Several events during the normal development of the mammalian neocortex depend on N-cadherin, including the radial migration of immature projection neurons into the cortical plate. Remarkably, radial migration requires the N-cadherin extracellular domain but not N-cadherin-dependent homophilic cell-cell adhesion, suggesting that other N-cadherin-binding proteins may be involved. We used proximity ligation and affinity purification proteomics to identify N-cadherin-binding proteins. Both screens detected MycBP2 and SPRY domain protein Fbxo45, two components of an intracellular E3 ubiquitin ligase.
KEYWORDS: F-box proteins, cadherin signaling, cell migration, neural development
ABSTRACT
Several events during the normal development of the mammalian neocortex depend on N-cadherin, including the radial migration of immature projection neurons into the cortical plate. Remarkably, radial migration requires the N-cadherin extracellular domain but not N-cadherin-dependent homophilic cell-cell adhesion, suggesting that other N-cadherin-binding proteins may be involved. We used proximity ligation and affinity purification proteomics to identify N-cadherin-binding proteins. Both screens detected MycBP2 and SPRY domain protein Fbxo45, two components of an intracellular E3 ubiquitin ligase. Fbxo45 appears to be secreted by a nonclassical mechanism, not involving a signal peptide and not requiring transport from the endoplasmic reticulum to the Golgi apparatus. Fbxo45 binding requires N-cadherin SPRY motifs that are not involved in cell-cell adhesion. SPRY mutant N-cadherin does not support radial migration in vivo. Radial migration was similarly inhibited when Fbxo45 expression was suppressed. The results suggest that projection neuron migration requires both Fbxo45 and the binding of Fbxo45 or another protein to SPRY motifs in the extracellular domain of N-cadherin.
INTRODUCTION
The complex layered structure of the mammalian neocortex arises through the coordinated generation, specification, migration, and connection of different types of neurons (1–3). Projection neurons are born and specified in the pallial ventricular zone (VZ) but journey long distances before undergoing terminal differentiation. The journey occurs in stages. First, newborn neurons enter the subventricular zone/intermediate zone, become multipolar, and migrate randomly (4). At the top of the intermediate zone, signals induce multipolar neurons to migrate radially outward into the cortical plate (CP), becoming bipolar and attaching to radial glia as they move. They then pass beyond earlier-born neurons and reach the top of the CP, where they undergo terminal translocation, stop migrating, and differentiate. This pattern of migration gives rise to the classic “inside-out” lamination of the neocortex, with first-born neurons positioned inside later neurons. Genetic disruption of neuron migration is associated with neurodevelopmental disorders, including lissencephaly, epilepsy, and schizophrenia.
Classical cadherins, including neuronal cadherin (N-cadherin [NCad]) and epithelial cadherin (E-cadherin [ECad]), are calcium-dependent cell-cell adhesion molecules (5). NCad is important for cell-cell adhesion in the neuroepithelium of the VZ (6, 7). NCad also regulates projection neuron migration at two stages: first, when neurons enter the CP (8–10), and second, during terminal translocation (11–13). NCad is upregulated on the cell surface at both these stages in response to an extracellular signal, reelin. Reelin stimulates signaling pathways involving Src kinases, Dab1, phosphatidylinositol (PI) 3-kinase, Crk/CrkL, C3G, and the small GTPases Rap1 and RalA (14–21). These pathways increase NCad surface expression, in collaboration with Rab GTPase and drebrin-like vesicular trafficking (9, 11, 22, 23). How NCad regulates neuron migration is unclear, however.
Because cadherins are best known for cell-cell adhesion (5), neuron migration may require homophilic binding of NCad on migrating neurons to NCad on other neurons, axons, or radial glia. Indeed, cultured neurons will polarize toward an external source of NCad (24). NCad may also be involved in attaching bipolar neurons in the CP to radial glia (25). However, our recent work revealed that an NCadW161A mutant, which cannot form “strand-swap” trans homodimers or support cell-cell adhesion (26–29), can support neuron migration into the CP (30). We further found that CP entry requires NCad binding to, and activation of, fibroblast growth factor receptors (FGFRs) in cis (on the same cell) (30). ECad does not support CP entry, even though it binds FGFRs. Mechanistically, NCad, but not ECad, protected FGFRs from degradation, and the first two of five extracellular calcium-binding domains on NCad (EC1 and EC2) were critical. These results leave open the possibility that additional proteins binding NCad, but not ECad, regulate neuron migration.
Fbxo45 (F box/SPRY domain-containing protein 45) is a little-studied protein that is highly expressed in the nervous system and is required for cortical lamination, axonal outgrowth, and synaptic connectivity (31–33). Most F-box proteins bind Skp1, Cul1, and Rbx1 to form an SCF (Skp1–Cul1–F-box) E3 ubiquitin ligase complex. Fbxo45 is atypical in that it does not bind Cul1 or Rbx1 and instead associates with MycBP2/PAM (Myc-binding protein 2/protein associated with Myc), forming an Fbxo45-Skp1-MycBP2 complex that has E3 ligase activity in vitro (32). The SPRY domain of Fbxo45 potentially interacts with substrates. Curiously, NCad was detected in an Fbxo45 interaction screen (34). Furthermore, knockdown of Fbxo45 decreased NCad expression and impaired the differentiation of neuronal stem cells (34), suggesting that Fbxo45 interaction with NCad is involved in brain development.
Here, we set out to identify secreted proteins that interact with the ectodomain of NCad and may regulate the radial polarization of multipolar neurons. Two different unbiased proteomics approaches detected Fbxo45 and MycBP2 as major binding partners for the extracellular domain of NCad. We found that the Fbxo45 SPRY domain binds to SPRY motifs in the EC1 region of NCad that are missing from ECad. Mutation of these motifs does not inhibit cell-cell adhesion but does inhibit neuron migration into the cortical plate in vivo. Fbxo45 appears to be secreted by an unconventional mechanism independent of a signal peptide. Cell-autonomous knockdown of Fbxo45 inhibited entry into the cortical plate. These results suggest that Fbxo45 is secreted and is required for normal neuron migration during brain development, potentially by binding the extracellular domain of NCad.
RESULTS
BioID identifies Fbxo45 and MycBP2 as extracellular NCad-interacting neuronal proteins.
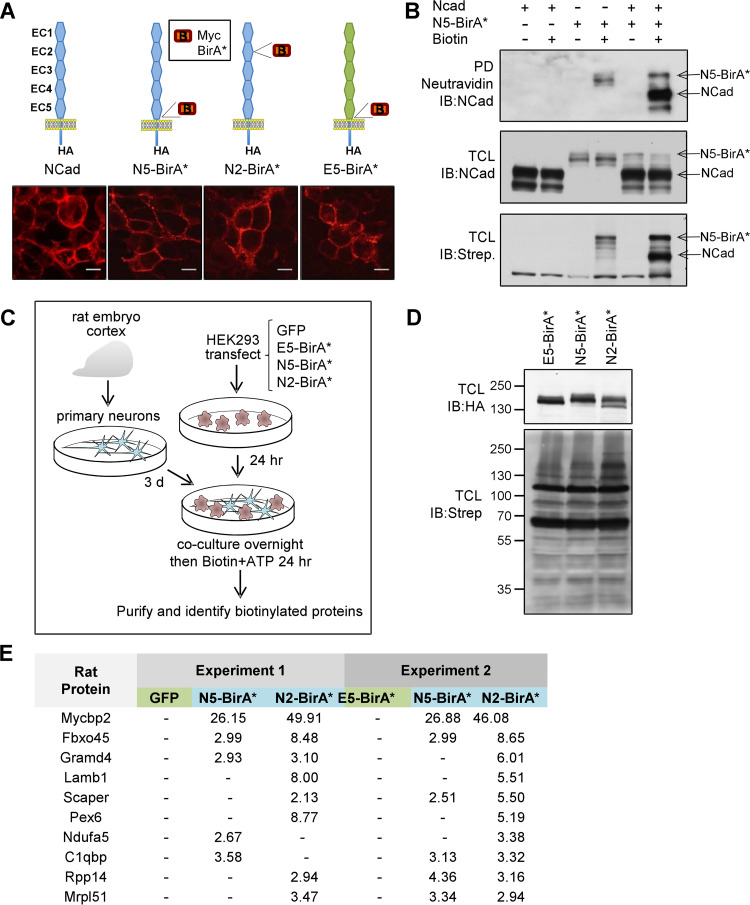
We used two proteomics approaches to identify NCad binding proteins: proximity-dependent biotin identification (BioID) (35), in which mutant BirAR118G (BirA*) transfers biotin onto a nearby amino group, and affinity purification followed by mass spectrometry. For BioID, we fused Myc-tagged BirA* in the extracellular domain of hemagglutinin (HA)-tagged NCad (NCad-HA), expressed the fusion protein on HEK293T cells, and cultured the cells with primary rat embryonic cortical neurons. The mixed cell population was incubated with biotin and ATP, and biotinylated proteins were isolated and identified. The biotinyl-AMP generated by BirA* has a working distance of ∼10 nm (36), which is about half the length spanned by the five extracellular cadherin (EC) repeats (37) (diagramed in Fig. 1A). To detect proteins that might interact with either end of the NCad ectodomain, we inserted Myc-BirA* either in the middle of EC2 (N2-BirA*) or between EC5 and the transmembrane domain (TM) (N5-BirA*) (Fig. 1A). Insertion of small protein domains at these sites does not interfere with adhesion (37). As a control, the EC1-to-EC5 region (EC1-5) of N5-BirA* was replaced with EC1-5 of ECad, to create E5-BirA*. All fusion proteins appeared to traffic normally to the cell surface (Fig. 1A). In addition, NCad was biotinylated when cells cotransfected with NCad and N5-BirA* were labeled with ATP and biotin (Fig. 1B). These results suggest that N5-BirA* coclusters with NCad at the surface.
