Abstract
Mood disorders are the most prevalent mental conditions encountered in psychiatric practice. Numerous patients suffering from mood disorders present with treatment-resistant forms of depression, co-morbid anxiety, other psychiatric disorders and bipolar disorders. Standardized essential oils (such as that of Lavender officinalis) have been shown to exert clinical efficacy in treating anxiety disorders. As endocannabinoids are suggested to play an important role in major depression, generalized anxiety and bipolar disorders, Cannabis sativa was suggested for their treatment. The endocannabinoid system is widely distributed throughout the body including the brain, modulating many functions. It is involved in mood and related disorders, and its activity may be modified by exogenous cannabinoids. CB1 and CB2 receptors primarily serve as the binding sites for endocannabinoids as well as for phytocannabinoids, produced by cannabis inflorescences. However, ‘cannabis’ is not a single compound product but is known for its complicated molecular profile, producing a plethora of phytocannabinoids alongside a vast array of terpenes. Thus, the “entourage effect” is the suggested positive contribution derived from the addition of terpenes to cannabinoids. Here, we review the literature on the effects of cannabinoids and discuss the possibility of enhancing cannabinoid activity on psychiatric symptoms by the addition of terpenes and terpenoids. Possible underlying mechanisms for the anti-depressant and anxiolytic effects are reviewed. These natural products may be an important potential source for new medications for the treatment of mood and anxiety disorders.
Keywords: Cannabidiol, terpenes, mood disorders, depression, anxiety, entourage
1. INTRODUCTION
1.1. Mood Disorders-Depression Disorder, Anxiety Disorder and Bipolar Disorder
Major depressive and anxiety disorders are the most prevalent mental conditions encountered in the psychiatric practice. Depression is a disease affecting hundreds of millions of people all over the world, and approximately 20% of the population will suffer from depression during their lifetime. It affects a vast spectrum of symptoms and has a tremendous effect on society due to the severe distress and disruption of life and, if left untreated, can be life-threatening [1]. Often closely related to depressive states is the presence of anxiety disorders, which are highly comorbid with other mental and physical disorders [2]. Bipolar disorder (BD) is characterized by switching between depressive and manic mood states, however, Bipolar 1 can be diagnosed even with one episode of mania. It is a relatively common condition afflicting approximately 1% of the general population and is considered a chronic disease that may require lifetime treatment. Similarly to anxiety and depressive disorders; the underlying pathophysiology of BD is unknown [3]. Numerous patients with these disorders are treatment-resistant with relatively high degrees of comorbidity with other psychiatric disorders such as obsessive-compulsive disorder. Resistance to treatment accompanied by incapability to work and frequent hospitalizations creates extensive costs for the health care and economic national systems. First-line medications for mood disorders are mostly based on the monoaminergic system (the noradrenergic, serotonergic, and dopaminergic neurotransmitters). Indeed, mounting evidence has shown that this system is responsible for many emotional and behavioral symptoms, among which are mood, vigilance, motivation, fatigue, and psychomotor agitation or retardation. Yet, the exact mechanisms are not completely understood [4] and research supports multiple neurochemical targets beyond the monoaminergic system. The treatments available tend to ease symptoms in about 60-70% of cases leaving numerous patients who do not find relief and the rate of remission is discouragingly low [5, 6]. Moreover, many who are responsive suffer from negative side effects such as dry mouth, abdominal pain, sexual dysfunction, increased anxiety, expressions of violence and even suicide [7]. There is no universally effective treatment or a cure for these disorders. Consequently, new pharmacological approaches are needed to both alleviate the wide-range of adverse effects and to treat those who do not respond to medications currently available. Recent studies, including randomized controlled trials, indicate that certain phytocannabinoids produced by certain Cannabis sativa varieties have the potential to alleviate anxiety and other mental disorders [5-9]. Here, we review the literature on the effects of cannabinoids and discuss the possibility of enhancing cannabinoid activity on psychiatric symptoms by the addition of terpenes and terpenoids. The method was a search in Pubmed and Google Scholar for relevant literature, including contradicting results, from the past 25 years by crossing the keywords cannabinoids, terpenes and anxiety, depression and bipolar disorders.
1.2. Cannabis Secondary Metabolites and their Biological Affinity
Cannabis sativa produces hundreds of different compounds, including a plethora of phytocannabinoids [10]. Out of the ±150 cannabinoids that were identified in C. sativa to-date, the most studied phytocannabinoids are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). These are produced in the plant in their carboxylated form as Δ9-tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA) and are decarboxylated, mainly under high temperatures, into their activated forms, THC and CBD respectively.
Research shows that phytocannabinoids bind to endogenous receptors in mammals, mainly of two types of G-protein-coupled cannabinoid receptors - CB1 and CB2 [11, 12]. However, there are additional receptors suggested to bind with phytocannabinoids including a G-coupled binding receptor GPCR55 [13, 14] and a nuclear, peroxisome proliferator‐activated receptors (PPARs), with three subtypes α, β (δ) and γ [15]. The endocannabinoid receptors are distributed all over the human body in different organs and cell types. These receptors are present in the central nervous system (CNS), as well as in arteries, heart, spleen, the urinary and reproductive systems, in the gastrointestinal tracts and the endocrine glands [16].
CB1 and CB2 receptors were originally designed to bind with endocannabinoids [17]. Tens of different endocannabinoids (endogenous compounds detected in mammalian tissues) are known, however, the most studied are anandamide (AEA) and 2-arachidonoylglycerol (2-AG). The endocannabinoid system consists of enzymes which are able to both catalyze endocannabinoid biosynthesis and metabolism, and downstream signaling pathways in response to CB1 and CB2 receptor activation [17]. Endocannabinoids participate in various processes in mammals, including activity and disease of the immune, neuronal and reproductive systems. They regulate metabolic activity and disorders, the cardiovascular system, the digestive tract and bladder [17]. Endocannabinoids are also involved in mental activity and disorders [17]. As cannabinoid receptors are distributed in many biophysical systems, their presence and absence are suggested to be involved in various functions in humans either directly or obliquely. Recent research indicates that the endocannabinoid system has a biological role in, and might be a potential target for the treatment of, certain psychiatric disorders [18, 19].
Some of the phytocannabinoids mimic the activity of endocannabinoids via binding to the endocannabinoid receptors. For example, THC which is the major psychoactive compound in the cannabis plant, and its metabolite 11-OH-THC is even more potent [20, 21]. These cannabinoids are agonists of endogenous cannabinoid CB1 receptors that are spread in large amounts in the spinal cord and peripheral nerves and in the brain, mainly in the cerebral cortex, including the cingulate cortex, hippocampus, basal amygdala, corpus striatum and other areas involved in mood disorders [22]. Unlike THC, CBD has relatively low affinity for the endocannabinoid receptors, however, there is evidence that it can interact with CB1 and CB2 receptors at reasonably low concentrations. In line with its low affinity for these receptors, most research with CBD has been directed at understanding CB1- and CB2-independent modes of action [23].
