1 dog.
Regarding laboratory tests, all dogs showed moderate to severe non-regenerative anemia (range: 7.0–24.7%) (Table 2). The white blood cell (WBC) count was within the reference range in 16 dogs. Five dogs showed leukocytosis (range: 23–50 × 103/µl). The platelet count was within the reference range in 9 dogs. Eleven dogs showed thrombocytosis (range: 520–2,260 × 103/µl), and 1 dog showed thrombocytopenia (130 × 103 /µl). Regarding blood biochemistry, the elevation of C-reactive protein (CRP) was commonly observed (14 dogs, range: 0.9–6.9 mg/dl). Thirteen dogs showed elevated alkaline phosphatase (ALP, range: 259–8,186 U/l), of which 9 were treated with corticosteroids before their admission to UT-VMC. Nine dogs showed elevated alanine transaminase (ALT, range: 93–907 U/l), of which 6 were treated with corticosteroids before their admission to UT-VMC. Autoagglutination test was negative in all the 19 examined dogs, and direct Coombs test was positive in 1 of the 19 dogs.
Table 2. The results of complete blood count in Miniature Dachshunds at first admission.
| Median (Range) | Reference range | |
|---|---|---|
| Hematocrit (%) | 19.1 (7−24.7) | 37.3−61.7 |
| RBC (×106/µl) | 2.7 (0.9−4.3) | 5.85−8.67 |
| Hemoglobin (g/dl) | 6.2 (1.9−8.4) | 13.1−20.5 |
| Reticulocyte (×103/µl) | 15.8 (5.3−55.9) | <60.0 |
| WBC (×103/µl) | 11.3 (5.9−50.4) | 5.05−16.76 |
| Platelet (×103/µl) | 524 (127−2,268) | 148−484 |
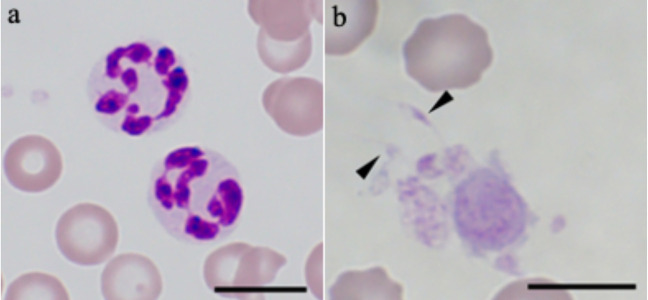
In the examinations of peripheral blood smears, hypersegmented neutrophils were found in 12 dogs (Fig. 1a). Ten of these 12 dogs were treated with corticosteroids before admission to UT-VMC. In addition, spindle-shaped platelets were found in 8 dogs (Fig. 1b).
Fig. 1.
Hypersegmented neutrophils (a) and platelets with spindle shape (b) observed in peripheral blood smears stained with Wright-Giemsa stain. Arrowheads indicate spindle-shaped platelets. Bar=10 µm.
Radiographic examinations for the thorax and ultrasonographic examinations for the abdomen were performed in all 21 dogs. While 12 dogs showed no abnormalities, 5 showed mild splenomegaly, 2 showed mild hepatomegaly, and 2 showed both mild splenomegaly and hepatomegaly.
In the examinations of bone marrow smears, the cellularity could not be evaluated in 10 dogs due to the dilution by peripheral blood. For the remaining 11 dogs, the cellularity was normocellular in 5 and hypercellular in 6. In addition, 1,000 nucleated cells could be counted in 17 dogs. The median M-E ratio was 1.1 (range: 0.2–14), and the median blast cell ratio was 1% (range: 0.2–2.6%). Erythroid cells were hypercellular in 6 dogs, normocellular in 5 dogs, and hypocellular in 6 dogs. The median erythroid maturation ratio was 0.7 (range: 0.08–3.84), and it was above the reference range in 16 dogs. Myeloid cells were hypercellular in 6 dogs, normocellular in 5 dogs, and hypocellular in 6 dogs. The median granulocyte maturation ratio was 0.1 (range: 0.05–0.27), and it was above the reference range in 5 dogs. Megakaryocytes were hypercellular in 2 dogs, normocellular in 6 dogs, and hypocellular in 3 dogs.
