Abstract
Objective:
To systematically review the literature on the therapeutic use of amphetamine, lisdexamfetamine and methylphenidate in elderly population with and without dementia.
Methods:
We conducted two researches on the PubMed, Scopus and Embase using the keywords (“elderly”) AND (“amphetamine” OR “methylphenidate” OR “lisdexamfetamine”) and then (“Alzheimer” OR “dementia”) AND (“amphetamine” OR “methylphenidate” OR “lisdexamfetamine”).
Results:
Twenty-nine papers met all the eligibility criteria. The results are encouraging as 81.5% of the studies showed clinical improvement of the investigated condition.
Conclusion:
Amphetamines and methylphenidate are probably effective strategies for different conditions in the elderly population. However, further studies are needed to provide more robust evidence on efficacy, dosage and safety for this population.
Keywords: Stimulant, amphetamine, methylphenidate, lisdexamfetamine, dementia, elderly
1. INTRODUCTION
Amphetamine was discovered in 1910, and it was available in the market in the 1930s as an over-the-counter medication for a number of conditions [1], including cognitive performance enhancer, mood elevation, and appetite suppression [2]. Due to its increased recreational use, amphetamine became highly regulated after America’s first amphetamine epidemic in the 1960s [3]. Currently, amphetamine and methylphenidate are approved by the Food and Drug Administration (FDA) for the treatment of attention-deficit/hyperactivity disorder (ADHD) and narcolepsy [1, 2].
Amphetamines are stimulants that exert their effects by increasing the extracellular levels of monoamines, therefore extending their signaling effects. There are three mechanisms by which amphetamines increase monoamine availability in the synaptic cleft: i) displacement of the monoamines stored in vesicles in the presynaptic terminal by the inhibition of vesicular monoamine transporter 2 (VMAT2) [4], ii) the inhibition of monoamine reuptake, and iii) the inhibition of the catalytic enzyme monoamine oxidase (MAO) which is responsible for breaking down these neurotransmitters [1, 5].
Amphetamine derivatives, including methylphenidate, act as reuptake inhibitors of dopamine (DA) and norepinephrine (NE) in presynaptic neurons, increasing their release into the extraneuronal space, but they do not influence MAO or VMAT2 [6]. As amphetamine and methylphenidate share some mechanisms of action [7, 8], they can cause similar effects in the central nervous system. Both increase DA and NE availability in corticostriatal systems [7], which are responsible for emotion regulation, risky decision making, and reward/reinforcement processes [7, 9]. These effects support the use of these substances in different clinical scenarios, such as ADHD [10], narcolepsy [11], Parkinson’s disease [12], fall prevention [13], late-life depression treatment augmentation [14-16], apathy [17] and catatonia [18].
The elderly population has grown significantly [19, 20] and is expected to keep growing due to the steady increase in life expectancy [21]. This population has special needs once they are at increased risk for chronic conditions, including neurodegenerative diseases [22-24]. DA levels decrease about 10% per decade since early adulthood and this decline has been associated with progressive impairment of cognitive and motor performance [25]. Moreover, there are several aging-related conditions, such as behavioral and cognitive syndromes, in which amphetamines could contribute to their management [26].
Although amphetamines might benefit elderly patients, the available evidence on their efficacy and the risks associated with the use of such complex drugs must be considered. Therefore, the current manuscript presents a systematic review about the use of amphetamine, lisdexamfetamine, and methylphenidate in the elderly population. Case reports, case series, and trials that aimed at evaluating the use of these drugs in elderly patients are described.
2. METHODS
We systematically reviewed articles reporting the use of stimulant drugs among elderly subjects. Three databases were used as sources: PubMed, Scopus, and Embase. Two different searches were conducted. The first one aimed at identifying the conditions in which these drugs have been used in the elderly population. The keywords were (“elderly”) AND (“amphetamine” OR “methylphenidate” OR “lisdexamfetamine”). The MeSH browser searched those terms as “elderly” [All Fields] AND (“amphetamine” [All Fields] OR “methylphenidate”[All Fields] OR “lisdexamfetamine”[All Fields]). The second search aimed at identifying the studies of stimulants in elderly patients with dementia. In this search, the keywords were (“Alzheimer” OR “dementia”) AND (“amphetamine” OR “methylphenidate” OR “lisdexamfetamine”). The MeSH browser searched those terms as (“Alzheimer”[All Fields] OR “dementia”[All Fields]) AND (“amphetamine”[All Fields] OR “methylphenidate”[All Fields] OR “lisdexamfetamine”[All Fields]). In both searches, almost all filters, when present, were disabled, leaving only two: “English” and “article”. The inclusion criteria were: (1) manuscript available in English, (2) original reports and not a review, (3) at least one stimulant drug was cited as a pharmacological strategy, (4) full papers and case reports (not letters, conference abstracts, notes, consensus or guidelines), (5) manuscript targeting elderly population (65 years or older), and (6) published after 1990. This year was chosen because the mechanisms of action of amphetamines were better understood after the 1990s [1]. From this decade, the studies were guided by pharmacological knowledge about the amphetamines and the conditions that could benefit from the drugs. Besides, before 1990, the Diagnostic and Statistical Manual of Mental Disorders (DSM)-III was the reference for psychiatric diagnosis, and only in 1994, DSM-IV was published (26). Since different versions of the the DSM modified the diagnostic criteria, it is plausible to assume that the most recent version is closer to the current diagnosis based on DSM-5.
A reference management software (EndNote X7 for Windows from Thomson Reuters, 2013) was used for screening purposes. This systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [27].
3. RESULTS
The flow diagrams of the searches are presented in Figs. 1 and 2. For the search concerning elderly and stimulants (Fig. 1), over six hundred papers were initially retrieved from the databases, 278 were duplicates, i.e. they were found in more than one database. Two hundred and sixty were excluded after careful analysis of the title and abstract. Thereafter, 114 were screened, in which 54 were published before 1990. Only 14 out of the 60 remaining papers met the eligibility criteria to be included in this systematic review. All of them are focused on the use of methylphenidade for different conditions: depression (7), catatonia (1), Parkinson’s disease (1), falls (1), age-related cognitive decline (1) and anorexia nervosa (1). Two case reports focused on the adverse effects of methylphenidate in elderly subjects.
