Abstract
Agri-food waste biomass is the most abundant organic waste and has high valorisation potential for sustainable bioproducts development. These wastes are not only recyclable in nature but are also rich sources of bioactive carbohydrates, peptides, pigments, polyphenols, vitamins, natural antioxidants, etc. Bioconversion of agri-food waste to value-added products is very important towards zero waste and circular economy concepts. To reduce the environmental burden, food researchers are seeking strategies to utilize this waste for microbial pigments production and further biotechnological exploitation in functional foods or value-added products. Microbes are valuable sources for a range of bioactive molecules, including microbial pigments production through fermentation and/or utilisation of waste. Here, we have reviewed some of the recent advancements made in important bioengineering technologies to develop engineered microbial systems for enhanced pigments production using agri-food wastes biomass/by-products as substrates in a sustainable way.
Keywords: Agri-food waste, fermentation, microbial pigments, bioengineering, engineered microbes, waste biomass
1. INTRODUCTION
Petro-derived colourants have fixed their roots in the modern food colouring industry. However, because of their severe harmful effects on human health like allergenicity, carcinogenicity, hyperactivity and toxicological nature, utilization of petro-derived colourants has been banned as food colourants in the food industry. Therefore, it is pre-requisite to develop safe, natural, economically cheaper colourants. Natural pigments are safe because these are obtained from natural sources, non-allergic, non-carcinogenic, non-toxic, biodegradable, natural antioxidants and have no risk as a pollutant to the environment. The market demand for bio-colourants/pigments is increasing at an accelerated rate [1], and it is the fastest-growing sector in the area of food and cosmetics industries with a forecast of $387.4 million global market volume for anthocyanins expected in 2021 [2]. In this context, microorganisms favour the trend by producing natural colourants from bio-resources or agri-food wastes. To cope up with the huge demands for natural colourants and keep the command on change in climate due to generation of high bio-waste of agri-food wastes, there have been great efforts to develop natural pigments with the help of microorganisms from agri-biomass. Waste biomass from the fruits and vegetable industry can be microbially processed and exploited for various natural pigments for food applications. Over the past few years, due to rapid advances in bioengineering including those of biotechnological tools and synthetic biology strategies, considerable development has been witnessed in the microbial production of natural bio/-chemicals obtained via the help of microorganisms [3]. Among the natural components, microbial production of pigments has realized great success, especially microbial pigments production using wastes biomass. In this review, we focus on the advancement in the utilization of waste biomass, current status of the microbial pigments, bioengineering practices and their market trend in food colouring and pigments industry. Major bottlenecks in the production of engineered microbes by using biotechnological tools, implementation of utilization strategies for agri-waste and commercialization as natural pigments for food applications are discussed in this review.
2. MICROBIAL SOURCES FOR NATURAL PIGMENTS
Natural colourants are gaining importance over synthetic colours due to the latter’s ill-effect on human health. Natural pigments are usually derived from plants (fruits, vegetables, leaves, stem or flowers, etc.), and also from insects, microorganisms and minerals [1]. Production of natural pigments from microbial sources is gaining much importance due to faster recovery, greater intensity of colour, higher yield of pigment, and cheaper production methods. Microorganisms such as bacteria, yeast and molds produce several kinds of natural pigments (Table 1) depending on their sources of
Table 1. Microbial strains for pigment production.
| Microorganisms | Pigment Color | Applications | References |
|---|---|---|---|
| Bacteria | |||
| Achromobacter | Pink, Orange, Red | Anticancer; Antimalarial | [11] |
| Helicobacter pylori | Red | Antiplasmodial | [12] |
| Bacillus sp. | Pink, Orange, Yellow | Antibacterial | [13] |
| Brevibacterium sp. | Orange | Antioxidation | [14] |
| Corynebacterium michigannisse | Creamish, Grayish, | Anticancer | [9] |
| Pseudomonas sp. | Brown, Yellow-green, | Antibiotic | [15] |
| Arthrobacter | Yellow | Cytotoxic | [16] |
| Rhodococcus maris | Deep red | Antibiotic | [15] |
| Streptomyces sp. | Blue, red, Yellow | Antibiotic | [9] |
| Aspergillus glaucus | Dark red | Anticancer | [9] |
| Staphylococcus roseus | Reddish-pink | Anticancer; Algicidal | [17] |
| Molds | |||
| Helminthosporium catenarium | Red Dark maroon |
Protection from UV irradiation Anti-inflammatory |
[18] |
| Helminthosporium cynodontis | Bronze color | Antibiotic | [18] |
| Helminthosporium gramineum | Red Dark maroon |
Protection from UV irradiation | [15] |
| Cystofilobasidium infirmominiatum | Red | Antibacterial | [19] |
| Aspergillus sp. | Red, Orange | Antibacterial | [18] |
| Blakeslea trispora | Cream | Tambjamines (BE-18591, Antibiotic) | [18] |
| Neurospora sp. | Orange-red | Anticancer | [20] |
| Helminthosporium avenae | Bronze color | Antibiotic | [18] |
| Monascus purpureus | Red, orange, yellow | Anticancer | [21] |
| Penicillium cyclopium | Orange | Antiproliferative | [22] |
| Penicillium nalgiovense | Red | Antiproliferative | [23] |
| Penicillium chrysogenum | Yellow | Algicidal | [24] |
| Yeasts | |||
| Cryptococcus sp. | Red | Algicidal | [18] |
| Phaffia rhodozyma | Orange | Antiprotozoan | [25] |
| Rhodotorula sp. | Orange/red, Yellow | Anticancer | [25] |
| Yarrowia lipolytica | Brown | Antiprotozoan | [26] |
| Algae | |||
| Dunaliella salina | Red, Orange | Antiprotozoan | [27] |
origin. Pigments extracted from microbial sources have a different range of commercial applications, as shown in Table 1. Microorganisms produce a wide variety of natural colours such as anthocyanins, carotenoids, quinins, violacein, monascins, melanins, etc. [4]. These pigments not only colour foods, but also enrich the antioxidant potential of food commodities, and help to serve as functional food ingredients in various food preparations. Currently, some food-grade microbial pigments are in food market such as Arpink red (Penicillium oxalicum), astaxanthin (Xanthophyllomyces dendrorhous), riboflavin (Ashbya gossypii), β-carotene/ lycopene (Blakeslea trispora) [5]. The first approval for the applications of lycopene produced from Blakeslea trispora was sought under regulation (EC) No 258/97 of the European Council concerning novel food ingredients. It was found that in food supplements, lycopene from Blakeslea trispora could replace the lycopene synthetic sources to avoid a rise in ingesting levels. In many countries, lycopene is permitted to use as a food pigment in processed foods while Australia and New Zealand were the first to permit. To summarize, lycopene from Blakeslea trispora has been considered as safe by the European Food Safety Authority (EFSA) and to be nutritionally at the same level as those of natural dietary based lycopenes [6]. However, the main concern remains that purposeful usage of pigments produced from microbes as a food ingredient may result in a substantial increase in the daily uses of pigments compared to the intakes solely from natural dietary sources [7-10].
2.1. Bacteria
Bacteria are potential generators of carotenoids pigments, especially β-carotene. Several research reports have demonstrated the feasibility of bacterial strains for the production of microbial pigment. For the production of lycopene Streptomyces chrestomyceticus, for zeaxanthin and lutein Flavobacterium sp. and for canthaxanthin Brevibcaterium, Corynebacterium, Rhodococcus maris sp. are usually employed at industrial scale [19]. Various bacterial strains like Serratia marcescens, Vibrio psychroerythrous, Vibrio gazogenes, Serratia rubidaea, Pseudomonas magneslorubra, Streptoverticillium rubrireticuli, Alteromonas rubra, Streptomyces longisporus and Rugamonas rubra have potential to produce microbial pigments [6, 28].
2.2. Yeast
Yeasts are unicellular eukaryotic microbes ubiquitously occurring in plants, animals, water, and soil. Due to their unicellular nature, yeasts are a good source of microbial pigments and utilize low-cost media as compared to bacteria and molds [29]. Yeasts are useful in the production of biotechnologically important carotenoids pigments such as β-carotene, astaxanthin and torulen, etc. Nutrient media, yeast strains and technological methods also affect the production yield, ease of separation, degree of purity and type of pigments. Potential pigments generating yeast strains include Rhodotorula glutinis, Phaffia rhodozyma, Yarrowia lipolytica, Xanthophyllomyces dendrorhous and Phaffia rhodozyma, etc. [6]. Carotenogenic red yeasts include so many sp. such as Rhodotorula, Phaffia, Rhodosporidium, Sporobolomyces, Cystofilobasidium, Sporidiobolus and Kockvaella, etc. [30]. Besides carotenoids, red yeasts serve as a potential biological source of other bio-nutrients such as ergosterol, Coenzyme Q10, unsaturated fatty acids, proteins, vitamins, minerals and nucleic acids, etc. [31].
2.3. Molds
Molds can be a potential source to generate efficient pigments [7]. Monascus purpureus is the most commonly used mold to produce bio-colours through solid-state fermentation. Monascus sp. produces three kinds of pigments such as ankaflavins and monascin (yellow), rubropunctatin and monascorubin (orange), and rubropunctamine and monascorubramine (red) pigments [32]. Other species of molds are also potential producers of different natural colouring components such as Ashbya gossypi (riboflavin), Aspergillus ruber (physcion), Blakslea trispora (lycopene), Monascus sp (monascin, rubropunctatin, monascorubramine), Penicillium oxalicum (arpink red), and Penicillium melinii (atrovenetin), etc. [4].