The dysplastic morphological features were also examined in bone marrow smears, and the number of dogs with dysplastic features and the frequencies of each feature in the smears are shown in Table 3. In erythroid cells, fragmented nucleus was most frequently observed (15 dogs; Fig. 2a), and the median frequency of this morphologic feature was 1.8%. In myeloid cells, ring neutrophil was the most frequent and observed in 5 dogs (Fig. 2b), and the median frequency was 0.15%. In megakaryocytes, dysmorphic megakaryocytes with multiple nuclei were most commonly observed (13 dogs; Fig. 2c), and the median frequency was 12%. One or more dysplastic morphological features were observed in all 21 dogs. Phagocytosis of intact erythroid precursors by intact, normal-appearing macrophages, which was described in PIMA [12], was not observed in any of the 21 dogs.
Table 3. The number of Miniature Dachshunds with dysplastic features and the frequencies of these features in bone marrow smears.
| Number of cases | Median frequency (Range) (%) |
||
|---|---|---|---|
| Erythroid linage | |||
| Fragmented nuclei | 15 | 1.8 (0.2−5.1) | |
| Megaloblastic cells | 8 | 0.6 (0.2−6.2) | |
| Binucleate cells | 3 | 0.5 (0.2−2.0) | |
| Myerloid linage | |||
| Ring neutrophils | 5 | 0.2 (0.1−0.3) | |
| Giant metamyelocytes and neutrophils | 3 | 8.0 (5.8−8.3) | |
| Pseudo-Pelger-Huet cell morphology | 1 | 0.6 | |
| Megakaryocytic linage | |||
| Dysmorphic megakaryocytes with multiple nuclei | 13 | 12 (4−52) | |
| Mononucleate megakaryocytes | 5 | 4 (4−12) | |
| Micro-megakaryocytes | 4 | 4 (4−8) | |
Fig. 2.
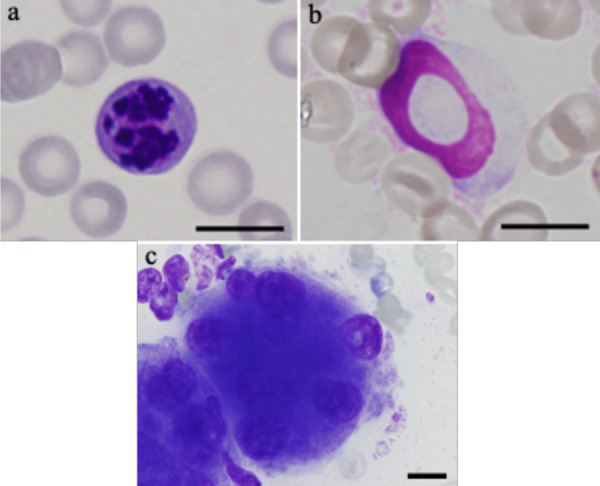
Fragmented nucleus in the erythroid lineage (a), ringed neutrophil in the myeloid linage (b), and dysmorphic megakaryocyte with multiple nuclei (c) observed in bone marrow smears stained with Wright-Giemsa stain. Bar=10 µm.
Histopathological examinations of bone marrow core samples were conducted in 19 dogs. Fibrocyte augmentation was observed in 3 dogs, and moderate to severe fibrosis was observed in 6 dogs (Fig. 3).
Fig. 3.
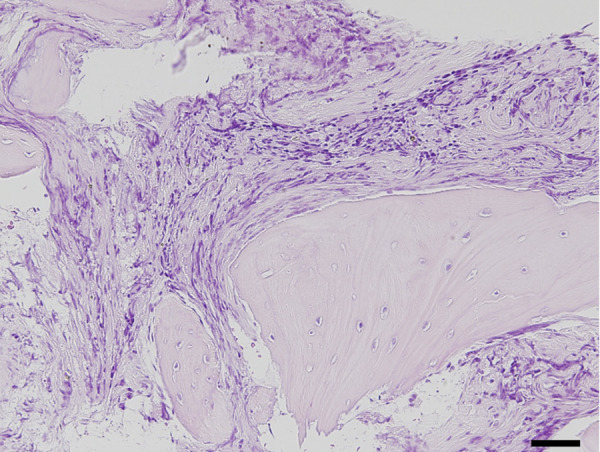
Severe fibrosis observed in bone marrow core biopsy stained by hematoxylin-eosin stain. Bar=50 µm.