Fig. (1).
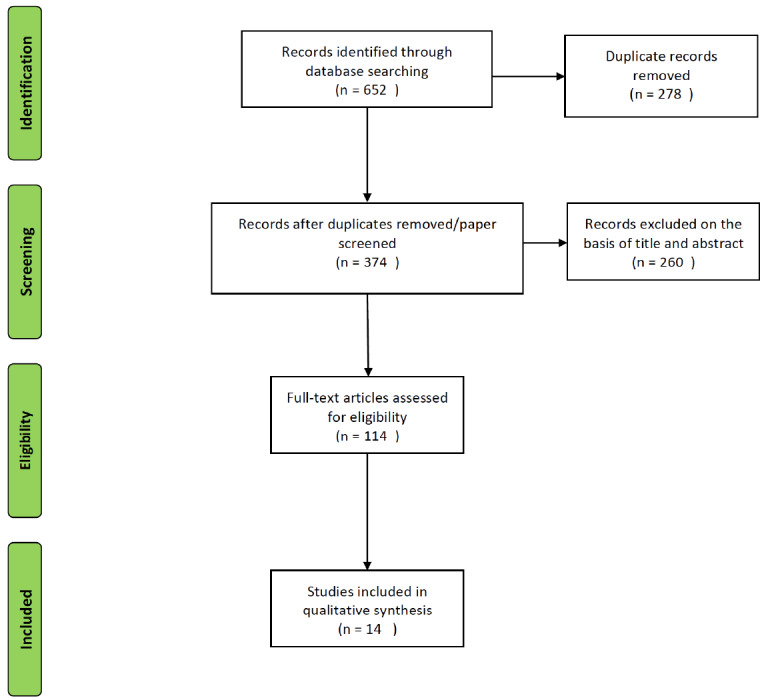
Flow chart of the search on the use of stimulants in the elderly.
Fig. (2).
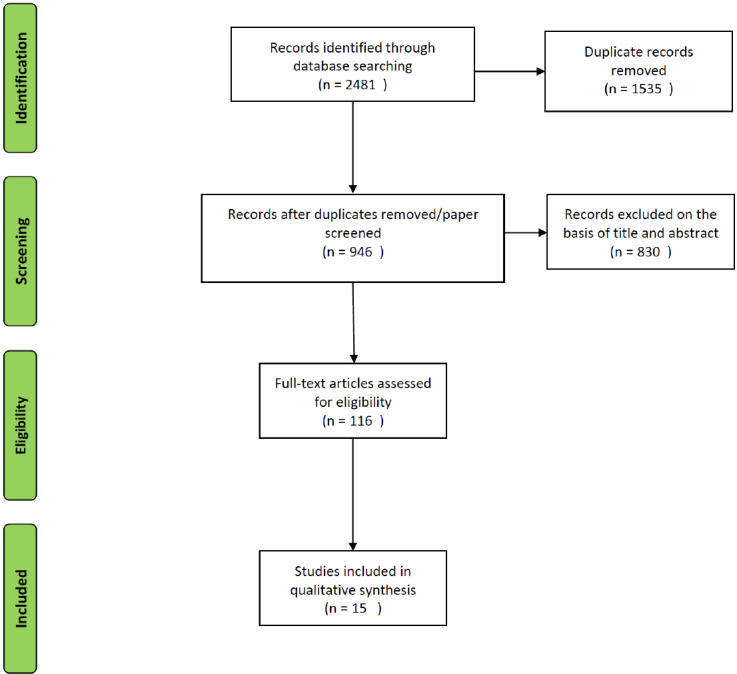
Flow chart of the search on the use of stimulants in dementia.
Regarding dementias and stimulants (Fig. 2), the initial search resulted in 2,481 papers, 1,535 were duplicates and 107 were published before 1990. Out of the 839 papers left, 15 meet the eligibility criteria.
Therefore, a total of 29 papers were included in this review. Tables 1, 2 and 3 show studies addressing the use of stimulants in depression (Table 1), dementia (Table 2) and other conditions (Table 3).
Table 1. Therapeutic use of stimulants in elderly with major depression.