Overall, there is a rising interest in naturally derived pigments such as flavonoids (anthocyanins), carotenoids, and some tetrapyrroles (phycobiliproteins and chlorophylls), due to their non-toxic, and ecofriendly properties making them a safer alternative. Several species of bacteria, fungi and algae have been commercially utilized for the production of pigments [33]. Several naturally occurring bacteria have highly developed pathways for synthesizing products naturally. These pathways can be metabolically engineered into other bacterial strains for enhanced production of pigments in a natural manner [34]. Recent developments in bioengineering have led to the advanced production of microbial pigments such as anthocyanins, carotenoids from genetically manageable microorganisms (e.g., Saccharomyces cerevisiae, Escherichia coli, Corynebacterium glutamicum, Bacillus subtilis and Pseudomonas putida) [35, 36]. There are two major classes of pigments having industrial applications in foods, feed and cosmetics, etc.
3. MAJOR CLASSES OF PIGMENTS AND THEIR VALUE-ADDITION
3.1. Carotenoids
Microorganisms are potential sources of natural colorants. Besides colour-giving nature of natural microbial pigments, they possess potent antioxidative, anti-cancer properties and pro-vitamin-activities. Carotenoids having pro-vitamin-A activity and natural colourant properties act as potential bioactive components having positive health effects of reducing the risks of modern lifestyle diseases such as cancer, macular degeneration, cardiac diseases and cataract [37]. Carotenoids production can be enhanced via fermentation of Agri-industrial wastes such as those obtained from apple pomace (via microbe like Rhodotorula sp.) [19], corn syrup (by Rhodotorula glutinis and Debaromyces castellii) [38], banana peel (by Monascus purpureus). Cyanobacteria and algae are potent sources of biocolorants, such as β-carotene and astaxanthin, having great marketable importance in food and pharmaceutical industries [39].
The transformation of agri-food wastes into valuable extracts, which form the fermentable substrate for microorganisms for the production of the pigment, is nowadays in practice industrially. Taskin and Erdal utilized the loquat kernel waste extract as a nutrient substrate for the yeast culture i.e. Rhodotorula glutinis MT-5 for the biosynthesis of carotenoids. They reported that among 10 isolates tested, MT-5 yeast isolate was reported with a maximum concentration of the carotenoids (72.36 mg/L) [40].
Miura et al. investigated the effects of genetic variations on carotenoids content by adding genes from Erwinia uredovora (crtE, crtB, crtI, and crtY) and Agrobacterium auranticum (crtZ and crtW) to Candida utilis (food grade yeast) for the microbial strains. crt represents the gene band that plays an important role in the bio-synthesis of the carotenoids pigments. They reported that the transgenic yeast showed higher content of β-carotene, lycopene and astaxanthin [41]. To harvest the pigment from culture biomass, cell disruption is a must to get better bioavailability of the fat-soluble pigments [42]. Several research reports investigated the improved microbial production of carotenoids, but very few are commercialized [43]. Though many practices are being applied currently to have a commercially viable process for microbial pigment production. Among the astaxanthin producing microbes, Phaffia rhodozyma and Xanthophyllomyces dendrorhous are the best having commercialized for the pigment production. These strains have certain good properties to be commercialized: produce a natural form of pigment; can be utilized in different types of nutritional sources and synthesize pigment in anaerobic conditions [44, 6].
β-carotene is biotechnologically synthesized by utilizing several strains of Rhodotorula yeasts at a commercial scale. Due to its excessive growth rate and unicellular nature, this yeast is used conveniently for massive-scale fermentation. The rate of manufacturing individual carotenoid depends on the processing conditions and due to these conditions, Rhodotorula glutinis produces different kinds of pigments like β-carotene, torulene and torularhodin. Sakaki et al. generated genetically modified specially mutated strains of Rhodotorula and reported an accelerated increase in the amount of pigments synthesized, like torulene and β-carotene [45]. Marova et al. screened three yeasts: Sporobolomyces roseus, Rhodotorula glutinis, and Rhodotorula mucilaginosa for carotenoids synthesis. To get enhanced pigment yield, these strains are used in mixed culture and combined with whey and potato extract as carbon and nitrogen sources. They reported that the Rhodotorula strains were more promising in pigment production as compared to Sporobolomyces sp. The highest pigment yield (46 mg/L of β-carotene) was observed using R. glutinis in whey medium and Rhodotorula mucilaginosa in potato extract (56 mg/L of β-carotene) [31]. Similarly, Lukacs et al. reported the yield of astaxanthin (36g/L) in a fed-batch fermentation when used Phaffia rhodozyma. This carotene-rich yeast biomass can be dried easily and could be a potent nutritional supplement in the food and feed industry [44]. Latha and Jeevaratnam produced carotenoids by yeast using cheap industrial waste or by-products as economically viable nutrient sources. From the study, it was concluded that among all the tried waste samples, dry tapioca powder, waste mango pulp and ethanol showed positive growth of red yeast. Red yeast did not show growth in whey which indicated that yeast was unable to assimilate the lactose for growth. The yield of carotenoids in waste mango pulp and dry tapioca powder ranged from 2.5 to 3.0 mg/L [46, 47].
A plethora of researches have been published on the fungal-carotenoids using oleaginous fungi that produces carotenoids-rich oil such as Blakeslea trispora, Mucor circinelloides, Neurospora crassa, Mucor hiemalis, and Phycomyces blakesleeanus, etc. [6]. Usually, fungal-carotenoids are rich in β-carotene contents. Cerda-Olmedo investigated the biotechnological production of carotenoids from a dimorphic fungal mutant of Blakeslea trispora and Mucor circinelloides and observed that these strains could be a useful medium to produce carotenoids [48]. Kantifedaki et al. investigated the production of fungal pigments by Monascus purpureus and Penicillium purpurogenum using orange processing waste. During solid-state fermentation, Monascus purpureus was observed to be more efficient in the production of carotenoids yielding 9 absorbance units/g of dry weight, when compared to Penicillium purpurogenum. Feedstock and method of fermentation can directly influence the production yield of pigments [39].
3.1.1. Bioengineered Microbial Carotenoids
Carotenoids are one of the major natural pigments serving many nutritional and physiological purposes. They occur naturally in plants, bacteria, fungi, and algae usually exhibiting the colors yellow, red or orange. Carotenoids are deployed extensively as cosmetics additives, health supplements, food colorants, nutraceuticals and animal feeds. Biosynthesis of carotenoids, such as β-carotene, is made possible to be hosted in non-carotenogenic hosts due to the availability of genetically engineered pathways.
Microbes play an important role when it comes to the enhanced production of these important molecules of interest. Production of microbial carotenoids (Table 2) can be improved further by combining various bioengineering engineering strategies and techniques to fulfil the increasing demand of natural pigments. One such example is, Multivariate modular metabolic engineering (MMME) - a systematic approach which has been recently developed to modulate secondary metabolism [49]. Biotechnological innovation through bioengineering approaches has the potential for sustainable industrial level bioproduction of carotenoids driven by leading research breakthroughs (Table 2). In order to obtain higher carotenoid yields, microbial biosynthesis of carotenoids is divided into four separate modules based on MMME approach (Fig. 1) [50, 51]. To date, many strategies have been employed to engineer the carotenoid biosynthesis module, including the selection of novel enzymes, engineering key enzymes, localized expression of key enzymes, optimization of gene expression and increasing carotenoid storage in producing cells (Fig. 2).
Table 2. Pigment production in microorganisms using various metabolic engineering strategies.
| Host Strain | Description | Output | Engineering Methods | References |
|---|---|---|---|---|
| Molds | ||||
| Blakeslea trispora | Produces carotenoids natively | Lycopene, 256 mg/L | Fermentation optimization using lycopene cyclase inhibitor | [75] |
| β-Carotene, 704.1 mg/L | Controlled oxygen transfer rate | [76] | ||
| Bacteria | ||||
| Corynebacterium glutamicum | Carotenoid native producer | β-Carotene, 7 mg/L | Integration of crt pathway genes accompanied by removal of crt | [61] |
| Escherichia coli | Non-native genetically modifiable producer | β-Carotene, 2.1g/L | Engineering dominant pathway (tricarboxylic acid cycle (TCA), PPP for carbon flux and MEP pathway for DMAPP and IPP supply | [59] |
| Lycopene, 0.5g/g DCW | Lycopene synthesis pathway expression regulation | [56] | ||
| Lycopene, 220 mg/L | Introduction of isopentenol utilization pathway (IUP) | [77] | ||
| Rhodobacter sphaeroides | Carotenogenic genes phototroph | Lycopene, 10mg/g DCW | Augmentation of MEP pathway, replacement of crtI and blocking PPP pathway | [65] |
| Yarrowia lipolytica | Non-native genetically modifiable producer | β-Carotene, 4g/L | Cyclic integration of multiple copy pathway genes | [73] |
| β-Carotene, 6.5g/L | Heterologous carotenoids pathway (crt pathway) optimization via promoter-gene pairs |
[74] | ||
| Yeast | ||||
| Saccharomyces cerevisiae | Non-native genetically modifiable producer | Astaxanthin, 218 mg/L | Genome evolution by atmospheric and room temperature plasma (ARTP) Increasing pool of acetyl-CoA |
[78] |
| Lycopene, 56 mg/g DCW | Genome manipulation for optimization of lycopene synthesis pathway | [79] | ||
| Lycopene, 2.3g/L | Enzymatic modulation for the biosynthesis of lycopene | [80] | ||
| β-carotene, 2.3 g/L | Combined metabolic modulation | [81] | ||
Fig. (1).