1.3. Involvement of Cannabinoids in the Treatment of Mood Disorders
Although both THC and CBD have therapeutic potential, it is CBD that was suggested to exhibit relatively high potency in relieving mood disorders: depression, anxiety and bipolar disorder [8, 9]. THC, a direct agonist of CB1 and CB2 receptors [24], has been shown in rats to have antidepressant-like effects on males more than on females [25]. In humans, it has been shown that THC has potential efficacy in the reduction of depression in joint administration with CBD [26]. CBD is a non-psychoactive component and demonstrated positive effects on several psychiatric disorders [27, 28], neurologic disorders like multiple-sclerosis [29] and epilepsy [29] and it is considered to possess neuroprotective characteristics [30]. The multiple pathways in which CBD exerts its effects are only partially understood [31]. CBD has repeatedly exerted antidepressant-like and anxiolytic-like effects in animal models [32-34]. These studies utilize standardized behavioral tests (such as elevated plus maze (EPM) for anxiety-like behavior and forced swim test (FST) for testing depressive-like behavior) to assess changes in symptom severity and activity of drugs. Indeed, fluorinated CBD derivatives showed high potency for behavioral effects in the EPM and FST [24-26, 35, 36].
1.4. Major Depression
Pre-clinical, animal model studies with CBD show that antidepressant-like effects can occur after either a single acute dose or chronic administration. Acute anti-depressant-like effects as modeled by a reduction in helplessness-like behavior were demonstrated in male and female Wistar-Kyoto (WKY) and male Flinders sensitive line (FSL) rats, both genetic model of depression, using the FST [37]. Increased convergent validity of CBD as an antidepressant treatment was demonstrated, using the tail suspension test (TST) another behavioral test indicating levels of helplessness. These effects were independently reinforced by modeling an additional depressive-like symptom using the behavioral test, the saccharin preference test (SPT) which is used to assess the levels of anhedonia. A recent study showed that CBD (30 mg/kg) induces a pro-hedonic effect in male WKY rats in SPT [33]. Interestingly, Zanelati et al., (2010) found an inverted U-shaped dose-dependent curve in mice after acute administration of CBD (3, 10, 30, 100 mg/kg, i.p) given 30 min prior to FST [34]. Results demonstrate that CBD reduced immobility in FST similar to the tricyclic antidepressant imipramine (both at 30 mg/kg) [34].
Other studies demonstrated CBD's anti-depressant effects after chronic administration. Rats administered 30 mg/kg for 14 days showed reduced immobility time and increased swimming time in FST without CBD affecting their locomotor activity in the open field test [38]. Similarly, single or repeated (15 days) admission of 20 mg/kg CBD reduced immobility time compared with those of imipramine [39].
Few studies provide biological evidence for the anti-depressant effect of CBD. For example, chronic treatment with 15 mg/kg CBD increased BDNF levels, a neuropeptide that plays an important role in depression, in the rat amygdala [38]. Moreover, 5-HT1A receptor antagonist (WAY100635) blocked the anti-depressant effect of CBD in the FST [34, 40], revealing the involvement of 5-HT1A receptors and BDNF in the observed effects of CBD. Both depression and anxiety are mediated through 5-HT1A receptors. It has been shown that CBD acts as a 5-HT1A agonist and the beneficial effect in mice of CBD in these two disorders may be mediated by this activation [34]. Despite this significant body of evidence, the prevalent nature of the psychiatric disorder and the struggle to advocate a relief for those suffering from mood disorders, to the best of our knowledge no controlled clinical study published yet has investigated whether CBD can decrease depressive symptoms in patients.
1.5. Anxiety Disorders
As the effects of cannabinoids on anxiety disorders have been extensively studied, we will review them only briefly. It has been repeatedly shown that CBD and THC decrease anxiety, the most common psychiatric disorder, in both animals and humans [41-48].
Knockout mice without the CB1 receptor exhibited increased anxiety-like behavior in light/dark box [49]. It has been suggested that amygdala, hippocampus, hypothalamus and cingulate cortex are candidate brain sites and pathways of the anxiolytic action of CBD [49]. Beyond the 5-HT1A receptor, several additional mechanisms have been proposed for anxiolytic effects of cannabinoids, including the free-acid amidhydrolase (FAAH) inhibition, COX-2 inhibition and TRPV1 blockade characteristics [50]. Note, that in contrast to several positive reports [51, 52], a systematic review and meta-analysis failed to find an anxiolytic effect of cannabinoids [53, 54].
1.6. Bipolar Disorder (BD)
Earlier studies have suggested that oxidative stress may play a role in the pathophysiology of BD. In a rat model of BD, it has been concluded that CBD protects against D-AMPH-induced oxidative protein damage and increases BDNF levels in the reversal model and these effects vary depending on the brain regions evaluated and doses of CBD administered [55]. The protective effects of CBD against glutamate toxicity may have a mood-stabilizing action similar to some other antiepileptic drugs of validated value in BD [22, 56, 57]. However, the empirical results reported are not always positive. In an animal study, CBD was not able to prevent or reverse the hyperlocomotion induced by D-AMPH, an animal model for mania-like behavior [55]. Based on small size studies on BD and taken together a recent review concluded that CBD may not be effective in manic episodes [58].
The conflicting results on the efficacy of CBD in BD still do not have clear implications for treatment. Obviously, the role of CBD and THC, their doses and complementary relative amounts, need further study. Several possible explanations were suggested for the diverse effects of cannabinoids. Some researchers thought that the cognitive and stress-related effects of cannabinoid signaling are dependent on the particular situation [59]. It was suggested that cannabinoids do not affect brain centers involved in the control of a particular behavior, but affect the way in which environmental stimuli are interpreted in the brain [59]. Another possible explanation might be that cannabinoid activity is dependent on the animal species and cannabis chemotype and its given composite molecular constituents [60, 61].
1.7. Possible Underlying Mechanisms
Anxiolytic and antidepressant effects may be found as a result of the interaction between cannabinoid and NA/5-HT systems [62]. Others suggest that the anxiolytic effect results from the presynaptic CB1 receptor-mediated inhibition of acetylcholine released by preganglionic sympathetic neurons [63], although CBD's attachment to the CB-1 and CB-2 receptors is controversial. Biochemical studies indicate that CBD may enhance endogenous signaling of the endocannabinoid anandamide indirectly. This is by inhibiting the intracellular degradation of anandamide catalyzed, by the enzyme fatty acid amide hydrolase [64]. In addition, another arousal mechanism that is mediated by cannabinoids is the inhibition of adrenaline secretion in adrenal glands and this may account for the decrease in plasma adrenaline concentration found following cannabinoid administration in rabbits [63]. However, while known to modulate neuroendocrine function, the precise acute and chronic dose-related effects of cannabinoids in humans remain to be studied carefully [65].