|
Author/
Year |
Design of Study | Drug | Therapeutic Use | Instrument Tool | Main Result |
|---|---|---|---|---|---|
| Pickett, 1990 | 5 year retrospective patient record study (N=129) | Dextroamphetamine (average maximal daily dose of 8.2 mg/day) and methylphenidate (maximal daily dose averaging 9mg/day) | Geriatric depressive disorders secondary to medical illness | DSM III, Lack of objective tool to assess improvement | A hundred and twenty-nine patients using dextroamphetamine and 25 patients on methylphenidate for depression were reviewed. One hundred and five patients (81%) experienced some improvement on psychostimulant treatment and 85 patients (66%) were rated as markedly or moderately improve. |
| Lazarus, 1992 | 3 weeks no randomized, no placebo controlled Clinical Trial (N=10) | Methylphenidate (mean dose of 17mg daily) | Poststroke depression | DSM III R, HAM-D | According to score on Hamilton Rating Scale for Depression, 80% (8 of 10) of the patients demonstrated a full or partial response. Results indicate that methylphenidate can be safe and effective in poststroke depression. |
| Lazarus, 1994 | Retrospective comparison for at least an average of 14.21 days (N=58) | Methylphenidate (máximum dose of 26.4 mg/day) vs northriptyline (máximum dose of 26.4 mg/day) | Poststroke depression | DSM III R to check if the patient no longer met the criteria after treatment | 28 patients had been treated with methylphenidate for an average of 14.21 days, and 30 with nortriptyline for an average of 39.36 days. Improvement rates were similar between the groups but the speed of response was dramatically better in methylphenidate (2.4 days vs 27 days in nortriptyline). |
| Wallace, 1995 | 8 days double-blind, placebo-controlled cross over trial (N=16) | Methylphenidate (10 mg/day per 2 days, then 20mg/day per 2 days) vs placebo (for 4 days) | Older, depressed, medically ill patients | DSM III R, HAM-D, Mini-Mental State | The benefit of methylphenidate over placebo was statistically and clinically significant. Treatment and order affected the results. Depressive symptoms were more effectively improved when patients received first methylphenidate and than placebo. |
| Lavretsky,2001 | Open label Clinical Trial for at least 8 weeks (N= 10) | Methylphenidate (mean dose was 12.5 mg daily) and Citalopram (mean dose was 26mg daily) | Major depression | DSM IV, HAM-D, CGI (clinical global impression scale) ECG | Patients were separated in 3 groups: citalopram plus methylphenidate since day 0, citalopram and addition of methylphenidate on day 3, citalopram and addition of methylphenidate after 3 weeks. Combination showed rapid onset of action, effectiveness, and was well tolerated in elderly patients with co existing medical conditions. |
| Lavretsky, 2003 | 10 weeks open-label, structured trial (N= 11) | Methylphenidate (ranged between 5 and 20 mg daily) plus citalopram (ranged between 20 and 40mg daily) | Major depression | DSM IV, HAM-D | All patients took citalopram and methylphenidate. Nine of them completed the study, 6 participants met criteria for accelerated response, and 2 patients responded after 3 weeks. One patient was a non responder. |
| Lavretsky, 2006 | 10 weeks double-blind, placebo controlled pilot trial (N= 16) |
Methylphenidate (15mg/day) plus citalopram (20-40 mg/daily) vs citalopram plus placebo | Major depression | HAM-D, MMSE, CVRF, CIRS-G, UKU side effects | Citalopram plus methylphenidate demonstrated rapid improvement (HDRS equal or less than 10 by day 21) when compared with citalopram plus placebo. It is helpful for patients who need a fast improvement and mainly in resistant depression. |
| Prowler, 2010 | Case Report | Methylphenidate (20mg daily) | Major depression with catatonia | No reported | Elderly patient with catatonic depression was treated with methylphenidate for 4 days with rapid improvement of catatonia. Methylphenidate can be useful in elderly with catatonic depression, apathetic and medically ill patients. |
| Madhusoodanan, 2014 | Case Report | Methylphenidate (5mg daily) |
Major depression |
CGI-S, CGI-I | Augmentation of mirtazapine with methylphenidate in hospitalized patient, showed significant improvement in 2 weeks. Methylphenidate can be helpful in elderly depressed patients for faster improvement, decrease morbidity and shortening of inpatient treatment. |
| Lavretsky,2015 | 16 week randomized, double-blind, placebo- controlled trial (N=143) | Methylphenidate (mean of daily dose was 16mg) plus citalopram (mean of daily dose was 32mg) vs citalopram plus placebo |
Major depression |
HAM-D | Citalopram plus methylphenidate was superior in enhance mood, well being, and remission rate compared with Methylphenidate plus placebo, citalopram plus placebo or either drugs alone. |
| Madhusoodanan, 2016 | Case Report | Methylphenidate (15 mg daily) |
Major depression |
CGI-S, HAM-D, GDS | Methylphenidate augmentation for treatment-resistant depression in an elderly patient with a meningioma showed significant improvement in 2 weeks. |
Table 2. Therapeutic use of Stimulants in elderly patients with dementia.
| Author/Year | Design of Study | Drug | Therapeutic Use | Instrument Tool | Main Result |
|---|---|---|---|---|---|
| Igor Galynker, 1996 | Open Label Clinical Trial (N= 27) |
Methylphenidate (ranging from 10 to 20 mg/day) | Negative Symptoms in Patients With Dementia | SANS, PANSS-N, HAM-D, MMSE, CGI | Negative symptoms in Alzheimers and Vascular type of dementia improved with methylphenidate. |
| Goforth, 2004 | Case Report | Methylphenidate (18mg/day) | Frontotemporal Dementia | QEEG and SPECT correlated with clinical findings | Personality went better to a near premorbid state. His mood and affect improved and impulsivity decreased with methylphenidate sutained release. |
| Herrmann, 2008 | 4 weeks crossover trial (N=25) | Methylphenidate (10 mg per day for 3 days and 20 mg per day for 11 days) | Apathy in Alzheimer Disease | MMSE, NPI, AES, Computerized behavioral tasks, CGI | Patients were allocated in 2 weeks of methylphenidate or placebo and the crossed over for more 2 weeks of study. According AES, most of the participants manifested improvement with methylphenidate compared with placebo. |
| Padala, 2010 | 12 weeks open label clinical trial (N=23) | Methylphenidate (10mg/day) | Improve Apathy and Functional Status in dementia of the Alzheimer Type | AES, GDS, MMSE, ADL, IADL, CGI-S, CGI-I | All patients were on methylphenidate. Improvement in apathy, depression, MMSE score, and functional status. No correlation between changes in the AES and depression scores was reported. |
| Baeyens, 2011 | 2 Case Reports | Methylphenidate (10 or 20 mg/day) | Hypothermia in patients with Lewy Bodies Dementia | MMSE | Both cases reported demonstrated improvement in hypothermia, alertness and cognitive function. |
| Rosenberg, 2013 | 6 week randomized, placebo- controlled trial (N= 60) |
Methylphenidate (20mg/day) | Apathy in Alzheimer disease | AES, ADMET, NPI, MMSE | Patients were randomly assigned to methylphenidate or placebo group. Significant reduction in apathy, improved CGI-C and NPI, showed CGI-C better then placebo, and MMSE results seems to be favorable on this group. |
| Padala, 2018 | 12 week double-blind, randomized, placebo-controlled trial (N= 60) | Methylphenidate (20mg daily) | Apathy in Community- Dwelling Older Veterans With Mild Alzheimer’s Disease |
AES, 3MS,MMSE, CGI-I,CGI-S | Patients were randomly assigned for methylphenidate or placebo group for a 12 week study. Methylphenidate improved functional status, cognition, caregiver burden, apathy, depression, and CGI scores. |
Table 3. Therapeutic use of stimulants in elderly patients without dementia.
