Abstract
Oxidative stress-induced series of related degenerative diseases have received widespread attention. To screen new lactic acid bacteria (LAB) strains to resist oxidative stress, traditional Chinese fermented vegetables were used as a resource library to screen of LAB. The Lactobacillus fermentum JX306 strain, which showed high scavenging activity of DPPH free radical and hydrogen radical, and a strong lipid peroxidation inhibition rate in vitro was selected. L. fermentum JX306 was also examined for its antioxidant capacity in D-galactose-induced aging mice. The results showed that L. fermentum JX306 could significantly decrease malondialdehyde (MDA) levels and improve the activity of glutathione peroxidase (GSH-Px), and total antioxygenic capacity (TOC) in the serum, kidney, and liver. Meanwhile, the strain could remarkably upregulate the transcriptional level of the antioxidant-related enzyme genes, such as peroxiredoxin1 (Prdx1), glutathione reductase (Gsr), glutathione peroxidase (Gpx1), and thioredoxin reductase (TR3) encoding genes in the liver. Besides, histopathological observation proves that this probiotic strain could effectively inhibit oxidative damage to the liver and kidney in aging mice. Therefore, this unique antioxidant strain may have a high application value in the functional food industry and medicine industry.
Key words: lactic acid bacteria, antioxidant activity, Lactobacillus fermentum, traditional Chinese fermented vegetables, D-galactose-induced aging mice
Introduction
Oxidative stress refers to an imbalance, which is caused by high levels of reactive oxygen species (ROS) and low levels of antioxidant activity (Preiser 2012). The excessive ROS can damage enzymes, fatty acids, proteins, nucleic acids, and other physiological substances of cells, which leads to structure and function disorders (Dizdaroglu et al. 1992; Wu et al. 2014). The oxidative stress can cause various diseases, including amyotrophic lateral sclerosis, asthma, allergies, and diabetes as well as further accelerate aging (Nyström 2003; Kurien et al. 2006; Lin and Beal 2006). When the entire antioxidant defense system cannot protect all biological macromolecules from the effects of oxidative damage, it is necessary to increase the defense capacity of the antioxidant system in order to protect human health (Wang et al. 2017). In recent years, LAB have received increasing attention because of their long history of safe use and their potential health benefits, such as improving stool consistency, immune modulation, and antagonism towards the pathogens. Another attractive feature of the LAB is their antioxidant capacity. Increasing experimental evidence indicates that probiotic LAB exerts beneficial antioxidative effects by ROS scavenging, chelating transition metal ions, and activating certain enzyme activities. Therefore, using LAB to scavenge the excess of free radicals, inhibit oxidative damage, and prevent the related restrictive diseases can be a potential treatment option (Mishra et al. 2015; Wang et al. 2017; Lin et al. 2018a).
The most studied probiotics are Lactobacillus and Bifidobacterium. Compared with other probiotic strains such as L. rhamnosus, L. casei, and L. plantarum, the study on L. fermentum is one of the less-studied potential probiotic strain and still in a developing stage (Lin et al. 2017). L. fermentum has beneficial effects on the cholesterol level, effectiveness of the immune response, and reduction of the gastrointestinal and upper respiratory tract infections in infants (Wang et al. 2009; Pan et al. 2011; Maldonado et al. 2012; Russo et al. 2015). It is worth noting that the antioxidant properties of L. fermentum have received extensive attention. Using the oxygen radical absorbance capacity (ORAC) method, a significant in vitro antioxidant capacity of L. fermentum LF31 has been shown (Persichetti et al. 2014). L. fermentum ME-3, as a well-known anti-oxidant probiotic strain, inhibited oxidative damage to the body and reduced the risk of intestinal infection in patients (Mikelsaar and Zilmer 2009). L. fermentum Suo could eliminate the chain reaction of oxygen free radicals and lipid peroxidation as well as inhibit HCl/ethanol-induced oxidative damage in the gastric tissue (Suo et al. 2016). L. fermentum MTCC589 could improve antioxidant enzyme activity, resist the reinfection of Escherichia coli, and reduce the immune aging of mice (Sharma et al. 2014).
In this study, 481 of LAB strains from Chinese traditional fermented vegetables were screened to isolate the probiotic strains with antioxidant activity. After the characterization of the antioxidant properties of the LAB strains in vitro, L. fermentum strain JX306 with high antioxidant activity was selected, and these properties were further studied in vivo using a D-galactose-induced aging mice model.
Experimental
Materials and Methods
Bacterial strains and culture conditions. A total of 481 isolates (Table SI) were used in this study, which were obtained from 35 Chinese traditional fermented vegetable samples collected from different areas of China. They were identified using the methods described earlier (Wu et al. 2009). All the strains were stored at –80°C in MRS broth with 20% glycerol. For all subsequent experiments, the strains were incubated in MRS broth at 37°C for 18 h. The intact cells were obtained by centrifugation (8,000 g for 10 min at 4°C) and then washed with sterilized isotonic saline (0.85%) three times. The final concentration of intact cells was adjusted to 4.0 × 108 CFU/ml.
