Abstract
Despite advances in diagnosis and treatment, tuberculosis (TB) continues to be one of the essential health problems throughout the world. Turkey is considered to be endemic for TB. In this study, we analyzed the distribution of Mycobacterium species, compare the diagnostic methods, and susceptibilities to anti-tuberculosis drugs of TB isolates. The aim was to document the current status and to provide a frame of reference for future studies. In this study, 278 Mycobacterium species isolated from 7,480 patients between September 2015 and June 2019 were included. Löwenstein-Jensen medium (LJ) and MGIT 960 were used for the isolation of strains. Susceptibility to 1st-line anti-tuberculosis drugs was determined. Positivity rates in clinical samples were as follows: 1.4% for direct microscopic acid-fast bacilli (AFB) detection, 3.4% for growth on the LJ, and 3.7% for growth on MGIT-960. Two hundred thirty-three isolates were identified as Mycobacterium tuberculosis complex (MTBC) and 45 were non-tuberculous mycobacteria (NTMs). Eleven of the NTMs (24.4%) were Mycobacterium fortuitum group isolates, and eight NTMs (17.7%) were Mycobacterium abscessus complex isolates. A number of patients diagnosed with tuberculosis peaked twice between the ages of 20–31 and 60–71. A hundred and eighty-two MTBC isolates (78.1%) were susceptible to all 1st-line anti-tuberculosis drugs, while 51 isolates (21.9%) were resistant to at least one drug tested. The multidrug-resistant tuberculosis rate was 13.7% among resistant strains and 3% in all strains. The liquid cultures were better for detection of both MTBC and NTMs isolates. The data demonstrate that MTBC continues to be challenge for this country and indicates the need for continued surveillance and full-spectrum services of mycobacteriology laboratory and infectious diseases.
Key words: tuberculosis, Mycobacterium species, Mycobacterium tuberculosis complex, culture, drug resistance
Introduction
Tuberculosis (TB), as an infectious disease is caused by Mycobacterium species in humans (Miotto et al. 2018). The etiological agent of the disease is most often Mycobacterium tuberculosis (MTB), and less commonly other species in the Mycobacterium tuberculosis complex (MTBC). Mycobacterium species other than these species are classified as non-tuberculosis mycobacteria (NTMs) (Waters and Ratjen 2016). In recent years, the incidence of diseases caused by NTMs has increased due to the increasing number of immunosuppressed patients (Samli and Ilki 2016).
Members of MTBC are usually transmitted to susceptible persons through airborne means such as coughing, sneezing, or speaking by patients suffering from infectious tuberculosis (Churchyard et al. 2016). The successful establishment of an infection in the lungs is influenced by various factors such as phagocytosis of the bacilli, their intracellular multiplication, latency, and active lung infection (Banuls et al. 2015). However, there is no clear evidence that NTMs are transmitted from either person to person or from animals to people. Therefore, the transmission of NTMs is probably through individual contacts with the source of infection (Churchyard et al. 2016).
Since the treatment of Mycobacterium species depends on the correct identification of the isolate, mycobacteriological tests that are leading to the identification are crucial. Methods that include direct microscopic acid-fast bacilli detection (AFB), growth on the solid or liquid media, and biochemical tests continue to play significant roles in the identification of mycobacteria (Kurtoglu et al. 2011).
Treatment and prophylaxis of tuberculosis differ from most other bacterial infections. In general, patients should receive multiple antibiotic treatments for at least 6 to 9 months (Ozmen et al. 2017). The World Health Organization (WHO) recommends the combined use of 1st-line drugs in the initial treatment of tuberculosis. However, anti-tuberculosis drug resistance remains a significant problem. Of the primary anti-tuberculosis drugs, isolates showing at least isoniazid (INH) and rifampin (RIF) resistance are defined as Multiple Drug-Resistant Tuberculosis (MDR-TB) (WHO 2019).
In this study, we aimed to document Mycobacterium species isolated in a large metropolitan tertiary care hospital in Turkey. The laboratory procedures used to isolate the organisms were also compared. Similarly, the resistance rates to the 1st-line anti-tuberculosis drugs were determined. The ultimate aim was to compare these data with the earlier studies and to provide the frame of reference for future studies.
Experimental
Materials and Methods
Ethical approval was provided from the Ethics Committee of Medical School, Bezmialem University, Istanbul, Turkey (10.09.2019/16–302).