All dogs were treated with prednisolone, and median of the largest dose of prednisolone in each case was 1.8 mg/kg/day (range: 1.3–3.4 mg/kg/day). Immunosuppressive drugs were also used in some cases as follows: cyclosporine (median: 7.5 mg/kg/day, range: 4–12 mg/kg/day) in 15 dogs, mycophenolate mofetil (median: 21 mg/kg/day, range: 20–30 mg/kg/day) in 6 dogs, leflunomide (median: 3.7 mg/kg/day, range: 3.4–4 mg/kg/day) in 2 dogs, and azathioprine (median: 1.5 mg/kg/day, range: 1–2 mg/kg/day) in 2 dogs. In addition, antibiotics were used in 20 dogs, menatetrenone (median: 1.4 mg/kg/day, range: 0.7–2.1 mg/kg/day) in 14 dogs, and hydroxycarbamide (12.5 mg/kg/day) in 1 dog.
One dog could not be followed for 2 months and one died from acute enteritis at 7 weeks after diagnosis. Therefore, treatment responsiveness and prognosis could be investigated in 19 dogs. Three dogs responded to corticosteroid alone, whereas 6 responded to corticosteroid and immunosuppressive drugs, and the median duration from treatment initiation to response was 78 days (range: 61–150 days). Ten dogs did not respond until 6 months after treatment initiation. Based on these observations, 9 dogs were assigned to the treatment-responsive group and 10 to the treatment-resistant group. During the study period, 5 dogs died from variable causes. Three dogs died from acute hemolytic transfusion reaction during blood transfusion on day 78, 82 and 90, respectively. One dog died on day 507 and was diagnosed with systemic histiocytosis on autopsy. The cause of death was unclear in the remaining 1 dog.
Comparison of clinical and clinicopathological features between treatment-responsive and -resistant MDs
Based on the response to treatment, 9 MDs were assigned to the treatment-responsive MD group and 10 were assigned to the treatment-resistant MD group. No significant difference was observed in age and sex distribution between the two groups. The number of platelets of MDs in the treatment-resistant group was significantly higher than that of MDs in the treatment-responsive group (P=0.004, Fig. 4). When evaluating peripheral blood smears, we found that hypersegmented neutrophils tended to be more frequently observed in the treatment-resistant MD group than in the treatment-responsive MD group (P=0.07, Table 4). No significant difference was observed in the proportions of dogs treated with corticosteroid before diagnosis between the two groups. Spindle-shaped platelets were significantly more frequent in the treatment-resistant MD group than in the treatment-responsive MD group (P=0.02).
Fig. 4.
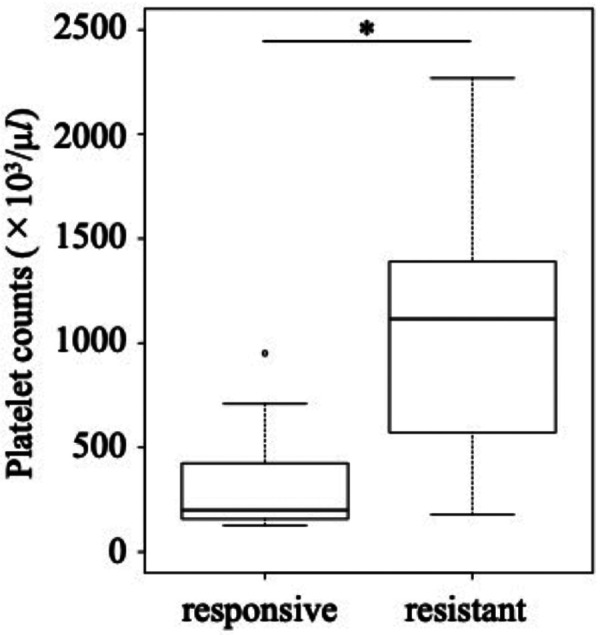
Comparison of platelet counts between treatment-responsive and treatment-resistant Miniature Dachshunds. *P<0.05.