In vitro determination of antioxidant activity of LAB strains. Primary screening of LAB with antioxidant capacity. The scavenging capacity against DPPH· of 481 LAB strains was evaluated based on the method described by Wang et al. (2017) with some modification. Briefly, 1.0 ml of LAB suspension was added to 2.0 ml of DPPH· solution (0.2 mM in ethanolic) and shaken well before incubation for 30 min in the dark at room temperature. In the control group, LAB suspension was replaced by sterile saline, and the DPPH free radical solution was replaced with ethanol solution in the blank group. After centrifugation at 8,000 g for 10 min, the absorbance of the supernatants was measured at 517 nm. The specific method for measuring DPPH· free radical scavenging capacity was as follows:
Hydroxyl radical scavenging. The method for the measurement of hydroxyl radical scavenging ability of the preliminarily screened LAB strains was based on Lin’s test method (Lin et al. 2018a) with some modifications. Briefly, 1 ml of the LAB suspension was added to 2.5 ml mixture containing 0.5 ml of O-phenanthroline (2.5 mM), 1.0 ml of PBS (10 mM, pH = 7.4), 0.5 ml of FeSO4 (2.5 mM), and 0.5 ml of H2O2 (20 mM). The mixture was shaken well and incubated in a water bath at 37°C for 1.5 h. The control group used sterile saline instead of LAB suspension. After centrifugation at 8,000 g for 10 minutes, the absorbance of the supernatant was measured at 510 nm. The specific details of the measurement of hydroxyl radical scavenging ability of LAB cells were as follows:
Lipid peroxidation inhibition rate. The lipid peroxidation inhibition rate of the primary screening LAB strains was determined based on a Kullisaar’s test method (Kullisaar et al. 2003) with some modifications. Briefly, 1.0 ml of the LAB suspension was added to a solution containing 0.5 ml of deionized water, 0.2 ml of FeSO4 (0.01%, w/v), 0.02 ml of ascorbic acid (0.01%, w/v), and 1.0 ml of linoleic acid emulsion (20 ml linoleic acid emulsion includes 0.2 ml of tween 20, 0.1 ml of linoleic acid, and 19.7 ml of sterile saline). The mixture was incubated at 37°C for 12 h. Then, 0.2 ml of 4% TCA, 2 ml of thiobarbituric acid (TBA, 0.8%), and 0.2 ml of butylated hydroxytoluene (BHT, 0.4%) were added to the mixture and shaken well. After reaction in a heated water bath at 100°C for 30 min, the solution was rapidly cooled by using an ice bath and extracted by 2 ml butyl alcohol. The supernatant was obtained by centrifugation at 8,000 g for 10 min, and the absorbance at 532 nm was determined as Asample. The absorbance of the mixture without the LAB cells was determined as Acontrol. The specific calculation method of the lipid peroxidation inhibition rate was as follows:
Tolerance of the selected strains to simulated bile. The tolerance of the six LAB strains to simulated bile was evaluated based on the test method described by Argyri et al. (2013). LAB cells (4 × 108 CFU/ml) were collected and resuspended into 1 ml simulated bile (a sterile saline solution containing 1 mg/ml pancreatic enzymes, and 0.5% (w/v) of bile salt. The LAB cells were incubated at 37°C for 1 h, and then the samples were diluted and plated on MRS solid plates. After 48 h of incubation at 37°C, the survival rate (SR) of cells was determined by a plate count method. The survival rate was determined as follows:
where, S1 is the initial number of cells, S2 is the final number of cells.
Tolerance of the selected strains to simulated gastric and intestinal fluids. Tolerance of the selected six strains to simulated gastric and intestinal fluids was tested based on the method described by Huang and Adams (2004). Briefly, LAB cells (4 × 108 CFU/ml) were collected and resuspended into 1 ml of the simulated gastric fluid or intestinal fluid. The simulated gastric and intestinal fluids were made in the same way as in the previous study (Huang and Adams 2004). LAB strains in the simulated gastric fluid were incubated at 37°C for 3 h, and LAB strains in the simulated intestinal fluid were incubated at 37°C for 24 h. Then, LAB strains were diluted and spread on MRS plates. After 48 h of incubation at 37°C respectively, the survival rate was determined as shown above for the SR(%) equation.
Assay antioxidant activity of L. fermentum JX306 in vivo using the D-galactose-induced aging mice model. Animal experiment designs. Sixty male KM mice (20 ± 2 g), purchased from Jinan Pengyue Animal Experimental Center (Jinan, China), were randomly divided into six groups after one-week adaptation: the normal control group (NC), model control group (MC), positive control group (PC), low-dose group (LD), middle-dose group (MD), and high-dose group (HD). The treatments of each group were shown in Table I. After feeding for eight weeks, mice were sacrificed. The specific methods and details of animal experiments have been approved by the Ethics Committee of Shandong Agriculture University and comply with relevant guidelines in the European Union (Directive 2010/63/EU).
Table I.
Groups of experimental animals.
| Group | Number | Experimental treatments | |
|---|---|---|---|
| Oral administration | Intraperitoneal injection | ||
| NC | 10 | Normal saline (0.85%, 20 ml/kg BW) | Normal saline (0.85%, 20 ml/kg BW) |
| MC | 10 | Normal saline (0.85%, 20 ml/kg BW) | D-galactose (30 g/l, 200 mg/kg BW) |
| PC | 10 | Vitamin C (30 g/l, 200 mg/kg BW) | D-galactose (30 g/l, 200 mg/kg BW) |
| LD | 10 | L. fermentum JX306 (108 CFU/day) | D-galactose (30 g/l, 200 mg/kg BW) |
| MD | 10 | L. fermentum JX306 (109 CFU/day) | D-galactose (30 g/l, 200 mg/kg BW) |
| HD | 10 | L. fermentum JX306 (1010 CFU/day) | D-galactose (30 g/l, 200 mg/kg BW) |
Determination of serum, liver, and kidney antioxidative parameters. Blood samples of each group were obtained by moving mice eyeballs after 12 h of the final administration. Subsequently, the samples were centrifuged (8,000 g, 10 min at 4°C) to obtain the serum samples. The serum samples were stored at – 80°C for further analysis. The liver and kidney samples of each mouse (0.1 g) were added to 0.9 ml of sterile saline and fully homogenized. After centrifugation at 8,000 g for 10 min at 4°C, the supernatants of each group were gathered for further analysis. Four oxidative stress products, including malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC) were determined in the serum, liver, and kidney samples according to the details in the kit (Nanjing Jiancheng Bioengineering Institute, China).
Changes of the antioxidant-related genes’ transcription in the liver. The total RNA of liver tissue in each group was extracted according to the specific details in the RNAiso Plus (Takara, China). The purity and quality of total RNA were determined by OD260/OD280 and agar gel electrophoresis. Then, the reversed transcription to synthesize cDNA was performed according to the details in the PrimeScriptII First Strand cDNA Synthesis Kit (Takara, China), and considering the needs of the next experiment, the cDNA was placed at –20°C. The 7500 Fast Real-Time PCR System (ABI, USA) and SYBR Green PCR Kit (TransGen, China) were used for operating the RT-PCR. The PCR procedure contained a denaturation step at 94°C for 10 min, followed by 40 cycles of denaturation at 94°C for 5 s, annealing, and extension at 60°C for 30 s.