Our facility is a 700-bed academic hospital providing tertiary health care in Istanbul city centre, and 7,480 patients with pre-diagnosis of tuberculosis admitted to Bezmialem University Medical Faculty Hospital between September 2015 and June 2019 were included in the study. Between the dates indicated, 7,480 clinical specimens were sent to the Clinical Microbiology Laboratory with a preliminary diagnosis of tuberculosis. Two hundred seventy-eights of these samples were positive for Mycobacterium species in at least one of the methods studied. Only the first positive isolates from the same patient were included in the study.
Homogenization and decontamination of sputum, bronchoalveolar lavage (BAL), fasting gastric juice, urine, and abscess materials were performed according to published guidelines. Sterile body fluids such as cerebrospinal fluid (CSF), peritoneal and pleural fluid, and tissue samples were tested without decontamination. N-acetyl-L-cysteine-4% sodium hydroxide-1.47% sodium citrate (NALC-NaOH) method was used for decontamination and homogenization of the samples. Five to ten ml of each clinical sample was mixed with an equal volume of NALC-NaOH solution. The mixture was transferred to tubes and vortexed for not more than 30 seconds. The tubes containing the mixture were allowed to stand at room temperature for 15 minutes. Fifty ml of phosphate buffer (0.067 M, pH = 6.8) was added to each tube containing the mixture, and the tubes were centrifuged at 3,000 g for 15 minutes. The resulting precipitates were diluted with 1–2 ml of phosphate buffer (pH = 6.8), and smears were prepared from these precipitates. The smears were stained by Erlich-Ziehl-Neelsen (EZN) (GBL Rose Biology Laboratory, Istanbul) method and examined microscopically under immersion oil.
Isolation of Mycobacterium species. For all the samples, both liquid and solid media are used for culture. Therefore, any isolate growing either on one of these media were used in identification studies. In some patients, multiple specimens were submitted for culture, and multiple cultures were positive for growth. In those patients, the very first isolate was used in the study.
For the liquid culture of mycobacteria, a Mycobacterium Growth Indicator Tube (BD MGIT-960, BD, Sparks, MD, USA) system was utilized, and the procedure suggested by the manufacturer was followed. Briefly, oleic acid-albumin-dextrose-catalase (OAOC) was added to the culture medium and as antimicrobial agents, polymyxin B (50 U/ml), azlocillin (10 mcg/ml), nalidixic acid (20 mcg/ml), trimethoprim antibiotic mixture (PANTA, Becton Dickinson, Sparks, MD, USA; 5.0 mcg/ml), and amphotericin B (5.0 mcg/ml) were added. 0.5 ml of the samples from decontaminated clinical mixtures was inoculated into the culture medium, and the culture bottles were incubated for six weeks at 37°C. Samples were considered negative if no growth signal was obtained by the end of this incubation period.
Simultaneous cultivation of the samples on solid media was carried out on Löwenstein-Jensen (LJ) slants (Becton Dickinson, USA). LJ tubes were evaluated daily for 42 days, and the cultures were terminated by 42nd day if no colonies observed. The presence of acid-resistant bacilli was screened by EZN staining from positive cultures.
Identification of Mycobacterium species. When MGIT-960 instrument signaled positivity, firstly, an EZN stain was performed to screen for AFB, and the positive tubes were inoculated on blood agar medium (Becton Dickinson, USA) containing 5% sheep blood, and the growth was examined to rule out a contaminating bacterial species. Samples were also inoculated onto the LJ medium for future susceptibility testing. After the growth on either solid or liquid media, the identification was performed using the MPT64 immunochromatographic test (BD MGIT TBc Identification Test, Becton Dickinson, Sparks, USA).
In the study, M. tuberculosis isolates were identified at the ‘complex’ level, and no further identification was performed, and therefore, isolates were designated as M. tuberculosis complex (MTBC). Non-tuberculous mycobacteria (NTMs) species were identified by the Turkish Public Health Laboratory through a commercial molecular assay (GenoType Mycobacterium CM, Hain Lifescience GmbH, Nehren, Germany).
Antimycobacterial susceptibility tests. The susceptibilities of MTBC strains to isoniazid (INH), Streptomycin (SM), Rifampicin (RIF), and Ethambutol (ETM) were investigated. For this aim, a commercial susceptibility test method (BD BACTEC MGIT-960 SIRE) was used. The concentrations of the drugs in the testing kit were as follows: SM at 1.0 μg/ml, INH at 0.1 μg/ml, RIF at 1.0 μg/ml, ETM at 5.0 μg/ml. The definitions of the multidrug resistance were that of the commercial test kit (BD BACTEC MGIT-960 SIRE).