2. INVOLVEMENT OF TERPENES IN THE TREATMENT OF MOOD DISORDERS
In addition to cannabinoids, other secondary metabolites of cannabis have shown anxiolytic effects. A comprehensive preclinical study showed that plant-derived chemicals like alkaloids, terpenes, flavonoids, phenolic acids, lignans, cinnamates, and saponins possess anxiolytic properties using various animal models of anxiety-like behavior [66]. Terpenes are an important class of compounds produced by C. sativa, contributing its characteristic aroma [22]. Each cannabis strain bears a typical terpenoid profile, differing from other strains both qualitatively and quantitatively according to their relative amounts and the assemblage of the given terpenes present [67]. Terpenes and terpenoids are not unique to cannabis as many other flower-producing plants also produce them.
Terpenes are organic compounds built up from hydrocarbon building blocks called isoprenes [68]. Isoprene is a molecule that consists of five carbon atoms attached to eight hydrogen atoms (C5H8) [69]. Terpenes are classified according to the number of pairs of isoprenes used to build the individual terpene – mono, sesqui, di etc. composing either 10-carbon monoterpenes, 15-carbon sesquiterpenes or 20-carbon diterpenes, respectively [69]. These three groups (mono-, di-, and sesqui-terpenes) are the most abundant terpenes in the essential oils of plants, cannabis included. Sesquiterpenes and bigger terpenes tend to degrade into monoterpenes with time or upon exposure to heat or UV and visible light. In plants, several terpene synthase enzymes are responsible for the synthesis of all the different terpenes building a vast array of terpenes [70, 71]. The term “terpenes” is often extended to include also terpenoids, the oxygenated derivatives of terpenes. These compounds are relatively more volatile and have a higher susceptibility to degradation than terpenes [67].
Importantly, terpenes were suggested not only to convey the smell of the different cannabis flowers but also to have some therapeutic abilities either by themselves or as co-activating agents, enhancing the beneficial activity of phytocannabinoids on humans [72]. Although most cannabis treatments for mood disorders involve the use of whole-inflorescences rather than a single-compound, and despite that cannabis inflorescences accumulate hundreds of milligrams of terpenes alongside cannabinoids (from 8% up to 20% of the cannabinoids content; Namdar et al., 2018), the involvement of terpenes as potential treatment for anxiety and depression has been under-studied.
In general, of the 400 terpenes known in cannabis, very few have been examined at the functional level [73]. A positive effect of terpenes on various psychiatric endophenotypes has been shown [67, 74]. For instance, propolis essential oil that contains several terpenes, such as cinnamyl alcohol, α- and β-Caryophyllene, Cadinene, Guaiol and Eudesmol revealed, in restraint-stressed mice, significant improvement in anxiety-like behavior [75]. The essential oil, as in many other studies on terpenes, had no effect on locomotor activity and significantly antagonized the hyperfunction of hypothalamic–pituitary–adrenal (HPA) axis, indicating that propolis essential oil contains therapeutic effects on anxiety [75]. Likewise, crocins - related hydrophilic carotenoids found in flowers, given to rats in doses of 30 and 50 mg/kg reduced mCPP-induced amplified self-grooming. These effects of crocins could not be attributed to changes in locomotor activity [76].
Standardized essential oils (such as that of Lavender officinalis) have been shown to exert clinical efficacy in treating anxiety disorders. This supports the assessment that these natural products are an important potential source for new anxiolytic drugs. A systematic review of essential oils, their bioactive constituents, and anxiolytic-like activity in animal models has been published [77]. According to the authors, the highest potent essential oil is that of Lavendula angustifolia, which has already been tested in controlled clinical trials with positive anxiolytic results. Different research groups, using different routes of administration supported the results that Lavender and Citrus aurantium essential oils have significant anxiolytic-like effects in several animal models such as the open field, elevated plus maze and marble burying tests as well. Other promising essential oils are those of Citrus synesis and bergamot oil, which showed some clinical anxiolytic actions. The potential antidepressant activity of these essential oils is of interest.
In a randomized, double-blind, double-dummy trial, 539 adults with Generalized Anxiety Disorder according to DSM-5 criteria and a Hamilton Anxiety Scale (HAMA) total score of 518 points, participated and received 160 or 80 mg Silexan (a lavender essential oil), 20 mg paroxetine (an antidepressant of the selective serotonin reuptake inhibitor, SSRI), or placebo once a day for 10 weeks. Silexan produced more anxiolytic effects than paroxetine [78], supporting its potential as a non-addictive alternative to benzodiazepines. However, in an animal model study, Linalool (a major component of lavender) did not show anxiolytic efficacy and its activity was not through the GABAA receptor [79].
Lima et al., (2013) reported on anxiolytic-like effects of (+)-limonene in an elevated plus-maze model (EPM) of anxiety in mice. Both concentrations of 0.5% and 1.0%, (+)-limonene, administered to mice by inhalation, significantly modified all the parameters measured in the EPM test in the same direction as intraperitoneally administered diazepam [80].
De Almeida et al., (2014) evaluated anxiolytic activity of (+)-limonene epoxide (EL) through the marble-burying test (MBT) assay and the antioxidant potential in vitro and in vivo in hippocampus of adult mice [81]. Subjects were treated with EL, ascorbic acid, diazepam or placebo of saline. The results showed the greatest reduction in marble burying in the subjects treated with EL, suggesting an anxiolytic effect of EL.
Only a few studies have addressed antidepressant effects of terpenes and in those, not all terpenes demonstrated similar effect as in anxiety-related illnesses. For example, the stimulant-like or CNS depressant-like effects of 20 essential oils were compared with CNS acting drugs [81]. Lavender and hyssop oils showed potential value for treating depression [81]; recall that the anxiolytic efficacy of Lavender is not consistently supported.
2.1. Potential Mechanisms
Plant essential oils, among which are Achillea wilhemsii, Alpinia zerumbet, Citrus aurantia, and Spiranthera odoratissima and Lavendula angustifolia, appear to exert anxiolytic-like effects without GABA/benzodiazepine activity, thus acting in different routes in their mechanisms of action from the benzodiazepines (for references for all the above see De Sousa et al., 2015) [77]. Accordingly, the pharmacological effect of inhaled D-limonene (1%) mentioned above, in the study by Lima et al., (2013) [80], was not blocked by flumazenil - a selective benzodiazepine receptor antagonist which did block the effects of diazepam. Thus, terpenes appear to exert their effects by different brain pathways than benzodiazepines. Achieving an anxiolytic effect in a mechanism which does not connect to the benzodiazepines pathway may offer new treatments that minimize the risks of addiction.
The treatment with EL, which reduced marble-burying (see above) decreased the lipid peroxidation level and nitrite content, supporting antioxidant activity. Furthermore, the EL increased activity of the enzymes catalase and superoxide dismutase in mice hippocampus indicates that EL may operate through antioxidant upregulation of these enzymes. This research reinforces the potential of treatment with terpenes to avoid the risk of addiction [81]. Thus, the decrease of oxidative stress by EL, and potentially by other terpenes may underlie their anti-anxiety effects.