Table 4. Comparisons of the clinical and clinicopathological features between treatment-responsive and -resistant Miniature Dachshunds.
| Responsive (n=9) | Resistant (n=10) | P-value | ||
|---|---|---|---|---|
| CBC | ||||
| Hematocrit (%) | 19.1 (7−24.6) | 18.1 (10.8−23.1) | 0.858 | |
| Reticulocyte (×103/µl) | 20.9 (8.9−52.4) | 13.8 (5.3−55.9) | 0.360 | |
| WBC (×103/µl) | 13.3 (5.9−49.2) | 11.3 (7.9−50.4) | 0.780 | |
| Neutrophil (×103/µl) | 10.4 (4.4−44.1) | 9.3 (6.1−42.4) | 0.842 | |
| Lymphocyte (×103/µl) | 1.6 (0.7−4.0) | 1.2 (0.3−2.4) | 0.095 | |
| Platelet (×103/µl) | 200 (127−951) | 1,116 (178−2,268) | 0.004 | |
| Biochemistry | ||||
| ALT (U/l) | 63 (35−102) | 113 (18−907) | 0.243 | |
| ALP (U/l) | 319 (29−1,008) | 590 (173−8,186) | 0.278 | |
| BUN (mg/dl) | 16.3 (8−39.3) | 14.8 (9.8−27.2) | 1 | |
| Creatinine (mg/dl) | 0.5 (0.2−0.7) | 0.2 (0.1−1.2) | 0.092 | |
| CRP (mg/dl) | 1.2 (0.7−6.9) | 0.9 (0.3−6.9) | 0.344 | |
| Periperal blood smear | ||||
| Hyper-segmented neutrophil | 3 | 8 | 0.07 | |
| Spindle shape platelet | 1 | 7 | 0.02 | |
| Bone marrow smear | ||||
| Granulocyte maturation ratio | 0.19 (0.06−0.27) | 0.07 (0.05−0.12) | 0.01 | |
CBC, complete blood count; WBC, white blood cell; ALT, alanine transaminase; ALP, alkaline phosphatase; BUN, blood urea nitrogen; CRP, C-reactive protein.
When evaluating bone marrow samples, we could examine 8 treatment-responsive MDs and 8 treatment-resistant MDs. The granulocyte maturation ratio was significantly higher in the treatment-responsive MD group than in the treatment-resistant group (P=0.01), and all dogs with granulocyte maturation ratio above the reference range were included in the treatment-responsive group. On the other hand, there was no significant difference in the M-E ratio, erythroid maturation ratio, and the frequencies of erythroid cells with fragmented nuclei, ring neutrophils, and dysmorphic megakaryocytes with multiple nuclei. Mild to severe myelofibrosis was observed only in 4 treatment-resistant MDs, although there was no significant difference in the frequency of myelofibrosis between the two groups (P=0.087). Treatment-responsive dogs showed significantly longer survival times compared with -resistant dogs (Fig. 5, P=0.03).
Fig. 5.
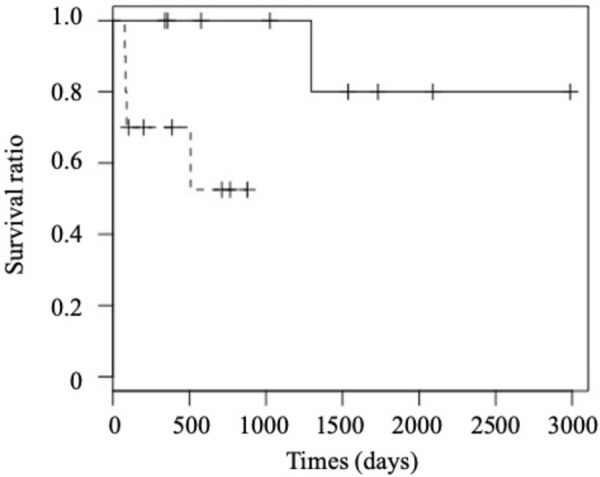
Comparison of overall survival between treatment-responsive (solid line) and treatment-resistant (dotted line) Miniature Dachshunds.