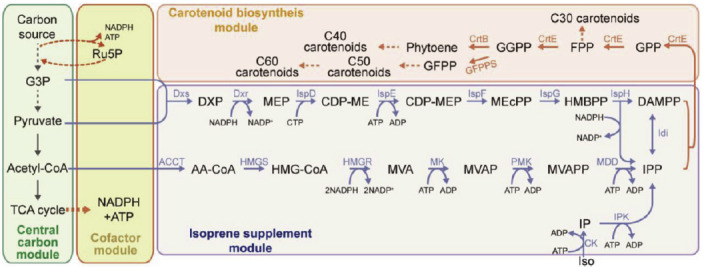
Scheme of carotenoid biosynthesis (Cheng et al.) [52]. Open Access under a Creative Commons CC-BY-NC-ND license. No changes or alterations were made in the figure. https://www.sciencedirect.com/science/article/pii/S221403011930046X#fig1. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
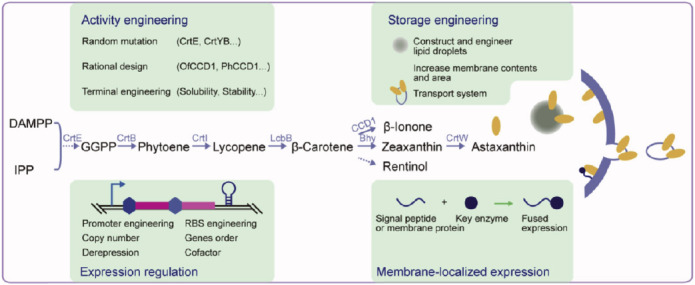
Strategies for engineering the carotenoid biosynthesis module (Cheng et al.) [52]. Open Access under a Creative Commons CC-BY-NC-ND license. No changes or alterations were made in the figure. https://www.sciencedirect.com/science/article/pii/S221403011930046X#fig3. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Some of the pathway design strategies deployed are the alteration of the host framework, optimization of heterologous crt pathway, and augmentation of dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP) precursors by rate-limiting enzyme overexpression of mevalonic acid pathway (MVA) or methylerythritol 4-phosphate (MEP) pathway. MVA pathway is measured to be a good process for the isoprenoids production [52, 53], and MVA heterologous expression pathway in engineered E. coli increases β-carotene output to 465 mg/L [54]. Balanced enhancement of 4-hydroxy-3-methy1but-2-en-l-yl diphosphate (IspH) and 4-hydroxy-3-methylbut-2-en-1-yl diphosphate synthase (ferredoxin) (IspG) in MEP pathway may eliminate pathway intermediates accumulation, thus enhancing the yield of lycopene and β-carotene [55]. Colourimetric behaviour of carotenoids is beneficial for conceptualizing new strategies to optimize crt pathways in synthetic biology [56, 57]. A novel multigene pathway is developed using well-understood genetic components of lycopene synthesis giving an output of 448 mg/g dry cell weight (DCW) [56]. Multiplex automated
genome engineering (MAGE) strategy can be used to rapidly enhance the capability of Escherichia coli to accommodate lycopene production [58]. By engineering the dominant metabolic components of carbon assimilation pathways (Embden-Meyerhof pathway (EMP) and pentose phosphate pathway (PPP)) in E. coli, nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) and adenosine triphosphate (ATP) provisioning essential for producing β-carotene are improved, yielding 2.1 g/L of β-carotene [59].
The basic principle of bioengineering approaches is to maximize carbon substrate-based flux targeting compound of interest while at the same time diminishing flux responsible for unnecessary by-products. The availability of new methods and technologies has enabled the genetic modification of microorganisms for the production of carotenoids. Corynebacterium glutamicum, which natively produces C50 decaprenoxanthin, is a common species used for the production of amino acid [60]. Production of lycopene, β-carotene and decaprenoxanthin can be significantly enhanced by suppression of crt caused by the deletion of crtR in C. glutamicum [61]. σ-factor overexpression can also enhance the production of carotenoids using C. glutamicum [62]. Integrations of the lysine pathway and crt pathway accompanied by the omission of crtR in C. glutamicum yielded l-lysine, 1.5g/L and β-carotene, 7mg/L using xylose as substitute input [61]. Synthesis of spheroidenone and spheroidene can be
done using crt genes of Rhodobacter sphaeroides, a facultative anaerobic phototroph purple bacterium [63]. R. sphaeroides unique folded membrane shape is favourable for carotenoid accumulation [64].
Substitution of endogenous neurosporene hydroxylase (CrtC) with heterologous phytoene desaturase (CrtI) in R. sphaeroides followed by blocking of pentose phosphate pathway (PPP) accompanied by enlargement of MEP pathway yielded 10.32 mg/g DCW of lycopene [65]. Synthesis of staxanthin was increased to 1.7 times by overexpression of rate-limiting geranylgeranyl pyrophosphate (GGPP) synthase in Xanthophyllomyces dendrorhous [66]. Production of astaxanthin was increased by 40% by decreasing feedback suppression of ergosterol to MVA pathway by removing diploid CYP61 genes encoding sterol desaturase [67]. Mutagenic treatment of β-carotene hydroxylase and its introduction yielded zeaxanthin production of 0.5 mg/g DCW [68]. Many compounds can be derived in a cost-effective manner by using an oleaginous yeast Yarrowia lipolytica [69]. Many genetic methodologies to engineer Y. lipolytica have been developed in past years making it a favourable host for carotenoids production via the MVA pathway [70]. A heterologous lycopene pathway via deletion of peroxisomal β-oxidation pathway was engineered in Y. lipolytica to enhance lipid body sizes, favouring the deposit of lycopene in lipid bodies resulting in higher yield [71]. Lycopene production of 21.1 mg/g DCW was attained via suppression of auxotrophy in Y. lipolytica PO1f strain and overexpression of MVA pathway [72]. Development of an effectual pathway for β-carotene under optimized fed-batch culture using multiple gene copies and strong promoters for synthesis yielded 4g/L of β-carotene [73]. Using a combinational methodology founded on Golden Gate assembly and titer of 6.5 g/L in fed-batch culture, Larroude et al. optimized promoter-gene pairs of heterologous crt pathway, resulting in β-carotene production of 90 mg/g DCW by the best strain. This shows the potential of Y. lipolytica to be a very good host strain for carotenoids production [74]. To-date bioengineering approaches are being widely used to optimize expression pathways for carotenoid overproduction in microbial systems [52]. Engineered microbial systems are valuable for industrial production of carotenoids in a sustainable and cost-effective manner as compared to chemical synthesis or plants based extraction.
3.2. Anthocyanins
Anthocyanins are ubiquitous, glycosylated, water-soluble plant pigments providing diverse colors (red, blue and purple colour) to flowers, leaves and fruits. Biologically, these are secondary metabolites of plants and protect them from oxidative stress, irradiation damage and pathogenic microbes. These belong to the group of polyphenols in the subcategory, flavonoids. Increasing preferences of natural colours directly influence the growing demand of anthocyanins as coloring compounds, dietary supplements, natural antioxidants, etc. [46]. Research efforts are being made to improve the production efficiency and stability of anthocyanins. In this context, the expression of bioengineered microorganisms for the sustainable production of pigments is a promising alternative [82]. Engineered anthocyanins can be produced by the approaches including biosynthetic enzymes gene coding, regulatory genetic engineering, Uridine diphosphate-glucose (UDP-glucose) regulation mechanism, transportation engineering. Theses metabolic engineering strategies permit the sustainable and successful production of microbial anthocyanins for food and cosmetics industries [2]. Also, by combining the biology and enzyme engineering approaches along with gene sequencing, DNA synthesis, and modeling of the metabolic network, bioengineering can play a pivotal role in the microbial production of valuable nutritious components for commercial demand [46]. Pigments produced by certain strains of Serratia marcescens have been used to produce prodigiosin, a bright red pigment. Prodigiosin has antimicrobial, immune-suppressive and anti-proliferative activity especially as a potent inhibitor for Staphylococcus aureus, Bacillus cereus, Candida albicans, Candida parapsilosis and Cryptococcus sp. [83].
3.2.1. Bioengineering for the Production of Microbial Anthocyanins
Extensive research in the recent years has characterized anthocyanin pathways in plant secondary metabolism in terms of chemical/enzymes as well as genetic terms. Microbes have been used to produce anthocyanin production platforms, leading to scaleup and bioprocess technologies for industrial production [84].
Anthocyanin biosynthetic pathway in Escherichia coli, combined with the addition of growth media with precursors like catechin, naringenin, coumaric acid, or eriodyctiol results in anthocyanin production [82, 85]. Many engineering methodologies such as optimizing process parameters of cultivation, redirection of carbon metabolism to malonyl-CoA, greater accessibility of UDP-glucose have further improved the production of anthocyanin. At a titer of 350 mg/L, these enhancements resulted in the output of anthocyanin cyanidin 3-O-glucoside (C3G) [85]. In another research, a polyculture of four-strain E. coli was utilized at a titer of 9.5 mg/L to yield de novo production of pelargonidin 3-O-glucoside [86]. Levisson et al. engineered Saccharomyces cerevisiae to demonstrate de novo production of pelargonidin 3-O-glucoside from glucose by the introduction of anthocyanin biosynthetic genes from Gerbera hybrida and Arabidopsis thaliana. Although the yields were not high, this method was able to bring out the bottlenecks in the enhancement of anthocyanin production, which could be utilized to develop better strategies [87].
