Abstract
Human epidermal growth factor receptor 2 (HER2) overexpression has been reported in various human cancers. HER2-targeted therapies showed clinical responses in humans with HER2-positive tumors. The incidence of canine primary lung cancer (cPLC) is increasing, but there are no effective systemic therapies for dogs with late-stage cPLC. HER2-targeted therapy could be an option for cPLC, but HER2 expression in cPLC remains unknown. We evaluated HER2 expression in cPLC. Immunohistochemical analysis revealed that 3 samples (19%) scored 3+; 8 (50%), 2+; 5 (31%); and 1+ and 0 (0%), 0. Of the cPLC tissues, 69% were HER2 positive (scored ≥2+). These data would lead to further evaluation of the role of HER2 in cPLC as a mechanism of malignancy and therapeutic target.
Keywords: Canis familiaris, lung cancer, oncogene protein human epidermal growth factor receptor 2
Human epidermal growth factor receptor 2 (HER2) is a receptor tyrosine kinase and one of the members of the human epidermal growth factor receptor family [2]. HER2 amplification and protein overexpression have been reported in various human cancers, including breast, gastric, and lung cancers [5, 11, 14, 19]. HER2 has been shown as both a diagnostic marker and therapeutic target in these tumors [5]. HER2-targeted therapies, such as lapatinib and trastuzumab, have been developed and showed clinical responses in humans with HER2-positive cancers [1, 8, 22].
In human medicine, lung cancer is the leading cause of cancer-related death worldwide [11]. Approximately 85% of human lung cancers are non-small cell lung cancers (NSCLCs), which consisted of three distinct histologic subtypes: squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma [15]. HER2 was overexpressed in a proportion of patients with NSCLC and related to tumorigenesis of NSCLC [13]. HER2 protein overexpression is observed in 6–35% of patients with NSCLC, and HER2 gene amplification is noted in 10–20% of such patients [13]. Subsets of patients with high HER2 immunohistochemical (IHC) expression (score of 3+) were found to have a good response to HER2-targeted antibody, trastuzumab [7, 9]. Moreover, several emerging approaches to target HER2-positive NSCLC have been established, and clinical trials are ongoing to evaluate the efficacy of new HER2-targeted therapies, such as trastuzumab emtansine and margetuximab [11].
Canine primary lung cancer (cPLC) is relatively uncommon in dogs compared to humans, with an estimated incidence of 1.5–4.2 cases per 10,000 dogs [3, 4]. However, its incidence is increasingly reported because of improved diagnostic procedures, increased pet longevity, and environmental changes [17]. Surgical excision is a more likely effective therapy for dogs, in which surgical remission could be achieved, and the median survival time is 330 days in these cases but 28 days in dogs that could not be rendered free of visible disease [16]. Although systemic therapy is required for dogs with late-stage cPLC, chemotherapy has been largely unrewarding in the gross disease setting [21]. Treatment with molecular- and antigen-targeted therapies could be beneficial, but such targeted therapies have yet to be thoroughly examined in dogs with cPLC [21].
As with human cancer, HER2 overexpression has been reported in dogs with canine mammary gland carcinoma, transitional cell carcinoma, and apocrine gland anal sac adenocarcinoma [6, 18, 24], and HER2-targeted therapies, such as lapatinib and HER2-targeting recombinant Listeria vaccines, have shown an anti-tumor effect on canine HER2-positive cancers [12, 18]. HER2 could be a therapeutic target and diagnostic marker for HER2-positive cPLC, but HER2 expression in cPLC is still unclear. This study aimed to evaluate HER2 mRNA and protein expression in cPLC tissues.
To evaluate mRNA expression of HER2, fresh cPLC (n=6) and normal lung (n=4) tissues were obtained from dogs with cPLC and healthy beagles, respectively. The cPLC tissues were obtained from dogs with cPLC at the Veterinary Medical Center of Tokyo between April 2017 and December 2018. To investigate the IHC expression of HER2, paraffin-embedded tissues of cPLC (n=16) were analyzed. The tissues were surgically removed between January 2011 and December 2016. The tissues samples were diagnosed by veterinary pathologists certified by the Japanese College of Veterinary Pathologists at the Department of Veterinary Pathology at the University of Tokyo. All patients underwent clinical staging by thoracic radiography and computed tomography.
Permission for the collection and use of resected tissue for this study was obtained from the owners. Healthy dog tissue was obtained from healthy beagles euthanized for other experiments. All procedures were approved by the Animal Care and Use Committee at the University of Tokyo (approval number, P17-074).