FIG 1.
Characterization of BirA* fusion proteins and BioID screen for NCad trans-interacting proteins. (A) Structure and localization of cadherin fusion proteins. (Top) Cadherin expression constructs. NCad, ECad, and myc-BirA* are color-coded blue, green, and red, respectively. Each construct has a C-terminal HA tag. (Bottom) Representative immunofluorescence images of the respective constructs expressed in HEK293T cells. Bars, 10 μm. (B) Biotinylation of NCad by N5-BirA*. HEK293T cells were transfected with NCad or N5-BirA* separately or together. PD, pulldown with NeutrAvidin beads, followed by immunoblotting (IB) with an NCad antibody. TCL, total-cell lysates probed with an NCad antibody or streptavidin (Strep.)-HRP. (C) Strategy for BioID screen. HEK293T cells were first transfected with the indicated constructs and then cocultured with DIV3 rat cortical neurons in the presence of biotin and ATP. Cell lysates were purified using streptavidin beads. (D) Total-cell lysates were analyzed by Western blotting with anti-HA to detect the fusion proteins and with streptavidin-HRP to detect biotinylated proteins. (E) Samples were analyzed by on-bead trypsin digestion and LC–MS-MS. Shown are the highest-abundance rat neuron proteins (peptide spectrum matches) biotinylated in the presence of N5-BirA*, N2-BirA*, and the negative controls GFP (experiment 1) or E5-BirA* (experiment 2). –, undetected.
To identify neuronal proteins that interact with the NCad extracellular domain, HEK293T cells were transiently transfected with N5-BirA*, N2-BirA*, or negative-control plasmids carrying green fluorescent protein (GFP) (experiment 1) or E5-BirA* (experiment 2). The transfected cells were cultured with rat embryonic cortical neurons and were labeled with biotin and ATP (Fig. 1C). Cell lysates were collected, and samples were analyzed for protein expression (Fig. 1D, top) and biotinylation (Fig. 1D, bottom). In all cases, there was extensive biotinylation of many proteins. The remaining sample was purified using streptavidin beads and was digested with trypsin before liquid chromatography-tandem mass spectrometry (LC–MS-MS) analysis. The rat protein database was searched for the peptides identified in order to distinguish rat neuron proteins from human HEK293T cell proteins (Fig. 1E; see also Tables S1 and S2 in the supplemental material). MycBP2 and Fbxo45 were the highest-ranked rat neuron proteins in both experiments (Fig. 1E; Table S1). MycBP2 was also the highest-ranked human protein in both experiments (Table S2). The results suggest that Fbxo45 and MycBP2 are major proteins expressed by rat neurons that interact with the ectodomain of NCad. However, 8 of the top 10 proteins, including Fbxo45 and MycBP2, are predicted intracellular proteins, so it is not clear whether they were released by cell death or by an active process.
Affinity purification of neuronal proteins that bind to NCad.
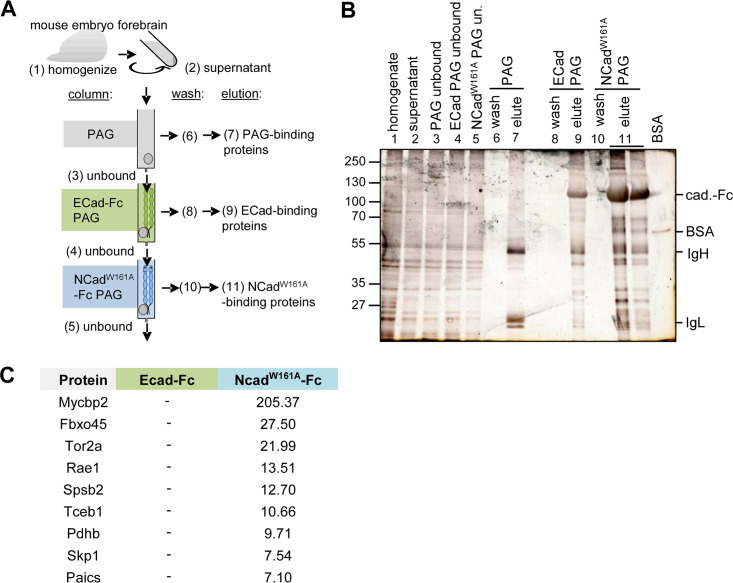
As an independent approach to detecting proteins that might regulate neuron migration, we affinity purified proteins from embryonic mouse brain homogenate using the recombinant NCadW161A ectodomain, which does not form strand-swap dimers, as bait. The ectodomain was fused to the human immunoglobulin constant region (Fc), expressed in HEK293T cells, and purified from the culture supernatant using a protein A/G (PAG) column. An ECad-Fc fusion protein was prepared similarly as a negative control. Neuronal proteins were then purified as illustrated in Fig. 2A. Mouse embryonic forebrains were homogenized; insoluble material was removed by centrifugation; and the supernatant was passed over PAG. Flowthrough from the PAG column was then passed over ECad-Fc in order to remove proteins that bind to ECad. The unbound material was then passed over NCadW161A-Fc in order to select proteins that bind to NCadW161A. All three columns were washed and were eluted at a low pH. As expected, PAG retained large amounts of immunoglobulin heavy and light chains (Fig. 2B, lane 7, IgH and IgL), while ECad-Fc and NCadW161A-Fc eluates contained major amounts of ∼110-kDa proteins, corresponding to the cadherin-Fc fusions (Fig. 2B, lanes 9 and 11). Following preparative SDS-polyacrylamide gel electrophoresis, ECad-Fc and NCadW161A-Fc-associated proteins running above and below the ∼110-kDa cadherin-Fc bands were trypsinized and identified by LC–MS-MS. The 10 most abundant proteins that bound to NCadW161A but not to ECad were predicted to be intracellular and may be secreted or released from dead or broken cells (Fig. 2C; Table S3). MycBP2 and Fbxo45 were the major proteins. Skp1 was also detected. Our results are consistent with NCad binding to an Fbxo45-Skp1-MycBP2 complex that is secreted or released from cells.
FIG 2.
Affinity purification screen for brain proteins that bind the NCad but not the ECad extracellular domain. (A) Strategy for affinity purification of NCadW161A-specific embryonic brain proteins. Numbers in parentheses correspond to lane numbers in panel B. PAG, protein A/G-Sepharose. (B) Silver-stained SDS-polyacrylamide gel of samples from the purification. (C) Highest-abundance proteins detected binding to NCadW161A and ECad (peptide spectrum matches). Fractions 9 and 11 were separated by preparative SDS-polyacrylamide gel electrophoresis, and regions above and below the cadherin-Fc band were excised. Proteins in these regions were analyzed by in-gel trypsin digestion and were identified by LC–MS-MS.
The EC1-2 region of NCad interacts with the SPRY domain of Fbxo45.
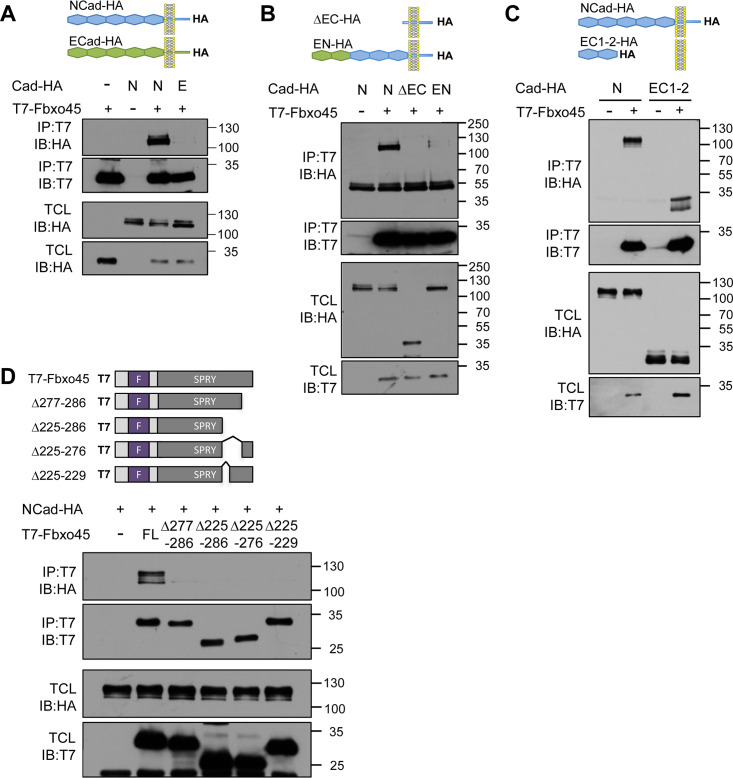
To confirm that Fbxo45 binds to NCad but not to ECad, Fbxo45 was tagged with T7 and cotransfected with HA-tagged cadherins. As expected, Fbxo45 coimmunoprecipitated with NCad but not with ECad (Fig. 3A). When the ectodomain of NCad was deleted (ΔEC) or the EC1-2 domains of NCad were switched to ECad (EN), Fbxo45 binding was inhibited (Fig. 3B). Moreover, NCad EC1-2 was sufficient to bind Fbxo45 (Fig. 3C). Taken together, our data suggest that the EC1-2 domains of NCad, but not those of ECad, are necessary and sufficient to bind Fbxo45. This finding is consistent with our observation that Fbxo45 was more efficiently biotinylated if BirA* was fused into EC2 than if it was fused into the juxtamembrane region (Table S1, compare N2-BirA* and N5-BirA*), as well as with a previous report (34).