| Author/Year | Design of Study | Drug | Therapeutic Use | Instrument Tool | Main Result |
|---|---|---|---|---|---|
| Gurian, 1990 | Case Report (N = 2) |
Methylphenidate (2.5 mg daily) | “To elevate mood” in very old subjects (91 and 104 years-old) |
DSM III R | Both patients months using methylphenidate were followed at least 8. They responded to low doses of methylphenidate, improving anhedonia, apathy, fatigue and loss of appetite. |
| Pobee, 1996 | Case Report | Methylphenidate (dose has not been reported) | Anorexia Nervosa | Not reported | Trial with methylphenidate failed to improve anorexia. |
| Sonde, 2001 | Double-Blind Placebo- Controlled (N=40) | Amphetamine (10 mg) vs placebo |
Impact of amphetamine added to physiotherapy after stroke |
FM motor performance score ADL with Barthel’s index | There was no significant difference between amphetamine or placebo in addition to physiotherapy on stroke related outcomes. |
| Ayache, 2001 | Case Report | Methylphenidate (5mg daily) | Respiratory insufficiency | Not reported | Methylphenidate accelerated the extubation process in a patient with respiratory insufficiency. |
| Turner, 2003 | Double-blind Pilot Trial (N= 6) | Methylphenidate (20 or 40 mg single dose) vs placebo | Improvement of age-related cognitive decline | CANTAB (PAL, SWM, SSP, NTOL, RVIP, IDED tasks) | There was no cognitive improvement after a single dose of methylphenidate. |
| Devos, 2007 | 3 months of a repeated-measures design was applied with one factor and four levels (N= 17) | Methylphenidate (1mg/Kg of methylphenidate separated in 3 doses per day) | Elderly with Parkinson | SWS test, the Tinetti Scale, the Unified Parkinson’s Disease Rating Scale (UPDRS) part III score and the Dyskinesia Rating Scale | Long term, high doses of methylphenidate, regardless L dopa use, improve gait and motor skills in elderly with Parkinson disease undergoing STN stimulation. |
| Sonde, 2007 | 3 months randomized, double-blind, placebo controlled clinical trail (N= 25) |
Amphetamine (20 or 10 mg/day) and L-dopa (100 or 50 mg/day) vs placebo vs L dopa alone | Stroke rehabilitation | FM motor performance score Barthel's ADL index | Despite not reaching statistical significance, there was an improvement trend in the groups using amphetamines compared with placebo or L dopa. |
| Espay, 2011 | 6 months randomized, placebo- controlled, double-blind (N= 27) | Methylphenidate (64.4 mg/day was the mean dose) | Gait impairment in Parkinson disease | ESS, FOGQ, GDS, H&Y MADRS, UPDRS, EQ-5D | Patients were randomly allocated to methylphenidate or placebo for 3 months and than crossed over for more 3 months of follow up. The use of methylphenidate didn’t improve gait and deteriorate motor function and quality of life. |
| Shorer, 2013 | Double-Blind Randomized Control Trial (N= 30) | Methylphenidate (10mg single dose) | Improving Falls | Single task: standing still; Dual task: standing still performing memory task; Single task: narrow base walking; Dual task: narrow base plus performing cognitive tasks | Single dose of methylphenidate was able to improve gait function in older adults, particularly when tasks demand high executive control such as in complex dual tasks. |
The most frequent use was the combination of methylphenidate with citalopram in four trials for depression, showing significant improvement of the patients’ symptoms, as assessed by the Hamilton Depression Rating Scale [28], and good tolerability [29-32]. Other eleven trials were conducted for different conditions: post-stroke depression [33, 34], Parkinson’s disease [35, 36], falls [13] age-related cognitive decline [36], post-stroke rehabilitation [38, 39], and medically-ill patients with depressive symptoms [40-42].
Regarding the studies focused on patients with dementia, all participants had clinical improvement (Table 3). Four studies described trials aiming to evaluate the efficacy of methylphenidate in the treatment of apathy in patients with Alzheimer’s disease [43-46]; one study evaluated the efficacy of methylphenidate in the treatment of hypothermia in patients with dementia with Lewy’s bodies [47]; one case study assessed the efficacy of methylphenidate in the treatment of frontotemporal dementia [48] and finally, one trial assessed the efficacy of methylphenidate in patients with dementia and negative symptoms [49] which included reduced interest in self-care, work and home tasks, social and family interaction [50].
Twenty-three out of the 27 (85.2%) studies that investigated the therapeutic effects of methylphenidate and amphetamine found a positive response to these drugs in a variety of conditions. However, four studies (14.8%) failed to show clinical improvement after methylphenidate or amphetamine: one randomized, placebo-controlled, double-blind study with methylphenidate to treat gait impairment in Parkinson’s disease [36]; one double-blind, placebo-controlled study with amphetamine in post-stroke patients [39]; one double-blind clinical trial with methylphenidate for age-related cognitive decline [37]; and a case report with methylphenidate and anorexia in an elderly woman [51].
4. DISCUSSION
In this systematic review, the majority of studies (81.5%) evaluating the therapeutic efficacy of amphetamines or methylphenidate on different conditions in the elderly had positive results. Among these studies, there were several case reports and case series alongside clinical trials. These trials had heterogeneous designs with different sample sizes, follow-up times and assessment tools. Despite promising, due to the low quality of these studies, the available evidence for the use of amphetamines in the elderly must be seen as very limited.