Study design and statistical analysis. This study was conducted as a retrospective clinical study. Data analysis was performed using SPSS 20.0 software pack (SPSS Inc., USA).
Results
In 278 (3.7%) of 7,480 clinical specimens from patients with suspected tuberculosis, one type of Mycobacterium species was isolated. Of the patients with Mycobacterium species, 123 (44.2%) were female, 155 (55.8%) were male.
The type of specimens from which MTBC’s were isolated was as follows: 198 respiratory tract samples (sputum, BAL and tracheal aspirate), 29 tissue specimens (lymph node biopsy, etc.), 22 sterile body fluids (pleura, peritoneum, CSF), 11 fasting gastric fluid, 11 abscesses, and seven urine samples.
In our study, the AFB positivity was 1.4% (104/7,480), and the culture positivity (LJ and/or MGIT-960) was 3.7% (278/7,480) in the clinical samples of patients with suspected tuberculosis. The culture positivity was 3.4% for LJ (254/7,480) and 3.7% for MGIT-960 (278/7,480). The contamination rate in cultures was 9% (675/7,480) for LJ and 7% (525/7,480) for MGIT-960. In the study, all strains identified as MBTC or NTMs were identified as the same species by both methods.
During the study period, PCR assay was performed for 24 patients’ samples upon request from the ordering clinics. A commercial automated Real-time PCR assay (QIAGEN Benelux B.V. The Netherlands) was used at a reference laboratory. Out of these 24 samples, five (20.8%) were positive. PCR positive samples were also positive in liquid (MGIT-960) and solid (LJ) culture methods as studied in house, but only three were positive by AFB. One of the samples negative by PCR (19 patients) was positive by the AFB staining, and 17 out of these 19 specimens were positive on solid media (LJ); all 19 were positive in liquid (MGIT-960) culture.
Two hundred thirty-three (MTBC) and 45 (NTMs) strains were positive by liquid (MGIT-960) or solid (LJ) culture methods. NTMs distribution was as follows: 11 (24.5%) isolates were of Mycobacterium fortuitum group, eight (17.8%) of Mycobacterium abscessus complex, five (11.1%) of Mycobacterium simiae, five (11.1%) of Mycobacterium lentifilavum, four (8.9%) of Mycobacterium chelonae, four (8.9%) of Mycobacterium intracellulare, three (6.7%) of Mycobacterium gordonae, two (4.4%) of Mycobacterium avium spp., two (4.4%) of Mycobacterium kansasii, and one (2.2%) of Mycobacterium heckeshornense.
The most MTBC isolates were from respiratory specimens; 120 from sputum, 35 from BALs, and four from tracheal aspirates. The remaining MTBC were recovered from different specimen types. Of the total 45 isolated NTMs strains, 37 were isolated from sputum samples. The number and the types of specimens from which mycobacteria were isolated are presented in Table I.
Table I.
Number, species, AFB positivity and distribution of Mycobacterial strains according to clinical samples.
| AFB | MTBC | NTMs | ||
|---|---|---|---|---|
| Respiratory | 198 | 98 | 159 | 39 |
| Sputum | 157 | 89 | 120 | 37 |
| Bronchoalveolar lavage | 37 | 7 | 35 | 2 |
| Tracheal aspirate | 4 | 2 | 4 | – |
| Tissue | 22 | 3 | 21 | 1 |
| Lymph Node Biopsy | 12 | 2 | 12 | – |
| Other Tissues | 10 | 1 | 9 | 1 |
| Sterile body fluid | 22 | – | 22 | – |
| Pleura | 13 | – | 13 | – |
| BOS | 6 | – | 6 | – |
| Peritoneum | 3 | – | 3 | – |
| Abscess | 18 | 3 | 17 | 1 |
| Fasting gastric fluid | 11 | – | 9 | 2 |
| Urine | 7 | – | 5 | 2 |
| Total | 278 | 104 | 233 | 45 |
In the study, the AFB was observed in 98 respiratory patient samples. We performed AFS for the patient respiratory samples as well as the growing microorganisms in the culture. However, 198 respiratory samples grew Mycobacterium species that were confirmed as acid-fast organisms by staining.
In all 104 patients, the AFB positive samples (respiratory, tissue, sterile body fluid), at least one type of Mycobacterium species was isolated on either solid or in liquid culture methods. Sixty-six (63.5%) of the AFB positive samples were obtained from male subjects, 38 (36.5%) were from females, and the mean age was 43.16 years old (females, mean age 37.55, males – 46.4 years old).