FIG 3.
NCad EC1-2 interacts with the SPRY domain of Fbxo45. (A to C) Various forms of NCad-HA were cotransfected with T7-Fbxo45 into HeLa cells. Cell lysates were immunoprecipitated with a T7 antibody, and samples of total-cell lysates (TCL) and immunoprecipitates (IP) were analyzed by Western blotting. (A) Fbxo45 interacts with NCad but not with ECad. (B) NCad EC1-2 is necessary for interaction with Fbxo45. (C) NCad EC1-2 is sufficient for interaction with Fbxo45. (D) The SPRY domain of Fbxo45 is necessary for interaction with NCad. All deletion mutations in the SPRY domain disrupt Fbxo45 binding to NCad.
We next investigated the NCad-binding region of Fbxo45. Fbxo45 contains an F-box domain (residues 33 to 83) that binds Skp1 and a SPRY domain (residues 92 to 283) that binds ubiquitin ligase substrates (32). We deleted different regions of the SPRY domain and assayed binding to NCad (Fig. 3D). Remarkably, none of the mutants tested was able to bind NCad, even when we deleted as few as 10 residues from the C terminus (Δ277-286) or 5 residues between positions 225 and 229 (Δ225-229). These regions correspond to β strands 11 and 15 in the predicted structure of the Fbxo45 SPRY domain (38). Our results implicate the folded structure of the Fbxo45 SPRY domain in binding to NCad.
Fbxo45 binds to SPRY-binding motifs in NCad.
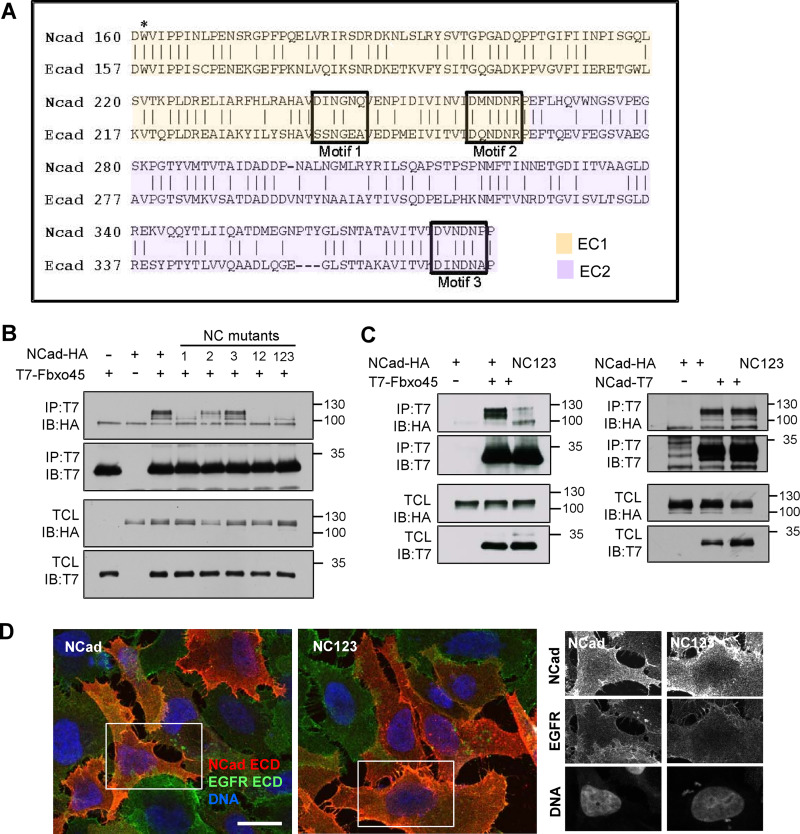
We reasoned that the Fbxo45 SPRY domain may bind to one or more of three canonical SPRY motifs [(D/E)(I/L)NXN] (39) that are present in NCad EC1-2 but not in ECad (Fig. 4A). Chung et al. (34) reported previously that deletion of NCad motifs 2 and 3 abolished Fbxo45 binding. However, motifs 2 and 3 chelate calcium ions in the folded structure (27, 40, 41). Deleting these motifs might cause misfolding and indirectly disrupt Fbxo45 binding. We therefore made conservative mutations, replacing SPRY motif 1, 2, or 3 with the corresponding sequence from ECad, thus generating chimeras NC1, NC2, and NC3. These chimeras were then tested for coimmunoprecipitation with Fbxo45 (Fig. 4B). Substitution of motif 1 (D242S, I243S, N246E, and Q247A) almost completely inhibited interaction, while substitution of motif 2 (M261Q) or motif 3 (V376I and P380A) was less inhibitory. Substituting motifs 1 and 2 (NC12) or motifs 1, 2, and 3 (NC123) abolished binding (Fig. 4B). However, NC123 still bound NCad (Fig. 4C) and was expressed on the surfaces of transfected cells, colocalizing with endogenous epidermal growth factor receptor (EGFR) (Fig. 4D).
FIG 4.
SPRY motifs in NCad bind Fbxo45. (A) Alignment of mouse NCad and ECad EC1-2 sequences. The alignments start with the first residues of the cleaved cadherins, while the numbering corresponds to the primary translation products. NCad motifs 1 to 3 correspond to the SPRY recognition motif [(D/E)(I/L)NXN]. Note that motifs 2 and 3 also bind calcium. W161, which is critical for the strand-swapped trans dimer, is marked with an asterisk. (B) Identification of the Fbxo45 binding site. NCad-HA chimeras containing ECad residues from motif 1, 2, or 3 were cotransfected into HeLa cells with T7-Fbxo45. Cell lysates were immunoprecipitated using a T7 antibody. (C) NC123 fails to bind Fbxo45 but still binds to NCad. (D) NC123 traffics to the cell surface. HeLa cells were transfected to express NCad-HA or NC123-HA, fixed, and immunostained with antibodies to the extracellular domains (ECD) of NCad and EGFR. Boxed areas correspond to individual channels shown on the right. Bar, 20 μm.
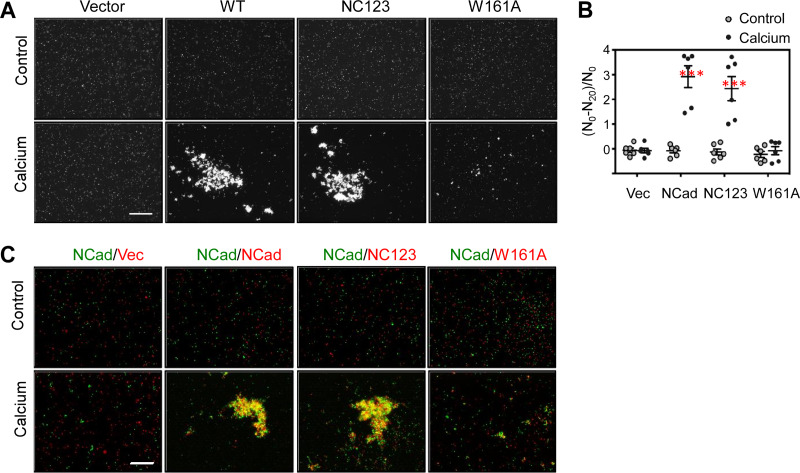
To test whether the Fbxo45-binding sites were required for homophilic adhesion, CHO-K1 cells, which lack cadherins (42), were transfected with GFP and wild-type or mutant NCad. Transfected cells were allowed to aggregate in the presence or absence of calcium. Calcium-dependent aggregation was stimulated by wild-type NCad or NC123 but not by NCadW161A (Fig. 5A and B). Moreover, mCherry-labeled cells expressing NC123, but not those expressing NCadW161A, aggregated with GFP-labeled cells expressing wild-type NCad in the presence of calcium (Fig. 5C). Thus, NC123 binds to NCad but not to Fbxo45, while NCadW161A binds to Fbxo45 but not to NCad.
FIG 5.
SPRY motif mutant NC123 supports calcium-dependent cell-cell interaction. (A) Aggregation assay. The indicated constructs were transfected into CHO-K1 cells together with GFP as a marker for transfected cells. Cell suspensions were allowed to aggregate in the absence or presence of calcium. Bar, 800 μm. (B) Quantification of data from replicate experiments (n = 6). N0, particle number at 0 min; N20, particle number at 20 min. ***, P < 0.001. (C) Aggregation assay with two different cell populations to investigate trans interaction between wild-type NCad and mutants. Green cells, coexpressing wild-type NCad and GFP, were mixed with red cells, coexpressing wild-type or mutant NCad and mCherry. Bar, 400 μm.
Fbxo45 reaches the cell surface through a nonclassical secretion pathway.