The primers (Table SII) for the amplification of the genes encoding for peroxiredoxin-1 (Prdx1), glutathione peroxidase (Gsr), glutathione peroxidase (Gpx), and thioredoxin reductase (TR3), used for quantitative-PCR, were as described previously (Zhao et al. 2018). The gene encoding for β-actin was considered as a reference gene. The transcriptional levels of four genes encoding antioxidant enzymes Prdx1, Gsr, Gpx, and TR3 in the liver were evaluated according to the standard curve of quantitative analysis. The specific result of the relative expression of the gene is expressed by the formula 2−ΔΔCT.
Histological analysis. Liver and kidney tissues were fixed with 10% formalin for 24 h and then embedded in conventional paraffin. For each paraffin block, thin sections (4–5 μm thickness) were gained, and then HE stained, and professional pathologists were invited to interpret the section results. Subsequently, these sections were used for light microscopic evaluation.
Statistical analysis. The software SPSS 20 was used to perform the statistical evaluation. Mean ± standard deviations were used to interpret the data. One-way ANOVA was operated to analyze the data. Significant differences between experimental groups were determined using LSD’s and Tukey’s tests. The data are significantly different with p < 0.05.
Results
Screening of antioxidant LAB strains. In this study, 481 strains isolated from Chinese traditional fermented vegetable samples were firstly screened for their DPPH free radical scavenging ability. As shown in Table II, six LAB strains were selected based on their high DPPH radical scavenging capacity. The strongest scavenging effects on the DPPH radical scavenging capacity were found for L. fermentum 306 (37.29%).
Table II.
The selected LAB strains with high antioxidant activity.
| Strains | DPPH scavenging rate (%) | Hydrogen radicals scavenging rate (%) | Inhibition rate of lipid peroxidation (%) |
|---|---|---|---|
| L. fermentum JX306 | 37.29 ± 1.75a | 37.90 ± 0.29a | 28.14 ± 2.97a |
| L. fermentum GZ394 | 34.92 ± 3.57a | 35.02 ± 1.70b | 23.89 ± 1.60b |
| L. plantarum SC34 | 23.09 ± 4.00c | 34.50 ± 1.44b | 12.69 ± 0.23d |
| L. plantarum GZ328 | 27.83 ± 2.25b | 29.21 ± 1.60c | 20.85 ± 2.07bc |
| Pediococcus pentosaceus GZ430 | 16.22 ± 0.89d | 23.32 ± 1.62d | 18.01 ± 2.09c |
| Leuconostoc mesenteroides YN295 | 15.26 ± 0.67d | 34.31 ± 1.18b | 14.43 ± 1.29d |
An average value within a list with different superscript alphabets differ (p < 0.05)
Data are shown as means ± SD from triplicate results
The antioxidant activity of the strains selected was further evaluated by HO· scavenging ability, and the lipid peroxidation inhibition rate. As shown in Table II, among the strains selected, L. fermentum JX306 exhibited the highest HO· scavenging capability, and the strongest lipid peroxidation inhibition activity with the HO· scavenging rate of 37.29%, and lipid peroxidation inhibition rate of 37.9%.
Tolerance to bile salts and the simulated gastric and intestinal fluids. Prerequisites for the application of LAB strains for commercial use include resistance to bile salt-mediated growth inhibition (Jamalifar et al. 2010), and their survival in an acidic, alkaline gastrointestinal environment. Oral lactic acid bacteria must overcome these adverse conditions to live to the intestines and, therefore, play a beneficial health effect. Thus, the tolerance of the strains selected for simulated bile, gastric fluid, and intestinal fluid was determined, as shown in Table III, L. fermentum JX306 showed the highest survival rates after incubation in these three simulated solutions. The survival rates were 78.28% for simulated bile, 53.05% for simulated gastric fluid, and 42.07% for simulated intestinal fluid, respectively.
Table III.
The survival rate (%) of LAB in simulated bile, gastric fluid, and intestinal fluid.
| Strains | Simulated bile | Simulated gastric fluid | Simulated intestinal fluid |
|---|---|---|---|
| L. fermentum JX306 | 78.28 ± 0.18a | 53.05 ± 1.75a | 42.07 ± 6.52a |
| L. fermentum GZ394 | 74.61 ± 4.67a | 30.57 ± 3.68b | 27.26 ± 4.95b |
| L. plantarum SC34 | 65.09 ± 6.33b | 5.34 ± 1.92c | 12.23 ± 1.85e |
| L. splantarum GZ328 | 74.48 ± 1.79a | 1.51 ± 0.22e | 9.75 ± 0.45f |
| Pediococcus pentosaceus GZ430 | 22.99 ± 3.12d | 3.62 ± 1.11d | 15.26 ± 2.34d |
| Leuconostoc mesenteroides YN295 | 33.89 ± 0.96c | 5.34 ± 0.79c | 17.28 ± 0.56c |
Average value within a list with different superscript alphabets differ (p < 0.05)
Data are shown as means ± SD from triplicate results
Therefore, based on the antioxidant activities and the survival responses of the strains selected to the simulated conditions in the human gastrointestinal tract, the strain of L. fermentum JX306 was finally selected for in vivo assay to examine their antioxidant profiles in the D-galactose-induced aging mice model.
Antioxidant activities of L. fermentum JX306 in the D-galactose-induced aging mice model. High levels of ROS lead to oxidative damage. D-galactose has low toxicity and slow action and could stimulate the body to produce a large number of free radicals. It has been used as a mature model to simulate the body’s oxidative stress process. In this study, the antioxidant activities of L. fermentum JX306 were verified in vivo in the D-galactose-caused oxidative damaged mice. After intraperitoneal injection of D-galactose for eight weeks, compared with the NC group, the MC group exhibited obvious signs of aging. The skin color of the mice was dull, the skin elasticity was poor, and worse emotions such as irritability and lethargy appeared. It is worth noting that no mouse death occurred during the entire test. The weight of the mice was analyzed in detail without statistical differences in all groups (p > 0.05) (data not shown).