The most important results come from clinical trials with citalopram and methylphenidate for the treatment of depression in elderly people without dementia. In 2015, Lavretsky et al., conducted the first randomized placebo controlled trial designed to test the efficacy and tolerability of methylphenidate and citalopram in geriatric depression as a combined strategy in comparison with either citalopram or methylphenidate as monotherapy or placebo [52]. There was a significant improvement in depression severity and cognitive performance in the three treatment groups compared to placebo. The improvements in depression severity and in the Clinical Global Impression score were more prominent in the citalopram plus methylphenidate group compared with the other two treatment groups (i.e., citalopram plus placebo or methylphenidate plus placebo). Sixteen participants out of 143 (11.2%) dropped out because of side effects (seven in the citalopram plus placebo and methylphenidate plus placebo groups and two in the citalopram plus methylphenidate group). The authors concluded that stimulants can be used as a safety alternative when other drugs fail in the treatment of resistant late-life depression [52]. It is worth mentioning that major depressive disorder occurs in up to 5% of community-dwelling older adults, while 8 to 16% of older adults have clinically significant depressive symptoms [53]. Depressive disorders are frequent in this population group, and treatment resistance is not unusual [54, 55]. Besides representing an augmentation strategy, methylphenidate add-on therapy seems to accelerate improvement in depression compared to citalopram alone [30].
Methylphenidate also improved gait and postural instability in both aged subjects and [56] and patients with Parkinson’s disease [35]. Moreover, methylphenidate could decrease the risk of falls as it can improve executive and motor functioning [57, 58]. Aging is associated with an increased risk of falls, which are among the main causes of morbidity and disability in the elderly [59, 60]. More than one-third of individuals aged 65 years or older fall each year, and these events are recurrent in half of such cases [59]. These numbers are even worse for patients with Parkinson’s disease, in which the reported frequency of one or more falls in a year is above 40% [61, 62].
Regarding patients with dementia, a series of comorbid conditions improved with methylphenidate (Table 2). The use of methylphenidate to treat apathy was investigated in four out of the seven studies in dementia. Actually, apathy is the most frequent neuropsychiatric symptom in Alzheimer’s disease [63]. Apathy seems to be caused by the dysfunction of the dopaminergic reward system, justifying the therapeutic use of stimulants [64]. This condition has been associated with caregiver distress, decreased quality of a patient’s life, and increased morbidity [65, 66].
Despite the potential benefits of amphetamines and their derivatives, these drugs may exhibit different cardiovascular effects, including increased blood pressure and heart frequency [67], thus limiting their use in aged populations. As aging is associated with hypertension and atherosclerosis [68], amphetamine and derivatives can potentially increase cardiovascular risk and related outcomes such as myocardial infarction, stroke [70] and sudden death in at-risk groups [71]. Therefore, the use of such drugs must be cautious, requiring a careful cardiovascular assessment before and during its use. In 2007, the FDA added a label on psychostimulants warning about the risk of these possible cardiovascular events [72]. Methylphenidate, amphetamine and lisdexamfetamine labels inform that these drugs have not been studied in the geriatric population.
The limited number of studies regarding the use of amphetamine and its derivatives in people aged 65 years or more make it challenging to accurately predict safety and adverse effects [72]. Moreover, amphetamines and methylphenidate can aggravate other conditions typically found in the elderly, such as sleep disorders, anorexia, anxiety, agitation [73], and interact with other medications, challenging the management of the patient.
CONCLUSION
In conclusion, the available studies show encouraging results on amphetamines and methylphenidate in a variety of age-related conditions. However, due to the overall quality of these studies, further investigation, especially randomized placebo-controlled trials, is warranted to provide more significant data on the efficacy and safety of amphetamines and derivatives for the geriatric population. Any effective strategy to manage the conditions covered in this review may cause a positive social and economic impact as elderly people will be a significant part of the world population by 2050.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 3MS
Modified Mini-Mental State
- ADL
Activities of Daily Living
- ADMET
Alzheimer’s Disease Cooperative Study Clinical Global Impression of Change
- AES
Apathy Evaluation Scale
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- CGI
Clinical Global Impression
- CGI
Clinical Global Impression Scale
- CGI-I
Clinical Global Impression Scale-Improvement
- CGI-S
Clinical Global Impression Scale-Severity
- CIRS-G
Cumulative Illness Rating Scale-Geriatric
- CVRF
Cerebrovascular Risk Factor Prediction Chart
- DSM III R
Diagnostic and Statistical Manual of Mental Disorders III, Revised
- DSM III
Diagnostic and Statistical Manual of Mental Disorders III
- DSM IV
Diagnostic and Statistical Manual of Mental Disorder IV
- ECG
Electrocardiogram
- EQ-5D
EuroQoL 5-dimension
- ESS
Epworth Sleepiness Scale
- FDA
Food and Drug Administration
- FM
Fugl-Meyer
- FOGQ
Freezing of Gait Questionnaire
- GDS
Geriatric Depression Scale
- H&Y
Hoehn & Yahr
- HAM-D
Hamilton Depression Rate Scale
- IADL
Instrumental Activities of Daily Living