When the age distribution of the patients was examined, a bi-modal distribution was observed. The number of patients diagnosed with tuberculosis peaked twice between the ages of 20–31 and 60–71. Similarly, isolations of MTBC peaked twice in the patients of 20–31 and 60–71 age range. The isolation of NTMs species was also prominent in the 0–11 age range. The distribution of Mycobacterial strains according to age groups is presented in Fig. 1.
Fig. 1.
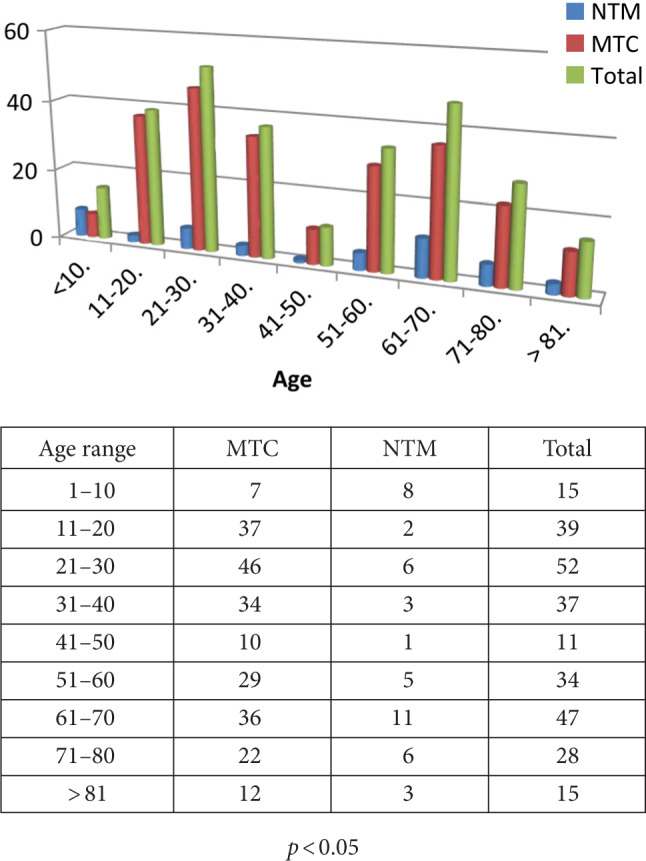
Distribution of Mycobacterial strains according to age groups.
Of the 233 MTBC strains isolated in the study, 182 (78.1%) were susceptible to all 1st-line anti-tuberculosis drugs, while 51 isolates (21.9%) were resistant to at least one of the 1st-line anti-tuberculosis drugs tested (Table II). Total drug resistance rates for INH, SM, RIF, and ETM were 16.7% (n = 39), 14.6% (n = 34), 3% (n = 7), and 0.8% (n = 2), respectively. The highest rates of resistance were observed to the pair of INH + SM (n: 22, 9.4%), and the highest rates of resistance to three drugs were against INH + SM + RIF (n = 73%). Seven rifampin-resistant strains were also resistant to INH and SM. One of the strains was resistant to four 1st-line anti-tuberculosis drugs. In our study, the rate of MDR-TB was 13.7% (7/51) among resistant strains and 3% (7/233) among all strains isolated.
Table II.
Number and proportion of resistant MTBC strains by years.
| 2015 (n : 14) |
2016 (n : 45) |
2017 (n : 66) |
2018 (n : 78) |
2019 (n : 30) |
Total (n : 233, %) |
||
|---|---|---|---|---|---|---|---|
| Sensitive to all drugs | 11 | 42 | 49 | 61 | 19 | 182 | (78.1) |
| INH | 2 | 3 | 13 | 12 | 9 | 39 | (16.7) |
| SM | 2 | 1 | 11 | 12 | 8 | 34 | (14.6) |
| RIF | 1 | – | 2 | 2 | 2 | 7 | (3) |
| EMB | – | – | – | 1 | 1 | 2 | (0.8) |
| İNH + SM | 2 | 1 | 8 | 7 | 5 | 23 | (9.9) |
| INH + RIF + SM (MDR-TB) | 1 | 2 | 2 | 2 | 7 | (3) | |
AFB – Acid-fast bacilli; MTC – Mycobacterium tuberculosis complex; NTMs – Non-tuberculosis mycobacteria
In the present study, 21.4% of MTBC isolates in 2015, 6.7% in 2016, 25.8% in 2017, 21.8% in 2017, 36.7% in 2018, and 36.7% in 2017 were tested against 1st-line anti-tuberculosis drugs. As the number of strains isolated in 2017 and 2018 increased, so did the number of resistant strains. However, this increase was not statistically significant (p < 0.05). The number and ratio of isolated resistant MTBC strains by years are presented in Table II.