Anthocyanin pathway engineering involves the co-expression of enzymes from plants. It is typically challenging to enable heterologous expression of plant genes in prokaryotes, thus necessitating the modification of genes/ enzymes before their functional expression. Consequently the screening of enzymes and microbial strain selection from a variety of species can be an important method to improve anthocyanins production from microbes [88]. Production of anthocyanin can be elevated using other methods such as the translational combination of several enzymes successively in multiple steps. For each enzyme, the local concentrations of substrates get maximized as a result of such fusions while at the same time unstable intermediates get degraded, allowing for the efficient occurrence of multiple reactions [89]. This method, when applied to E. coli has yielded improved translation of catechin to cyanidin 3-O-glucoside due to translational combination of N-terminus of ANS from Petunia to flavonoid 3-glucosyltranferase (F3GT) from Arabidopsis [90]. The formation of the fused protein complex converts the unstable intermediate anthocyanidin at a faster rate, thus catalysing successive biochemical reactions.
Engineered microorganisms yield products that often have been found to be toxic to the host strains, thereby limiting their scalable usage. One way to mitigate this challenge and achieve large-scale production is to move the yielded outputs from the cytoplasm to extracellular environments, where these toxic products can be removed through targeted efflux pumps. For example, overexpression of yeast alcohol dehydrogenase (YadH), a cyanidin 3-O-glucoside-associated efflux pump caused an increase in anthocyanins production by 15% [85]. Furthermore, the promotion of a titer of cyanidin 3-Oglucoside was achieved via removal of TolC efflux pump, considered to be accountable for secretion of catechin. Besides modifying already present transporters in host microorganisms, transporters from plants can also be introduced as an alternate method to further enhance the transportation of products and substrates to accelerate the production of anthocyanin in engineered species. Novel approaches have also been extended to the microbial biosynthesis of methylated anthocyanins as shown in Fig. (3) [82]. The production of peonidin 3-O-glucoside (an O-methylated anthocyanin) from catechin was achieved in E. coli with the introduction of Petunia hybrida ANS, Arabidopsis thaliana F3GT, and Vitis vinifera anthocyanin O-methyltransferase (AOMT), and a resulted titer of 56 mg/L was reported upon pathway optimization [91].
Fig. (3).
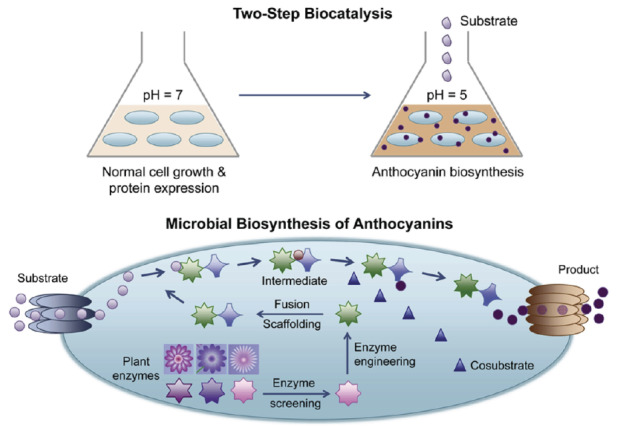
The strategies applied in metabolic engineering of E. coli for the biosynthesis of anthocyanins (Zha and Koffas) [82]. Open Access under a Creative Commons CC-BY-NC-ND license. No changes or alterations were made in the figure. https://www.sciencedirect.com/science/article/pii/S2405805X17300686#fig3. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. MICROBIAL PIGMENTS FROM WASTE BIOMASS OR AGRI-WASTE/BY-PRODUCTS
Agri-industrial waste can be a good fermentable substitute and the main source of microbial nutrients for the exploitation of natural pigments useful in food industries (Table 3).
Table 3. Microbial pigments from agri-industrial by-products/waste.
| Waste Biomass | Microbes | Target Pigment | Extraction Method | Yield | Characterization | Application | Findings | References |
|---|---|---|---|---|---|---|---|---|
| Bacteria | ||||||||
| Shrimp waste |
Lactobacillus plantarum B4496, Lactobacillus acidophilus B4495, Lactococcus lactis B634, Pediococcus acidilactici CFR2182 |
Carotenoids | Fermentation | 4.2mg /100g | Spectral analysis | _ | • Carotenoid recovery varied between 72.4 and 78.5% | [95] |
| Apple pomace | Sarcina sp. | Carotenoids | Fermentation | 12.87mg/100g | Spectral analysis | _ | • Potassium nitrate with apple pomace at pH of 5.5 gave maximum production of carotenoids | [96] |
| Waste Biomass | Microbes | Target Pigment | Extraction Method | Yield | Characterization | Application | Findings | References |
| Bacteria | ||||||||
| Rice powder | Bacillus clausii | β-carotene | Fermentation | 69.4 mg/g | Spectral and FTIR | _ | • Maximum yield of β-carotenoid 48.9% using Bacillus clausii | [97] |
| Palm date waste | Lactobacillus plantarum | Carotenoids | Fermentation | 54.89 mg/kg dry cell | HPLC | _ | • Palm date waste is a good source for carotenoid production | [98] |
| Liquid pineapple waste | Chryseobacterium artocarpi CECT 8497 | Flexirubin | Fermentation | 540 mg/L | Chromatography, electrospray ionization mass spectrometry (ESI-MS) and Attenuated total reflection (ATR) | Pigment incorporated as a natural colorant in soap making | • Higher pigment production observed | [99] |
| Molds | ||||||||
| Rice broken | Monascus ruber MTCC2326 | Red and yellow pigments | Solid-state fermentation, 80% ethanol | 20mg/100g red and 33mg/100g yellow pigments |
Column Chromatography, High-performance thin-layer chromatography (HPTLC), Gas chromatography-mass spectrometry (GC-MS) | Flavoured milk (1.2% MFR) | • Application of pigments was found to be acceptable in flavoured milk | [100] |
| Dry milled corncob | Penicillium resticulosum | Red pigment | Fermentation | 497mg/L | Spectral analysis | _ | • Pigment was stable at pH 2-9 | [101] |
| Sugar-beet molasses | Rhodotorula mucilaginosa NRRL-2502 | Carotenoids | Fermentation | 89.0 mg/L | Spectral analysis | _ | • Whey produces higher carotenoids than molasses | [102] |
| Whey | Rhodotorula mucilaginosa NRRR-2502 | Carotenoids | Fermentation | 35mg/g of dry weight | Spectral analysis | _ | • Whey produces higher carotenoids than molasses | [102] |
| Orange waste |
Monascus purpureus
(mold), Penicillium purpurogenum |
Carotenoids | Solid-state fermentation | 9 units/g of dry weight | HPLC | _ | • Monascus purpureus was observed more efficient | [39] |
| Waste Biomass | Microbes | Target Pigment | Extraction Method | Yield | Characterization | Application | Findings | References |
| Yeast | ||||||||
| Whey | Rhodotorula glutinis | β-carotene | Fermentation | 46 mg/L of β-carotene | Reverse phase high performance liquid chromatography (RP-HPLC) | _ | • Highest yield observed in R. glutinis on whey medium and R. mucilaginosa on potato medium | [103] |
| Potato extract | Rhodotorula mucilaginosa | β-carotene | Fermentation | 56 mg/L of β-carotene | RP-HPLC | _ | • Highest yield observed in R. glutinis on whey medium and R. mucilaginosa on potato medium | [103] |
| Fermented radish brine | Rhodotorula glutinis | β-carotene | Batch Fermentation | 201 μg/L | HPLC | _ | • 15% higher than those in initial condition | [29] |
| Hydrolyzed Mung bean waste flour and sweet potato extract | Rhodotorula glutinis TISTR | Carotenoids | Batch Fermentation | 3.48mg/L | Spectral analysis | _ | • Agri-industrial waste is a good substrate for microbes to obtain high pigment yields | [104] |
| Jackfruit seed | Monascus purpureus | Red and yellow pigments | Solid-state fermentation, 70% Ethanol | 1.304 U/g and 0.497 U/g for red and yellow pigments | Spectral analysis | _ | • Fructose supplementation resulted in about 4.5% increase in yield | [105] |
| Kinnow peel waste with pea pod, taro leaves, green gram waste, soya, okra | Monascus purpureus | Pigment | Submerged fermentation; ethanol, methanol, Dimethyl-sulphoxide | 7.35 AU/g with 90% ethanol | Spectral analysis | _ | • Kinnow peel powder served as a good substrate, • Maximum pigment was observed with 90% ethanol |
[106] |
| Waste Biomass | Microbes | Target Pigment | Extraction Method | Yield | Characterization | Application | Findings | References |
| Yeast | ||||||||
| Whey, waste mango pulp, dry tapioca powder | Red yeast Rhodotorula glutinis DFR-PDY | Carotenoids | 1N HCl in a water bath at 70°C for 1h, and then acetone: methanol (1:1) was added | 5mg/L | TLC, and HPLC | Popcorn, biscuit, cake icing and ice creams | • Pigment was found to be more stable in sesame oil • Food products mixed with different concentrations of pigment observed with satisfactory color appeal |
[47] |
| Sugar cane molasses | Rhodotorula glutinis 32 | Carotenoids | Fed- batch Fermentation | 183mg/L | TLC and HPLC | _ | • Total carotenoid increased upto 30% in β-carotene | [107] |
| Whey ultrafiltrate | Rhodotorula rubra GED5 and Kluyveromyces lactis MP11 | Carotenoids | Batch Fermentation | 10.2 mg/L | Spectral analysis | _ | • Whey ultrafiltrate is a good substrate for carotenoids synthesis | [108] |
| Agricultural substrate (Glycerol, corn steep liquor and parboiled rice water) | Sporidiobolus pararoseus | Carotenoids and β-carotene |
Fermentation | 843μg/L and 396 μg/L |
Spectral analysis | _ | • Max. total carotenoids concentration observed after 96 h | [109] |
| Fruit waste (pineapple, pomegranate and orange) | Rhodotorula rubra | Carotenoids | Fermentation | 2.98 mg/L | UV-Visible and Fourier-transform infrared spectroscopy (FT-IR) | _ | • Cheaper fruit waste extract is a good substrate for carotenoids production | [110] |
| Waste loquat kernels | Rhodotorula glutinis MT-5 | β-carotene | Fermentation | 62.73 mg/L | Spectral analysis | _ | • MT‐5 was found best to produce carotenoid | [40] |
Bio-colorants produced by microorganisms are safe, non-toxic, cheaper, environmentally friendly and with potential antioxidant properties [92]. Production of bio-colorants (carotenoids, anthocyanins, chlorophylls, melanin, phycocyanins and xanthophylls etc.) through fermentation of agri-food wastes by utilizing bacteria, fungi, yeast and algae are now in trend [93]. Agri-industries generate huge wastes and by-products such as those obtained as fruit pomace, peels, seeds, pea pod powder, corn steep liquor, whey, bran, molasses, etc. that can be used as a possible source/substrate (carbon, nitrogen and minerals) for the microbial production of the pigments [4]. Several microbes such as Serratia, Pseudomonas, Bacillus, Vibrio, Sarcina, Streptomyces, Achromobacter, Yarrowia, Monascus, Penicillium, Phaffia, Rhodotorula, etc. have potential to produce a range of blue, red and yellow bio-pigments [94]. Microbial pigments should cover some important features like they should be non-pathogenic, non-toxic, give efficient colour yield, tolerant against adverse processing conditions such as salt concentration, pH, and temperature [33].