The total RNA was extracted and reverse transcribed using a transcriptase (ReverTra Ace, Toyobo, Osaka, Japan). Real-time polymerase chain reaction (PCR) was performed using a premix reagent (THUNDERBIRD SYBR qPCR Mix, Toyobo), specific primers for HER2 (forward primer, CAGCCCTGGTCACCTACAA; reverse primer, CCACATCCGTAGACAGGTAG), and a real-time PCR system (StepOnePlus, Thermo Fisher Scientific, Waltham, MA, USA). mRNA expression was analyzed using the standard curve method, and expression value was normalized using gapdh (forward primer, TGACACCCACTCTTCCACCTTC; reverse primer, CGGTTGCTGTAGCCAAATTCA) as an internal control.
The cPLC tissues were retrospectively evaluated by IHC analysis. All samples were fixed in 10% neutral buffered formalin, embedded in paraffin wax, and cut into 4-µm-thick serial sections. Paraffin-embedded tumor sections were dewaxed and rehydrated in xylene and graded ethanol. Then, antigen retrieval was performed using Dako Target Retrieval Solution, pH 9.0 (Agilent Technologies, Santa Clara, CA, USA), by microwaving for 15 min at 750 W. After washing with Tris-buffered saline containing 0.1% Tween 20 (TBS-t), endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol for 10 min. Then, specimens were washed with TBS-t and incubated in 8% skimmed milk for 1 hr at 20–28°C to reduce nonspecific binding before overnight incubation with primary antibodies, which included mouse IgG1 anti-HER2 monoclonal antibodies (clone, CB-11, Leica Biosystems, Wetzlar, Germany) diluted 1:40, at 4°C in a humidified chamber. A negative control was incubated with purified mouse IgG1, κ isotype antibody (clone, MG1-45, BioLegend, San Diego, CA, USA), under identical conditions. After washing with TBS-t, sections were incubated with a horseradish peroxidase (HRP)-conjugated anti-mouse antibody (Envision+ System-HRP Labeled Polymer; K4001; Agilent Technologies) for 30 min at 20–28°C. Thereafter, the sections were washed with TBS-t, incubated with 3,3′ diaminobenzidine (Dojindo Laboratories, Rockville, MD, USA) solution for 3 min, and counterstained with Mayer’s hematoxylin. In the negative control, sections were subjected to the same procedures. The results were quantified based on the recent guidelines proposed by the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) [23].
The statistical method and software used were Fisher’s exact test (R Development Core Team, 2018) and the beeswarm R package (version 0.2.3, 2016). A P-value <0.05 was considered statistically significant.
mRNA expression levels of HER2 were evaluated in cPLC and normal lung tissues. Variation in the mRNA expression of HER2 in cPLC tissues was higher than that in normal tissues and high HER2 mRNA expression was more frequently observed in cPLC tissues compared to normal lung tissues (Fig. 1). Of 6 cPLC samples, 5 expressed higher HER2 mRNA expression than any normal samples (P<0.05).
Fig. 1.
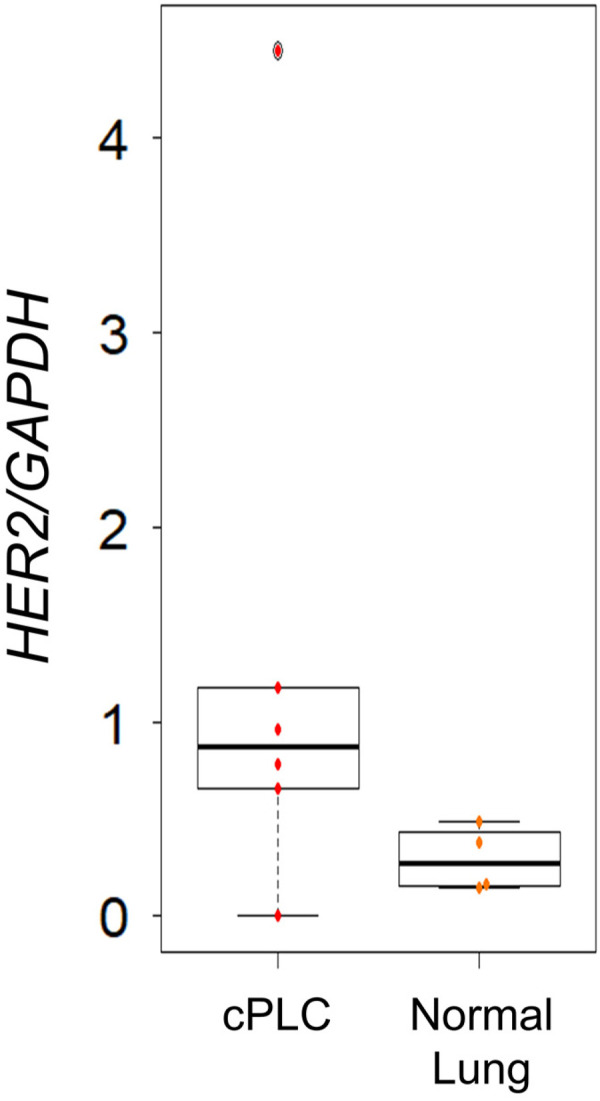
Human epidermal growth factor receptor 2 (HER2) mRNA expression in canine primary lung cancer (cPLC) (n=6) and normal lung tissues (n=4). Of 6 cPLC samples, 5 expressed higher HER2 mRNA than any normal samples (P<0.05).