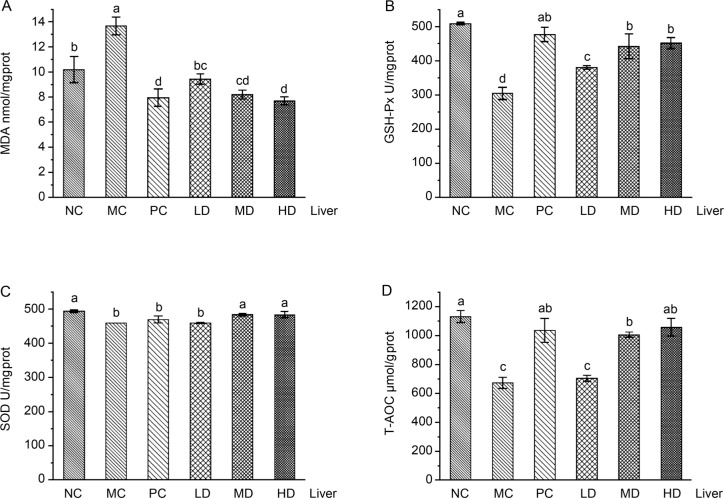
The level of antioxidant products in the D-galactose-induced aging mice liver. Compared with the NC group, the levels of GSH-Px and T-AOC antioxidant products in the MC group showed a significant downward trend, while the activity of MDA showed an upward trend. (p < 0.05, Fig. 1). Oral treatment with L. fermentum JX306 strain could change significantly adverse effects, which were caused by the three oxidative stress products (Fig. 1). The MDA levels decreased with the increase of LAB dose, and it reached 9.43 nmol/mg protein, 8.19 nmol/mg protein, and 7.70 nmol/mg protein in the LD, MD, and HD group, respectively, which were 31.01%, 40.03%, and 43.65% lower than the results in MC group. It should be noted that the MDA levels in a high-dose group were significantly decreased compared to the normal control group, and comparable to the PC group. In the LAB groups, the GSH-Px and T-AOC levels increased with the increase of LAB dose, and it reached 451.80 U/mgprot, and 1057.13 μmol/gprot in a HD group respectively, which were 48.40% and 57.13% higher than the results in MC group. As shown in Fig. 1, the hepatic SOD activity was not significantly influenced by D-gal, vitamin C or L. fermentum JX306 strain application.
Fig. 1.
Effect of L. fermentum JX306 on GSH-Px, SOD, T-AOC activities, and MDA concentration in the liver of mice with oxidative stress induced by D-galactose. (A) MDA; (B) GSH-Px; (C) SOD; (D) T-AOC. All data are presented as mean ± SD (n = 3). Bars with different letters were significantly different (p < 0.05).
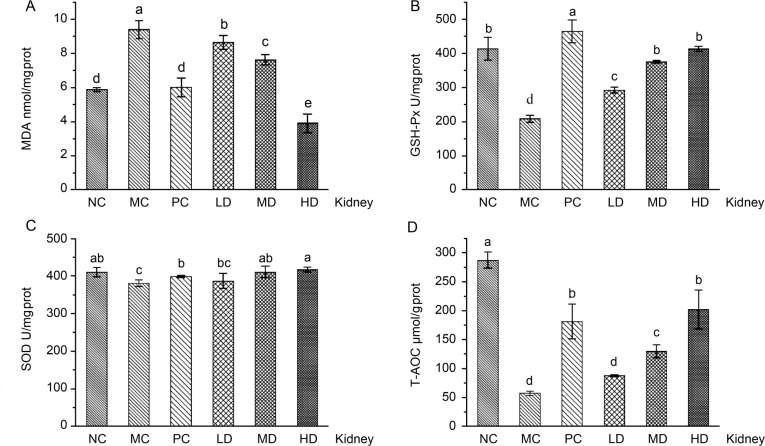
The level of antioxidant products in the D-galactose-induced aging mice kidney. When compared with the NC group, the injection of D-galactose to mice from the MC group generated a lot of free radicals. The excessive radicals hurt all biological macromolecules and cause oxidative damage to tissues and cells; thus, they led to the decrease of GSH-Px and T-AOC activities and the increase of MDA levels (p < 0.05, Fig. 2). The administration of L. fermentum JX306 could effectively reverse the adverse changes, which were caused by D-galactose (p < 0.05). The T-AOC levels boosted with the increase of the LAB dose and reached 87.80 μmol/g protein, 129.80 μmol/g protein, and 202.27 μmol/g protein in the LD, MD, and HD group respectively. These levels were 53.12%, 126.34%, and 252.76% higher than the results demonstrated for the MC group. It is worth mentioning that the activity of GSH-Px with high-dose of L. fermentum JX306 was higher, and there was no statistically significant difference when compared to the NC group. This result showed that the high-dose of L. fermentum JX306 had the most significant effect in inhibiting oxidative damage, which was caused by excessive free radicals. The MDA levels decreased with the increase of the LAB dose, and reached 8.64 nmol/mg protein, 7.62 nmol/mg protein, and 3.91 nmol/mg protein in the LD, MD, and HD group, respectively. These levels were 7.92%, 18.79%, and 58.32% lower when compared to the MC group. It should be noted that the MDA levels in the high-dose group were significantly decreased in comparison to the normal control group and PC group. The administration of L. fermentum JX306 did not significantly affect SOD activity in the kidney when compared to that of the MC group.
Fig. 2.
Effect of L. fermentum JX306 on GSH-Px, SOD, T-AOC activities, and MDA concentration in the kidney of mice with oxidative stress induced by D-galactose. (A) MDA; (B) GSH-Px; (C) SOD; (D) T-AOC. All data are presented as mean ± SD (n = 3). Bars with different letters were significantly different (p < 0.05).