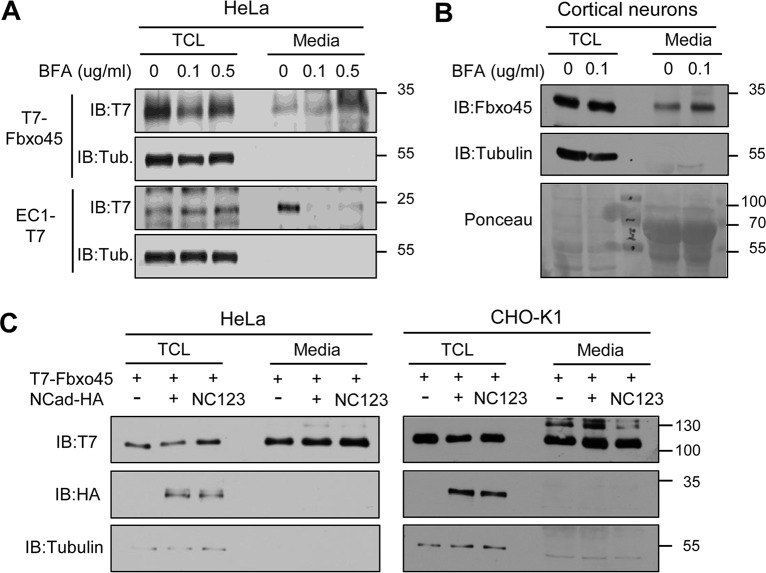
The binding of Fbxo45 to NCad EC1-2 raises the question of how Fbxo45 reaches the cell exterior. Transfected cells released T7-Fbxo45 or Fbxo45-T7 but not tubulin, suggesting that Fbxo45 is actively secreted and not released from broken cells (Fig. 6A and data not shown). While Fbxo45 lacks an N-terminal endoplasmic reticulum (ER) translocation signal for conventional secretion (43), some proteins that lack signal peptides are secreted by unconventional pathways (44–47). To test whether Fbxo45 is secreted by an unconventional pathway, we used brefeldin A (BFA), which inhibits conventional but not unconventional secretion (48). BFA slightly increased the secretion of T7–Fbxo45 but inhibited the secretion of a similar-sized N-terminal signal sequence protein, EC1–T7 (Fig. 6A). This suggests that Fbxo45 is secreted by an unconventional, BFA-insensitive route.
FIG 6.
Fbxo45 is secreted by an unconventional pathway. (A) T7-Fbxo45 is secreted by a brefeldin A (BFA)-insensitive route. HeLa cells were transfected with NCad EC1-T7 or T7-Fbxo45 and were incubated with various concentrations of BFA in serum-free medium for 24 h. Total-cell lysates (TCL) and concentrated medium were harvested; then 0.5% of the TCL and 15% of the medium were analyzed by Western blotting. Tub., tubulin control for cell lysis. (B) Mouse primary cortical neurons secrete Fbxo45 by an unconventional pathway. DIV2 cultures were incubated with serum-free Neurobasal medium containing 0 or 0.1 μg/ml of BFA for 20 to 24 h. The medium and TCL were subjected to Western blotting for Fbxo45 and tubulin. Ponceau staining shows equal loading. (C) Coexpression of NCad or NC123 with Fbxo45 does not affect Fbxo45 secretion by HeLa or CHO-K1 cells.
To test whether untagged, endogenous Fbxo45 is secreted, primary mouse cortical neurons were incubated with serum-free Neurobasal medium with or without BFA for 20 to 24 h. The medium was collected, and Fbxo45 was detected with a specific antibody (34). Fbxo45, but not tubulin, was detected in the neuron culture medium, and the level of secretion was increased by BFA, suggesting unconventional secretion (Fig. 6B).
NCad could potentially bind Fbxo45 in an intracellular vesicle and help ferry Fbxo45 to the surface. Therefore, we tested whether coexpression of NCad with Fbxo45 would stimulate Fbxo45 secretion. Neither NCad nor NC123, which does not bind Fbxo45, affected the secretion of Fbxo45 from HeLa or cadherin-deficient CHO-K1 cells (Fig. 6C). Taken together, these results suggest that Fbxo45 is secreted by an unconventional, NCad-independent pathway.
Neuron migration into the cortical plate requires Fbxo45 and NCad SPRY motifs.
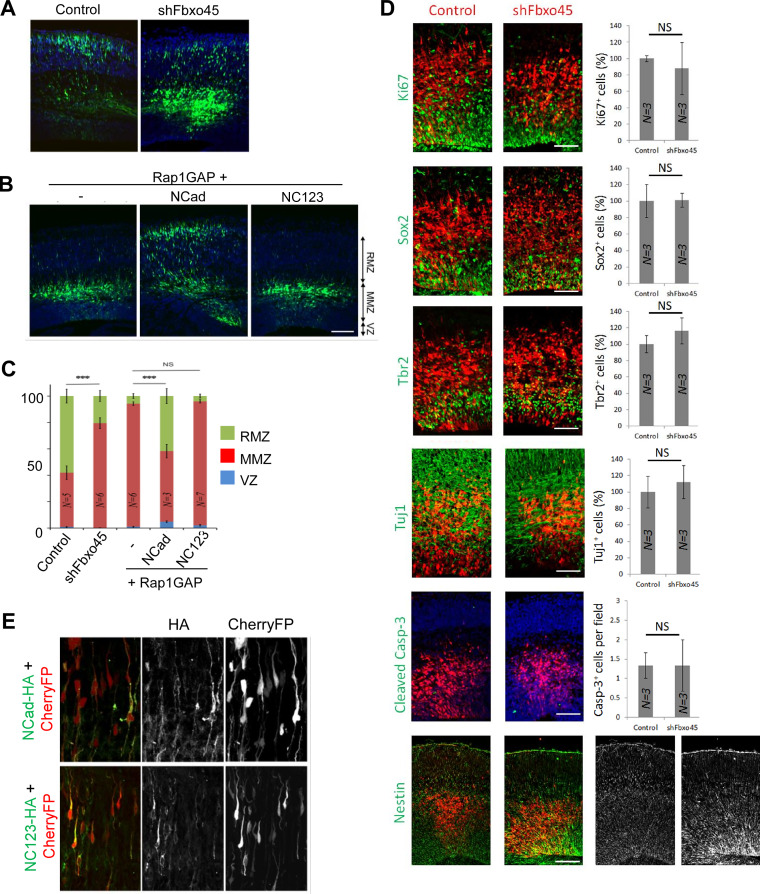
Since ECad EC1-2 cannot substitute for NCad EC1-2 for either Fbxo45 binding or neuron migration (30), Fbxo45 may regulate neuron migration. We tested whether Fbxo45 regulates neuron migration by performing in utero electroporation of GFP and Fbxo45 short hairpin RNA (shRNA) at embryonic day 14.5 (E14.5) and measuring the positions of GFP-expressing neurons 3 days later. Under these conditions, Fbxo45 shRNA (shFbxo45) significantly (P = 0.0003) inhibited the movement of neurons from the multipolar migration zone (MMZ) to the radial migration zone (RMZ) (Fig. 7A and C). A similar arrest was observed with another shRNA targeting a different sequence in Fbxo45 (not shown). Immunofluorescence of cortical sections 2 days after electroporation (E16.5), when neuron migration was not yet delayed, showed no effects on proliferation (Ki67), differentiation (Sox2, Tbr2, or Tuj1), apoptosis (cleaved caspase-3), or the radial glia fibers (nestin) (Fig. 7D). These results suggest that Fbxo45 is needed for neuron entry into the cortical plate, but they do not specify whether Fbxo45 is required inside or outside migrating neurons.
FIG 7.
Fbxo45 and NCad SPRY motifs are required for neuron entry into the radial migration zone in vivo. (A) Fbxo45 shRNA inhibits neuron migration from the multipolar migration zone (MMZ) to the radial migration zone (RMZ), with little effect on the ventricular zone (VZ). E14.5 mouse embryos were microinjected and electroporated in utero with plasmids expressing GFP alone (control) or with Fbxo45 shRNA. Three days later, sections were prepared, and the positions of GFP-expressing neurons were recorded. Representative sections are shown. (B) NC123 does not support the migration of Rap1GAP-expressing neurons into the RMZ. E14.5 mouse embryos were microinjected and electroporated in utero with plasmids expressing GFP and Rap1GAP together with either a vector (–), NCad-HA, or NC123-HA. The results were analyzed as for panel A. Bar, 100 μm. (C) Quantification of the results shown in panels A and B. Results are expressed as percentages of GFP-expressing neurons in the VZs, MMZs, and RMZs of embryos following electroporation with different constructs. N, number of embryos. (D) In utero electroporation was performed at E14.5, and results were analyzed 2 days later. Inhibition of Fbxo45 did not affect cell division (Ki67), apical (Sox2) or basal (Tbr2) progenitor cells, neuronal commitment (Tuj1), cell survival (cleaved caspase-3), or the integrity of radial glia fibers (nestin). Bars, 50 μm for Ki67, Sox2, Tbr2, and Tuj1; 100 μm for caspase-3 and nestin. (E) NCad-HA and NC123-HA were expressed at similar levels by in utero electroporation. Sections of embryo brains electroporated with plasmids expressing Cherry fluorescent protein (CherryFP) (red) and either NCad-HA (top) or NC123-HA (bottom) were visualized with an anti-HA antibody (green).
To test whether Fbxo45 binding to the extracellular domain of NCad may regulate neuron migration, we asked whether NC123 could overcome the migration delay caused by Rap1 GTPase-activating protein (Rap1GAP), which inhibits NCad upregulation on the neuron surface (49). Embryos were electroporated in utero at E14.5 to express GFP, Rap1GAP, and either a vector, NCad-HA, or NC123-HA. Three days later, embryos were euthanized, and the positions of the GFP-expressing neurons were visualized. As shown in Fig. 7B and quantified in Fig. 7C, Rap1GAP delayed neuron entry into the radial migration zone, and this delay was rescued by overexpressing NCad, as expected (P = 1.3 × 10−6). However, NC123 did not rescue migration, suggesting that the NCad Fbxo45-binding site is required (P = 0.41). To confirm that NC123 was expressed in this experiment, sections were stained for the HA tag on NCad-HA and NC123-HA. The wild-type and mutant proteins were expressed at similar levels (Fig. 7E). These results suggest that NCad interaction with Fbxo45 or other proteins through its SPRY motifs is required for neurons to migrate into the cortical plate from the multipolar zone.