In the IHC analysis, 16 cPLC tissue samples were collected from 16 dogs (5 castrated male, 3 intact male, 7 spayed female, and 1 intact female dogs) of different breeds (5 Chihuahuas, 4 Miniature Dachshunds, 2 Shih Tzu, 1 Beagle, 1 English Pointer, 1 Saluki, and 1 Toy Poodle). The mean age was 12.3 ± 1.3 years (population standard deviation), ranging from 10.2 to 15.1 years. Three tumors were located on right cranial, 3 on the right middle, 2 on the right caudal, 1 on the left cranial, and 7 on the left caudal area (Table 1).
Table 1. Characteristics of dogs and the corresponding human epidermal growth factor receptor 2 expression.
| No. | Breeda) | Age (years) |
Sexb) | Location | TNM classification | Classification | IHCc) score | ||
|---|---|---|---|---|---|---|---|---|---|
| T | N | M | |||||||
| 1 | CH | 13.2 | MC | Right cranial | T1 | N0 | M0 | Adenocarcinoma | 1+ |
| 2 | CH | 11.5 | FS | Right middle | T1 | N0 | M0 | Adenocarcinoma | 1+ |
| 3 | TP | 11.3 | MC | Left caudal | T1 | N1 | M0 | Adenocarcinoma | 1+ |
| 4 | SAL | 11.8 | M | Left caudal | T1 | N1 | M0 | Adenocarcinoma | 1+ |
| 5 | SHI | 10.2 | FS | Left caudal | T1 | N0 | M0 | Adenocarcinoma | 2+ |
| 6 | CH | 12.3 | MC | Left caudal | T1 | N0 | M0 | Adenocarcinoma | 2+ |
| 7 | MLT | 13.6 | MC | Left caudal | T1 | N0 | M0 | Adenocarcinoma | 2+ |
| 8 | SHI | 11.8 | MC | Left cranial | T1 | N0 | M0 | Adenocarcinoma | 2+ |
| 9 | MD | 11.2 | FS | Right middle | T1 | N0 | M0 | Adenocarcinoma | 2+ |
| 10 | CH | 11.2 | M | Right cranial | T1 | N0 | M0 | Adenocarcinoma | 2+ |
| 11 | CH | 14.2 | F | Right cranial | T1 | N0 | M0 | Adenocarcinoma | 2+ |
| 12 | MD | 15.1 | M | Right middle | T1 | N0 | M0 | Adenocarcinoma | 3+ |
| 13 | MD | 12.3 | FS | Left caudal | T2 | N0 | M1 | Adenocarcinoma | 3+ |
| 14 | BG | 13.0 | FS | Right caudal | T1 | N0 | M0 | Alveolar carcinoma | 3+ |
| 15 | EP | 12.8 | FS | Right caudal | T1 | N0 | M0 | Bronchial cell carcinoma | 2+ |
| 16 | MD | 12.3 | FS | Left caudal | T1 | N0 | M0 | Squamous cell carcinoma | 1+ |
a) CH, Chihuahua; TP, Toy Poodle; SAL, Saluki; SHI, Shih Tzu; MLT, Maltese; MD, Miniature Dachshund; BG, Beagle; EP, English Pointer. b) M, male; MC, male castrated; F, female; FS, female spayed. c) IHC, immunohistochemistry.