Potential mechanisms for the antidepressant-like effects of linalool and β-pinene on mice were tested using FST [82]. The terpene activity was compared with that of a Serotonin-Reuptake Inhibitor (SRI) drug, fluoxetine, which is a widely used anti-depressant, and other antagonist drugs. It was found that WAY100635, a 5-HT1A receptor antagonist, blocked the antidepressant-like effect of linalool and β-pinene. In contrast, pretreatment of mice with serotonin synthesis inhibitor PCPA did not modify reductions in the immobility time elicited by the two monoterpenes. However, Yohimbine, an α2 adrenergic receptor antagonist, was found to modify the observed effect of linalool on immobility time. In the case of β-pinene Propranolol, a non-selective β adrenergic receptor antagonist, and DSP-4, a noradrenergic neurotoxin, both reversed the anti-immobility effect. In addition, SCH23390, a D1 receptor antagonist, blocked the antidepressant-like effect of β-pinene. Taken together, the results show that linalool and β-pinene produce an antidepressant-like effect through interaction with the monoaminergic system [82]. Note, these findings suggest caution when planning combined treatment with monoaminergic antidepressants and terpenes. In addition, further studies of underlying mechanisms are warranted.
3. CANNABINOIDS, TERPENES AND THE IMMUNE SYSTEM
Abundant evidence points to an elevation in inflammatory markers in affective disorders [83-86]. Hence, new therapeutic agents may consider this pathway.
Cannabinoids have been shown to modulate a variety of immune cell functions in humans and animals [87-91]. It seems that cannabinoids and their agonists can exert both immunomodulatory and neuroprotective effects [92]. For example, CBD can inhibit immune cell migration and thus induce anti-inflammatory effects [91]. In addition, JWH-015, a synthetic CB2-selective agonist triggered apoptosis in thymocytes in vitro and inhibited the proliferative response of T and B cells to mitogens through the induction of apoptosis [93].
Although the information on the immunomodulatory effects of terpenes is scarce [94], some terpenes also are immunosuppressive agents [95]. For example, terpenes extracted from Zanthoxylum rhoifolium, a South American tree, significantly improved NK cell cytotoxicity in vitro and in vivo in tumors [96] and the terpenes isolated from Ganoderma applanatum present similar protective effect by possessing antioxidant abilities [97].
In this context, it would be useful to mention that 'treatment resistance' may actually be an indicant of neuroprogression and the changing biological underpinnings of affective disorders over recurrent episodes (see e.g., [98]).
Hence, in cases of treatment resistance, novel treatment approaches, as discussed in this review, may address the risk of neuroprogression.
4. CANNABINOIDS AND THE MICROBIOTA-GUT-BRAIN AXIS
Interestingly, the microbiota has been shown to be important effectors of normal healthy brain function. Nearly 100 trillion bacteria inhabit the human intestine. These were recognized previously to be important for human metabolism and development and function of the human immune system. Yet, in recent studies, it was established that gut microbiota is also important for the function of the CNS [99]. More specifically, they may affect stress-related behaviors, depression disorder, anxiety disorder and possibly also bipolar disorder by activating signaling systems and neural pathways in the CNS [100].
One of the possible mechanisms for this intriguing connection was demonstrated in a mouse model and is correlated to the enhancement of the protective effect of caspase-1 inhibition; caspase-1 maturation is part of the inflammasome signaling modulate that activates pro-inflammatory interleukins IL-1β and IL-18 [101].
May cannabis consumption affect the microbiota-gut-brain axis? Only few evidences up to date support such a connection. In mice, THC altered the microbiota, including an increase in the presence of Akkermansia muciniphilia bacterial strain that is associated with reducing insulin resistance, weight loss, and improving intestinal barrier function [102]. However, whether THC or other cannabis-derived compounds may directly affect the microbiota-gut-brain axis remains to be determined.
5. THE “ENTOURAGE EFFECT” OF CANNABINOIDS AND TERPENES FOR THE TREATMENT OF MOOD DISORDERS-PROPOSAL FOR FUTURE RESEARCH
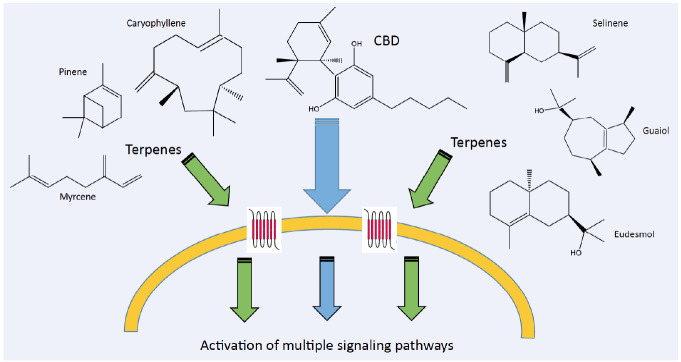
The “entourage effect”, is the suggested positive contribution derived from the addition of terpenes to the effect of cannabinoids [72] (see Fig. 1). This means that the entirety of the effect is greater than the sum effects of its contributing parts. The entourage effect in Cannabis was first postulated by Mechoulam and Ben-Shabat [103]. Their findings led them to the development of the hypothesis, that other inactive biological products, accompanying the primary endogenous cannabinoids, increase its activity. Russo [104] described the concept of botanical synergy, in which a dominant molecule is supported by other plant derivatives – cannabinoids, terpenes, flavonoids and other inactive substances, to achieve a maximal pharmacological effect. Russo reviews several studies, in which a whole plant extract had a superior effect to purified cannabinoid.
Fig. (1).
Activation pathways of CBD and accompanying terpenes and terpenoids in human cells. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Synergistic interactions may be found between different cannabinoids (i.e.,“intra-entourage”) [105] and between cannabinoids and terpenes (i.e.,“inter-entourage”) [106]. Thus, cannabis appliance should be optimized to contain mixtures of these C. sativa-derived combined components. Another possibility is to use the whole extract with all compounds that produce the greatest synergistic activity [107].
Additionally, among popular recreational users, it is commonly believed that the Cannbais indica strains are rich in Myrcene [67], a terpene known to induce relaxation and decrease anxiety, while Cannabis sativa strains are mostly rich in limonene and other terpenes, shown to be related to alertness and arousing behavior. It is assumed that combining terpenes with cannabinoids enhances the mood-stabilizing effects attributed to the main two cannabinoids, THC and CBD. Although terpenes are present in cannabis inflorescence’ extract in relatively low amounts, their contribution to the therapeutic effect of the cannabinoids may be significant [72]; however, this observation has yet to be verified, clinically.