The number of M. tuberculosis isolates at different age groups was presented in Fig. 1. Accordingly, the numbers of TB isolated at different age groups demonstrated a bi-model distribution peaking at third and seventh decade of life. Possible differences between various age groups were analyzed by the Chi-square test, and in both decades, the rates of M. tuberculosis isolations were significantly different.
Discussion
TB remains a major public health problem throughout the world. According to the 2018 report of the World Health Organization, it was estimated that in 2017 approximately 10 million people suffered from TB, and approximately 1.5 million people have died (WHO 2019). The determination of the presence of the etiological agent and correct identifications are important to prevent the spread of tuberculosis. Therefore, it is necessary to isolate, determine the type, and to assess the drug resistance of Mycobacterium species (Kurtoglu et al. 2011).
Direct microscopic examination of smears is rapid and relatively practical and, therefore, is preferred despite low rates of sensitivity in detecting bacilli (Kurtoglu et al. 2011). Patients positive on direct microscopic examination are considered to be both more contagious and exhibit a fast progression to clinical disease. Consequently, a direct microscopic examination is recommended, especially in patients with pulmonary tuberculosis (Tarylan et al. 2015).
In our study, the AFB positivity was 1.4% (104/7,480), and culture positivity (LJ and/or MGIT-960) was 3.7% (278/7,480) in patients with suspected tuberculosis. Overall, MTBC detection rate was 3.1% (233/7,480). Similarly, MTBC detection rate was between 2.1% and 11% in many studies (Baylan et al. 2002; Dundar et al. 2009; Kurtoglu et al. 2011).
Today, culture is the standard gold method for the detection of mycobacteria (Kunduracioglu et al. 2013). World Health Organization recommends the use of liquid media for M. tuberculosis culture (WHO 2007). In other studies, it has been reported that the liquid medium was superior in recovering mycobacteria over solid media (Hwang et al. 2014; Kwak et al. 2017). In the current investigation, mycobacteria were detected in 3.7% (278/7,480) liquid cultures (MGIT-960) and 3.4% (254/7,480) solid (LJ) cultures.
Age distribution of TB patients is an important parameter reflecting the control of TB in the community. While the disease peaks in advanced ages in populations that implement effective TB control programs, in populations where the control programs are lax, the disease peaks at younger ages (Karatas et al. 2019). In some studies, the TB is seen mostly in advanced ages (Coffman et al. 2017); however, in an earlier Turkish study (Karatas et al. 2019), it was reported two peaks of the TB prevalence in the age group 15–24 and over 65 years old. Similarly, we noticed that the number of patients diagnosed with tuberculosis peaked twice between the ages of 20–31 and 60–71 years. These results suggest that the Turkish population is composed of mixed communities where both loose and effective TB control efforts are exerted.
The isolation rate of mycobacteria (NTMs) other than the M. tuberculosis complex is increasing throughout the world (Mbeha et al. 2014). To date, more than 160 NTMs species have been identified, and approximately one third is associated with diseases in humans. It has been shown to cause serious clinical consequences, especially in people with immunodeficiency (Liu et al. 2016). It has been reported that the distribution of NTMs species varies according to geographical regions and demographic characteristics of affected patients (Spaulding et al. 2017).
In our study, NTMs were isolated from 45 patients. Among NTMs, M. fortuitum group (11 isolates), and M. abscessus complex (eight isolates) were the most common mycobacteria. In the US study, Spaulding et al. (Spaulding et al. 2017) reported that other than MAC, M. abscessus/M. chelonae, and M. fortuitum were the most common isolated NTMs species. In other investigations, the frequencies among NTMs species varied significantly according to geographical regions. In a study conducted in China, Liu et al. (2016) found that more than half (59.66%) of 523 NTMs isolates were M. avium, and M. intracellulare. In this study, rapid growers such as M. abscessus and M. fortuitum were more frequent. The number MAC isolates were not as high as the others (Liu et al. 2016; Spaulding et al. 2017). Many factors could be accounted for this difference. The discrepancies in the number of people with immunodeficiency could be one factor since immunodeficiency seems to be an underlying risk factor for MAC infection. TB is a disease that should be treated with proper drug regimens to be administered to patients. The increased drug resistance complicates the treatment and impairs control of the disease. In many studies, resistance rates to commonly used 1st-line anti-tuberculosis drugs have been reported to be between 10–25% for INH, 3–16% for RIF, 0.7–19% for SM, and 0.7–10% for ETM (Karadag et al. 2004; Saral et al. 2007; Dundar et al. 2009; Aydin et al. 2011; Perincek et al. 2011). Similarly, in this report, resistances to 1st-line anti-tuberculosis drugs of INH, SM, RIF and ETM were 16.7%, 14.6%, 3%, and 0.8%, respectively. These data argue that the rate of resistance in this country follows trends not unlike those of other countries.