HER2-positive cPLC tissues demonstrated strong and broad staining of the circumferential membrane of cPLC cells and negative or weakly positive staining on peri-tumor normal cells and normal lung tissues via IHC staining (Fig. 2). We analyzed the HER2 expression score based on the ASCO/CAP guidelines (Table 2) to evaluate the staining pattern. Representative staining patterns of each score are shown in Fig. 2. HER2 expression score analysis demonstrated that 3 samples (19%) scored 3+; 8 samples (50%), 2+; 5 samples (31%), 1+; and 0 sample (0%), 0 (Table 3 and Supplementary Fig. 1). In total, 69% of cPLC tissues were HER2 positive (scored ≥2+). There was no relationship between HER2 expression scores and clinical characteristics (Table 1).
Fig. 2.
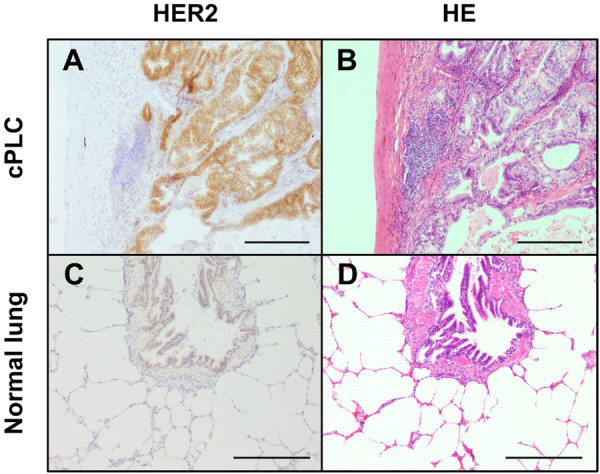
Representative staining pattern of human epidermal growth factor receptor 2 (HER2) in canine primary lung cancer (cPLC) (A) and normal lung tissue (C). Circumferential membrane staining is complete and intense in cPLC. (B, D) Hematoxylin and eosin staining of cPLC and normal tissues. Scale bar, 200 µm.
Table 2. Human epidermal growth factor receptor 2 scoring methods proposed by the American Society of Clinical Oncology/College of American Pathologists.
| Score | Description |
|---|---|
| 3+ | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells |
| 2+ | Circumferential membrane staining that is incomplete and/or
weak/moderate and in >10% of tumor cells or Complete and circumferential membrane staining that is intense and in <10% of tumor cells |
| 1+ | Incomplete membrane staining that is faint/barely perceptible and in >10% of tumor cells |
| 0 | No staining observed or Membrane staining that is incomplete and is faint/barely perceptible and in <10% of tumor cells |
Table 3. Human epidermal growth factor receptor 2 scores in canine primary lung cancer tissue samples.
| Negative | Positive | |||
|---|---|---|---|---|
| Score | 0 | 1+ | 2+ | 3+ |
| Number (%) | 0 (0%) | 5 (31%) | 8 (50%) | 3 (19%) |
In the current study, high HER2 mRNA expression was frequently found in cPLC tissues and circumferential membrane staining of canine HER2 in cPLC. HER2 expression was strongly detected in 69% of cPLC tissues, while HER2 was negative in normal lung tissues. Because most cases were resectable early-stage diseases and the number of patients was limited, we could not observe a correlation between HER2 expression and clinical characteristics, such as TNM classification, clinical stage, and histologic subtype. Further studies are required to investigate the association between HER2 expression and malignancies in cPLC.
Our results demonstrated that approximately 70% of cPLC overexpressed HER2. Recently, Lorch et al. reported that 38% of canine pulmonary adenocarcinoma possessed somatic HER2 point mutation mainly in hotspot V659E transmembrane domain and constitutively activated HER2 downstream signaling [10]. Importantly, they demonstrated that no cases having HER2 point mutation showed overexpression of HER2 mRNA and protein, as similar to typical finding that HER2 amplification/overexpression and gene mutations are mutually exclusive [10]. They also showed one out of HER2 wild type four tissues overexpressed HER2 protein [10]. The discrepancy in frequency of HER2 protein overexpression between the reports and our results could be because of the difference in clones of antibody, populations and/or scoring systems. Overall, through our and Lorch et al. findings, majority of cPAC possessed HER2 abnormality including overexpression and gene mutations, which would be beneficial findings for diagnosis and development of a novel therapy.