Further research is warranted to investigate the potential therapeutic value of adding terpenes to treatment with CBD, with or without additional THC for the benefit of patients suffering from depression, anxiety or BD. The understanding that terpenes hold important biological and behavioral abilities is a novel concept, which brings forth many difficulties when testing it in natural situations [73]. However, the value of such entourage effects, enhancing the beneficial influence of cannabis, is very high as the side effects and additional risks of other conventional treatments for psychiatric disorders are considerable. The effects of combining this original treatment with the conventional pharmacological approach also require further investigation.
CONCLUSION
To our best knowledge, such innovative combinations between terpenes and cannabinoids have not been considered earlier in existing scientific research. The use of various cannabis-derived compounds opens the arena to the option of avoiding the adverse effects of the available antidepressants and mood stabilizers while treating mood disorders. This may be particularly important for patients who are non-responsive or non-adherent to conventional treatment.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- BDNF
Brain-derived neurotrophic factor
- CBCs
cannabichromenes
- CBDs
cannabidiols
- CBGs
cannabigerols
- CBNDs
cannabinodiols
- CBNs
cannabinols
- CNS
central nervous system
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders
- DSP-4
N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine
- EL
(+)-limonene epoxide
- EPM
elevated plus maze
- FLX
fluoxetine
- FST
forced swimming test
- FSL
Flinders sensitive line rats
- GPCR
G-protein-coupled cannabinoid receptors
- HAMA
Hamilton Anxiety Scale
- HUF-101
fluorinated CBD derivative 1
- HUF-102
fluorinated CBD derivative 2
- HUF-103
fluorinated CBD derivative 3
- MBT
marble burying test
- mCPP
Meta-chlorophenyl-piperazine
- OCD
Obsessive-compulsive disorder
- OFC
orbitofrontal cortex
- PCPA
para-chlorophenylalanine
- RCT
randomized control trial
- SCH23390
R(+)-SCH-23390 hydrochloride
- SSRI
serotonin reuptake inhibitors
- SPT
saccharin preference test
- THCs
tetrahydrocannabinols
- TST
tail suspension test
- URB597
indirect cannabinoid agonist
- WAY100635
N-[2-[4-(2-Methoxyphenyl)-1-pipera- zinyl]ethyl]-N-2-pyridinylcyclohexane- carboxamide maleate salt
- WIN55,212-2
[D-Ala2, D-Leu5]-Enkephalin acetate salt
- WKY
Wistar-Kyoto rats
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bondy B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2002;4(1):7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Portman M.E., Riskind J.H., Rector N.A. Encycl. Hum. Behav. Second Ed.; 2012. Generalized anxiety disorder. pp. 215–220. [DOI] [Google Scholar]
- 3.Geddes J. Bipolar disorder bipolar disorder. Lithium. 2003;346:6–8. doi: 10.1016/S0140-6736(15)00241-X. [DOI] [Google Scholar]
- 4.Coplan J.D., Gopinath S., Abdallah C.G., Berry B.R. A Neurobiological hypothesis of treatment-resistant depression- mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front. Behav. Neurosci. 2014;8:189. doi: 10.3389/fnbeh.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pigott H.E., Leventhal A.M., Alter G.S., Boren J.J. Efficacy and effectiveness of antidepressants: Current status of research. Psychother. Psychosom. 2010;79(5):267–79. doi: 10.1159/000318293. [DOI] [PubMed] [Google Scholar]
- 6.Fornaro M., Kardash L., Novello S., Fusco A., Anastasia A., De Berardis D., Perna G., Carta M.G. Progress in bipolar disorder drug design toward the development of novel therapeutic Targets: A clinician’s perspective. Expert Opin. Drug Discov. 2018;13(3):221–228. doi: 10.1080/17460441.2018.1428554. [DOI] [PubMed] [Google Scholar]
- 7.Cascade E., Kalali A.H., Kennedy S.H. Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont.) 2009;6(2):16–18. [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams D.I. The therapeutic effects of cannabis and cannabinoids: An update from the national academies of sciences, engineering and medicine report. Eur. J. Intern. Med. 2018;49:7–11. doi: 10.1016/j.ejim.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Soares V.P., Campos A.C. Evidences for the anti-panic actions of cannabidiol. Curr. Neuropharmacol. 2017;15(2):291–299. doi: 10.2174/1570159X14666160509123955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanuš L.O., Meyer S.M., Muñoz E., Taglialatela-Scafati O., Appendino G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Reports. 2016;33(12):1357–1392. doi: 10.1039/c6np00074f.. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned CDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 12.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 13.Wilsey B., Marcotte T., Tsodikov A., Millman J., Bentley H., Gouaux B., Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J. Pain. 2008;9(6):506–521. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter B.E., Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013;29(3):574–577. doi: 10.1016/j.yebeh.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Sullivan S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007;152(5):576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grotenhermen F. Pharmacology of cannabinoids. Neuroendocrinolo. Lett. 2004;25(1-2):14–23. [PubMed] [Google Scholar]
- 17.Pertwee R.G. Endocannabinoids and their pharmacological actions. Handb. Exp. Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1.. [DOI] [PubMed] [Google Scholar]
- 18.Ashton C.H., Moore P.B. Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr. Scand. 2011;124(4):250–261. doi: 10.1111/j.1600-0447.2011.01687.x. [DOI] [PubMed] [Google Scholar]
- 19.Karhson D.S., Hardan A.Y., Parker K.J. Endocannabinoid signaling in social functioning: An RDoC perspective. Transl. Psychiatry. 2016;6(9):e905. doi: 10.1038/tp.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo E.B., McPartland J.M. Cannabis and cannabis extracts: greater than the sum of their parts? J. Cannabis Ther. 2001 doi: 10.1300/J175v01n03_08. [DOI] [Google Scholar]
- 21.Maykut M.O. Health consequences of acute and chronic marihuana use. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1985 doi: 10.1016/0278-5846(85)90085-5. [DOI] [PubMed] [Google Scholar]
- 22.Ashton C.H., Moore P.B., Gallagher P., Young A.H. Cannabinoids in bipolar affective disorder: A review and discussion of their therapeutic potential. J. Psychopharmacol. 2005 doi: 10.1177/0269881105051541. [DOI] [PubMed] [Google Scholar]