DISCUSSION
NCad plays important roles during brain development, including stabilizing the ventricular zone, regulating neuron migration, and coordinating axonal, dendritic, and synaptic differentiation (6, 7, 11–13, 22, 49). While NCad-dependent cell-cell homophilic adhesion is presumably important for many of these processes, we found recently that NCad-dependent migration into the cortical plate does not require NCad homophilic adhesion (30). Our new results suggest that NCad may need Fbxo45 to trigger migration into the cortical plate. Fbxo45 appears to be secreted by an unconventional pathway, circumventing the usual ER-to-Golgi apparatus route, and binds through its SPRY domain to SPRY consensus motifs in NCad EC1. These motifs are required for NCad to support neuron migration into the cortical plate but are dispensable for cell-cell adhesion. While these results do not exclude the possibility that another protein binds the same residues in NCad as does Fbxo45, a parsimonious explanation is that neuron migration into the cortical plate is stimulated when Fbxo45 is secreted and binds NCad.
A previous study detected NCad binding to Fbxo45 and proposed that the interaction occurred inside the cell (34). However, the specific mechanism was not addressed. Our results suggest that Fbxo45 is actively secreted from cells by a nonclassical, signal peptide-independent mechanism that is insensitive to BFA. Several other proteins undergo unconventional secretion, including the cytokine interleukin-1 (50), thioredoxin (51), fibroblast growth factors 1 (52) and 2 (53), α-synuclein (54, 55), galectins (56), phosphoglucose isomerase/autocrine motility factor (57), and heat shock proteins (47). However, the mechanisms of unconventional secretion are still unclear. The lack of conserved signals, the relatively small amounts of proteins secreted, and the apparent variety of mechanisms have presented technical challenges (45, 47, 58). One mechanism, known as autophagy-mediated secretion, involves chaperone-mediated autophagy (CMA) to translocate proteins into lysosomes, followed by lysosome fusion with the plasma membrane (59). A loose consensus for CMA targeting contains a glutamine preceded or followed by four residues including at least one basic (K or R), one acidic (D or E), and one hydrophobic (I, L, V, or F) residue. Fbxo45 contains such a sequence (residues 225 to 229) (QIGER). However, deletion of this sequence did not reproducibly inhibit Fbxo45 secretion (data not shown). It is possible that Fbxo45 secretion involves CMA and that variability in secretion stems from variable levels of autophagy between experiments.
Mutational analysis of the Fbxo45-NCad interaction suggests that the Fbxo45 SPRY domain makes canonical interactions with three candidate SPRY motifs in NCad EC1-2 [(D/E)(I/L)NXN] (39) that are not conserved in ECad (Fig. 3 and 4). We found that swapping NCad motif 1 with the corresponding residues of ECad strongly inhibits Fbxo45 binding. This motif lies on the opposite surface from the EC1-EC1 interface in the strand-swap NCad dimer (41), so it is unlikely to regulate NCad transdimerization between cells. However, this surface has been implicated in cis interactions between adjacent cadherins on the same membrane (41). Mutations here cause measurable changes in mechanical coupling to the actin cytoskeleton but do not affect cell-cell adhesion (60). The same region of ECad is important for a proposed conformational change involved in inside-out activation (61, 62). Mutation of a nearby residue in ECad is found in some cancers and also inhibits inside-out activation (62). Therefore, trans interactions with Fbxo45 may affect cis interactions between NCad molecules on the same cell or may modulate NCad conformational activation.
Previous studies have shown that interfering with NCad surface expression by inhibiting reelin, Rap1, Rab proteins, and drebrin-like causes a delay in neuron entry into the cortical plate (22, 23, 49). We found that inhibiting Fbxo45 expression with shRNA caused a similar delay in entry into the cortical plate (Fig. 7A and C). There appeared to be no significant effects on neurogenesis, cell differentiation, apoptosis, or local cytoarchitecture. This suggests that Fbxo45 expression is needed at approximately the same stage as NCad. We were unable to identify mutations in Fbxo45 that inhibit secretion, so we could not directly test whether Fbxo45 secretion is important in vivo. Instead, we performed an indirect experiment, testing whether Fbxo45-binding sites are required for overexpressed NCad to overcome the effect of Rap1GAP, which reduces NCad surface levels and inhibits migration (49). Forced expression of exogenous NCad could rescue the migration defect, while the NC123 mutant could not (Fig. 7B and C). Therefore, the NCad Fbxo45-binding sites were required, in agreement with the binding of Fbxo45 (or another unidentified protein) to NCad before neurons enter the cortical plate.
The fact that Fbxo45 has intracellular and extracellular functions remains a puzzle. Inside the cell, Fbxo45 binds MycBP2 and Skp1 to stimulate ubiquitylation and turnover of Par-4, mTOR, Munc-13, and p73 (33, 63–65). This intracellular function is thought to regulate the epithelial-mesenchymal transition and synaptic function (33, 66). However, our results suggest that Fbxo45 is also secreted and acts outside the cell. Other unconventionally secreted proteins have different functions inside and outside the cell. Heat shock protein 70 (Hsp70) is an intracellular chaperone, but stress stimulates Hsp70 secretion, and extracellular Hsp70 activates macrophages (47, 67). Vasohibins catalyze tubulin detyrosination but, when secreted, regulate angiogenesis (68, 69). As another example, phosphoglucose isomerase is a key metabolic enzyme, but when secreted, it is known as autocrine motility factor and stimulates cell migration by binding to specific cell surface receptors (57). By analogy, we can hypothesize that Fbxo45 is secreted under specific biological conditions and that secreted Fbxo45 regulates nonadhesive functions of NCad.
In conclusion, Fbxo45 appears to be secreted by a nonclassical mechanism. Outside the cell, it binds to SPRY motifs in EC1-2 of NCad. Mutating these motifs inhibits NCad function in neuron migration without affecting cell-cell adhesion. Therefore, secreted Fbxo45, or a protein with overlapping binding requirements, likely regulates NCad during neuron migration. This suggests that Fbxo45 has different functions depending on whether it is intracellular or extracellular. Further studies are warranted to characterize the mechanism and function of the secreted form.
MATERIALS AND METHODS
BioID and expression plasmid cloning.
To generate N5-BirA*, a PCR product of myc-BirA* (plasmid no. 35700; Addgene) was ligated into XhoI- and SpeI-cleaved pCAG-mNCad-HA, which contains XhoI and SpeI sites introduced into the region between EC5 and the transmembrane domain (49). GFP-NCad contains GFP inserted at the same position. For N2-BirA* cloning, PCR products of myc-BirA* and NCad EC2 were sequentially ligated to pBluescript II KS(+), and the final insert was ligated to pCAG-NCad-HA using EcoRI and KpnI. E5-BirA* was created by ligating a PCR product of murine ECad EC1-5 to the transmembrane and intracellular regions of N5-BirA* using EcoRI and XhoI. The murine ECad template was a kind gift of Masatoshi Takeichi (70). pCAGGFP (Addgene) and pCAGCherryFP were used for cotransfection. The NC1, NC2, NC3, NC12, and NC123 mutations were created by standard site-directed mutagenesis using Kapa HiFi DNA polymerase (Kapa Biosystems) according to the manufacturer’s instructions. Murine Fbxo45 (Harvard Plasmid Database) was inserted into the pCAG vector possessing a T7 tag by use of BamHI and NotI. Fbxo45 deletion mutants were generated by site-directed mutagenesis. All constructs were confirmed by sequencing. pLKO.1 Fbxo45 shRNA plasmids shFbxo45b (TRCN0000201180; target sequence, GACATGGAGGATAAGACTTTA) and shFbxo45a (TRCN0000339817; target sequence, TGGAATCTGGTGGACAATAAT) were obtained from Sigma. When cotransfected with Flag-Fbxo45, these shRNAs inhibited expression by 58% and 53%, respectively.
Cell culture and transfection.
HEK293T, HeLa, and CHO-K1 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and penicillin-streptomycin (100 U/ml) and were transfected using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Unless otherwise stated, transfected cells were incubated 40 to 48 h before protein analysis or fixation for immunofluorescence. Cortical neurons from embryonic day 18 (E18) Sprague-Dawley rats (Charles River) or E15.5 to E16.5 CD-1 mice (Charles River) were prepared as reported previously (71) and were maintained in Neurobasal medium with B-27 Supplement (Thermo Fisher Scientific). All animal procedures were approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee. Neurons were transfected using Lipofectamine 2000. Hippocampal neurons were prepared as reported previously (71), transfected after 3 or 5 days in vitro (DIV), and fixed 2 days later.
HEK293T cell-neuron trans biotinylation (BioID).
HEK293T cells were transfected with pCAG plasmids carrying GFP (experiment 1), E5-BirA* (experiment 2), and N5-BirA* or N2-BirA* (both experiments). After 24 h, transfected cells were detached with 5 mM EDTA in phosphate-buffered saline (PBS), centrifuged at 1,000 rpm for 5 min, suspended in Neurobasal medium with 10% horse serum, penicillin-streptomycin (100 U/ml), and B-27 Supplement, and added to rat cortical primary cultures that had been prepared 3 days previously. About 10 × 106 to 18 × 106 HEK293 cells were added to 100 × 106 rat cortical neurons per sample. After overnight incubation, the medium was changed to fresh Neurobasal medium containing 5% horse serum, penicillin-streptomycin (100 U/ml), B-27 Supplement, 50 μM biotin, and 1 mM ATP. After 24 h, mixed cells were washed with cold PBS twice and were harvested in 1% Triton X-100 in PBS buffer with 1 mM EDTA and Complete protease inhibitor (Roche). The lysates were centrifuged at 14,000 rpm for 15 min, and the supernatant was mixed with Streptavidin Sepharose High Performance medium (Sigma-Aldrich) and was rotated for 5 h at 4°C. Streptavidin beads were washed three times with buffer 1 (20 mM Tris-HCl [pH 7.4], 0.1% NP-40, 1 M NaCl, 5 mM EDTA [pH 8.0]), followed by two washes each with buffer 2 (2 mM Tris-HCl [pH 7.4], 0.1% NP-40, 0.5 mM EDTA) and buffer 3 (1 M urea, 10 mM Tris-HCl [pH 7.4], 0.1% NP-40, 1 mM EDTA). A final wash was performed with 50 mM Tris-HCl, pH 7.4, to remove urea and detergents. The beads were equilibrated with 50 mM ammonium bicarbonate, pH 8.0, and were digested with trypsin overnight at 37°C with gentle agitation. Additional trypsin was added for a further 2 h at 37°C. Digestion was stopped by adding formic acid to a 1% final concentration. Beads were centrifuged at 4,000 rpm for 2 min, and the supernatant was transferred to a new tube. Beads were rinsed twice more with water, and all supernatants were combined and lyophilized. The peptides were cleaned using a ZipTip micro-C18 system (Millipore Corporation) and were analyzed by LC–MS-MS.