ROC curve analysis was also conducted to evaluate sensitivity, specificity and optimal cutoff value of the number of platelets to predict the resistance to treatment. The area under the ROC curve was 0.89, and a cut-off number of platelets of 1,115 × 103/µl was determined as a point where the sum of sensitivity and specificity values were the highest. When this cut-off value was used, the sensitivity and specificity to predict the resistance to treatments were 0.60 (6/10) and 1.00 (0/9), respectively. The median overall survival of dogs with platelets of >1,115 × 103/µl was 354 days (range: 82–766 days), whereas that of dogs with platelets of <1,115 × 103/µl were 878 days (range: 78–2,986 days). Although the difference in overall survival was not statistically significant, the overall survival of dogs with platelets of >1,115 × 103/µl tended to be shorter than that of dogs with platelets of <1,115 × 103/µl (P=0.052, Fig. 6).
Fig. 6.
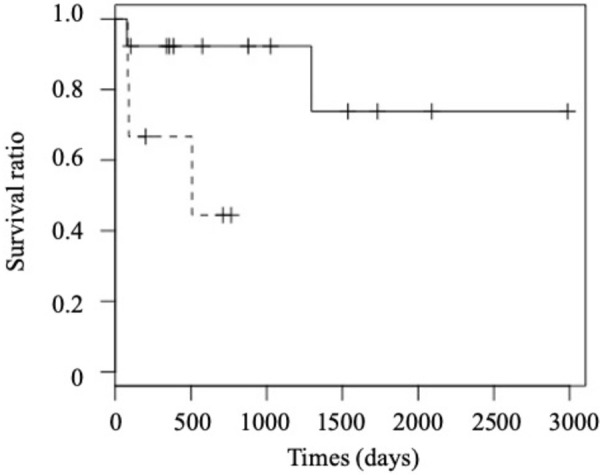
Comparison of overall survival between Miniature Dachshunds with platelets of <1,115 × 103/µl (solid line) and those with platelets of >1,115 × 103/µl (dotted line).
DISCUSSION
In this study, we investigated the breed predisposition of dogs diagnosed with non-neoplastic bone marrow disorders that induce anemia. As a result, MD was identified as a predisposed breed of non-neoplastic bone marrow disorders in Japan. In the United States, Miniature Dachshunds were reported as a predisposed breeds for PIMA [2]. It was reported that MD was a breed commonly predisposed to inflammatory colorectal polyps and sterile panniculitis, which were rare in other breeds [16, 23]. It is possible that breed-specific genetic aberration might be an underlying cause of non-neoplastic bone marrow disorders seen in MDs in Japan.
Next, we retrospectively investigated clinical and clinicopathological features of MDs diagnosed with non-neoplastic bone marrow disorders. On signalment, spayed females were significantly overrepresented in MDs. In previous studies, spayed females were also significantly predisposed to be diagnosed with NRIMA, PRCA, and PIMA [2, 17, 22], but the underlying causes have been unknown.
With regard to laboratory tests, autoagglutination tests were negative in all MDs, and direct Coombs test was positive only in 1 dog in MDs. Since it was previously reported that 57% of dogs diagnosed as having NRIMA or PRCA showed a positive direct Coombs test [17], the proportions of dogs with positive direct Coombs test results seemed to be low in the present study. Meanwhile, one or more dysplastic morphological features were found in the bone marrow of all dogs. However, the frequencies of these features in each smear were considered low because previous reports showed that dysplastic features were more than 10% or 25% of each lineage in canine MDS [3, 21]. Sixteen of 17 MDs showed an elevation in the erythroid maturation ratio. An increase in the erythroid maturation ratio or left-shifted erythroid maturation was reported in MDS, NRIMA, and PIMA [12, 17, 21]. Since clear phagocytosis of erythroid precursors by intact macrophage was not observed in any case, PIMA could be excluded as a diagnosis for MDs included in the present study. However, all dogs were considered to harbor clinicopathological features that were similar to both MDS and NRIMA, and it was unclear which disease was more suitable as the diagnosis for these MDs.
Thrombocytosis was observed in 11 of 21 MDs at initial admission. Additionally, the number of platelets was significantly increased in treatment-resistant MDs compared with treatment-responsive MDs, and a cut-off number of platelets of 1,115 × 103/µl might be useful to predict the resistance to treatment and prognosis. Hypersegmented neutrophils tended to be more frequently observed in treatment-resistant MDs compared with treatment-responsive MDs. Furthermore, spindle-shaped platelets were significantly increased in treatment-resistant MDs compared with treatment-responsive MDs. These observations indicated that bone marrow disorders in treatment-resistant MDs might harbor distinct features compared with -responsive dogs, and sensitivity to immunosuppressive treatments could be predicted based on the number of platelets and the morphological features in peripheral blood.