- IDED
Attentional set-shifting task
- MADRS
Montgomery Asberg Depression Rating Scale
- MMSE
Mini-Mental State Examination
- N
Number of participants in the trial
- NPI
Neuropsychiatric Inventory
- NTOL
Tower of London spatial planning task
- PAL
Paired Associates Learning
- PANSS-N
Positive and Negative Syndrome Scale for Schizophrenia
- QEEG
Quantitative Electroencephalography
- RVIP
Rapid visual information processing
- SANS
Scale for the Assessment of Negative symptoms
- SPECT
Single Photo Emission Computed Tomography
- SSP
Task and the spatial span
- SWM
Spatial Working Memory
- SWS
Stand-Walk-Sit Test
- UKU
Side effects rate scale
- UPDRS
Unified Parkinson’s Disease Rating Scale (I, II and III)
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
The Neuropsychiatry Program is funded by the Department of Psychiatry and Behavioral Sciences, University of Texas Health Houston.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Heal D.J., Smith S.L., Gosden J., Nutt D.J. Amphetamine, past and present--a pharmacological and clinical perspective. J. Psychopharmacol. (Oxford) 2013;27(6):479–496. doi: 10.1177/0269881113482532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rasmussen N. Amphetamine-type stimulants: The early history of their medical and non-Medical uses. Int. Rev. Neurobiol. 2015;120:9–25. doi: 10.1016/bs.irn.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen N. America’s first amphetamine epidemic 1929-1971: a quantitative and qualitative retrospective with implications for the present. Am. J. Public Health. 2008;98(6):974–985. doi: 10.2105/AJPH.2007.110593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panenka W.J., Procyshyn R.M., Lecomte T., MacEwan G.W., Flynn S.W., Honer W.G., Barr A.M. Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend. 2013;129(3):167–179. doi: 10.1016/j.drugalcdep.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Partilla J.S., Dempsey A.G., Nagpal A.S., Blough B.E., Baumann M.H., Rothman R.B. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J. Pharmacol. Exp. Ther. 2006;319(1):237–246. doi: 10.1124/jpet.106.103622. [DOI] [PubMed] [Google Scholar]
- 6.Wilens T.E. Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J. Clin. Psychopharmacol. 2008;28(3) Suppl. 2:S46–S53. doi: 10.1097/JCP.0b013e318173312f. [DOI] [PubMed] [Google Scholar]
- 7.Faraone S.V. The pharmacology of amphetamine and methylphenidate: Relevance to the neurobiology of attention-deficit/hyperactivity disorder and other psychiatric comorbidities. Neurosci. Biobehav. Rev. 2018;87:255–270. doi: 10.1016/j.neubiorev.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgkins P., Shaw M., Coghill D., Hechtman L. Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options. Eur. Child Adolesc. Psychiatry. 2012;21(9):477–492. doi: 10.1007/s00787-012-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18(1):7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly J.J., Glessner J.T., Elia J., Hakonarson H. ADHD & pharmacotherapy: Past, present and future: A review of the changing landscape of drug therapy for attention deficit hyperactivity disorder. Ther. Innov. Regul. Sci. 2015;49(5):632–642. doi: 10.1177/2168479015599811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lammers G.J. Drugs used in narcolepsy and other hypersomnias. Sleep Med. Clin. 2018;13(2):183–189. doi: 10.1016/j.jsmc.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Devos D., Moreau C., Delval A., Dujardin K., Defebvre L., Bordet R. Methylphenidate: a treatment for Parkinson’s disease? CNS Drugs. 2013;27(1):1–14. doi: 10.1007/s40263-012-0017-y. [DOI] [PubMed] [Google Scholar]
- 13.Shorer Z., Bachner Y., Guy T., Melzer I. Effect of single dose methylphenidate on walking and postural stability under single- and dual-task conditions in older adults--a double-blind randomized control trial. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(10):1271–1280. doi: 10.1093/gerona/glt035. [DOI] [PubMed] [Google Scholar]
- 14.Nelson J.C. The role of stimulants in late-life depression. Am. J. Psychiatry. 2015;172(6):505–507. doi: 10.1176/appi.ajp.2015.15030356. [DOI] [PubMed] [Google Scholar]
- 15.Madhusoodanan S., Goia D. Rapid resolution of depressive symptoms with methylphenidate augmentation of mirtazapine in an elderly depressed hospitalized patient: A case report. Clin. Pract. 2014;11(3):283–288. doi: 10.2217/cpr.14.32. [DOI] [Google Scholar]
- 16.Madhusoodanan S., Landinez J. Methylphenidate augmentation for treatment-resistant depression in an elderly patient with a meningioma. Ann. Longterm Care. 2016;24(9):33–36. [Google Scholar]
- 17.Padala P.R., Burke W.J., Bhatia S.C., Petty F. Treatment of apathy with methylphenidate. J. Neuropsychiatry Clin. Neurosci. 2007;19(1):81–83. doi: 10.1176/jnp.2007.19.1.81. [DOI] [PubMed] [Google Scholar]
- 18.Prowler M.L., Weiss D., Caroff S.N. Treatment of catatonia with methylphenidate in an elderly patient with depression. Psychosomatics. 2010;51(1):74–76. doi: 10.1016/S0033-3182(10)70662-9. [DOI] [PubMed] [Google Scholar]
- 19.From the centers for disease control and prevention. Public health and aging: trends in aging--United States and worldwide. JAMA. 2003;289(11):1371–1373. doi: 10.1001/jama.289.11.1371. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Trends in aging--United states and worldwide. MMWR Morb. Mortal. Wkly. Rep. 2003;52(6):101–104, 106. [PubMed] [Google Scholar]
- 21.Kaye J.A. Healthy brain aging. Arch. Neurol. 2002;59(11):1721–1723. doi: 10.1001/archneur.59.11.1721. [DOI] [PubMed] [Google Scholar]