In many studies, it has been reported that resistance to INH and RIF are often, but resistance to other anti-tuberculosis drugs may also develop (Dheda et al. 2014; Pienar et al. 2018). However, in some studies, similar to our study, resistance to INH and SM was higher, and RIF and ETM resistances were reduced. In these studies, there were significant differences between the resistance rates by region, and the primary anti-tuberculosis drugs with the highest resistance rates were INH and SM (Kurtoglu et al. 2011; Linger et al. 2014; Sani et al. 2015; Stagg et al. 2017). It is thought that higher rates of INH and SM resistance compared to other primary anti-tuberculosis drugs may be related to the more frequent use of these drugs in both prophylaxis and treatment. Similar arguments could be put forth for this country.
The Report of the Turkish Association of Anti-TB Campaign published in 2018 indicated that in 2016, the drug susceptibility test results were surveyed for 6,037 MTC, and resistance to at least one drug was detected in 19.2% isolates. According to this report, 3.3% of the patients who underwent drug susceptibility testing were found to have MDR-TB, and the rate of multidrug resistance was 2.1% in new patients and 14.2% in previously treated patients (Saglik Bakanligi T.C. 2018). According to WHO 2018 data, MDR-TB ratio is 3.5% of new TB cases and 18% of previously treated cases globally (WHO 2019). The rates of MDR-TB in the studies made in different regions of Turkey vary between 2.2–14.7% (Saral et al. 2007; Dundar et al. 2009; Aydin et al. 2011; Kurtoglu et al. 2011). In our study, resistance to more than one drug was detected in 51 (21.88%) isolates. MDR-TB ratio was 13.7% (7/51) among resistant strains and 3% (7/233) among all strains isolated, and this rate was consistent with other studies.
Today, the number of patients with tuberculosis has increased, possibly due to changes in recent years such as social and economic turmoil, increased number of migrant patients, changes in the practices healthcare system, and drug-resistant Mycobacterium isolates. Therefore, a correct and rapid diagnosis and supervised treatment concepts should be reviewed and applied to prevent susceptible strains from gaining resistance and stopping the further spread of resistant strains. Our data indicate that NTMs species will probably be more frequently encountered in the future. Furthermore, we can speculate that drug resistance will continue to be an important problem in patients with tuberculosis.
One of the limitations of this study was that the patients and the isolates were selected from a single centre albeit it was a large tertiary care centre. This might have influenced the information gathered. However, it should also be stated that the medical centre where the study is conducted is located in the heart of the old city of Istanbul, where the immigrants are concentrated.
Data from this study indicate that the frequency of isolations of M. tuberculosis complex in the population seems to form a bimodal distribution, one in the third decade of life and the other in the 7th. Obviously, our study is not designed to address this particular demographic aspect of M. tuberculosis infection in detail. However, we judge that providing such data would be a useful addition to a study aiming to capture a current picture of Mycobacterium species in an endemic country.