LC–MS-MS analysis for BioID.
LC–MS-MS analysis was performed with an Easy-nLC 1000 chromatograph coupled to an Orbitrap Elite mass spectrometer (both from Thermo Scientific). The LC system, configured in a vented format, consisted of a fused-silica nanospray needle (PicoTip emitter; inside diameter [i.d.], 75 μm; New Objective) packed in-house with 25 cm of Magic C18 AQ reverse-phase medium (particle size, 100 Å; Michrom Bioresources Inc.) and a trap (IntegraFrit capillary; i.d., 100 μm; New Objective) containing 2 cm of Magic C18 AQ medium (particle size, 200 Å). The peptide sample was diluted in 10 μl of 2% acetonitrile and 0.1% formic acid in water, and 8 μl was loaded onto the column and separated using a two-mobile-phase system consisting of 0.1% formic acid in water (phase A) and 0.1% acetic acid in acetonitrile (phase B). A 90-min gradient from 7% to 35% acetonitrile in 0.1% formic acid at a flow rate of 400 nl/min was used for chromatographic separations. The mass spectrometer was operated in a data-dependent MS-MS mode over the m/z range of 400 to 1,800. The mass resolution was set at 120,000. For each cycle, the 15 most abundant ions from the scan were selected for MS-MS analysis using 35% normalized collision energy. Selected ions were dynamically excluded for 30 s.
Cadherin-Fc fusion proteins.
DNA sequences encoding the ectodomains of ECad and NCadW161A were PCR amplified, cut with either MfeI and AgeI (for ECad) or EcoRI and AgeI (for NCad), and cloned into a plasmid (pcDNA3.1-ApoER2-Fc [15]) that had been cut with EcoRI and AgeI. The murine ECad template was a kind gift of Masatoshi Takeichi (70), and the murine NCadW161A template was from reference 30. The plasmids were confirmed by sequencing. Fusion proteins were produced in HEK293T cells. For each construct, five 6-cm plates were transfected with a total of 120 μg of DNA using the calcium phosphate method. The medium was removed 6 h later and was gently replaced with serum-free DMEM. Two days later, the medium was harvested and concentrated using Amicon Centricon YM-100 centrifugal filters. Protein concentration and purity were estimated by SDS-PAGE with a bovine serum albumin (BSA) standard. Approximately 250 μg of each fusion protein was mixed with 250 μl (packed volume) protein A/G Plus-agarose (Santa Cruz Biotechnology), and unbound proteins were washed away using PBS.
Purification of proteins interacting with NCadW161A and not with ECad.
Brains from 21 mouse embryos (E16.5) were homogenized with a Dounce homogenizer in a total volume of 8 ml of buffer A (100 mM NaCl, 20 mM HEPES [pH 7.5], 2 mM CaCl2, 0.2 mM EDTA, Complete protease inhibitor) containing 1% Triton X-100. The homogenate was centrifuged at 10,000 × g for 30 min at 4°C. The supernatant was adjusted to contain 0.3 μg/ml DNase I and was mixed with protein A/G Plus-agarose (packed volume, 120 μl) in a Bio-Spin minicolumn (Bio-Rad) for 30 min at 4°C. Unbound material was mixed with ECad-Fc bound to protein A/G Plus-agarose (packed volume, 440 μl; estimated 220 μg ECad-Fc) for 90 min at 4°C. Unbound material was mixed with NCadW161A-Fc bound to protein A/G Plus-agarose (packed volume, 440 μl; estimated 220 μg NCadW161A-Fc) for 2 h at 4°C. Finally, all three columns were washed eight times with 1 ml buffer A containing 0.1% Triton X-100, once with 1 ml of 20 mM HEPES (pH 7.5)–2 mM CaCl2, and once with 1 ml of H2O before being eluted twice with 1 ml of 0.1% formic acid in H2O. Eluates were neutralized with 100 μl of 1 M ammonium bicarbonate, frozen, and lyophilized. Lyophilized proteins were dissolved in SDS-PAGE sample buffer and were resolved by SDS-PAGE (with a precast 4%-to-15% gradient gel; Bio-Rad). The gel was stained with Simply Blue SafeStain (Invitrogen). Sections of the gel above and below the cadherin-Fc bands were excised and were analyzed by trypsin digestion and mass spectrometry. Briefly, the gel pieces were destained with 25 mM ammonium bicarbonate in 50% acetonitrile and were subsequently dehydrated using acetonitrile. The protein was digested overnight with 5 ng/μl trypsin (Promega Corporation) in 50 mM ammonium bicarbonate at 37°C. The peptides were extracted with 5% (vol/vol) formic acid in water and then with acetonitrile. The pooled extracts were dried under a vacuum and were cleaned using a ZipTip with C18 resin (Millipore Corporation) before MS analysis.
LC–MS-MS analysis for affinity purification.
LC–MS-MS analysis was performed with an Eksigent NanoLC-2D system (Eksigent/AB Sciex) coupled to an LTQ Orbitrap mass spectrometer (Thermo Scientific). The LC system, configured in a vented format (72), consisted of a fused-silica nanospray needle (PicoTip emitter; i.d., 75 μm; New Objective) packed in-house with 25 cm of Magic C18 AQ reverse-phase medium (particle size, 100 Å; Michrom Bioresources Inc.), and a trap (IntegraFrit capillary; i.d., 100 μm; New Objective) containing 2 cm of Magic C18 AQ medium (particle size, 200 Å). The peptide sample was diluted in 10 μl of 2% acetonitrile and 0.1% formic acid in water, and 8 μl was loaded onto the column and was separated using a two-mobile-phase system consisting of 0.1% formic acid in water (phase A) and 0.1% acetic acid in acetonitrile (phase B). A 60-min gradient from 7% to 35% acetonitrile in 0.1% formic acid at a flow rate of 400 nl/min was used for chromatographic separations. The mass spectrometer was operated in a data-dependent MS-MS mode over the m/z range of 400 to 1,800. The mass resolution was set at 60,000. For each cycle, the five most abundant ions from the scan were selected for MS-MS analysis using 35% normalized collision energy. Selected ions were dynamically excluded for 45 s.
MS data analysis.
Data were analyzed using Proteome Discoverer, version 1.4 (Thermo Scientific). The data were used to search the UniProt human (19 March 2014), rat (9 January 2015), and mouse (19 March 2014) protein databases, as well as a list of common contaminants, the common Repository of Adventitious Proteins (http://www.thegpm.org/crap/). Trypsin was set as the enzyme, with maximum missed cleavages set to 2. The precursor ion tolerance was set to 10 ppm, and the fragment ion tolerance was set to 0.6 Da. SEQUEST (73) was used for searching, and search results were run through Percolator (74) for scoring.
Immunoprecipitation and pulldown.
Cells were washed twice with cold PBS and were harvested in 1% Triton X-100 in PBS with protease/phosphatase inhibitors. The lysates were centrifuged at 14,000 rpm for 15 min, and supernatants were mixed with T7-Tag Antibody Agarose (EMD Millipore) for immunoprecipitation or with NeutrAvidin (Thermo Fisher Scientific) for 2 h. Agarose beads were washed three times with lysis buffer, eluted with SDS sampling buffer, and analyzed by SDS-PAGE and Western blotting. The following antibodies were used for immunoprecipitation and Western blotting: mouse N-cadherin (Thermo Fisher Scientific), horseradish peroxidase (HRP)-conjugated streptavidin (Jackson ImmunoResearch Laboratories, Inc.), mouse antitubulin (Santa Cruz Biotechnology, Inc.), mouse anti-T7 (EMD Millipore), mouse anti-HA (HA.11; BioLegend), rabbit anti-HA (Bethyl, Montgomery, TX), and mouse Flag M2 (Sigma-Aldrich). A rabbit Fbxo45 antibody was kindly provided by Kojo S. J. Elenitoba-Johnson (University of Pennsylvania).
Immunofluorescence.