- 22.Brown R.C., Lockwood A.H., Sonawane B.R. Neurodegenerative diseases: an overview of environmental risk factors. Environ. Health Perspect. 2005;113(9):1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niccoli T., Partridge L. Ageing as a risk factor for disease. Curr. Biol. 2012;22(17):R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters R. Ageing and the brain. Postgrad. Med. J. 2006;82(964):84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calipari E.S., Ferris M.J. Amphetamine mechanisms and actions at the dopamine terminal revisited. J. Neurosci. 2013;33(21):8923–8925. doi: 10.1523/JNEUROSCI.1033-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohan K.J., Rough J.N., Evans M., Ho S.Y., Meyerhoff J., Roberts L.M., Vacek P.M. A protocol for the Hamilton rating scale for depression: Item scoring rules, rater training, and outcome accuracy with data on its application in a clinical trial. J. Affect. Disord. 2016;200:111–118. doi: 10.1016/j.jad.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavretsky H., Kumar A. Methylphenidate augmentation of citalopram in elderly depressed patients. Am. J. Geriatr. Psychiatry. 2001;9(3):298–303. doi: 10.1097/00019442-200108000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Lavretsky H., Park S., Siddarth P., Kumar A., Reynolds C.F., III Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am. J. Geriatr. Psychiatry. 2006;14(2):181–185. doi: 10.1097/01.JGP.0000192503.10692.9f. [DOI] [PubMed] [Google Scholar]
- 31.Lavretsky H., Reinlieb M., St Cyr N., Siddarth P., Ercoli L.M., Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am. J. Psychiatry. 2015;172(6):561–569. doi: 10.1176/appi.ajp.2014.14070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavretsky H., Kim M.D., Kumar A., Reynolds C.F., III Combined treatment with methylphenidate and citalopram for accelerated response in the elderly: an open trial. J. Clin. Psychiatry. 2003;64(12):1410–1414. doi: 10.4088/JCP.v64n1202. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus L.W., Winemiller D.R., Lingam V.R., Neyman I., Hartman C., Abassian M., Kartan U., Groves L., Fawcett J. Efficacy and side effects of methylphenidate for poststroke depression. J. Clin. Psychiatry. 1992;53(12):447–449. [PubMed] [Google Scholar]
- 34.Lazarus L.W., Moberg P.J., Langsley P.R., Lingam V.R. Methylphenidate and nortriptyline in the treatment of poststroke depression: a retrospective comparison. Arch. Phys. Med. Rehabil. 1994;75(4):403–406. doi: 10.1016/0003-9993(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 35.Devos D., Krystkowiak P., Clement F., Dujardin K., Cottencin O., Waucquier N., Ajebbar K., Thielemans B., Kroumova M., Duhamel A., Destée A., Bordet R., Defebvre L. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2007;78(5):470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Espay A.J., Dwivedi A.K., Payne M., Gaines L., Vaughan J.E., Maddux B.N., Slevin J.T., Gartner M., Sahay A., Revilla F.J., Duker A.P., Shukla R. Methylphenidate for gait impairment in Parkinson disease: a randomized clinical trial. Neurology. 2011;76(14):1256–1262. doi: 10.1212/WNL.0b013e3182143537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner D.C., Robbins T.W., Clark L., Aron A.R., Dowson J., Sahakian B.J. Relative lack of cognitive effects of methylphenidate in elderly male volunteers. Psychopharmacology (Berl.) 2003;168(4):455–464. doi: 10.1007/s00213-003-1457-3. [DOI] [PubMed] [Google Scholar]
- 38.Sonde L., Lökk J. Effects of amphetamine and/or L-dopa and physiotherapy after stroke - a blinded randomized study. Acta Neurol. Scand. 2007;115(1):55–59. doi: 10.1111/j.1600-0404.2006.00728.x. [DOI] [PubMed] [Google Scholar]
- 39.Sonde L., Nordström M., Nilsson C.G., Lökk J., Viitanen M. A double-blind placebo-controlled study of the effects of amphetamine and physiotherapy after stroke. Cerebrovasc. Dis. 2001;12(3):253–257. doi: 10.1159/000047712. [DOI] [PubMed] [Google Scholar]
- 40.Pickett P., Masand P., Murray G.B. Psychostimulant treatment of geriatric depressive disorders secondary to medical illness. J. Geriatr. Psychiatry Neurol. 1990;3(3):146–151. doi: 10.1177/089198879000300304. [DOI] [PubMed] [Google Scholar]
- 41.Wallace A.E., Kofoed L.L., West A.N. Double-blind, placebo-controlled trial of methylphenidate in older, depressed, medically ill patients. Am. J. Psychiatry. 1995;152(6):929–931. doi: 10.1176/ajp.152.6.929. [DOI] [PubMed] [Google Scholar]
- 42.Ayache D.C., Junior R.F. Methylphenidate in a patient with depression and respiratory insufficiency. Int. J. Psychiatry Med. 2001;31(4):443–449. doi: 10.2190/CXNE-0UFR-7Q04-CQ3M. [DOI] [PubMed] [Google Scholar]
- 43.Padala P.R., Padala K.P., Lensing S.Y., Ramirez D., Monga V., Bopp M.M., Roberson P.K., Dennis R.A., Petty F., Sullivan D.H., Burke W.J. Methylphenidate for Apathy in Community-Dwelling Older Veterans With Mild Alzheimer’s Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Am. J. Psychiatry. 2018;175(2):159–168. doi: 10.1176/appi.ajp.2017.17030316. [DOI] [PubMed] [Google Scholar]
- 44.Padala P.R., Burke W.J., Shostrom V.K., Bhatia S.C., Wengel S.P., Potter J.F., Petty F. Methylphenidate for apathy and functional status in dementia of the Alzheimer type. Am. J. Geriatr. Psychiatry. 2010;18(4):371–374. doi: 10.1097/JGP.0b013e3181cabcf6. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg P.B., Lanctôt K.L., Drye L.T., Herrmann N., Scherer R.W., Bachman D.L., Mintzer J.E. Safety and efficacy of methylphenidate for apathy in Alzheimer’s disease: a randomized, placebo-controlled trial. J. Clin. Psychiatry. 2013;74(8):810–816. doi: 10.4088/JCP.12m08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann N., Rothenburg L.S., Black S.E., Ryan M., Liu B.A., Busto U.E., Lanctôt K.L. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J. Clin. Psychopharmacol. 2008;28(3):296–301. doi: 10.1097/JCP.0b013e318172b479. [DOI] [PubMed] [Google Scholar]
- 47.Baeyens H., Dekoninck J., Baeyens J.P. The experimental use of methylphenidate for hypothermia in patients with lewy body dementia. Eur. Geriatr. Med. 2011;2:177–178. doi: 10.1016/j.eurger.2011.05.014. [DOI] [Google Scholar]