An increasing number of HER2-targeted therapies are developed in human medicine and some drugs, such as lapatinib, afatinib, and trastuzumab, showed clinical benefit for patients with NSCLC [11]. Since cPLC share clinic pathologic features with NSCLC [10] and canine HER2 has 92% amino acid homology with human HER2 [20], HER2-targeted therapies, which showed anti-tumor effect against NSCLC, could exhibit clinical benefit for cPLC. In fact, one of the HER2-targeted therapies, neratinib, have shown an anti-tumor effect on HER2V659E cPLC cell line which displayed constitutive activation of HER2 downstream signaling in vitro [10]. Moreover, other types of HER2-targeted therapies, such as trastuzumab and HER2-targeting recombinant Listeria vaccines, have shown potential anti-tumor effect against cPLC cell lines and dogs with osteosarcoma [10, 12]. Because these therapies are independent on HER2 downstream signaling, they might be potential novel therapies for dogs with HER2-positive cPLC. Above all, there are several therapeutic options targeting HER2 in cPLC, and our preliminary data would lead to further preclinical and clinical evaluation of these HER2 targeted therapies in cPLC.
Supplementary Material
REFERENCES
- 1.Awada G., Gombos A., Aftimos P., Awada A.2016. Emerging drugs targeting human epidermal growth factor receptor 2 (HER2) in the treatment of breast cancer. Expert Opin. Emerg. Drugs 21: 91–101. doi: 10.1517/14728214.2016.1146680 [DOI] [PubMed] [Google Scholar]
- 2.Casalini P., Iorio M. V., Galmozzi E., Ménard S.2004. Role of HER receptors family in development and differentiation. J. Cell. Physiol. 200: 343–350. doi: 10.1002/jcp.20007 [DOI] [PubMed] [Google Scholar]
- 3.Dorn C. R., Taylor D. O., Frye F. L., Hibbard H. H.1968. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J. Natl. Cancer Inst. 40: 295–305. [PubMed] [Google Scholar]
- 4.Dobson J. M., Samuel S., Milstein H., Rogers K., Wood J. L. N.2002. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 43: 240–246. doi: 10.1111/j.1748-5827.2002.tb00066.x [DOI] [PubMed] [Google Scholar]
- 5.English D. P., Roque D. M., Santin A. D.2013. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol. Diagn. Ther. 17: 85–99. doi: 10.1007/s40291-013-0024-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gama A., Alves A., Schmitt F.2008. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. Virchows Arch. 453: 123–132. doi: 10.1007/s00428-008-0644-3 [DOI] [PubMed] [Google Scholar]
- 7.Gatzemeier U., Groth G., Butts C., Van Zandwijk N., Shepherd F., Ardizzoni A., Barton C., Ghahramani P., Hirsh V.2004. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann. Oncol. 15: 19–27. doi: 10.1093/annonc/mdh031 [DOI] [PubMed] [Google Scholar]
- 8.Hurvitz S. A., Kakkar R.2012. Role of lapatinib alone or in combination in the treatment of HER2-positive breast cancer. Breast Cancer (Dove Med. Press) 4: 35–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langer C. J., Stephenson P., Thor A., Vangel M., Johnson D. H., Eastern Cooperative Oncology Group Study 2598.2004. Trastuzumab in the treatment of advanced non-small-cell lung cancer: is there a role? Focus on Eastern Cooperative Oncology Group study 2598. J. Clin. Oncol. 22: 1180–1187. doi: 10.1200/JCO.2004.04.105 [DOI] [PubMed] [Google Scholar]
- 10.Lorch G., Sivaprakasam K., Zismann V., Perdigones N., Contente-Cuomo T., Nazareno A., Facista S., Wong S., Drenner K., Liang W. S., Amann J. M., Sinicropi-Yao S. L., Koenig M. J., La Perle K., Whitsett T. G., Murtaza M., Trent J. M., Carbone D. P., Hendricks W. P. D.2019. Identification of recurrent activating HER2 mutations in primary canine pulmonary adenocarcinoma. Clin. Cancer Res. 25: 5866–5877. doi: 10.1158/1078-0432.CCR-19-1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mar N., Vredenburgh J. J., Wasser J. S.2015. Targeting HER2 in the treatment of non-small cell lung cancer. Lung Cancer 87: 220–225. doi: 10.1016/j.lungcan.2014.12.018 [DOI] [PubMed] [Google Scholar]