Thrombocytosis is observed in cases with ET, which is characterized by hyperplasia of megakaryocytic lineage [14]. Since 2 of 21 dogs showed hyperplasia of megakaryocyte lineage in the present study, it was possible that they might be affected with ET. However, various kinds of dysplastic features were also observed on bone marrow smears in these dogs, and it was thought that ET was not suitable as a definitive diagnosis for these dogs.
Hypersegmented neutrophils are evaluated as a dysplastic feature in human MDS patients [24]. Although hypersegmented neutrophils were reported to be observed after administration of corticosteroids [11], no significant difference was observed in the frequency of corticosteroid administration between treatment-responsive MDs and treatment-resistant MDs. Subsequently, it is reasonable to consider hypersegmented neutrophils as dysplastic features in the present study. Spindle-shaped platelets have been poorly reported, but similar platelets were reported in human hematopoietic neoplasms [8]. Therefore, it is possible that treatment-resistant MDs harbored a bone marrow disorder that was similar to MDS.
The granulocyte maturation ratio was significantly higher in treatment-responsive MDs compared with treatment-resistant MDs. An increase in this ratio has been observed in various conditions, such as infection, corticosteroid treatment, hemorrhage, metabolic acidosis, and postoperative states [9], but the causes of this increase in treatment-responsive MDs were unclear in the present study.
In human medicine, patients are diagnosed as MDS-unclassifiable (MDS-U) when they show persistent cytopenia(s), no significant unequivocal dysplastic features in hematopoietic cells, blast cell ratios of less than 5% in bone marrow, and MDS-defining cytogenetic abnormalities [13]. Considering these features, it is possible that bone marrow disorders of MDs in the present study were similar to MDS-U. However, the diagnosis of MDS-U was difficult in these dogs because cytogenetic abnormalities relating to MDS have not been elucidated in dogs.
Both anemia and thrombocytosis were observed in some MDs especially in treatment-resistant group. In human medicine, myelodysplastic and myeloproliferative neoplasms (MDS/MPNs) are defined as a heterogeneous group diagnosed based on both features of MDS and MPN. Considering the clinicopathological features of treatment-resistant MDs, part of these dogs might be affected with disorders similar to MDS/MPN-unclassifiable (MDS/MPN-u), which is one of the types of MDS/MPNs [1].
Interestingly, it was reported that non-regenerative anemia and thrombocytosis, which were similar to the observations in treatment-resistant MDs, were observed in a mouse model of human MDS where the ASXL1 gene was mutated [15]. Additionally, the frequencies of dysplastic features in this mouse model were reported as relatively low [15], which were also similar to the observations in treatment-resistant MDs. Taken together, genetic aberration might be related to the pathogenesis of the bone marrow disorder seen in treatment-resistant MDs.
One of the limitations of the present study is that a standardized treatment protocol could not be conducted for all dogs because this study was preliminary retrospective one. An additional limitation is that the present study was conducted at a single facility. It is also a limitation that the evaluations of peripheral blood and bone marrow smears were not conducted by a third person who is board-certified and blinded to the outcomes of the dogs. Furthermore, the number of dogs included in the present study was relatively low, and some comparisons might not have enough statistical power to show significant differences. Therefore, prospective studies at multiple facilities using larger sample sizes are needed.
In conclusion, we found that, in Japan, MD was predisposed to bone marrow disorder that induced anemia. The investigations of the clinical and clinicopathological features of non-neoplastic bone marrow disorders in MDs revealed that bone marrow disorders of treatment-resistant MDs might harbor distinct features compared with other dogs. In addition, it is possible that the sensitivity to immunosuppressive treatments in MDs could be predicted based on the number of platelets and dysplastic features in the peripheral blood. In the future, cytological evaluations of peripheral blood and bone marrow smears by board-certified and blinded specialists are essential to elucidate these findings, and further studies, including examinations of genome aberrations, are needed to elucidate the pathophysiology of bone marrow disorders observed in MDs.