Engineering microorganisms for the production of natural biocolorants by utilizing bio-waste is a promising and sustainable approach to satisfying industrial needs for biocolours. Pigments from Monascus spp. are of microbial source and can be utilized as nourishment grade biocolorants. Here Jackfruit seed was utilized as a substrate enhanced with carbon sources like mannitol, lactose, starch and fructose and nitrogen sources like yeast extracts, peptone, ammonium sulfate and ammonium nitrate for the generation of colors by Monascus purpureus in strong state fermentation (SSF) [99]. Nanosized natural food pigments obtained from microbial sources can expand security, the shelf life for food and feed [111]. Here we present the capability of microbial pigments utilized as food colourants and its benefits, investigating the potential techniques for overproduction of pigments in microbial frameworks, along with the strategies to improve the stability of pigments in relation to their formulations. Advances in bioengineering have empowered the large scale production of microorganisms with commercial interest. With a more detailed understanding of the biosynthetic pathway for microbial pigments, production can help in understanding the detours in the pigment production and counter-acting the barriers [112, 113]. Recently nanotechnology has additionally been adequately utilized in the food sector, including in the formulation of pigments [114]. The enhanced pigment production was observed in Monascus purpureus added with fructose as sole carbon substrate and yeast extract as the main nitrogen source [99]. Several red yeast strains (Sporobolomyces roseus, Rhodotorula glutinis, Rhodotorula mucilaginosa) were selected in a relative screening study. To enhance the yield of pigments with improved biomass, several process parameters were tested for increasing the production of β-carotene by Rhodotorula glutinis on whey medium (46 mg/L) and Rhodotorula mucilaginosa on potato medium and 5% salt (56 mg/L of β-carotene). All strains had the option to utilize the wide variety of waste substrates including whey, potato extract and yeast extract and the best production medium was optimised as culture medium with yeast extract [97]. Similarly, Kot et al. regulated the possibilities of concurrent biosynthesis of carotenoids by the Rhodotorula yeast strains in the culture media with glycerol waste and deproteinized wastewater from potato processing. It was observed that wastewater from potato processing and glycerol could be utilized as a media component for the yeast strains, Rhodotorula mucilaginosa, Rhodotorula glutinis, and Rhodotorula gracilis, respectively [115].
CONCLUSION AND FUTURE ASPECTS
Food colour manufacturing industries are facing multiple challenges. Some of them include decrease in demand for synthetic pigments due to their ill-health effects, and developing new natural pigments with potential bioactivity and maximum stability for all industrial applications. Thus, there is an incredible interest to replace synthetic pigments with natural microbial engineered pigments in foods, feed and cosmetics industries. Microbial sources are specifically helpful as they can be scaled-up and can be more promptly controlled than plants or insects. Improvement and integration, like strain advancement in microbial fermentation, metabolic, and protein designing and engineering, can have a generous effect in both the quality and amount of common food pigments. The majority of the studies on carotenoid production are centered around the carbon flux mechanism. Bioengineering is valuable for designing and can improve pigment yields, empowering the regulatory pathways. Microbial cell factories can be produced by using heterologous expression of biosynthetic pathways from known or novel pigment producers. In addition, physiological engineering might be another technique for improving carotenoid creation. Nevertheless further research is required to upgrade pigment characteristics, similar to composition and yield, by finding the most advanced parameters for development, utilization of genetically engineered organisms to enhance the production of pigments. Usage of agri-food-forestry wastes and residues to produce pigments via microbial technology can definitely help in minimising environmental contamination. In recent years, there has been a developed interest among the scientific community and industrialists on exploring various underutilized raw materials such as those of agri-industrial residues to produce value-added products. Thus, agri-food wastes possess huge potential to be used for microbial production of pigments which is expected to find wide applications in food, feed and other relevant industries.
ACKNOWLEDGEMENTS
Authors thank Dr. Urvashi Kuhad, Assistant Professor, Department of English, Ram Lal Anand College, University of Delhi South Campus, New Delhi, India who has helped in language editing.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
MS, VKG and RB acknowledge ERA Chair for Food (By-) Products Valorization Technologies of the Estonian University of Life Sciences (VALORTECH) which has received funding from the European Union’s Horizon 2020 research and innovation program (under grant agreement No. 810630).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Sen T., Barrow C.J., Deshmukh S.K. Microbial pigments in the food industry-challenges and the way forward. Front. Nutr. 2019;6:7. doi: 10.3389/fnut.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelhagen I., Wulff-Vester A.K., Wendell M., Hvoslef-Eide A-K., Russell J., Oertel A., Martens S., Mock H.P., Martin C., Matros A. Colour bio-factories: towards scale-up production of anthocyanins in plant cell cultures. Metab. Eng. 2018;48:218–232. doi: 10.1016/j.ymben.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huccetogullari D., Luo Z.W., Lee S.Y. Metabolic engineering of microorganisms for production of aromatic compounds. Microb. Cell Fact. 2019;18(1):41. doi: 10.1186/s12934-019-1090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panesar R., Kaur S., Panesar P.S. Production of microbial pigments utilizing agro-industrial waste: a review. Curr. Opin. Food Sci. 2015;1:70–76. doi: 10.1016/j.cofs.2014.12.002. [DOI] [Google Scholar]
- 5.Dufosse L. Microbial pigments. Reference module in life sciences. Oxford: Elsevier; 2017. pp. 1–16. [Google Scholar]
- 6.Dufosse L. Microbial production of food grade pigments. Food Technol. Biotechnol. 2006;44(3):313–321. [Google Scholar]
- 7.Gupta V.K., Mach R., Sreenivasaprasad P. Fungal biomolecules: sources, applications and recent developments. UK: Wiley-Blackwell; 2015. [Google Scholar]
- 8.Gupta V.K., Treichel H., Antonio de Oliveira L., Shapaval L., Tuohy M.G. Microbial functional foods and nutraceuticals. UK: Wiley-Blackwell; 2017. [Google Scholar]
- 9.Konuray G., Erginkaya Z. Antimicrobial and antioxidant properties of pigments synthesized from microorganisms. In: Mendez-Vilas A., editor. The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs. FORMATEX; 2015. pp. 27–33. [Google Scholar]
- 10.Numan M., Bashir S., Mumtaz R., Tayyab S., Rehman N.U., Khan A.L., Shinwari Z.K., Al-Harrasi A. A possible immunosuppressant, cycloprodigiosin hydrochloride, obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 2018;237(3):543–547.. doi: 10.1006/bbrc.1997.7186. [DOI] [PubMed] [Google Scholar]
- 11.Kawauchi K., Shibutani K., Yagisawa H., Kamata H., Nakatsuji S., Anzai H., Yokoyama Y., Ikegami Y., Moriyama Y., Hirata H. A possible immunosuppressant, cycloprodigiosin hydrochloride, obtained from Pseudoalteromonas denitrificans. Biochem. Biophys. Res. Commun. 1997;237(3):543–547. doi: 10.1006/bbrc.1997.7186. [DOI] [PubMed] [Google Scholar]
- 12.Asker D., Ohta Y. Production of canthaxanthin by Haloferax alexandrinus under non-aseptic conditions and a simple, rapid method for its extraction. Appl. Microbiol. Biotechnol. 2002;58(6):743–750. doi: 10.1007/s00253-002-0967-y. [DOI] [PubMed] [Google Scholar]
- 13.Khaneja R., Perez-Fons L., Fakhry S., Baccigalupi L., Steiger S., To E., Sandmann G., Dong T.C., Ricca E., Fraser P.D., Cutting S.M. Carotenoids found in Bacillus. J. Appl. Microbiol. 2010;108(6):1889–1902. doi: 10.1111/j.1365-2672.2009.04590.x. [DOI] [PubMed] [Google Scholar]
