Abstract
Objective:
Many people with psychotic experiences do not develop psychotic disorders, yet those who seek help demonstrate high clinical complexity and poor outcomes. In this systematic review and meta-analysis, we evaluated the effectiveness and cost-effectiveness of psychological interventions for people with psychotic experiences.
Method:
We searched 13 databases for studies of psychological interventions for adults with psychotic experiences, but not psychotic disorders. Our outcomes were the proportion of participants remitting from psychotic experiences (primary); changes in positive and negative psychotic symptoms, depression, anxiety, functioning, distress, and quality of life; and economic outcomes (secondary). We analysed results using multilevel random-effects meta-analysis and narrative synthesis.
Results:
A total of 27 reports met inclusion criteria. In general, there was no strong evidence for the superiority of any one intervention. Five studies reported on our primary outcome, though only two reports provided randomised controlled trial evidence that psychological intervention (specifically, cognitive behavioural therapy) promoted remission from psychotic experiences. For secondary outcomes, we could only meta-analyse trials of cognitive behavioural therapy. We found that cognitive behavioural therapy was more effective than treatment as usual for reducing distress (pooled standardised mean difference: −0.24; 95% confidence interval = [−0.37, −0.10]), but no more effective than the control treatment for improving any other outcome. Individual reports indicated that cognitive behavioural therapy, mindfulness-based cognitive therapy, sleep cognitive behavioural therapy, systemic therapy, cognitive remediation therapy, and supportive treatments improved at least one clinical or functional outcome. Four reports included economic evaluations, which suggested cognitive behavioural therapy may be cost-effective compared with treatment as usual.
Conclusion:
Our meta-analytic findings were primarily null, with the exception that cognitive behavioural therapy may reduce the distress associated with psychotic experiences. Our analyses were limited by scarcity of studies, small samples and variable study quality. Several intervention frameworks showed preliminary evidence of positive outcomes; however, the paucity of consistent evidence for clinical and functional improvement highlights a need for further research into psychological treatments for psychotic experiences.
PROSPERO protocol registration number:
CRD42016033869
Keywords: Psychosis, ultra-high risk, at-risk mental state, psychotic experiences, psychological intervention
Introduction
High-risk criteria for psychotic disorders (Broome et al., 2005; Cannon et al., 2008; Cornblatt et al., 2002; Miller et al., 2002; Yung et al., 1996, 2003) are predicated on the presence of sub-threshold psychotic symptoms, also called psychotic experiences (PEs), and the presumption that preventing or delaying transition to a full psychotic disorder syndrome is a primary therapeutic target. However, most people with PEs never develop a psychotic disorder (Hui et al., 2013; McGorry et al., 2018; Perez et al., 2018), but have high clinical complexity, poor response to treatment (Perlis et al., 2011; Valiji Bharmal et al., 2015; Wigman et al., 2014), sub-optimal clinical and functional outcomes, and increased risk of self-harm (Fusar-Poli et al., 2012; Granö et al., 2011; Hui et al., 2013; Hutton et al., 2011; Kelleher et al., 2012; Yates et al., 2019). Despite evidence of these poor outcomes, many people with PEs do not meet the increasingly high thresholds for secondary care mental health services, while in primary mental healthcare settings their PEs often go unnoticed or untreated even though their depression and anxiety scores are higher, on average, than those of individuals without PEs (Hui et al., 2013; Perez et al., 2018).
Research on psychological interventions for people with PEs has mainly focused on delaying or preventing transition to psychotic disorder. Despite this focus, a recent network meta-analysis of transition rates among people at high risk for psychosis found no evidence to support the effectiveness of needs-based interventions (NBIs), cognitive behavioural therapy (CBT), integrated psychological interventions, or family-focused therapy (FFT) in comparison with each other (Davies et al., 2018a). A subsequent network meta-analysis of intervention effects further found that no one specific intervention was more effective than others with regard to reducing attenuated positive psychotic symptoms (Davies et al., 2018b). Yet, Nelson et al. (2018) have proposed several limitations of these reviews, citing the omission of (1) trial evidence demonstrating positive group-level effects of these interventions and (2) key clinical (e.g. depression and general psychopathology) and functional outcomes that clearly have important implications for the treatment of people with PEs.