Acknowledgments
We would like to thank J. K. Chambers and K. Uchida for their helps to conduct pathological examinations of bone marrow core specimens. This study was supported by the Japan Society for the Promotion of Science, KAKENHI [grant number 17H05043 and 17H03921]. Hirotaka T. received 17H05043 and Hajime T. received 17H03921. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Arber D. A., Orazi A., Hasserjian R., Thiele J., Borowitz M. J., Le Beau M. M., Bloomfield C. D., Cazzola M., Vardiman J. W.2016. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127: 2391–2405. doi: 10.1182/blood-2016-03-643544 [DOI] [PubMed] [Google Scholar]
- 2.Assenmacher T. D., Jutkowitz L. A., Koenigshof A. M., de A Lucidi C., Scott M. A.2019. Clinical features of precursor-targeted immune-mediated anemia in dogs: 66 cases (2004-2013). J. Am. Vet. Med. Assoc. 255: 366–376. doi: 10.2460/javma.255.3.366 [DOI] [PubMed] [Google Scholar]
- 3.Blue J. T.2003. Myelodysplasia: differentiating neoplastic from nonneoplastic syndromes of ineffective hematopoiesis in dogs. Toxicol. Pathol. 31Suppl: 44–48. [DOI] [PubMed] [Google Scholar]
- 4.Bowen D., Culligan D., Jowitt S., Kelsey S., Mufti G., Oscier D., Parker J., UK MDS Guidelines Group2003. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br. J. Haematol. 120: 187–200. doi: 10.1046/j.1365-2141.2003.03907.x [DOI] [PubMed] [Google Scholar]
- 5.Brazzell J. L., Weiss D. J.2006. A retrospective study of aplastic pancytopenia in the dog: 9 cases (1996-2003). Vet. Clin. Pathol. 35: 413–417. doi: 10.1111/j.1939-165X.2006.tb00157.x [DOI] [PubMed] [Google Scholar]
- 6.Breuer W., Darbes J., Hermanns W., Thiele J.1999. Idiopathic myelofibrosis in a cat and in three dogs. Comp. Haematol. Int. 9: 17–24. doi: 10.1007/BF02585517 [DOI] [Google Scholar]
- 7.Cheson B. D., Greenberg P. L., Bennett J. M., Lowenberg B., Wijermans P. W., Nimer S. D., Pinto A., Beran M., de Witte T. M., Stone R. M., Mittelman M., Sanz G. F., Gore S. D., Schiffer C. A., Kantarjian H.2006. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108: 419–425. doi: 10.1182/blood-2005-10-4149 [DOI] [PubMed] [Google Scholar]
- 8.Hattori A., Sanada M., Kojima T., Ihzumi T., Koike T., Nagayama R., Shibata A.1981. Studies on platelet shape and function 9th report bipolar platelets. Blood Vessels 12: 182–187. doi: 10.2491/jjsth1970.12.182 [DOI] [Google Scholar]
- 9.Honda T., Uehara T., Matsumoto G., Arai S., Sugano M.2016. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin. Chim. Acta 457: 46–53. doi: 10.1016/j.cca.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 10.Jain N. C., Blue J. T., Grindem C. B., Harvey J. W., Kociba G. J., Krehbiel J. D., Latimer K. S., Raskin R. E., Thrall M. A., Zinkl J. G.1991. Proposed criteria for classification of acute myeloid leukemia in dogs and cats. Vet. Clin. Pathol. 20: 63–82. doi: 10.1111/j.1939-165X.1991.tb00571.x [DOI] [PubMed] [Google Scholar]
- 11.Liles W. C., Dale D. C., Klebanoff S. J.1995. Glucocorticoids inhibit apoptosis of human neutrophils. Blood 86: 3181–3188. doi: 10.1182/blood.V86.8.3181.3181 [DOI] [PubMed] [Google Scholar]