- 14.Guyomarc’h F., Binet A., Dufosse L. Production of carotenoids by Brevibacterium linens: variation among strains, kinetic aspects and HPLC profiles. J. Ind. Microbiol. Biotechnol. 2000;24(1):64–70. doi: 10.1038/sj.jim.2900761. [DOI] [Google Scholar]
- 15.Kim D., Lee J.S., Park Y.K., Kim J.F., Jeong H., Oh T.K., Kim B.S., Lee C.H. Biosynthesis of antibiotic prodiginines in the marine bacterium Hahella chejuensis KCTC 2396. J. Appl. Microbiol. 2007;102(4):937–944. doi: 10.1111/j.1365-2672.2006.03172.x. [DOI] [PubMed] [Google Scholar]
- 16.Galaup P., Sutthiwong N., Leclercq-Perlat M.N., Valla A., Caro Y., Fouillaud M., Guerard F., Dufosse L. First isolation of Brevibacterium sp. pigments in the rind of an industrial red- smear-ripened soft cheese. Int. J. Dairy Technol. 2015;68(1):144–147. doi: 10.1111/1471-0307.12211. [DOI] [Google Scholar]
- 17.Mukherjee G., Singh S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process Biochem. 2011;46(1):188–192. doi: 10.1016/j.procbio.2010.08.006. [DOI] [Google Scholar]
- 18.Joshi V.K., Attri D., Bala A., Bhushan S. Microbial pigments. Indian J. Biotechnol. 2003;2:362–369. [Google Scholar]
- 19.Herz S., Weber R.W., Anke H., Mucci A., Davoli P. Intermediates in the oxidative pathway from torulene to torularhodin in the red yeasts Cystofilobasidium infirmominiatum and C. capitatum (Heterobasidiomycetes, Fungi). Phytochemistry. 2007;68(20):2503–2511. doi: 10.1016/j.phytochem.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Gerber N.N. Prodigiosin-like pigments. CRC Crit. Rev. Microbiol. 1975;3(4):469–485. doi: 10.3109/10408417509108758. [DOI] [PubMed] [Google Scholar]
- 21.Mapari S.A., Meyer A.S., Thrane U., Frisvad J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009;8(1):24. doi: 10.1186/1475-2859-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez R., Fierro F., Garcia-Rico R.O., Laich F. Mold-fermented foods: Penicillium spp. as ripening agents in the elaboration of cheese and meat products. Mycofactories. Bentham Science Publishers; 2011. pp. 73–98. [Google Scholar]
- 23.Yadav S., Manjunatha K., Ramachandra B., Suchitra N., Prabha R. Characterization of pigment producing Rhodotorula from dairy environmental samples. Asian. J. Dairying Foods. Res. 2014;33(1):1–4. doi: 10.5958/j.0976-0563.33.1.001. [DOI] [Google Scholar]
- 24.Carreira A., Ferreira L.M., Loureiro V. Production of brown tyrosine pigments by the yeast Yarrowia lipolytica. J. Appl. Microbiol. 2001;90(3):372–379. doi: 10.1046/j.1365-2672.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- 25.Arun N., Singh D. Differential response of Dunaliella salina and Dunaliella tertiolecta isolated from brines of Sambhar Salt Lake of Rajasthan (India) to salinities: a study on growth, pigment and glycerol synthesis. J. Mar. Biol. Assoc. India. 2013;55(1):65–70. doi: 10.6024/jmbai.2013.55.1.01758-11. [DOI] [Google Scholar]
- 26.Davoli P., Weber R.W. Carotenoid pigments from the red mirror yeast Sporobolomyces roseus. Mycologist. 2002;16(3):102–108. doi: 10.1017/S0269915X02001027. [DOI] [Google Scholar]
- 27.Houbraken J., Frisvad J.C., Seifert K.A., Overy D.P., Tuthill D.M., Valdez J.G., Samson R.A. New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia. 2012;29:78–100. doi: 10.3767/003158512X660571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta C., Garg A.P., Prakash D., Goyal S., Gupta S. Microbes as potential source of biocolours. Pharmacologyonline. 2011;2:1309–1318. [Google Scholar]
- 29.Malisorn C., Suntornsuk W. Optimization of β-carotene production by Rhodotorula glutinis DM28 in fermented radish brine. Bioresour. Technol. 2008;99(7):2281–2287. doi: 10.1016/j.biortech.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 30.Libkind D., Sommaruga R., Zagarese H., van Broock M. Mycosporines in carotenogenic yeasts. Syst. Appl. Microbiol. 2005;28(8):749–754. doi: 10.1016/j.syapm.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Marova I., Certik M., Breierov E. Biomass - Detection, Production and Usage. IntechOpen; 2011. Production of enriched biomass by carotenogenic yeasts - application of whole-cell yeast biomass to production of pigments and other lipid compounds. pp. 345–384.. [DOI] [Google Scholar]
- 32.Pattanagul P., Pinthong R., Phianmongkhol A., Leksawasdi N. Review of angkak production (Monascus purpureus). Warasan Khana Witthayasat Maha Witthayalai Chiang Mai. 2007;34(3):319–328. [Google Scholar]
- 33.Babitha S. In: Microbial pigments. Biotechnology for Agroindustrial Utilization; Singh-Nee Nigam, P. Pandey A., editor. Netherlands: Springer; 2009. pp. 147–162. [Google Scholar]
- 34.Ruffing A., Chen R.R. Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis. Microb. Cell Fact. 2006;5:25–33. doi: 10.1186/1475-2859-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niu F.X., Lu Q., Bu Y.F., Liu J.Z. Metabolic engineering for the microbial production of isoprenoids: Carotenoids and isoprenoid-based biofuels. Synth. Syst. Biotechnol. 2017;2(3):167–175. doi: 10.1016/j.synbio.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C., Zada B., Wei G., Kim S.W. Metabolic engineering and synthetic biology approaches driving isoprenoid production in Escherichia coli. Bioresour. Technol. 2017;241:430–438. doi: 10.1016/j.biortech.2017.05.168. [DOI] [PubMed] [Google Scholar]
- 37.Ray R.C., Shetty K., Ward O.P. Solid-state fermentation and value-added utilization of horticultural processing wastes. In: Ray R.C., Ward O.P., editors. Microbial biotechnology in horticulture. Vol. 3. Science Publishers; New Hampshire, USA: 2008. pp. 231–272.. [DOI] [Google Scholar]
- 38.Buzzini P. Batch and fed-batch carotenoid production by Rhodotorula glutinis-Debaryomyces castellii co-cultures in corn syrup. J. Appl. Microbiol. 2001;90(5):843–847. doi: 10.1046/j.1365-2672.2001.01319.x. [DOI] [PubMed] [Google Scholar]
- 39.Kantifedaki A., Kachrimanidou V., Mallouchos A., Papanikolaou S., Koutinas A.A. Orange processing waste valorisation for the production of biobased pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 2018;185:882–890. doi: 10.1016/j.jclepro.2018.03.032. [DOI] [Google Scholar]
- 40.Taskin M., Erdal S. Production of carotenoids by Rhodotorula glutinis MT-5 in submerged fermentation using the extract from waste loquat kernels as substrate. J. Sci. Food Agric. 2011;91(8):1440–1445. doi: 10.1002/jsfa.4329. [DOI] [PubMed] [Google Scholar]
- 41.Miura Y., Kondo K., Saito T., Shimada H., Fraser P.D., Misawa N. Production of the carotenoids lycopene, β-carotene, and astaxanthin in the food yeast Candida utilis. Appl. Environ. Microbiol. 1998;64(4):1226–1229. doi: 10.1128/AEM.64.4.1226-1229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frengova G.I., Beshkova D.M. Carotenoids from Rhodotorula and Phaffia: yeasts of biotechnological importance. J. Ind. Microbiol. Biotechnol. 2009;36(2):163–180. doi: 10.1007/s10295-008-0492-9. [DOI] [PubMed] [Google Scholar]
- 43.Bhosale P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004;63(4):351–361. doi: 10.1007/s00253-003-1441-1. [DOI] [PubMed] [Google Scholar]
- 44.Lukacs G., Linka B., Nyilasi I. Phaffia rhodozyma and Xanthophyllomyces dendrorhous: Astaxanthin-producing yeasts of biotechnological importance. Acta Aliment. 2006;5:99–107. doi: 10.1556/AAlim.35.2006.1.11. [DOI] [Google Scholar]
- 45.Sakaki H., Nakanishi T., Tada A., Miki W., Komemushi S. Activation of torularhodin production by Rhodotorula glutinis using weak white light irradiation. J. Biosci. Bioeng. 2001;92(3):294–297. doi: 10.1016/S1389-1723(01)80265-6. [DOI] [PubMed] [Google Scholar]
- 46.Zha J., Koffas M.A.G. Anthocyanin production in engineered microorganisms. Biotechnol. Natural Products. Springer Cham; 2017. pp. 81–97. [Google Scholar]
- 47.Latha B.V., Jeevaratnam K. Purification and characterization of the pigments from Rhodotorula glutinis DFR-PDY isolated from natural source. Global J. Biotechnol. Biochem. 2010;5(3):166–174. [Google Scholar]