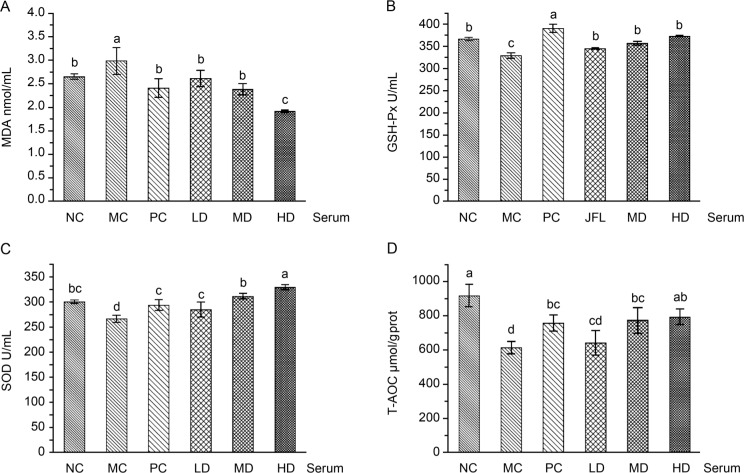
Effect of antioxidant products in the serum from the D-galactose-induced aging mice. As shown in Fig. 3, a significant decrease of T-AOC activities and an increase of MDA levels were observed in the serum of the D-galactose induced aging mice’s control group when compared to those of the normal control group. L. fermentum JX306 administration reversed the changes in T-AOC and MDA levels. The T-AOC levels enlarged with the increase of the LAB dose and reached 641.25 μmol/g protein, 772.53 μmol/g protein, and 793.53 μmol/g protein in the LD, MD, and HD group, respectively. These values were 4.45%, 25.83%, and 29.25% higher than the results in the MC group. The MDA levels decreased with the increase of LAB dose, and reached 1.92 nmol/mg protein in the HD group, and were 35.75%, 27.64%, and 20.44% lower than the results in the MC group, NC, and PC group, respectively. The GSH-Px levers in sera of L. fermentum JX306 administration mice showed only a slight increase. The serum SOD activities were not significantly influenced by D-galactose, vitamin C or L. fermentum JX306.
Fig. 3.
Effect of L. fermentum JX306 on GSH-Px, SOD, T-AOC activities and MDA concentration in the serum of mice with oxidative stress induced by D-galactose. (A) MDA; (B) GSH-Px; (C) SOD; (D) T-AOC. All data are presented as mean ± SD (n = 3). Bars with different letters were significantly different (p < 0.05).
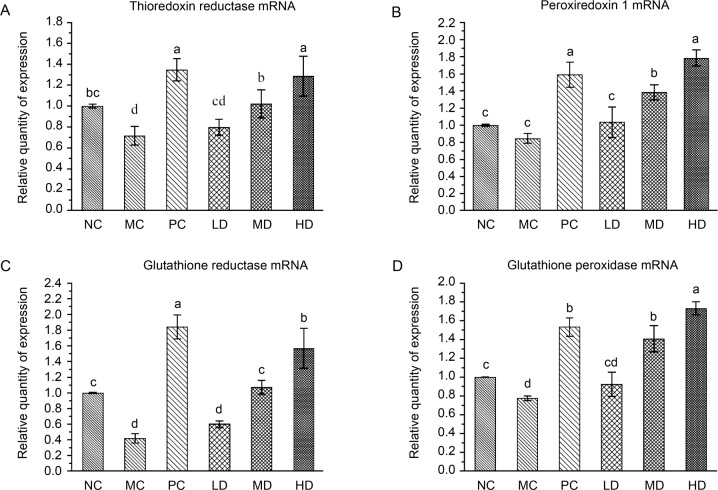
Effect of L. fermentum JX306 on the relative gene expression in the liver of D-galactose-induced aging mice. In order to further explore the related antioxidant mechanism of L. fermentum JX306 at the gene level, we have completed a quantitative analysis of the relative expression of the key antioxidant genes in the thioredox system (TRX) and the glutathione system (GSH). As shown in Fig. 4, when compared with the normal group, D-galactose treatment significantly reduced the relative gene expression levels of Prdx1, Gsr, Gpx, and TR3 (p < 0.05). However, it is worth noting that this phenomenon was significantly alleviated by L. fermentum JX306 intervention, and the relative gene expression levels of Prdx1, Gsr, Gpx, and TR3 were all significantly increased. Therefore, oral treatment with L. fermentum JX306 strain could significantly increase the transcription level of antioxidant genes in the liver and could play a key role in inhibiting oxidative damage.
Fig. 4.
Effect of L. fermentum JX306 on the expression of the genes encoding for peroxiredoxin-1 (Prdx1), glutathione peroxidase (Gsr), glutathione peroxidase (Gpx), and thioredoxin reductase (TR3) in the liver of D-galactose induced aging mice. (A) Thioredoxin reductase mRNA; (B) Peroxiredoxin1 mRNA; (C) Glutathione reductase mRNA; (D) Glutathione peroxidase mRNA. All data are presented as mean ± SD (n = 3). Bars with different letters were significantly different (p < 0.05).
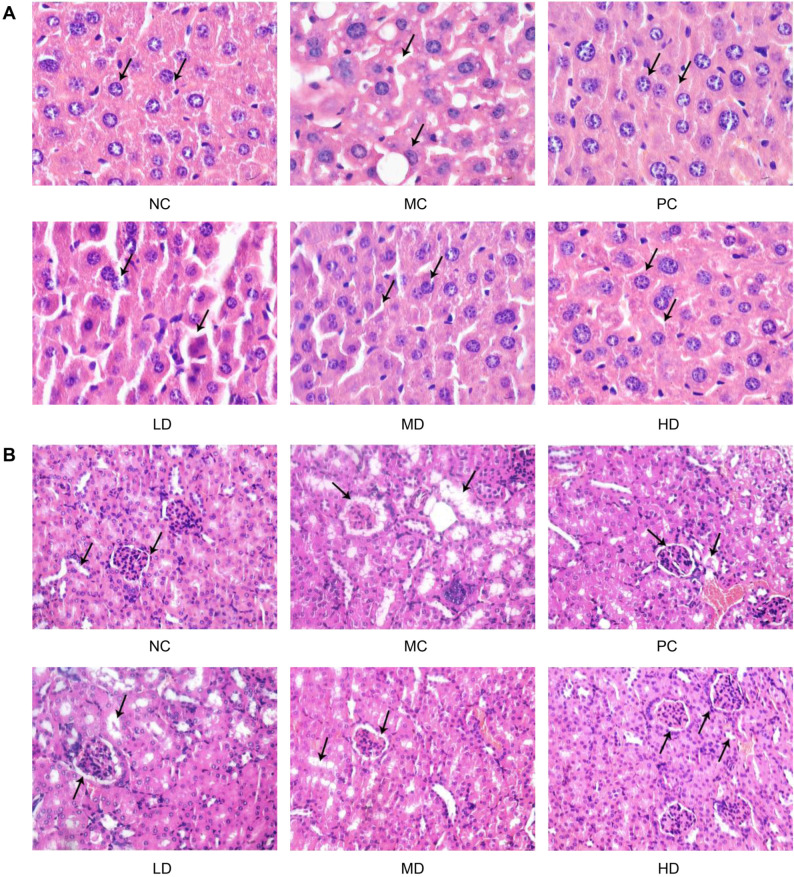
Histopathological changes in mice livers and kidneys. As shown in Fig. 5, the HE sections showed that L. fermentum JX306 had an inhibitory effect on the oxidative damage caused by D-galactose in liver and kidney tissues. In the NC group, cells in the liver indicated large and round cell nucleus, nucleoli conspicuous, and entire cytoplasm (Fig. 5A). The renal tubules and glomerulus of the kidney showed an intact morphological structure (Fig. 5B). While for the MC group, the histological picture of the kidney indicated that glomeruli were severely damaged, as did tubulointerstitial lesions. A loss of brush borders and vacuolation of renal tubules were also observed. A histological picture of the liver showed necrotic spots, edema degeneration, and vacuoles degeneration. However, analysis of the three groups treated with L. fermentum JX306 indicated that D-galactose-induced pathologic changes could be alleviated with a dose-related effect. As especially observed in the HD group, the severe oxidative damages caused by D-galactose were strikingly improved to the level of the normal group.