- 23.Pertwee R.G. The pharmacology of cannabinoid receptors and their ligands: An overview. Intl. J. Obesity. 2006;30(Suppl. 1):S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 24.Micale V., Di Marzo V., Sulcova A., Wotjak C.T., Drago F. Endocannabinoid system and mood disorders: Priming a target for new therapies. Pharmacol. Ther. 2013;138(1):18–37. doi: 10.1016/j.pharmthera.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Silva L., Black R., Michaelides M., Hurd Y.L., Dow-Edwards D. Sex and age specific effects of delta-9-Tetrahydrocannabinol during the periadolescent period in the rat: The unique susceptibility of the prepubescent animal. Neurotoxicol. Teratol. 2016;58:88–100. doi: 10.1016/j.ntt.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Cuttler C., Spradlin A., McLaughlin R. J. A Naturalistic examination of the perceived effects of cannabis on negative affect. J. Affect. Disord. 2018;235:198–205. doi: 10.1016/j.jad.2018.04.054. [DOI] [PubMed] [Google Scholar]
- 27.Iseger T.A., Bossong M.G. A Systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr. Res. 2015;162(1-3):153–161. doi: 10.1016/j.schres.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 28.Murphy M., Mills S., Winstone J., Leishman E., Wager-Miller J., Bradshaw H., Mackie K. Chronic adolescent Δ9-Tetrahydrocannabinol treatment of male mice leads to long-term cognitive and behavioral dysfunction, which are prevented by concurrent cannabidiol treatment. Cannabis Cannabinoid Res. 2017;2(1):235–246. doi: 10.1089/can.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izquierdo G. Multiple sclerosis symptoms and spasticity management: New data. Neurodegener. Dis. Manag. 2017;7(6s):7–11. doi: 10.2217/nmt-2017-0034.. [DOI] [PubMed] [Google Scholar]
- 30.Campos A.C., Fogaça M.V., Sonego A.B., Guimarães F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016;112:119–127. doi: 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 31.Morales P., Reggio P. H., Jagerovic N. An overview on medicinal chemistry of synthetic and natural derivatives of cannabidiol. Front. Pharmacol. 2017;8:422. doi: 10.3389/fphar.2017.00422.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blessing E.M., Steenkamp M.M., Manzanares J., Marmar C.R. Cannabidiol as a potential treatment for anxiety disorders. Neurotherapeutics. 2015;12(4):825–836. doi: 10.1007/s13311-015-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoval G., Shbiro L., Hershkovitz L., Hazut N., Zalsman G., Mechoulam R., Weller A. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology. 2016;73(2):123–129. doi: 10.1159/000443890. [DOI] [PubMed] [Google Scholar]
- 34.Zanelati T.V., Biojone C., Moreira F.A., Guimarães F.S., Joca S.R.L. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT 1A receptors. Br. J. Pharmacol. 2010;159(1):122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breuer A., Haj C. G., Fogaça M. V., Gomes F. V., Silva N. R., Pedrazzi J. F., Bel E. A. D., Hallak J. C., Crippa J. A., Zuardi A. W., Mechoulam R., Guimarães F.S. Fluorinated cannabidiol derivatives: Enhancement of activity in mice models predictive of anxiolytic, antidepressant and antipsychotic effects. PLoS One. 2016;11(8):e0162087. doi: 10.1371/journal.pone.0158779.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morales P., Hurst D.P., Reggio P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Products. 2017;103:103–113. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shbiro L., Hen-Shoval D., Hazut N., Rapps K., Dar S., Zalsman G., Mechoulam R., Weller A., Shoval G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019;201:59–63. doi: 10.1016/j.physbeh.2018.12.019.. [DOI] [PubMed] [Google Scholar]
- 38.Réus G.Z., Stringari R.B., Ribeiro K.F., Luft T., Abelaira H.M., Fries G.R., Aguiar B.W., Kapczinski F., Hallak J.E., Zuardi A.W., Crippa J.A., Quevedo J. Administration of cannabidiol and imipramine induces antidepressant-like effects in the forced swimming test and increases brain-derived neurotrophic factor levels in the rat amygdala. Acta Neuropsychiatr. 2011;23(5):241–248. doi: 10.1111/j.1601-5215.2011.00579.x. [DOI] [PubMed] [Google Scholar]
- 39.Schiavon A.P., Bonato J.M., Milani H., Guimarães F.S., Weffort de Oliveira R.M. Influence of single and repeated cannabidiol Administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:27–34. doi: 10.1016/j.pnpbp.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Sartim A. G., Guimarães F. S., Joca S. R. L. Antidepressant-like Effect of cannabidiol injection into the ventral medial prefrontal cortex-possible involvement of 5-HT1A and CB1 receptors. Behav. Brain Res. 2016;303:218–27. doi: 10.1016/j.bbr.2016.01.033. [DOI] [PubMed] [Google Scholar]
- 41.Lisboa S.F., Gomes F.V., Terzian A.L.B., Aguiar D.C., Moreira F.A., Resstel L.B.M., Guimarães F.S. The endocannabinoid system and anxiety. Vitam. Horm. 2017;103:193–279. doi: 10.1016/bs.vh.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Marco E.M., Pérez-Alvarez L., Borcel E., Rubio M., Guaza C., Ambrosio E., File S.E., Viveros M.P. Involvement of 5-HT1A receptors in behavioural effects of the cannabinoid receptor agonist CP 55,940 in bale Rats. Behav. Pharmacol. 2004;15(1):21–27. doi: 10.1097/00008877-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Patel S. Pharmacological Evaluation of cannabinoid receptor ligands in a mouse model of anxiety: Further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 2006;318(1):304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- 44.Rubino T., Realini N., Castiglioni C., Guidali C., Viganó D., Marras E., Petrosino S., Perletti G., Maccarrone M., Di Marzo V., Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb. Cortex. 2008;18(6):1292–1301. doi: 10.1093/cercor/bhm161. [DOI] [PubMed] [Google Scholar]
- 45.Bortolato M., Campolongo P., Mangieri R.A., Scattoni M.L., Frau R., Trezza V., La Rana G., Russo R., Calignano A., Gessa G.L., Cuomo V., Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31(12):2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- 46.Kathuria S., Gaetani S., Fegley D., Valiño F., Duranti A., Tontini A., Mor M., Tarzia G., La Rana G., Calignano A., Giustino A., Tattoli M., Palmery M., Cuomo V., Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9(1):76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 47.García-Gutiérrez M.S., García-Bueno B., Zoppi S., Leza J.C., Manzanares J. Chronic blockade of cannabinoid CB 2 receptors Induces anxiolytic-like actions associated with alterations in GABA a receptors. Br. J. Pharmacol. 2012;165(4):951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bahi A., Al Mansouri S., Al Memari E., Al Ameri M., Nurulain S. M., Ojha S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014;135:119–24. doi: 10.1016/j.physbeh.2014.06.003.. [DOI] [PubMed] [Google Scholar]