In conclusion, this investigation demonstrates that tuberculosis is still a growing public health threat in Istanbul, Turkey. We do not know how much of this increase is the result of the recent high immigration rates. The drug resistance rates also seem to be on the rise. Therefore, rapid and accurate laboratory services are imperative in combatting such a growing public health menace. Our study demonstrates that the liquid culture is superior to the solid media for the recovery of Mycobacterium species and, hence, should be included in the routine mycobacteriology laboratory procedures. Collectively, these results underscore the need for the marshaling preventive public health efforts on this age-old concern.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
ORCID
Bilge Sumbul https://orcid.org/0002-8768-3777
Mehmet Ziya Doymaz https://orcid.org/0000-0003-2066-0252
Literature
- Aydın F, Kaklıkkaya N, Bayramoğlu G, Ozkul G, Buruk K, Dinç U, Köse T, Dede R.. [Resistance rates of Mycobacterium tuberculosis complex strains isolated from clinical specimens] (Turkish). Mikrobiyol Bul. 2011. Jan;45(1):36–42. [PubMed] [Google Scholar]
- Bañuls AL, Sanou A, Van Anh NT, Godreuil S.. Mycobacterium tuberculosis: ecology and evolution of a human bacterium. J Med Microbiol. 2015. Nov 01;64(11):1261–1269. 10.1099/jmm.0.000171 [DOI] [PubMed] [Google Scholar]
- Baylan O, Kısa O, Albay A, Dogancı L.. Mycobacterium tuberculosis Complex (MTC) strains isolated from tuberculosis cases in our mycobacteriology laboratory and their anti-tuberculosis drug susceptibilities in 2002. Gulhane Tip Derg. 2003;45(3):256–262. [Google Scholar]
- Churchyard G, Kim P, Shah NS, Rustomjee R, Gandhi N, Mathema B, Dowdy D, Kasmar A, Cardenas V.. What we know about tuberculosis transmission: an overview. J Infect Dis. 2017; 216(suppl_6):S629-S635. 10.1093/infdis/jix362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman J, Chanda-Kapata P, Marais BJ, Kapata N, Zumla A, Negin J.. Tuberculosis among older adults in Zambia: burden and characteristics among a neglected group. BMC Public Health. 2017. Dec;17(1):804 10.1186/s12889-017-4836-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheda K, Gumbo T, Gandhi NR, Murray M, Theron G, Udwadia Z, Migliori GB, Warren R.. Global control of tuberculosis: from extensively drug-resistant to untreatable tuberculosis. Lancet Respir Med. 2014. Apr;2(4):321–338. 10.1016/S2213-2600(14)70031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dundar D, Sonmez Tamer G.. Resistance Rates of Mycobacterium tuberculosis isolates to primary antituberculous agents. Klimik Derg. 2009;22(2):52–54. [Google Scholar]
- Hwang SM, Hwang KC, Hong YJ, Lee HR, Kim TS, Park KU, Song J, Lee JH, Kim EC.. Improving antitubercular drug susceptibility testing with liquid media. Ann Clin Lab Sci. 2014. Spring; 44(2):123–130. [PubMed] [Google Scholar]
- Karadag A, Tokac M, Guvenli A, Sunbul M, Gunaydin M, Sanic A.. Resistance ratio to major anti-tuberculosis drugs of tuberculosis complex bacilli isolated from clinical samples. ANKEM Derg. 2004;18(4):189–192. [Google Scholar]
- Karataş M, Kuyucu T, Sevim T.. Evaluation of 1753 patients treated with the diagnosis of tuberculosis. Cumhuriyet Med J. 2019. Mar 27;41(1):34–41. 10.7197/223.vi.543017 [DOI] [Google Scholar]
- Kunduracioğlu A, Karasu I, Bïçmen C, Özsöz A, Erbaycu AE.. [Comparison of the performances of MTD Gene-Probe® test, BACTEC 960™ system and Löwenstein-Jensen culture methods in the diagnosis of smear-negative tuberculosis cases] (Turkish). Mikrobiyol Bul. 2013. Jul 29;47(3):417–431. 10.5578/mb.5728 [DOI] [PubMed] [Google Scholar]
- Kurtoglu MG, Kesli R, Terzi Y, Baykan M.. Investigation of the Susceptibilities of Mycobacterium tuberculosıs Complex strains to major anti-tuberculosis drugs with BACTEC MGIT 960 System. Nobel Med. 2011;7(1):42–48. [Google Scholar]
- Kurtoğlu MG, Ozdemir M, Keşli R, Ozkalp B, Baysal B.. [Isolation rate of Mycobacterium tuberculosis complex from patients with suspected tuberculosis and identification of the strains with BACTEC™ NAP and immunochromatographic TB Ag MPT64 Rapid™ Tests] (Turkish). Mikrobiyol Bul. 2011. Apr;45(2):266–273. [PubMed] [Google Scholar]