Fig. 5.
Effects of L. fermentum JX306 treatment on organic damages in the liver (A), and the kidney (B) of D-galactose induced aging mice.
Discussion
In recent years, due to long history of safe use and potential therapeutic benefits (Mokoena 2017; Garcia-Castillo et al. 2019; Zhao et al. 2019) for human health of probiotics, the antioxidative activity of LAB has attracted more and more attention (Amaretti et al. 2013; Tang et al. 2016; Zhao et al. 2018). Traditional Chinese fermented vegetables contain abundant LABs. In this study, for isolation of new LAB strains with high antioxidant activity, the variety of fermented vegetable samples from different areas of China were collected, and 481 LAB strains were isolated from these samples (Table SI). A DPPH free radical scavenging method can directly and rapidly reflect the antioxidant capacity of lactic acid bacteria and has been widely used to evaluate this activity (Antolovich et al. 2002; Ding et al. 2017; Lin et al. 2018b). After screening of the antioxidant activity of 481 LAB strains with a DPPH free radical scavenging method, six strains with a high DPPH radical scavenging rate were selected. It should be noted that these six strains were isolated from the fermented vegetable samples, which were collected from southwestern China. The L. fermentum JX306, isolated from Chinese sauerkraut in Jiangxi Province, showed the highest scavenging ability of DPPH radicals at a density of 108 CFU/ml (37.29%). Additionally, the strains of L. plantarum GZ328 and L. fermentum GZ394 (Table II), which showed high DPPH radical scavenging ability, were also isolated from Chinese sauerkraut. Therefore, the sauerkraut from southwestern China is a good source for the isolation of LAB with high antioxidant activity.
A D-galactose-induced oxidative stress model is a very mature model in many animal experiments (Ho et al. 2003). In this study, the antioxidant activities of L. fermentum JX306 were verified in vivo in a D-galactose-induced aging mice model. MDA is considered one of the by-products of the lipid peroxidation process, and its concentration is one of the most commonly used biomarkers to reflect the lipid peroxidation level (Nielsen et al. 1997). It was reported that L. plantarum CCFM10, L. plantarum AR501, and L. delbrueckii subsp. bulgaricus F17 can decrease the level of MDA and inhibit the generation of an excess of free radicals (Ding et al. 2017; Lin et al. 2018b; Zhao et al. 2018). A comprehensive analysis of the literature and our results shows that lactic acid bacteria could indeed relieve the D-galactose-induced oxidative damage. After oral administration of L. fermentum JX306 for eight weeks, the MDA levels in serum, liver, and kidney were significantly lower when compared to the MD group. It is worth emphasizing that MDA levels in the high-dose group were also significantly lower than those in the NC group. Therefore, L. fermentum JX306 has high lipid antioxidant activity.
Generally, excess of free radicals is mainly eliminated by redox systems in the body, such as the sulfur oxygen reduction (TRX) system and the glutathione (GSH) system, which can reduce and control the occurrence of the oxidative stress-related diseases (Yu et al. 2015; Lin et al. 2018a). GSH-Px is the most important antioxidant factor in the glutathione (GSH) system, which could directly scavenge free radicals and prevent cell damage (Esposito et al. 2000; Ding et al. 2017). The oral treatment with L. fermentum JX306 strain could significantly increase the antioxidant enzymatic activity of GSH-Px in the liver and kidney of mice. SOD is the key enzyme for hastening the reaction of superoxide anions to H2O2 (Nordberg and Arnér 2001). However, almost no difference was observed in SOD levels among these groups. Our results were supported by previous research (Zhao et al. 2018), and the low toxicity and slow action of D-galactose may be the reason that no significant differences in SOD levels among these groups were observed (Zhao et al. 2018). Total antioxidant capacity (T-AOC) represents the ability of non-enzymatic antioxidant systems to scavenge an excess of free radicals (Zhang et al. 2013). After intragastric administration of L. fermentum JX306, T-AOC levels showed an upward trend in the serum, liver, and kidney. Similar to our findings, the previous researches reported that intragastric administration of L. fermentum JX306, L. plantarum AR501 (Lin et al. 2018a), L. plantarum CCFM10, and L. plantarum RS15-3 (Zhao et al. 2019) significantly increased T-AOC in the liver.
Normal dose of D-galactose can be metabolized into glucose through the liver enzymatic hydrolysis, and excessive concentration of D-galactose will induce the production of reactive oxygen radicals, causing oxidative damage to liver tissues. To further elucidate the antioxidant mechanism, the changes in the relative level of mRNA encoding Prdx1, Gsr, Gpx, and TR3 in the liver were determined. GSH serves as the most abundant cellular thiol resource and provides a buffer system to maintain the cellular redox status. Prdx1, Gsr, and Gpx are all part of a glutathione peroxidase/glutathione/glutathione reductase antioxidant pathway (Wu et al. 2019). TR3 is a component of the TRX system (Arnér and Holmgren 2000). TRX can directly remove intracellular ROS, such as hydrogen peroxide and oxygen free radicals, and regulate intracellular oxidation-reduction balance (Nordberg and Arnér 2000). This test proved that D-galactose harms all body’s biological macromolecules, generates excessive free radicals, and causes oxidative damage. After oral administration of L. fermentum JX306, the expression of the genes encoding Prdx1, Gsr, Gpx, and TR3 was increased (Fig. 4), thus alleviating the D-galactose-induced liver injury in mice, and delaying the oxidative damage process. This was supported in this study by the histopathological analysis results. A high dose of L. fermentum JX306 was able to significantly improve the consequences of severe oxidative damage caused by D-galactose in the liver and kidney tissue.