- 12.Lucidi C. A., de Rezende C. L. E., Jutkowitz L. A., Scott M. A.2017. Histologic and cytologic bone marrow findings in dogs with suspected precursor-targeted immune-mediated anemia and associated phagocytosis of erythroid precursors. Vet. Clin. Pathol. 46: 401–415. doi: 10.1111/vcp.12502 [DOI] [PubMed] [Google Scholar]
- 13.Margolskee E., Hasserjian R. P., Hassane D., Tam W., Mathew S., Ok C. Y., Wang S. A., Oak J., Arber D. A., Orazi A.2017. Myelodysplastic Syndrome, Unclassifiable (MDS-U) with 1% blasts is a distinct subgroup of MDS-U with a poor prognosis. Am. J. Clin. Pathol. 148: 49–57. doi: 10.1093/ajcp/aqx043 [DOI] [PubMed] [Google Scholar]
- 14.Mizukoshi T., Fujino Y., Yasukawa K., Matumoto H., Matsumura S., Nagasaki T., Ohno K., Tsujimoto H., Shimoda T.2006. Essential thrombocythemia in a dog. J. Vet. Med. Sci. 68: 1203–1206. doi: 10.1292/jvms.68.1203 [DOI] [PubMed] [Google Scholar]
- 15.Nagase R., Inoue D., Pastore A., Fujino T., Hou H. A., Yamasaki N., Goyama S., Saika M., Kanai A., Sera Y., Horikawa S., Ota Y., Asada S., Hayashi Y., Kawabata K. C., Takeda R., Tien H. F., Honda H., Abdel-Wahab O., Kitamura T.2018. Expression of mutant Asxl1 perturbs hematopoiesis and promotes susceptibility to leukemic transformation. J. Exp. Med. 215: 1729–1747. doi: 10.1084/jem.20171151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohmi A., Tsukamoto A., Ohno K., Uchida K., Nishimura R., Fukushima K., Takahashi M., Nakashima K., Fujino Y., Tsujimoto H.2012. A retrospective study of inflammatory colorectal polyps in miniature dachshunds. J. Vet. Med. Sci. 74: 59–64. doi: 10.1292/jvms.11-0352 [DOI] [PubMed] [Google Scholar]
- 17.Stokol T., Blue J. T., French T. W.2000. Idiopathic pure red cell aplasia and nonregenerative immune-mediated anemia in dogs: 43 cases (1988–1999). J. Am. Vet. Med. Assoc. 216: 1429–1436. doi: 10.2460/javma.2000.216.1429 [DOI] [PubMed] [Google Scholar]
- 18.Turinelli V., Gavazza A., Stock G., Fournel-Fleury C.2015. Canine bone marrow cytological examination, classification and reference values: A retrospective study of 295 cases. Res. Vet. Sci. 103: 224–230. doi: 10.1016/j.rvsc.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 19.Weiss D. J.2006. A retrospective study of the incidence and the classification of bone marrow disorders in the dog at a veterinary teaching hospital (1996–2004). J. Vet. Intern. Med. 20: 955–961. [DOI] [PubMed] [Google Scholar]
- 20.Weiss D. J., Aird B.2001. Cytologic evaluation of primary and secondary myelodysplastic syndromes in the dog. Vet. Clin. Pathol. 30: 67–75. doi: 10.1111/j.1939-165X.2001.tb00261.x [DOI] [PubMed] [Google Scholar]
- 21.Weiss D. J., Smith S. A.2000. Primary myelodysplastic syndromes of dogs: a report of 12 cases. J. Vet. Intern. Med. 14: 491–494. doi: 10.1111/j.1939-1676.2000.tb02264.x [DOI] [PubMed] [Google Scholar]
- 22.Weiss D. J.2002. Primary pure red cell aplasia in dogs: 13 cases (1996–2000). J. Am. Vet. Med. Assoc. 221: 93–95. doi: 10.2460/javma.2002.221.93 [DOI] [PubMed] [Google Scholar]
- 23.Yamagishi C., Momoi Y., Kobayashi T., Ide K., Ohno K., Tsujimoto H., Iwasaki T.2007. A retrospective study and gene analysis of canine sterile panniculitis. J. Vet. Med. Sci. 69: 915–924. doi: 10.1292/jvms.69.915 [DOI] [PubMed] [Google Scholar]
- 24.Yoshida Y.1996. Physician education: myelodysplastic syndrome. Oncologist 1: 284–287. doi: 10.1634/theoncologist.1-4-284 [DOI] [PubMed] [Google Scholar]