- 49.Martin M., Ledent C., Parmentier M., Maldonado R., Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl.) 2002 doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 50.Patel S., Hill M. N., Cheer J. F., Wotjak C. T., Holmes A. The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci. Biobehav. Rev. 2017 doi: 10.1016/j.neubiorev.2016.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergamaschi M.M., Queiroz R.H.C., Chagas M.H.N., De Oliveira D.C.G., De Martinis B.S., Kapczinski F., Quevedo J., Roesler R., Schröder N., Nardi A.E., Martín-Santos R., Hallak J.E., Zuardi A.W., Crippa J.A. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–1226. doi: 10.1038/npp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crippa J.A.S., Nogueira D.G., Borduqui F.T., Wichert-Ana L., Duran F.L.S., Martin-Santos R., Vinícius S.M., Bhattacharyya S., Fusar-Poli P., Atakan Z., Santos F.A., Freitas-Ferrari M.C., McGuire P.K., Zuardi A.W., Busatto G.F., Hallak J.E. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011;25(1):121–130. doi: 10.1177/0269881110379283. [DOI] [PubMed] [Google Scholar]
- 53.Whiting P. F., Wolff R. F., Deshpande S., Di Nisio M., Duffy S., Hernandez A. V., Keurentjes J.C., Lang S., Misso K., Ryder S., Schmidlkofer S., Westwood M., Kleijnen J. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. doi: 10.1001/jama.2015.6358.. [DOI] [PubMed] [Google Scholar]
- 54.Wong S.S., Wilens T.E. Medical cannabinoids in children and Adolescents: A systematic review. Pediatrics. 2017;140(5):e20171818. doi: 10.1542/peds.2017-1818. [DOI] [PubMed] [Google Scholar]
- 55.Valvassori S.S., Elias G., De Souza B., Petronilho F., Dal-Pizzol F., Kapczinski F., Trzesniak C., Tumas V., Dursun S., Nisihara C.M.H., Chagas M.H., Hallak J.E., Zuardi A.W., Quevedo J., Crippa J.A. Effects of cannabidiol on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychopharmacol. 2011;25(2):274–280. doi: 10.1177/0269881109106925. [DOI] [PubMed] [Google Scholar]
- 56.Ashton H., Young A.H. GABA-ergic drugs: Exit stage left, enter stage right. J. Psychopharmacol. 2003;17(2):174–178. doi: 10.1177/0269881103017002004. [DOI] [PubMed] [Google Scholar]
- 57.Porter R., Ferrier N., Ashton H. Anticonvulsants as mood stabilisers. Adv. Psychiatr. Treat. 2007;•••:96–103. doi: 10.1192/apt.5.2.96. [DOI] [Google Scholar]
- 58.Crippa J.A., Guimarães F.S., Campos A.C., Zuardi A.W. Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front. Immunol. 2018;9:2009. doi: 10.3389/fimmu.2018.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haller J., Varga B., Ledent C., Barna I., Freund T.F. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur. J. Neurosci. 2004;19(7):1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- 60.Akinshola B.E., Chakrabarti A., Onaivi E.S. In-Vitro and in-Vivo action of cannabinoids. Neurochem. Res. 1999;24(10):1233–1240. doi: 10.1023/A:1020968922151. [DOI] [PubMed] [Google Scholar]
- 61.Navarro M., Hernández E., Muñoz R.M., Del Arco I., Villanúa M.A., Carrera M.R.A., Rodríguez De Fonseca F. Acute administration of the CB1cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8(2):491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- 62.Mendiguren A., Aostri E., Pineda J. Regulation of noradrenergic and serotonergic systems by cannabinoids: Relevance to cannabinoid-Induced effects. Life Sci. 2018;192:115–127. doi: 10.1016/j.lfs.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 63.Niederhoffer N., Hansen H.H., Fernandez-Ruiz J.J., Szabo B. Effects of cannabinoids on adrenaline release from adrenal medullary cells. Br. J. Pharmacol. 2001 doi: 10.1038/sj.bjp.0704359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bisogno T., Hanuš L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranganathan M., Braley G., Pittman B., Cooper T., Perry E., Krystal J., D’Souza D.C. The effects of cannabinoids on serum cortisol and prolactin in humans. Psychopharmacology (Berl.) 2009;203(4):737–744. doi: 10.1007/s00213-008-1422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farzaei M.H., Bahramsoltani R., Rahimi R., Abbasabadi F. Abdollahi, M. A systematic review of plant-derived natural compounds for anxiety disorders. Curr. Top. Med. Chem. 2016;16(17):1924–1942. doi: 10.2174/1568026616666160204121039. [DOI] [PubMed] [Google Scholar]
- 67.Aizpurua-Olaizola O., Soydaner U., Öztürk E., Schibano D., Simsir Y., Navarro P., Etxebarria N., Usobiaga A. Evolution of the cannabinoid and terpene content during the growth of cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016;79(2):324–331. doi: 10.1021/acs.jnatprod.5b00949. [DOI] [PubMed] [Google Scholar]
- 68.ElSohly M.A., Slade D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005;78(5):539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Wagner H. Synergy research: Approaching a new generation of phytopharmaceuticals. Fitoterapia. 2011;82(1):34–37. doi: 10.1016/j.fitote.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 70.Chen F., Tholl D., Bohlmann J., Pichersky E. The Family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66(1):212–29. doi: 10.1111/j.1365-313X.2011.04520.x.. [DOI] [PubMed] [Google Scholar]
- 71.Iijima Y. The Biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol. 2004;136(3):3724–3736. doi: 10.1104/pp.104.051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Russo E. B., Taming T. H.C. Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163(7):1344–64. doi: 10.1111/j.1476-5381.2011.01238.x.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gershenzon J., Dudareva N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007;3(7):408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 74.Perry N., Perry E. Aromatherapy in the management of psychiatric disorders. CNS Drugs. 2006;20(4):257–280. doi: 10.2165/00023210-200620040-00001. [DOI] [PubMed] [Google Scholar]
- 75.Li Y.J., Xuan H.Z., Shou Q.Y., Zhan Z.G., Lu X., Hu F.L. Therapeutic effects of propolis essential oil on anxiety of restraint-stressed mice. Hum. Exp. Toxicol. 2012;31(2):157–165. doi: 10.1177/0960327111412805. [DOI] [PubMed] [Google Scholar]
- 76.Georgiadou G., Tarantilis P.A., Pitsikas N. Effects of the active constituents of Crocus Sativus L. Crocins, in an animal model of Obsessive-compulsive disorder. Neurosci. Lett. 2012;528(1):27–30. doi: 10.1016/j.neulet.2012.08.081. [DOI] [PubMed] [Google Scholar]
- 77.De Sousa D.P., De Almeida S.H.P., Andrade L.N., Andreatini R. A systematic review of the anxiolytic-like effects of dssential oils in animal models. Molecules. 2015;20(10):18620–18660. doi: 10.3390/molecules201018620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasper S., Gastpar M., Müller W.E., Volz H.P., Möller H.J., Schläfke S., Dienel A. Lavender oil preparation silexan is effective in generalized anxiety disorder - A randomized, double-blind comparison to placebo and paroxetine. Int. J. Neuropsychopharmacol. 2014;17(6):859–869. doi: 10.1017/S1461145714000017. [DOI] [PubMed] [Google Scholar]