In this study, L. fermentum JX306 with high antioxidant activities was isolated from traditional Chinese fermented vegetable samples. The strain not only showed excellent antioxidant activities in vitro, but also effectively restrained the oxidative damage in D-galactose-induced aging mice. While administered orally, the strain could significantly decrease the MDA levels and improve activities of SOD, GSH-Px. and TOC in the serum, liver, and kidney. It could also markedly up-regulate the transcription levels of the genes encoding for antioxidant-related enzymes in the livers of D-galactose-induced aging mice. Besides, the strain could improve the D-galactose-induced histological lesions, and a dose-effect relationship was observed in this case. Thus, L. fermentum JX306 could be regarded as a potential strain for further exploiting antioxidant functional products to treat the oxidative-stress-related restrictive disease.
Supplementary materials are available on the journal’s website.
Acknowledgments
This experiment was supported by the National Natural Science Foundation of China (31772060) and Shandong Provincial Key Laboratory of Agricultural Microbiology Open Fund (SDKL2017015).
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Amaretti A, Di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A.. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biot. 2013; 97(2): 809–817. 10.1007/s00253-012-4241-7 [DOI] [PubMed] [Google Scholar]
- Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K.. Methods for testing antioxidant activity. Analyst (Lond). 2002. Jan 10;127(1):183–198. 10.1039/b009171p [DOI] [PubMed] [Google Scholar]
- Argyri AA, Zoumpopoulou G, Karatzas KAG, Tsakalidou E, Nychas GJE, Panagou EZ, Tassou CC.. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 2013. Apr;33(2):282–291. 10.1016/j.fm.2012.10.005 [DOI] [PubMed] [Google Scholar]
- Arnér ESJ, Holmgren A.. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000. Oct; 267(20): 6102–6109. 10.1046/j.1432-1327.2000.01701.x [DOI] [PubMed] [Google Scholar]
- Chaplin AV, Shkoporov AN, Efimov BA, Pikina AP, Borisova OY, Gladko IA, Postnikova EA, Lordkipanidze AE, Kafarskaia LI.. Draft genome sequence of Lactobacillus fermentum NB-22. Genome Announc. 2015. Aug 27;3(4):e00896-15. 10.1128/genomeA.00896-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Wang L, Zhang J, Ke W, Zhou J, Zhu J, Guo X, Long R.. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J Funct Foods. 2017. Aug;35:481–488. 10.1016/j.jff.2017.06.008 [DOI] [Google Scholar]
- Dizdaroglu M. Oxidative damage to DNA in mammalian chromatin. Mutation Research/DNAging. 1992. Sep;275(3–6):331–342. 10.1016/0921-8734(92)90036-O [DOI] [PubMed] [Google Scholar]
- Esposito LA, Kokoszka JE, Waymire KG, Cottrell B, MacGregor GR, Wallace DC.. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic Biol Med. 2000. Mar; 28(5):754–766. 10.1016/S0891-5849(00)00161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Castillo V, Komatsu R, Clua P, Indo Y, Takagi M, Salva S, Islam MA, Alvarez S, Takahashi H, Garcia-Cancino A, et al. Evaluation of the immunomodulatory activities of the probiotic strain Lactobacillus fermentum UCO-979C. Front Immunol. 2019. Jun 13;10:1376 10.3389/fimmu.2019.01376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SC, Liu JH, Wu RY.. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology. 2003;4(1):15–18. 10.1023/A:1022417102206 [DOI] [PubMed] [Google Scholar]
- Huang Y, Adams MC.. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol. 2004. Mar;91(3):253–260. 10.1016/j.ijfoodmicro.2003.07.001 [DOI] [PubMed] [Google Scholar]
- Jamalifar H, Bigdeli B, Nowroozi J, Zolfaghari HS, Fazeli MR.. Selection for autochthonous bifidobacteial isolates adapted to simulated gastrointestinal fluid. Daru. 2010;18(1):57–66. [PMC free article] [PubMed] [Google Scholar]
- Kant R, Blom J, Palva A, Siezen RJ, de Vos WM.. Comparative genomics of Lactobacillus . Microb Biotechnol. 2011. May;4(3):323–332. 10.1111/j.1751-7915.2010.00215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullisaar T, Songisepp E, Mikelsaar M, Zilmer K, Vihalemm T, Zilmer M.. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Br J Nutr. 2003. Aug;90(2):449–456. 10.1079/BJN2003896 [DOI] [PubMed] [Google Scholar]
- Kurien BT, Hensley K, Bachmann M, Scofield RH.. Oxidatively modified autoantigens in autoimmune diseases. Free Radic Biol Med. 2006. Aug 15;41(4):549–556. 10.1016/j.freeradbiomed.2006.05.020 [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF.. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006. Oct;443(7113):787–795. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- Lin Q, Li D, Qin H.. Molecular cloning, expression, and immobilization of glutamate decarboxylase from Lactobacillus fermentum YS2. Electron J Biotechnol. 2017. May;27:8–13. 10.1016/j.ejbt.2017.03.002 [DOI] [Google Scholar]
- Lin X, Xia Y, Wang G, Xiong Z, Zhang H, Lai F, Ai L.. Lactobacillus plantarum AR501 alleviates the oxidative stress of D-galactose-induced oxidative stress model liver by upregulation of Nrf2-mediated antioxidant enzyme expression. J Food Sci. 2018a;83(7): 1990–1998. 10.1111/1750-3841.14200 [DOI] [PubMed] [Google Scholar]
- Lin X, Xia Y, Wang G, Yang Y, Xiong Z, Lv F, Zhou W, Ai L.. Lactic acid bacteria with antioxidant activities alleviating oxidized oil induced hepatic injury in mice. Front Microbiol. 2018b. Nov 6;9: 2684 10.3389/fmicb.2018.02684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado J, Cañabate F, Sempere L, Vela F, Sánchez AR, Narbona E, López-Huertas E, Geerlings A, Valero AD, Olivares M, et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr. 2012. Jan;54(1):55–61. 10.1097/MPG.0b013e3182333f18 [DOI] [PubMed] [Google Scholar]