- 48.Cerdá-Olmedo E. Phycomyces and the biology of light and color. FEMS Microbiol. Rev. 2001;25(5):503–512. doi: 10.1111/j.1574-6976.2001.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 49.Ajikumar P.K., Xiao W-H., Tyo K.E., Wang Y., Simeon F., Leonard E., Mucha O., Phon T.H., Pfeifer B., Stephanopoulos G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330(6000):70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadav V.G., De Mey M., Lim C.G., Ajikumar P.K., Stephanopoulos G. The future of metabolic engineering and synthetic biology: towards a systematic practice. Metab. Eng. 2012;14(3):233–241. doi: 10.1016/j.ymben.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang C., Seow V.Y., Chen X., Too H-P. Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat. Commun. 2018;9(1):1858. doi: 10.1038/s41467-018-04211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C., Swofford C.A., Sinskey A.J. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2019;10:e00118. doi: 10.1016/j.mec.2019.e00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liao P., Hemmerlin A., Bach T.J., Chye M.L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016;34(5):697–713. doi: 10.1016/j.biotechadv.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Yoon S.H., Lee S.H., Das A., Ryu H.K., Jang H.J., Kim J.Y., Oh D.K., Keasling J.D., Kim S.W. Combinatorial expression of bacterial whole mevalonate pathway for the production of β-carotene in E. coli. J. Biotechnol. 2009;140(3-4):218–226. doi: 10.1016/j.jbiotec.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Li Q., Fan F., Gao X., Yang C., Bi C., Tang J., Liu T., Zhang X. Balanced activation of IspG and IspH to eliminate MEP intermediate accumulation and improve isoprenoids production in Escherichia coli. Metab. Eng. 2017;44:13–21. doi: 10.1016/j.ymben.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Coussement P., Bauwens D., Maertens J., De Mey M. Direct combinatorial pathway optimization. ACS Synth. Biol. 2017;6(2):224–232. doi: 10.1021/acssynbio.6b00122. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y., Zhu R.Y., Mitchell L.A., Ma L., Liu R., Zhao M., Jia B., Xu H., Li Y.X., Yang Z.M., Ma Y., Li X., Liu H., Liu D., Xiao W-H., Zhou X., Li B-Z., Yuan Y-J., Boeke J.D. In vitro DNA SCRaMbLE. Nat. Commun. 2018;9(1):1935. doi: 10.1038/s41467-018-03743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H.H., Isaacs F.J., Carr P.A., Sun Z.Z., Xu G., Forest C.R., Church G.M. Programming cells by multiplex genome engineering and accelerated evolution. Nature. 2009;460(7257):894–898. doi: 10.1038/nature08187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao J., Li Q., Sun T., Zhu X., Xu H., Tang J., Zhang X., Ma Y. Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab. Eng. 2013;17:42–50. doi: 10.1016/j.ymben.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Kogure T., Inui M. Recent advances in metabolic engineering of Corynebacterium glutamicum for bioproduction of value-added aromatic chemicals and natural products. Appl. Microbiol. Biotechnol. 2018;102(20):8685–8705. doi: 10.1007/s00253-018-9289-6. [DOI] [PubMed] [Google Scholar]
- 61.Henke N.A., Wiebe D., Pérez-García F., Peters-Wendisch P., Wendisch V.F. Coproduction of cell-bound and secreted value-added compounds: simultaneous production of carotenoids and amino acids by Corynebacterium glutamicum. Bioresour. Technol. 2018;247:744–752. doi: 10.1016/j.biortech.2017.09.167. [DOI] [PubMed] [Google Scholar]
- 62.Taniguchi H., Henke N.A., Heider S.A.E., Wendisch V.F. Overexpression of the primary sigma factor gene sigA improved carotenoid production by Corynebacterium glutamicum: application to production of β-carotene and the non-native linear C50 carotenoid bisanhydro bacterioruberin. Metab. Eng. Commun. 2017;4:1–11. doi: 10.1016/j.meteno.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naylor G.W., Addlesee H.A., Gibson L.C.D., Hunter C. The photosynthesis gene cluster of Rhodobacter sphaeroides. Photosynth. Res. 1999;62:121–139. doi: 10.1023/A:1006350405674. [DOI] [Google Scholar]
- 64.Chi S.C., Mothersole D.J., Dilbeck P., Niedzwiedzki D.M., Zhang H., Qian P., Vasilev C., Grayson K.J., Jackson P.J., Martin E.C., Li Y., Holten D., Neil Hunter C. Assembly of functional photosystem complexes in Rhodobacter sphaeroides incorporating carotenoids from the spirilloxanthin pathway. Biochim. Biophys. Acta. 2015;1847(2):189–201. doi: 10.1016/j.bbabio.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su A., Chi S., Li Y., Tan S., Qiang S., Chen Z., Meng Y. Metabolic redesign of Rhodobacter sphaeroides for lycopene production. J. Agric. Food Chem. 2018;66(23):5879–5885. doi: 10.1021/acs.jafc.8b00855. [DOI] [PubMed] [Google Scholar]
- 66.Hara K.Y., Morita T., Endo Y., Mochizuki M., Araki M., Kondo A. Evaluation and screening of efficient promoters to improve astaxanthin production in Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2014;98(15):6787–6793. doi: 10.1007/s00253-014-5727-2. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto K., Hara K.Y., Morita T., Nishimura A., Sasaki D., Ishii J., Ogino C., Kizaki N., Kondo A. Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes. Microb. Cell Fact. 2016;15(1):155. doi: 10.1186/s12934-016-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pollmann H., Breitenbach J., Sandmann G. Engineering of the carotenoid pathway in Xanthophyllomyces dendrorhous leading to the synthesis of zeaxanthin. Appl. Microbiol. Biotechnol. 2017;101(1):103–111. doi: 10.1007/s00253-016-7769-0. [DOI] [PubMed] [Google Scholar]
- 69.Zhu Q., Jackson E.N. Metabolic engineering of Yarrowia lipolytica for industrial applications. Curr. Opin. Biotechnol. 2015;36:65–72. doi: 10.1016/j.copbio.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Darvishi F., Ariana M., Marella E.R., Borodina I. Advances in synthetic biology of oleaginous yeast Yarrowia lipolytica for producing non-native chemicals. Appl. Microbiol. Biotechnol. 2018;102(14):5925–5938. doi: 10.1007/s00253-018-9099-x. [DOI] [PubMed] [Google Scholar]
- 71.Matthäus F., Ketelhot M., Gatter M., Barth G. Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 2014;80(5):1660–1669. doi: 10.1128/AEM.03167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartz C., Frogue K., Misa J., Wheeldon I. Host and pathway engineering for enhanced lycopene biosynthesis in Yarrowia lipolytica. Front. Microbiol. 2017;8:2233. doi: 10.3389/fmicb.2017.02233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao S., Tong Y., Zhu L., Ge M., Zhang Y., Chen D., Jiang Y., Yang S. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab. Eng. 2017;41:192–201. doi: 10.1016/j.ymben.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Larroude M., Celinska E., Back A., Thomas S., Nicaud J.M., Ledesma-Amaro R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol. Bioeng. 2018;115(2):464–472. doi: 10.1002/bit.26473. [DOI] [PubMed] [Google Scholar]
- 75.Mantzouridou F.T., Naziri E. Scale translation from shaken to diffused bubble aerated systems for lycopene production by Blakeslea trispora under stimulated conditions. Appl. Microbiol. Biotechnol. 2017;101(5):1845–1856. doi: 10.1007/s00253-016-7943-4. [DOI] [PubMed] [Google Scholar]
- 76.Mantzouridou F., Roukas T., Achatz B. Effect of oxygen rate on β-carotene production from synthetic medium by Blakeslea trispora in shake flask culture. Enzyme Microb. Technol. 2005;37:687–694. doi: 10.1016/j.enzmictec.2005.02.020. [DOI] [Google Scholar]
- 77.Chatzivasileiou A.O., Ward V., Edgar S.M., Stephanopoulos G. Two-step pathway for isoprenoid synthesis. Proc. Natl. Acad. Sci. USA. 2018;•••:1–6. doi: 10.1073/pnas.1812935116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin J., Wang Y., Yao M., Gu X., Li B., Liu H., Ding M., Xiao W., Yuan Y. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels. 2018;11:230. doi: 10.1186/s13068-018-1227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y., Xiao W., Wang Y., Liu H., Li X., Yuan Y. Lycopene overproduction in Saccharomyces cerevisiae through combining pathway engineering with host engineering. Microb. Cell Fact. 2016;15(1):113. doi: 10.1186/s12934-016-0509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kang C.W., Lim H.G., Yang J., Noh M.H., Seo S.W., Jung G.Y. Synthetic auxotrophs for stable and tunable maintenance of plasmid copy number. Metab. Eng. 2018;48:121–128. doi: 10.1016/j.ymben.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 81.Ma T., Shi B., Ye Z., Li X., Liu M., Chen Y., Xia J., Nielsen J., Deng Z., Liu T. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab. Eng. 2019;52:134–142. doi: 10.1016/j.ymben.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 82.Zha J., Koffas M.A.G. Production of anthocyanins in metabolically engineered microorganisms: Current status and perspectives. Synth. Syst. Biotechnol. 2017;2(4):259–266. doi: 10.1016/j.synbio.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gulani C., Bhattacharya S., Das A. Assessment of process parameters influencing the enhanced production of prodigiosin from Serratia marcescens and evaluation of its antimicrobial, antioxidant and dyeing potentials. Malays. J. Microbiol. 2012;8(2):116–122. doi: 10.21161/mjm.03612. [DOI] [Google Scholar]