- 12.Mason N. J., Gnanandarajah J. S., Engiles J. B., Gray F., Laughlin D., Gaurnier-Hausser A., Wallecha A., Huebner M., Paterson Y.2016. Immunotherapy with a HER2-targeting listeria induces HER2-Specific immunity and demonstrates potential therapeutic effects in a phase I trial in canine osteosarcoma. Clin. Cancer Res. 22: 4380–4390. doi: 10.1158/1078-0432.CCR-16-0088 [DOI] [PubMed] [Google Scholar]
- 13.Mazières J., Peters S., Lepage B., Cortot A. B., Barlesi F., Beau-Faller M., Besse B., Blons H., Mansuet-Lupo A., Urban T., Moro-Sibilot D., Dansin E., Chouaid C., Wislez M., Diebold J., Felip E., Rouquette I., Milia J. D., Gautschi O.2013. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 31: 1997–2003. doi: 10.1200/JCO.2012.45.6095 [DOI] [PubMed] [Google Scholar]
- 14.Ménard S., Fortis S., Castiglioni F., Agresti R., Balsari A.2001. HER2 as a prognostic factor in breast cancer. Oncology 61Suppl 2: 67–72. doi: 10.1159/000055404 [DOI] [PubMed] [Google Scholar]
- 15.Molina J. R., Yang P., Cassivi S. D., Schild S. E., Adjei A. A.2008. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 83: 584–594. doi: 10.1016/S0025-6196(11)60735-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogilvie G. K., Weigel R. M., Haschek W. M., Withrow S. J., Richardson R. C., Harvey H. J., Henderson R. A., Fowler J. D., Norris A. M., Tomlinson J., et al. 1989. Prognostic factors for tumor remission and survival in dogs after surgery for primary lung tumor: 76 cases (1975-1985). J. Am. Vet. Med. Assoc. 195: 109–112. [PubMed] [Google Scholar]
- 17.Sabattini S., Mancini F. R., Marconato L., Bacci B., Rossi F., Vignoli M., Bettini G.2014. EGFR overexpression in canine primary lung cancer: pathogenetic implications and impact on survival. Vet. Comp. Oncol. 12: 237–248. doi: 10.1111/vco.12002 [DOI] [PubMed] [Google Scholar]
- 18.Sakai K., Maeda S., Saeki K., Nakagawa T., Murakami M., Endo Y., Yonezawa T., Kadosawa T., Mori T., Nishimura R., Matsuki N.2018. Anti-tumour effect of lapatinib in canine transitional cell carcinoma cell lines. Vet. Comp. Oncol. 16: 642–649. doi: 10.1111/vco.12434 [DOI] [PubMed] [Google Scholar]
- 19.Shao X., Kuai X., Pang Z., Zhang L., Wu L., Xu L., Zhou C.2018. Correlation of Gli1 and HER2 expression in gastric cancer: Identification of novel target. Sci. Rep. 8: 397. doi: 10.1038/s41598-017-17435-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singer J., Weichselbaumer M., Stockner T., Mechtcheriakova D., Sobanov Y., Bajna E., Wrba F., Horvat R., Thalhammer J. G., Willmann M., Jensen-Jarolim E.2012. Comparative oncology: ErbB-1 and ErbB-2 homologues in canine cancer are susceptible to cetuximab and trastuzumab targeting. Mol. Immunol. 50: 200–209. doi: 10.1016/j.molimm.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vail D. M., Thamm D. H., Liptak J. M.2019. Withrow & MacEwen’s Small Animal Clinical Oncology, 6th ed., pp. 507–514. Elsevier, Amsterdam. [Google Scholar]
- 22.Vogel C. L., Franco S. X.2003. Clinical experience with trastuzumab (herceptin). Breast J. 9: 452–462. doi: 10.1046/j.1524-4741.2003.09602.x [DOI] [PubMed] [Google Scholar]
- 23.Wolff A. C., Hammond M. E. H., Hicks D. G., Dowsett M., McShane L. M., Allison K. H., Allred D. C., Bartlett J. M. S., Bilous M., Fitzgibbons P., Hanna W., Jenkins R. B., Mangu P. B., Paik S., Perez E. A., Press M. F., Spears P. A., Vance G. H., Viale G., Hayes D. F., American Society of Clinical OncologyCollege of American Pathologists.2013. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31: 3997–4013. doi: 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto S., Kato D., Kamoto S., Yamamoto K., Tsuboi M., Shinada M., Ikeda N., Tanaka Y., Yoshitake R., Eto S., Saeki K., Chambers J. K., Kinoshita R., Uchida K., Nishimura R., Nakagawa T.2019. Detection of human epidermal growth factor receptor 2 overexpression in canine anal sac gland carcinoma. J. Vet. Med. Sci. 81: 1034–1039. doi: 10.1292/jvms.19-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