HeLa cells were seeded onto 12-mm collagen IV-coated coverslips at 105 cells/well in a 24-well plate and were transfected immediately with 400 ng of pCAG-NCad-HA or pCAG-NC123-HA using Lipofectamine 2000. The medium was changed 6 h later, and 2 days later, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature. Coverslips were washed in PBS, blocked for 1 h in 1% BSA–5% normal goat serum in PBS, incubated with the primary antibody for 1 h, washed, incubated with secondary antibodies for 1 h, washed, and mounted in ProLong Glass mountant (Thermo Fisher Scientific). All procedures were performed at room temperature unless otherwise specified. Primary antibodies were as follows: rabbit anti-NCad (H-63) (recognizing the extracellular domain; Santa Cruz Biotechnology), mouse anti-EGFR (recognizing the extracellular domain; clone 528) (Calbiochem), and rat anti-HA (clone 3F10; Sigma). Secondary antibodies were as follows: Alexa Fluor 568-conjugated goat anti-rabbit IgG(H+L), Alexa Fluor 488-conjugated goat anti-mouse IgG(H+L), and Alexa Fluor 647-conjugated goat anti-rat IgG(H+L) (all from Jackson ImmunoResearch). No staining with an HA antibody was detected, showing that the cells were not permeabilized. NCad, EGFR, and HA antibodies all stained cytoplasmic vesicles if the cells were permeabilized with 0.1% Triton X-100 before blocking and antibody addition (data not shown).
Preparation of secreted proteins and total-cell lysates.
To collect secreted proteins, cells were incubated in serum-free DMEM (for HeLa cells) or Neurobasal medium (for primary cortical neurons) for 20 to 24 h at 37°C under 5% CO2. The conditioned medium was centrifuged at 3,000 rpm for 5 min to remove cell debris. Supernatants were collected and concentrated using Amicon Ultra filters (nominal molecular weight limit [NMWL], 10 kDa; EMD Millipore). After removal of the conditioned medium, cells were washed with cold PBS and were harvested in 1% Triton X-100 in PBS buffer with protease/phosphatase inhibitors. Triton-insoluble material was removed at 14,000 rpm for 15 min. Samples of the conditioned medium and cell lysates were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting.
Short-term aggregation assay.
CHO-K1 cells were cotransfected with GFP or mCherry and the NCad constructs indicated in Fig. 5. After 24 h, transfected cells were washed twice with prewarmed HEPES-buffered Ca2+, Mg2+-free Hanks’ solution (HCMF, consisting of 137 mM NaCl, 5.4 mM KCl, 0.63 mM Na2HPO4, 5.5 mM glucose, and 10 mM HEPES [pH 7.4]) and were placed in suspension using 0.01% trypsin–1 mM CaCl2 in HCMF. Suspended cells were centrifuged at 1,000 rpm for 5 min, resuspended in 0.5% soybean trypsin inhibitor in HCMF, washed three times with cold HCMF, and counted. A total of 200,000 cells in HCMF were added to 1% BSA-coated 24-well plates with or without 2 mM CaCl2. Cells were shaken at 80 rpm for 20 min at 37°C, and images were collected using 2× or 4× objectives. Cell aggregation assays were performed with three biological replicates. Data were quantified using Analyze Particles in ImageJ (75).
In utero electroporation.
In utero microinjection and electroporation were performed at E14.5 essentially as described elsewhere (76) using timed pregnant CD-1 mice (Charles River Laboratories). In brief, mice were anesthetized, and at the midline incision, the uterine horns were exposed. A plasmid solution was injected into the lateral ventricle using needles that were pulled from Wiretrol II glass capillaries (Drummond Scientific) and calibrated for 1-μl injections. DNA solutions were mixed in 10 mM Tris (pH 8.0) with 0.01% fast green. The embryo in the uterus was placed between the forceps-type electrodes (Nepa Gene) with 5-mm pads and was electroporated with five 50-ms pulses of 45 V using the ECM 830 electroporation system (Harvard Apparatus). The uterine horns were then placed back into the abdominal cavity in order for normal brain development to continue. Three days later, mice were euthanized, and embryo brains were sectioned and were visualized with GFP. Immunohistochemistry to detect HA-tagged NCad was performed essentially as described elsewhere (49). The following additional antibodies were used: Ki67 (catalog no. 556003; BD Biosciences), Sox2 (catalog no. 4900; Cell Signaling Technology), Tbr2 (ab23345; Abcam), Tuj1 (catalog no. MMS-435P; Covance), cleaved caspase-3 (catalog no. 9661; Cell Signaling Technology), and nestin (MAB353; Millipore).
Statistical analysis.
Statistical analysis was performed with Prism, version 7.0 (GraphPad, La Jolla, CA). Statistical significance was determined by a two-tailed unpaired t test. Data are reported as means ± standard errors of the means, and statistical significance was set at a P value of <0.05.
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to Barry Gumbiner and Kojo S. J. Elenitoba-Johnson for helpful discussions and for sharing reagents. We thank the staff of Fred Hutch Shared Resources, especially Phil Gafken, Yuko Ogata, David McDonald, Lena Schroeder, and Julio Vazquez, for proteomics and imaging; Alexander (Sasha) Strait for technical assistance; and members of the Cooper laboratory for encouragement.
This work was supported by grants R01 NS080194 and GM109463 from the U.S. Public Health Service and grants J.0129.15, J.0179.16, and T.0243.18 from the FNRS. FHCRC imaging and proteomics laboratories are supported by grant P01 CA015704 from the U.S. Public Health Service.
Y.N., E.C.-J., E.K., H.C., Y.J., and J.A.C. performed experiments. Y.N. and J.A.C. wrote and revised the manuscript with input from Y.J.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Marin O, Rubenstein JL. 2003. Cell migration in the forebrain. Annu Rev Neurosci 26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 2.Bielas S, Higginbotham H, Koizumi H, Tanaka T, Gleeson JG. 2004. Cortical neuronal migration mutants suggest separate but intersecting pathways. Annu Rev Cell Dev Biol 20:593–618. doi: 10.1146/annurev.cellbio.20.082503.103047. [DOI] [PubMed] [Google Scholar]
- 3.Ayala R, Shu T, Tsai LH. 2007. Trekking across the brain: the journey of neuronal migration. Cell 128:29–43. doi: 10.1016/j.cell.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Kriegstein AR, Noctor SC. 2004. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci 27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Takeichi M. 1990. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem 59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. 2007. N-cadherin mediates cortical organization in the mouse brain. Dev Biol 304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Takeichi M, Inuzuka H, Shimamura K, Matsunaga M, Nose A. 1990. Cadherin-mediated cell-cell adhesion and neurogenesis. Neurosci Res Suppl 13:S92–S96. doi: 10.1016/0921-8696(90)90036-3. [DOI] [PubMed] [Google Scholar]
- 8.Cooper JA. 2014. Molecules and mechanisms that regulate multipolar migration in the intermediate zone. Front Cell Neurosci 8:386. doi: 10.3389/fncel.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jossin Y. 2011. Polarization of migrating cortical neurons by Rap1 and N-cadherin: revisiting the model for the Reelin signaling pathway. Small GTPases 2:322–328. doi: 10.4161/sgtp.18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartner A, Fornasiero EF, Valtorta F, Dotti CG. 2014. Distinct temporal hierarchies in membrane and cytoskeleton dynamics precede the morphological polarization of developing neurons. J Cell Sci 127:4409–4419. doi: 10.1242/jcs.149815. [DOI] [PubMed] [Google Scholar]
- 11.Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U. 2011. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gil-Sanz C, Franco SJ, Martinez-Garay I, Espinosa A, Harkins-Perry S, Müller U. 2013. Cajal-Retzius cells instruct neuronal migration by coincidence signaling between secreted and contact-dependent guidance cues. Neuron 79:461–477. doi: 10.1016/j.neuron.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Garay I, Gil-Sanz C, Franco SJ, Espinosa A, Molnár Z, Mueller U. 2016. Cadherin 2/4 signaling via PTP1B and catenins is crucial for nucleokinesis during radial neuronal migration in the neocortex. Development 143:2121–2134. doi: 10.1242/dev.132456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell BW, Herrick TM, Cooper JA. 1999. Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev 13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, Cooper JA, Herz J. 1999. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron 24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 16.Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA. 2004. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol 14:606–610. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 17.Park TJ, Curran T. 2008. Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J Neurosci 28:13551–13562. doi: 10.1523/JNEUROSCI.4323-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voss AK, Britto JM, Dixon MP, Sheikh BN, Collin C, Tan SS, Thomas T. 2008. C3G regulates cortical neuron migration, preplate splitting and radial glial cell attachment. Development 135:2139–2149. doi: 10.1242/dev.016725. [DOI] [PubMed] [Google Scholar]
- 19.Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. 2002. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J Biol Chem 277:49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- 20.Hirota Y, Nakajima K. 2017. Control of neuronal migration and aggregation by Reelin signaling in the developing cerebral cortex. Front Cell Dev Biol 5:40. doi: 10.3389/fcell.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah B, Lutter D, Tsytsyura Y, Glyvuk N, Sakakibara A, Klingauf J, Puschel AW. 2017. Rap1 GTPases are master regulators of neural cell polarity in the developing neocortex. Cereb Cortex 27:1253–1269. doi: 10.1093/cercor/bhv341. [DOI] [PubMed] [Google Scholar]