- Mikelsaar M, Zilmer M.. Lactobacillus fermentum ME-3 – an antimicrobial and antioxidative probiotic. Microb Ecol Health Dis. 2009. Apr;21(1):1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J.. Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. 2015. Apr 15;63(14):3615–3626. 10.1021/jf506326t [DOI] [PubMed] [Google Scholar]
- Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: A mini-review. Molecules. 2017. Jul 26;22(8):1255 10.3390/molecules22081255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P.. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997. Jul 01; 43(7):1209–1214. 10.1093/clinchem/43.7.1209 [DOI] [PubMed] [Google Scholar]
- Nordberg J, Arnér ESJ.. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001. Dec;31(11):1287–1312. 10.1016/S0891-5849(01)00724-9 [DOI] [PubMed] [Google Scholar]
- Nyström T. The free-radical hypothesis of aging goes prokaryotic. Cell Mol Life Sci. 2003;60(7):1333–1341. 10.1007/s00018-003-2310-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan DD, Zeng XQ, Yan YT.. Characterisation of Lactobacillus fermentum SM-7 isolated from koumiss, a potential probiotic bacterium with cholesterol-lowering effects. J Sci Food Agric. 2011. Feb;91(3):512–518. 10.1002/jsfa.4214 [DOI] [PubMed] [Google Scholar]
- Persichetti E, De Michele A, Codini M, Traina G.. Antioxidative capacity of Lactobacillus fermentum LF31 evaluated in vitro by oxygen radical absorbance capacity assay. Nutrition. 2014. Jul;30 (7–8):936–938. 10.1016/j.nut.2013.12.009 [DOI] [PubMed] [Google Scholar]
- Preiser JC. Oxidative stress. J pen-Parenter Enter. 2012;36(2):147–154. [DOI] [PubMed] [Google Scholar]
- Russo P, Iturria I, Mohedano ML, Caggianiello G, Rainieri S, Fiocco D, Spano G.. Zebrafish gut colonization by mCherry-labelled lactic acid bacteria. Appl Microbiol Biot. 2015. Apr;99(8):3479–3490. 10.1007/s00253-014-6351-x [DOI] [PubMed] [Google Scholar]
- Sharma R, Kapila R, Kapasiya M, Saliganti V, Dass G, Kapila S.. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr Res. 2014. Nov;34(11):968–981. 10.1016/j.nutres.2014.09.006 [DOI] [PubMed] [Google Scholar]
- Suo H, Zhao X, Qian Y, Sun P, Zhu K, Li J, Sun B.. Lactobacillus fermentum Suo attenuates HCl/ethanol induced gastric injury in mice through its antioxidant effects. Nutrients. 2016. Mar 10;8(3): 155 10.3390/nu8030155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Xing Z, Hu W, Li C, Wang J, Wang Y.. Antioxidative effects in vivo and colonization of Lactobacillus plantarum MA2 in the murine intestinal tract. Appl Microbiol Biotechnol. 2016. Aug; 100(16): 7193–7202. 10.1007/s00253-016-7581-x [DOI] [PubMed] [Google Scholar]
- Tang W, Xing Z, Li C, Wang J, Wang Y.. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017. Apr;221:1642–1649. 10.1016/j.foodchem.2016.10.124 [DOI] [PubMed] [Google Scholar]
- Wafula EN, Brinks E, Becker B, Huch M, Trierweiler B, Mathara JM, Oguntoyinbo FA, Cho GS, Franz CMAP.. Draft genome sequence of Lactobacillus fermentum BFE 6620, a potential starter culture for African vegetable foods, isolated from fermented cassava. Genome Announc. 2017. Aug 17;5(33):e00801-17. 10.1128/genomeA.00801-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Yu H, Gao X, Li X, Qiao S.. Influence of Lactobacillus fermentum I5007 on the intestinal and systemic immune responses of healthy and E. coli challenged piglets. Antonie van Leeuwenhoek. 2009. Jun;96(1):89–98. 10.1007/s10482-009-9339-2 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W.. Antioxidant properties of probiotic bacteria. Nutrients. 2017. May 19;9(5):521 10.3390/nu9050521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KC, Cui JY, Liu J, Lu H, Zhong X, Klaassen CD.. RNA-Seq provides new insights on the relative mRNA abundance of antioxidant components during mouse liver development. Free Radic Biol Med. 2019. Apr;134:335–342. 10.1016/j.freeradbiomed.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Wang L, Wang J, Li H, Menghe B, Wu J, Guo M, Zhang H.. Isolation and preliminary probiotic selection of lactobacilli from koumiss in Inner Mongolia. J Basic Microbiol. 2009. Jun;49(3): 318–326. 10.1002/jobm.200800047 [DOI] [PubMed] [Google Scholar]
- Wu Y, Tang L, Chen B.. Oxidative stress: implications for the development of diabetic retinopathy and antioxidant therapeutic perspectives. Oxid Med Cell Longev. 2014;2014:1–12. 10.1155/2014/752387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Bai F, Liu Y, Yang Y, Yuan Q, Zou D, Qu S, Tian G, Song L, Zhang T, et al. Fibroblast growth factor (FGF21) protects mouse liver against d-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol Cell Biochem. 2015. May; 403(1–2):287–299. 10.1007/s11010-015-2358-6 [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu C, Li D, Zhao Y, Zhang X, Zeng X, Li S.. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol. 2013. Mar;54:270–275. 10.1016/j.ijbiomac.2012.12.037 [DOI] [PubMed] [Google Scholar]
- Zhao J, Tian F, Yan S, Zhai Q, Zhang H, Chen W.. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in D-galactose-induced oxidative stress model. Food Funct. 2018. Feb 21;9(2):917-924. 10.1039/c7fo0174g [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hong K, Zhao J, Zhang H, Zhai Q, Chen W.. Lactobacillus fermentum and its potential immunomodulatory properties. J Funct Foods. 2019. May;56:21–32. 10.1016/j.jff.2019.02.044 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials are available on the journal’s website.