- Kwak M, Lee WK, Lim YJ, Lee SH, Ryoo S.. Systematic review and meta-analysis of the nitrate reductase assay for drug susceptibility testing of Mycobacterium tuberculosis and the detection limits in liquid medium. J Microbiol Methods. 2017. Oct;141:1–9. 10.1016/j.mimet.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Linger Y, Kukhtin A, Golova J, Perov A, Lambarqui A, Bryant L, Rudy GB, Dionne K, Fisher SL, Parrish N, et al. Simplified microarray system for simultaneously detecting rifampin, isoniazid, ethambutol, and streptomycin resistance markers in Mycobacterium tuberculosis . J Clin Microbiol. 2014. Jun 01;52(6):2100–2107. 10.1128/JCM.00238-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Lian L, Jiang Y, Huang M, Tan Y, Zhao X, Zhang J, Yu Q, Liu J, Dong H, et al. Identification of species of nontuberculous mycobacteria clinical isolates from 8 provinces of China. BioMed Res Int. 2016;2016:1–10. 10.1155/2016/2153910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbeha B, Mine M, Motswaledi MS, Dewar J.. Nontuberculous mycobacteria, Botswana, 2011–2014. Emerg Infect Dis. 2019. Jul; 25(7):1401–1403. 10.3201/eid2507.181440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotto P, Zhang Y, Cirillo DM, Yam WC.. Drug resistance mechanisms and drug susceptibility testing for tuberculosis. Respirology. 2018. Dec;23(12):1098–1113. 10.1111/resp.13393 [DOI] [PubMed] [Google Scholar]
- Ozmen E, Aslan A, Ucar M, Aydin H, Yilmaz A.. Resistance ratios of Mycobacterium tuberculosis Complex strains isolated in Erzurum Regional Tuberculosis Laboratory against major anti-tuberculosis drugs. ANKEM Derg. 2017;31(2):53–58. [Google Scholar]
- Perincek G, Tabakoglu E, Otkun M, Ozdemir L, Ozdemir B.. Resistance rates of Anti-tuberculosis drugs in pulmonary tuberculosis patients producing Mycobacterium Tuberculosis . Turk Thorac J. 2011. Sep 1;12(3):111–113. 10.5152/ttd.2011.25 [DOI] [Google Scholar]
- Pienaar E, Linderman JJ, Kirschner DE.. Emergence and selection of isoniazid and rifampin resistance in tuberculosis granulomas. PLoS One. 2018. May 10;13(5):e0196322 10.1371/journal.pone.0196322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglik Bakanligi T.C. Turkiyede Verem Savaşi 2018 Raporu. Ankara (Turkey): Sağlık Bakanlığı Yayın; 2018. [Google Scholar]
- Şamlı A, İlki A.. Comparison of MALDI-TOF MS, nucleic acid hybridization and the MPT64 immunochromatographic test for the identification of Mycobacterium tuberculosis and non-tuberculosis Mycobacterium species. New Microbiol. 2016. Oct;39(4):259–263. [PubMed] [Google Scholar]
- Saral OB, Sucu N, Boz GA, Erdem M, Koksal İ.. Evaluation of combined drug resistance with Bactec method in 442 Mycobacterium tuberculosis strains. Turk Thorac J. 2007;8(3):174–178. [Google Scholar]
- Spaulding AB, Lai YL, Zelazny AM, Olivier KN, Kadri SS, Prevots DR, Adjemian J.. Geographic distribution of nontuberculous mycobacterial species identified among clinical isolates in the United States, 2009–2013. Ann Am Thorac Soc. 2017. Nov;14(11): 1655–1661. 10.1513/AnnalsATS.201611-860OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg HR, Lipman MC, McHugh TD, Jenkins HE.. Isoniazid-resistant tuberculosis: a cause for concern? Int J Tuberc Lung Dis. 2017. Feb 01;21(2):129–139. 10.5588/ijtld.16.0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavanaee Sani A, Shakiba A, Salehi M, Bahrami Taghanaki HR, Ayati Fard SF, Ghazvini K.. Epidemiological characterization of drug resistance among Mycobacterium tuberculosis isolated from patients in Northeast of Iran during 2012–2013. BioMed Res Int. 2015;2015:1–6. 10.1155/2015/747085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylan M, Yilmaz S, Kaya H, Demir M, Selimoglu-Sen H, Sezgi C, Abakay O, Tanrıkulu AC, Abakay A.. Tuberculosis control status of Diyarbakir province between the years 2005–2010. Dicle Med J. 2015;42(2):227–234. [Google Scholar]
- Waters V, Ratjen F.. Antibiotic treatment for nontuberculous mycobacteria lung infection in people with cystic fibrosis. Cochrane Database Syst Rev. 2016;12:CD010004 10.1002/14651858.CD010004.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Global tuberculosis report 2019. Geneva (Switzerland): World Health Organization; 2019. [Google Scholar]
- WHO The use of liquid medium for culture and DST [Internet]. Geneva (Switzerland): World Health Organization; 2007. [cited 2019 Dec 16]. Available from: https://www.who.int/tb/laboratory/policy_liquid_medium_for_culture_dst/en/ [Google Scholar]