- 79.Cline M., Taylor J.E., Flores J., Bracken S., McCall S., Ceremuga T.E. Investigation of the anxiolytic effects of linalool, a Lavender extract, in the gale Sprague-Dawley rat. AANA J. 2008;76(1):47–52. [PubMed] [Google Scholar]
- 80.Lima N.G.P.B., De Sousa D.P., Pimenta F.C.F., Alves M.F., De Souza F.S., MacEdo R.O., Cardoso R.B., De Morais L.C.S.L., Melo D. M. D. F. F.; De Almeida, R. N. Anxiolytic-like Activity and GC-MS Analysis of (R)-(+)-Limonene fragrance, a natural compound gound in goods and plants. Pharmacol. Biochem. Behav. 2013 doi: 10.1016/j.pbb.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 81.De Almeida A.A.C., De Carvalho R.B.F., Silva O.A., De Sousa D.P., De Freitas R.M. Potential antioxidant and anxiolytic effects of (+)-Limonene epoxide in mice after marble-burying test. Pharmacol. Biochem. Behav. 2014;118:69–78. doi: 10.1016/j.pbb.2014.01.006.. [DOI] [PubMed] [Google Scholar]
- 82.Guzmán-Gutiérrez S.L., Bonilla-Jaime H., Gómez-Cansino R., Reyes-Chilpa R. Linalool and β-Pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015 doi: 10.1016/j.lfs.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 83.Goldstein B.I., Kemp D.E., Soczynska J.K., McIntyre R.S. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: A systematic review of the literature. J. Clin. Psychiatry. 2009;70(8):1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 84.Jones K.A., Thomsen C. The Role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 85.Miller A. H. Depression and immunity: A role for T cells? Brain, Behav. Immun. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yirmiya R., Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011 doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 87.Walter L., Franklin A., Witting A., Wade C., Xie Y., Kunos G., Mackie K., Stella N. Nonpsychotropic cannabinoid receptors Regulate microglial cell migration. J. Neurosci. 2018;23(4):1398–1405. doi: 10.1523/jneurosci.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malfait A.M., Gallily R., Sumariwalla P.F., Malik A.S., Andreakos E., Mechoulam R., Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA. 2000;97(17):9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Börner C., Höllt V., Kraus J. Activation of human T cells induces Upregulation of cannabinoid receptor Type 1 transcription. Neuroimmunomodulation. 2007;14(6):281–286. doi: 10.1159/000117809. [DOI] [PubMed] [Google Scholar]
- 90.Borner C., Bedini A., Hollt V., Kraus J. Analysis of promoter regions regulating basal and interleukin-4-inducible expression of the human CB1 receptor gene in T Lymphocytes. Mol. Pharmacol. 2007;73(3):1013–1019. doi: 10.1124/mol.107.042945. [DOI] [PubMed] [Google Scholar]
- 91.Lunn C.A. A Novel Cannabinoid peripheral cannabinoid receptorselective inverse agonist blocks leukocyte recruitment in Vivo. J. Pharmacol. Exp. Ther. 2006;316(2):780–8. doi: 10.1124/jpet.105.093500.. [DOI] [PubMed] [Google Scholar]
- 92.Tanasescu R., Constantinescu C.S. Cannabinoids and the immune system: an overview. Immunobiology. 2010;215(8):588–597. doi: 10.1016/j.imbio.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Lombard C., Nagarkatti M., Nagarkatti P. CB2 Cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clin. Immunol. 2007;122(3):259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Provenza F.D., Villalba J.J. The role of natural plant products in modulating the immune system: An adaptable approach for combating disease in grazing animals. Small Rumin. Res. 2010;89:131–139. doi: 10.1016/j.smallrumres.2009.12.035. [DOI] [Google Scholar]
- 95.Okada Y., Matono N., Shiono M., Takai T., Hikida M., Ohmori H. Suppression of in Vitro cellular immune response by nitrogen-containing terpene alcohol derivatives. Biol. Pharm. Bull. 2011;19(11):1443–1446. doi: 10.1248/bpb.19.1443. [DOI] [PubMed] [Google Scholar]
- 96.da Silva S.L., Figueiredo P.M.S., Yano T. Chemotherapeutic Potential of the volatile oils from Zanthoxylum Rhoifolium Lam leaves. Eur. J. Pharmacol. 2007;576(1-3):180–188. doi: 10.1016/j.ejphar.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 97.Jeong Y.T., Yang B.K., Jeong S.C., Kim S.M., Song C.H. Ganoderma applanation: A promising mushroom for antitumor and Immunomodulating activity. Phytother. Res. 2008;22(5):614–619. doi: 10.1002/ptr.2294. [DOI] [PubMed] [Google Scholar]
- 98.Slyepchenko A., Maes M., Köhler C. A., Anderson G., Quevedo J., Alves G. S., Berk M., Fernandes B. S., Carvalho A. F. T helper 17 cells may drive neuroprogression in major depressive disorder: Proposal of an integrative model. Neurosci. Biobehav. Rev. 2016;64:83–100. doi: 10.1016/j.neubiorev.2016.02.002.. [DOI] [PubMed] [Google Scholar]
- 99.Foster J.A., McVey Neufeld K.A. Gut-brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Luna R.A., Foster J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015;32:35–41. doi: 10.1016/j.copbio.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Wong M.L., Inserra A., Lewis M.D., Mastronardi C.A., Leong L., Choo J., Kentish S., Xie P., Morrison M., Wesselingh S.L., Rogers G.B., Licinio J. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry. 2016;21(6):797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sherwin E., Dinan T.G., Cryan J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018;1420(1):5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 103.Ben-Shabat S., Fride E., Sheskin T., Tamiri T., Rhee M.H., Vogel Z., Bisogno T., De Petrocellis L., Di Marzo V., Mechoulam R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-Arachidonoyl-Glycerol cannabinoid activity. Eur. J. Pharmacol. 1998;17, 353(1):23–31. doi: 10.1016/S0014-2999(98)00392-6.. [DOI] [PubMed] [Google Scholar]
- 104.Russo E. B. The case for the entourage effect and conventional breeding of clinical cannabis: No “Strain,” no gain. Front. Plant Sci. 2019 doi: 10.3389/fpls.2018.01969.. [Epub a head of Print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berman P., Futoran K., Lewitus G.M., Mukha D., Benami M., Shlomi T., Meiri D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in cannabis. Sci. Rep. 2018;8(1):14280. doi: 10.1038/s41598-018-32651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nallathambi R., Mazuz M., Namdar D., Shik M., Namintzer D., Vinayaka A.C., Ion A., Faigenboim A., Nasser A., Laish I., Fred M.K., Hinanit K. Identification of synergistic interaction between cannabis-derived compounds for cytotoxic activity in colorectal cancer cell lines and colon polyps that induces apoptosis-related cell death and distinct gene expression. Cannabis Cannabinoid Res. 2018;31:120–135. doi: 10.1089/can.2018.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Koltai H., Poulin P., Namdar D. Promoting cannabis products to pharmaceutical drugs. Eur. J. Pharm. Sci. 2019;132:118–120. doi: 10.1016/j.ejps.2019.02.027. [DOI] [PubMed] [Google Scholar]