- 22.Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, Nakajima K, Nabeshima Y, Hoshino M. 2010. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron 67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Inoue S, Hayashi K, Fujita K, Tagawa K, Okazawa H, Kubo KI, Nakajima K. 2019. Drebrin-like (Dbnl) controls neuronal migration via regulating N-cadherin expression in the developing cerebral cortex. J Neurosci 39:678–691. doi: 10.1523/JNEUROSCI.1634-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gartner A, Fornasiero EF, Munck S, Vennekens K, Seuntjens E, Huttner WB, Valtorta F, Dotti CG. 2012. N-cadherin specifies first asymmetry in developing neurons. EMBO J 31:1893–1903. doi: 10.1038/emboj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai M, Guo Y, Ma J, Yu H, Zhao D, Fan W, Ju X, Sheikh MA, Malik YS, Xiong W, Guo W, Zhu X. 2015. Myosin X regulates neuronal radial migration through interacting with N-cadherin. Front Cell Neurosci 9:326. doi: 10.3389/fncel.2015.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pertz O, Bozic D, Koch AW, Fauser C, Brancaccio A, Engel J. 1999. A new crystal structure, Ca2+ dependence and mutational analysis reveal molecular details of E-cadherin homoassociation. EMBO J 18:1738–1747. doi: 10.1093/emboj/18.7.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. 1995. Structural basis of cell-cell adhesion by cadherins. Nature 374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 28.Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. 1998. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron 20:1153–1163. doi: 10.1016/s0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 29.Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. 2002. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 30.Kon E, Calvo-Jiménez E, Cossard A, Na Y, Cooper JA, Jossin Y. 2019. N-cadherin-regulated FGFR ubiquitination and degradation control mammalian neocortical projection neuron migration. Elife 8:e47673. doi: 10.7554/eLife.47673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida K. 2005. Characterization of estrogen-induced F-box protein FBXO45. Oncol Rep 14:531–535. [PubMed] [Google Scholar]
- 32.Saiga T, Fukuda T, Matsumoto M, Tada H, Okano HJ, Okano H, Nakayama KI. 2009. Fbxo45 forms a novel ubiquitin ligase complex and is required for neuronal development. Mol Cell Biol 29:3529–3543. doi: 10.1128/MCB.00364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tada H, Okano HJ, Takagi H, Shibata S, Yao I, Matsumoto M, Saiga T, Nakayama KI, Kashima H, Takahashi T, Setou M, Okano H. 2010. Fbxo45, a novel ubiquitin ligase, regulates synaptic activity. J Biol Chem 285:3840–3849. doi: 10.1074/jbc.M109.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung FZ, Sahasrabuddhe AA, Ma K, Chen X, Basrur V, Lim MS, Elenitoba-Johnson KS. 2014. Fbxo45 inhibits calcium-sensitive proteolysis of N-cadherin and promotes neuronal differentiation. J Biol Chem 289:28448–28459. doi: 10.1074/jbc.M114.561241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roux KJ, Kim DI, Raida M, Burke B. 2012. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol 196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DI, Birendra KC, Zhu W, Motamedchaboki K, Doye V, Roux KJ. 2014. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc Natl Acad Sci U S A 111:E2453–E2461. doi: 10.1073/pnas.1406459111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SA, Tai CY, Mok LP, Mosser EA, Schuman EM. 2011. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. Proc Natl Acad Sci U S A 108:9857–9862. doi: 10.1073/pnas.1019003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo JS, Suh HY, Park SY, Oh BH. 2006. Structural basis for protein recognition by B30.2/SPRY domains. Mol Cell 24:967–976. doi: 10.1016/j.molcel.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Kuang Z, Lewis RS, Curtis JM, Zhan Y, Saunders BM, Babon JJ, Kolesnik TB, Low A, Masters SL, Willson TA, Kedzierski L, Yao S, Handman E, Norton RS, Nicholson SE. 2010. The SPRY domain-containing SOCS box protein SPSB2 targets iNOS for proteasomal degradation. J Cell Biol 190:129–141. doi: 10.1083/jcb.200912087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. 2006. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 41.Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, Troyanovsky RB, Ben-Shaul A, Frank J, Troyanovsky SM, Shapiro L, Honig B. 2011. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure 19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emond MR, Biswas S, Blevins CJ, Jontes JD. 2011. A complex of Protocadherin-19 and N-cadherin mediates a novel mechanism of cell adhesion. J Cell Biol 195:1115–1121. doi: 10.1083/jcb.201108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viotti C. 2016. ER to Golgi-dependent protein secretion: the conventional pathway. Methods Mol Biol 1459:3–29. doi: 10.1007/978-1-4939-3804-9_1. [DOI] [PubMed] [Google Scholar]
- 44.Nickel W, Rabouille C. 2009. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra V. 2013. Unconventional protein secretion: an evolving mechanism. EMBO J 32:1660–1664. doi: 10.1038/emboj.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nickel W, Seedorf M. 2008. Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol 24:287–308. doi: 10.1146/annurev.cellbio.24.110707.175320. [DOI] [PubMed] [Google Scholar]
- 47.De Maio A. 2011. Extracellular heat shock proteins, cellular export vesicles, and the Stress Observation System: a form of communication during injury, infection, and cell damage. Cell Stress Chaperones 16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misumi Y, Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. 1986. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem 261:11398–11403. [PubMed] [Google Scholar]
- 49.Jossin Y, Cooper JA. 2011. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci 14:697–703. doi: 10.1038/nn.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, Dinarello CA. 1984. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A 81:7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. 1992. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem 267:24161–24164. [PubMed] [Google Scholar]
- 52.Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, Maciag T. 1992. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci U S A 89:10691–10695. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mignatti P, Morimoto T, Rifkin DB. 1992. Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J Cell Physiol 151:81–93. doi: 10.1002/jcp.1041510113. [DOI] [PubMed] [Google Scholar]
- 54.Lee HJ, Patel S, Lee SJ. 2005. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci 25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn KJ, Paik SR, Chung KC, Kim J. 2006. Amino acid sequence motifs and mechanistic features of the membrane translocation of alpha-synuclein. J Neurochem 97:265–279. doi: 10.1111/j.1471-4159.2006.03731.x. [DOI] [PubMed] [Google Scholar]
- 56.Sato S, Burdett I, Hughes RC. 1993. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Exp Cell Res 207:8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- 57.Funasaka T, Raz A. 2007. The role of autocrine motility factor in tumor and tumor microenvironment. Cancer Metastasis Rev 26:725–735. doi: 10.1007/s10555-007-9086-7. [DOI] [PubMed] [Google Scholar]
- 58.Nickel W. 2003. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur J Biochem 270:2109–2119. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 59.Kaushik S, Cuervo AM. 2012. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol 22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strale PO, Duchesne L, Peyret G, Montel L, Nguyen T, Png E, Tampe R, Troyanovsky S, Henon S, Ladoux B, Mege RM. 2015. The formation of ordered nanoclusters controls cadherin anchoring to actin and cell-cell contact fluidity. J Cell Biol 210:1033. doi: 10.1083/jcb.20141011109022015c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrova YI, Spano MM, Gumbiner BM. 2012. Conformational epitopes at cadherin calcium-binding sites and p120-catenin phosphorylation regulate cell adhesion. Mol Biol Cell 23:2092–2108. doi: 10.1091/mbc.E11-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petrova YI, Schecterson L, Gumbiner BM. 2016. Roles for E-cadherin cell surface regulation in cancer. Mol Biol Cell 27:3233–3244. doi: 10.1091/mbc.E16-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han S, Kim S, Bahl S, Li L, Burande CF, Smith N, James M, Beauchamp RL, Bhide P, DiAntonio A, Ramesh V. 2012. The E3 ubiquitin ligase protein associated with Myc (Pam) regulates mammalian/mechanistic target of rapamycin complex 1 (mTORC1) signaling in vivo through N- and C-terminal domains. J Biol Chem 287:30063–30072. doi: 10.1074/jbc.M112.353987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peschiaroli A, Scialpi F, Bernassola F, Pagano M, Melino G. 2009. The F-box protein FBXO45 promotes the proteasome-dependent degradation of p73. Oncogene 28:3157–3166. doi: 10.1038/onc.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen X, Sahasrabuddhe AA, Szankasi P, Chung F, Basrur V, Rangnekar VM, Pagano M, Lim MS, Elenitoba-Johnson KS. 2014. Fbxo45-mediated degradation of the tumor-suppressor Par-4 regulates cancer cell survival. Cell Death Differ 21:1535–1545. doi: 10.1038/cdd.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diaz VM, de Herreros AG. 2016. F-box proteins: keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin Cancer Biol 36:71–79. doi: 10.1016/j.semcancer.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. 2008. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 180:4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- 68.Nieuwenhuis J, Adamopoulos A, Bleijerveld OB, Mazouzi A, Stickel E, Celie P, Altelaar M, Knipscheer P, Perrakis A, Blomen VA, Brummelkamp TR. 2017. Vasohibins encode tubulin detyrosinating activity. Science 358:1453–1456. doi: 10.1126/science.aao5676. [DOI] [PubMed] [Google Scholar]
- 69.Aillaud C, Bosc C, Peris L, Bosson A, Heemeryck P, Van Dijk J, Le Friec J, Boulan B, Vossier F, Sanman LE, Syed S, Amara N, Coute Y, Lafanechere L, Denarier E, Delphin C, Pelletier L, Humbert S, Bogyo M, Andrieux A, Rogowski K, Moutin MJ. 2017. Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 358:1448–1453. doi: 10.1126/science.aao4165. [DOI] [PubMed] [Google Scholar]
- 70.Nose A, Nagafuchi A, Takeichi M. 1988. Expressed recombinant cadherins mediate cell sorting in model systems. Cell 54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- 71.Rumbaugh G, Sia GM, Garner CC, Huganir RL. 2003. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci 23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Licklider LJ, Thoreen CC, Peng J, Gygi SP. 2002. Automation of nanoscale microcapillary liquid chromatography-tandem mass spectrometry with a vented column. Anal Chem 74:3076–3083. doi: 10.1021/ac025529o. [DOI] [PubMed] [Google Scholar]
- 73.Eng JK, McCormack AL, Yates JR. 1994. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom 5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 74.Kall L, Canterbury JD, Weston J, Noble WS, MacCoss MJ. 2007. Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat Methods 4:923–925. doi: 10.1038/nmeth1113. [DOI] [PubMed] [Google Scholar]
- 75.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tabata H, Nakajima K. 2008. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ 50:507–511. doi: 10.1111/j.1440-169X.2008.01043.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.