- 48.Goforth H.W., Konopka L., Primeau M., Ruth A., O’Donnell K., Patel R., Poprawski T., Shirazi P., Rao M. Quantitative electroencephalography in frontotemporal dementia with methylphenidate response: a case study. Clin. EEG Neurosci. 2004;35(2):108–111. doi: 10.1177/155005940403500212. [DOI] [PubMed] [Google Scholar]
- 49.Galynker I., Ieronimo C., Miner C., Rosenblum J., Vilkas N., Rosenthal R. Methylphenidate treatment of negative symptoms in patients with dementia. J. Neuropsychiatry Clin. Neurosci. 1997;9(2):231–239. doi: 10.1176/jnp.9.2.231. [DOI] [PubMed] [Google Scholar]
- 50.Reichman W.E., Coyne A.C., Amirneni S., Molino B., Jr, Egan S. Negative symptoms in Alzheimer’s disease. Am. J. Psychiatry. 1996;153(3):424–426. doi: 10.1176/ajp.153.3.424. [DOI] [PubMed] [Google Scholar]
- 51.Pobee K.A., LaPalio L.R. Anorexia nervosa in the elderly: A multidisciplinary diagnosis. Clin. Gerontol. 1996;16(3):3–9. doi: 10.1300/J018v16n03_02. [DOI] [Google Scholar]
- 52.Lavretsky H., Reinlieb M., St Cyr N., Siddarth P., Ercoli L.M., Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am. J. Psychiatry. 2015;172(6):561–569. doi: 10.1176/appi.ajp.2014.14070889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor W.D. Clinical practice. Depression in the elderly. N. Engl. J. Med. 2014;371(13):1228–1236. doi: 10.1056/NEJMcp1402180. [DOI] [PubMed] [Google Scholar]
- 54.Unützer J., Park M. Older adults with severe, treatment-resistant depression. JAMA. 2012;308(9):909–918. doi: 10.1001/2012.jama.10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kok R.M., Reynolds C.F. III Management of Depression in Older Adults: A Review. JAMA. 2017;317(20):2114–2122. doi: 10.1001/jama.2017.5706. [DOI] [PubMed] [Google Scholar]
- 56.Shorer Z., Bachner Y., Guy T., Melzer I. Effect of single dose methylphenidate on walking and postural stability under single- and dual-task conditions in older adults--a double-blind randomized control trial. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(10):1271–1280. doi: 10.1093/gerona/glt035. [DOI] [PubMed] [Google Scholar]
- 57.Schweitzer J.B., Lee D.O., Hanford R.B., Zink C.F., Ely T.D., Tagamets M.A., Hoffman J.M., Grafton S.T., Kilts C.D. Effect of methylphenidate on executive functioning in adults with attention-deficit/hyperactivity disorder: normalization of behavior but not related brain activity. Biol. Psychiatry. 2004;56(8):597–606. doi: 10.1016/j.biopsych.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Soleimani R., Kousha M., Zarrabi H., Tavafzadeh-Haghi S.M., Jalali M.M. The impact of methylphenidate on motor performance in children with both attention deficit hyperactivity disorder and developmental Coordination Disorder: A randomized double-blind crossover clinical trial. Iran. J. Med. Sci. 2017;42(4):354–361. [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Aama T. Falls in the elderly: spectrum and prevention. Can. Fam. Phys. 2011;57(7):771–776. [PMC free article] [PubMed] [Google Scholar]
- 60.Agashivala N., Wu W.K. Effects of potentially inappropriate psychoactive medications on falls in US nursing home residents: analysis of the 2004 National Nursing Home Survey database. Drugs Aging. 2009;26(10):853–860. doi: 10.2165/11316800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Fasano A., Canning C.G., Hausdorff J.M., Lord S., Rochester L. Falls in Parkinson’s disease: A complex and evolving picture. Mov. Disord. 2017;32(11):1524–1536. doi: 10.1002/mds.27195. [DOI] [PubMed] [Google Scholar]
- 62.Grimbergen Y.A., Munneke M., Bloem B.R. Falls in Parkinson’s disease. Curr. Opin. Neurol. 2004;17(4):405–415. doi: 10.1097/01.wco.0000137530.68867.93. [DOI] [PubMed] [Google Scholar]
- 63.Nobis L., Husain M. Apathy in Alzheimer’s disease. Curr. Opin. Behav. Sci. 2018;22:7–13. doi: 10.1016/j.cobeha.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guimarães H.C., Levy R., Teixeira A.L., Beato R.G., Caramelli P. Neurobiology of apathy in Alzheimer’s disease. Arq. Neuropsiquiatr. 2008;66(2B):436–443. doi: 10.1590/S0004-282X2008000300035. [DOI] [PubMed] [Google Scholar]
- 65.Schulz R., O’Brien A.T., Bookwala J., Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35(6):771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 66.Mitrani V.B., Czaja S.J. Family-based therapy for dementia caregivers: clinical observations. Aging Ment. Health. 2000;4(3):200–209. doi: 10.1080/713649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinkellner T., Freissmuth M., Sitte H.H., Montgomery T. The ugly side of amphetamines: short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol. Chem. 2011;392(1-2):103–115. doi: 10.1515/bc.2011.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Indave B.I., Sordo L., Bravo M.J., Sarasa-Renedo A., Fernández-Balbuena S., De la Fuente L., Sonego M., Barrio G. Risk of stroke in prescription and other amphetamine-type stimulants use: A systematic review. Drug Alcohol Rev. 2018;37(1):56–69. doi: 10.1111/dar.12559. [DOI] [PubMed] [Google Scholar]
- 70.Westover A.N., Halm E.A. Do prescription stimulants increase the risk of adverse cardiovascular events? A systematic review. BMC Cardiovasc. Disord. 2012;12:41. doi: 10.1186/1471-2261-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kornfield R., Watson S., Higashi A.S., Conti R.M., Dusetzina S.B., Garfield C.F., Dorsey E.R., Huskamp H.A., Alexander G.C. Effects of FDA advisories on the pharmacologic treatment of ADHD, 2004-2008. Psychiatr. Serv. 2013;64(4):339–346. doi: 10.1176/appi.ps.201200147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gurian B., Rosowsky E. Low-dose methylphenidate in the very old. J. Geriatr. Psychiatry Neurol. 1990;3(3):152–154. doi: 10.1177/089198879000300305. [DOI] [PubMed] [Google Scholar]
- 73.Berman S.M., Kuczenski R., McCracken J.T., London E.D. Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol. Psychiatry. 2009;14(2):123–142. doi: 10.1038/mp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]