- 84.Petroni K., Tonelli C. Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Sci. 2011;181(3):219–229. doi: 10.1016/j.plantsci.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 85.Lim C.G., Wong L., Bhan N., Dvora H., Xu P., Venkiteswaran S., Koffas M.A. Development of a recombinant Escherichia coli strain for overproduction of plant pigment, anthocyanin. Appl. Environ. Microbiol. 2015;81(18):6276–6284. doi: 10.1128/AEM.01448-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones J.A., Vernacchio V.R., Collins S.M., Shirke A.N., Xiu Y., Englaender J.A., Cress B.F., McCutcheon C.C., Linhardt R.J., Gross R.A., Koffas M.A.G. Complete biosynthesis of anthocyanins using E. coli polycultures. MBio. 2017;8(3):e00617–e00621. doi: 10.1128/mBio.00621-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Levisson M., Patinios C., Hein S., de Groot P.A., Daran J-M., Hall R.D., Martens S., Beekwilder J. Engineering de novo anthocyanin production in Saccharomyces cerevisiae. Microb. Cell Fact. 2018;17(1):103. doi: 10.1186/s12934-018-0951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao S., Jones J.A., Lachance D.M., Bhan N., Khalidi O., Venkataraman S., Wang Z., Koffas M.A.G. Improvement of catechin production in Escherichia coli through combinatorial metabolic engineering. Metab. Eng. 2015;28:43–53. doi: 10.1016/j.ymben.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Springob K., Nakajima J., Yamazaki M., Saito K. Recent advances in the biosynthesis and accumulation of anthocyanins. Nat. Prod. Rep. 2003;20(3):288–303. doi: 10.1039/b109542k. [DOI] [PubMed] [Google Scholar]
- 90.Yan Y., Li Z., Koffas M.A.G. High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol. Bioeng. 2008;100(1):126–140. doi: 10.1002/bit.21721. [DOI] [PubMed] [Google Scholar]
- 91.Cress B.F., Leitz Q.D., Kim D.C., Amore T.D., Suzuki J.Y., Linhardt R.J., Koffas M.A.G. CRISPRi-mediated metabolic engineering of E. coli for O-methylated anthocyanin production. Microb. Cell Fact. 2017;16(1):10. doi: 10.1186/s12934-016-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abbas M., Ali A., Arshad M., Atta A., Mehmood Z., Tahir I.M., Iqbal M. Mutagenicity, cytotoxic and antioxidant activities of Ricinus communis different parts. Chem. Cent. J. 2018;12(1):3. doi: 10.1186/s13065-018-0370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mishra B., Varjani S., Varma G.K.S. Agro-industrial by-products in the synthesis of food grade microbial pigments: An eco-friendly alternative. Green Bio-processes. Singapore: Springer; 2019. pp. 245–265. [Google Scholar]
- 94.Rodrigues D.B., Flores E.M.M., Barin J.S., Mercadante A.Z., Jacob-Lopes E., Zepka L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014;65:144–148. doi: 10.1016/j.foodres.2014.06.037. [DOI] [Google Scholar]
- 95.Bhaskar N., Suresh P.V., Sakhare P.Z., Sachindra N.M. Shrimp biowaste fermentation with Pediococcus acidolactici CFR2182: Optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteination/demineralization and carotenoid recovery. Enzyme Microb. Technol. 2007;40(5):1427–1434. doi: 10.1016/j.enzmictec.2006.10.019. [DOI] [Google Scholar]
- 96.Joshi V.K., Attri D., Rana M.S. Optimization of apple pomace based medium and fermentation conditions for pigment production by Sarcina sp. Indian J. Nat. Prod. Resour. 2011;2(4):421–427. [Google Scholar]
- 97.Korumilli T., Mishra S. Carotenoid production by Bacillus clausii using rice powder as the sole substrate: pigment analyses and optimization of key production parameters. J. Biochem. Technol. 2014;5(4):788–794. [Google Scholar]
- 98.Elsanhoty R.M., Al-Turki I.A., Ramdan M.F. Screening of medium components by Plackettt-Burman design for carotenoid production using date (Phoenix dactylifera) wastes. Ind. Crops Prod. 2012;36:313–320. doi: 10.1016/j.indcrop.2011.10.013. [DOI] [Google Scholar]
- 99.Aruldass C.A., Aziz A., Venil C.K., Khasim A.R., Ahmad W.A. Utilization of agro-industrial waste for the production of yellowish orange pigment from Chryseobacteirum artocarpi CECT 8497. Int. Biodeter. Biodegrad. 2016;113:342–349. doi: 10.1016/j.ibiod.2016.01.024. [DOI] [Google Scholar]
- 100.Vidyalakshmi R., Paranthaman R., Murugesh S., Singaravadivel K. Microbial bioconversion of rice broken to food grade pigments. Global J. Biotechnol. Biochem. 2009;4:84–87. [Google Scholar]
- 101.Sopandi T., Wardah A., Surtiningsih T., Suwandi A., Smith J.J. Utilization and optimization of a waste stream cellulose culture medium for pigment production by Penicillium spp. J. Appl. Microbiol. 2013;114(3):733–745. doi: 10.1111/jam.12110. [DOI] [PubMed] [Google Scholar]
- 102.Aksu Z., Eren A.T. Carotenoids production by the yeast Rhodotorula mucilaginosa: Use of agriculture wastes as a carbon source. Process Biochem. 2005;40(9):2985–2991. doi: 10.1016/j.procbio.2005.01.011. [DOI] [Google Scholar]
- 103.Marova I., Carnecka M., Halienova A., Certik M., Dvorakova T., Haronikova A. Use of several waste substrates for carotenoid-rich yeast biomass production. J. Environ. Manage. 2012;95(Suppl.):S338–S342. doi: 10.1016/j.jenvman.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Tinoi J., Rakariyatham N., Deming R.L. Simplex optimization of carotenoid production by Rhodotorula glutinis using hydrolyzed mung bean waste flour as substrate. Process Biochem. 2005;40(7):2551–2557. doi: 10.1016/j.procbio.2004.11.005. [DOI] [Google Scholar]
- 105.Subhasree R.S., Babu P.D., Vidyalakshmi R., Mohan V.P. Effect of carbon and nitrogen sources on stimulation of pigment production by Monascus purpureus on Jackfruit Seeds. Int. J Microbiol. Res. 2011;2(2):184–187. [Google Scholar]
- 106.Panesar R. Bioutilization of kinnow waste for the production of biopigments using submerged fermentation. Int. J. Food Sci. Nutr. 2012;3(1):9–13. [Google Scholar]
- 107.Bhosale P., Gadre R.V. β-Carotene production in sugarcane molasses by a Rhodotorula glutinis mutant. J. Ind. Microbiol. Biotechnol. 2001;26(6):327–332. doi: 10.1038/sj.jim.7000138. [DOI] [PubMed] [Google Scholar]
- 108.Frengova G., Simova E., Beshkova D. Use of whey ultrafiltrate as a substrate for production of carotenoids by the yeast Rhodotorula rubra. Appl. Biochem. Biotechnol. 2004;112(3):133–141. doi: 10.1385/ABAB:112:3:133. [DOI] [PubMed] [Google Scholar]
- 109.Valduga E., Rausch Ribeiro A.H., Cence K., Colet R., Tiggemann L., Zeni J., Toniazzo G. Carotenoids production from a newly isolated Sporidiobolus pararoseus strain using agroindustrial substrates. Biocatal. Agric. Biotechnol. 2014;3(2):207–213. doi: 10.1016/j.bcab.2013.10.001. [DOI] [Google Scholar]
- 110.Tarangini K., Mishra S. Carotenoid production by Rhodotorulasp. on fruit waste extract as a sole carbon source and optimization of key parameters. Iran. J. Chem. Chem. Eng., (IJCCE) 2014;33(3):89–99.. [Google Scholar]
- 111.Jixian G., Yanfei R., Jianfei Z., Zheng L., Qiujin L., Huiqin L. Microbial synthesis preparation and application of red nanopigment dye liquor for cotton. Faming Zhuanli Shenqing.,CN106434757 A 20170222. 2017 [Google Scholar]
- 112.Lin C-H., Lin T-H., Pan T-M. Alleviation of metabolic syndrome by monascin and ankaflavin: the perspective of Monascus functional foods. Food Funct. 2017;8(6):2102–2109. doi: 10.1039/C7FO00406K. [DOI] [PubMed] [Google Scholar]
- 113.He X., Li Y., Lawson D., Xie D.Y. Metabolic engineering of anthocyanins in dark tobacco varieties. Physiol. Plant. 2017;159(1):2–12. doi: 10.1111/ppl.12475. [DOI] [PubMed] [Google Scholar]
- 114.Venil C.K., Zakaria Z.A., Ahmad W.A. Bacterial pigments and their applications. Process Biochem. 2013;48:1065–1079. doi: 10.1016/j.procbio.2013.06.006. [DOI] [Google Scholar]
- 115.Kot A.M., Błażejak S., Kieliszek M., Gientka I., Bryś J. Simultaneous production of lipids and carotenoids by the red yeast Rhodotorula from waste glycerol fraction and potato wastewater. Appl. Biochem. Biotechnol. 2019;189(2):589–607. doi: 10.1007/s12010-019-03023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