Recent meta-analyses have left a number of key gaps concerning interventions for people with PEs that must be filled in order to ensure that treatment decisions and clinical guidelines are based on the most relevant, accurate, and up-to-date evidence available. First, most reviews have limited their focus to ‘ultra-high risk’ or ‘clinical high risk’ populations, thereby omitting people with PEs who may not have these diagnoses. Second, there is presently no meta-analytic evidence addressing the question of which psychological interventions lead to remission from PEs and improvement in depression, anxiety, and general functioning, all of which are important features of at-risk states for psychosis that lead to disability (Byrne and Morrison, 2014; Fowler et al., 2018; Law and Morrison, 2014). Third, the psychological intervention that has been most investigated in the context of people with PEs is CBT, while the evidence concerning alternative approaches has yet to be collated (Nelson et al., 2009). Fourth, the cost-effectiveness of achieving therapeutic targets other than transition has received little attention. Fifth, no review has set limitations for the use of antipsychotics, despite the fact that international guidelines do not generally recommend their use for people at-risk for developing psychosis (Addington et al., 2017; Early Psychosis Guidelines Writing Group and EPPIC National Support Program, 2016; National Institute for Health and Care Excellence (NICE), 2014; Schmidt et al., 2015). Finally, no review has aimed to illuminate the key ingredients of effective psychological interventions for this population. To address these significant gaps in the literature and to inform the development of a new therapeutic framework, we conducted a systematic review and meta-analysis that aimed to (1) synthesise evidence about the effectiveness of and economic outcomes associated with psychological interventions for people with PEs and (2) identify common components of effective interventions.
Methods
This review was conducted as part of the Tailoring evidence-based psychological therapY for People with common mental disorder including Psychotic EXperiences (TYPPEX), a nationwide National Institute for Health Research (NIHR) Programme Grant for Applied Research (RP-PG-0616-20003) that aims to develop an effective therapeutic framework for service users with PEs in the UK Improving Access to Psychological Therapies (IAPT) primary mental healthcare setting (www.england.nhs.uk/mental-health/adults/iapt/). The programme focuses on clinical and functional outcomes other than transition to psychotic disorder, reflecting the low transition rate among individuals with PEs accessing primary mental healthcare services (Hui et al., 2013; Perez et al., 2018). The therapeutic framework will adhere to current international guidelines, which recommend psychological therapy – but not antipsychotic medication – for the treatment of individuals with PEs (NICE, 2014; Schmidt et al., 2015).
The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; www.crd.york.ac.uk/prospero), registration number: CRD42016033869 (22 May 2018 version), and a full protocol has been published prospectively elsewhere (Soneson et al., 2019). We follow the PRISMA (Liberati et al., 2009) reporting guidelines.
Data sources and searches
Two research assistants (E.S. and D.R.) collaborated with medical librarians at the University of Cambridge to create the search strategy (Supplemental Appendix A). The strategy combined terms for PEs, specific psychotic symptoms and psychological interventions, as well as database-specific subject headings. We searched MEDLINE, Embase and Health Management Information Consortium (HMIC) via Ovid; PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Education Resources Information Center (ERIC) and EconLit via EBSCO; British Nursing Index (BNI) via ProQuest; and all Cochrane databases from 1 January 2000 (or the earliest publication date included in the database, if after 2000) to 15 December 2018 (when we ran all searches). We additionally searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) for relevant trials and Google Scholar, EThOS, and Open Grey for grey literature and dissertations. We collected additional citations through hand-searching reference lists of included publications.
Study selection
We included studies that examined any psychological intervention in adults with PEs but not psychotic disorders. To be included in our review, studies were required to have used the presence of PEs as the main study entry criterion. Due to the variety of terms used to represent PEs, we included populations with the following diagnoses: at-risk mental state, ultra-high risk/clinical high risk, attenuated psychosis, psychotic-like experiences, unusual experiences, sub-threshold psychosis, prodromal psychosis and schizotypal disorders. We restricted our studies to adults (operationalised as studies in which participants’ mean age was ⩾16 years) to reflect the age of people attending adult mental health services (e.g. UK IAPT services).
We included all frameworks of psychological interventions provided their effects were studied in people with PEs (i.e. interventions did not need to target PEs specifically). We did not restrict intervention setting (and included online interventions). We excluded studies that combined psychological and pharmacological interventions (i.e. where medication was provided as part of the intervention protocol). For medication prescribed external to the intervention, we placed no restriction regarding the proportion of participants taking medication for depressive or anxiety disorders, but included only studies in which less than 25% of participants were prescribed antipsychotic medication. The decision to limit the proportion of the study population using antipsychotic medication aligns with international guidelines’ cautions against prescribing antipsychotics for people at high-risk for developing psychosis (Addington et al., 2017; Early Psychosis Guidelines Writing Group and EPPIC National Support Program, 2016; NICE, 2014; Schmidt et al., 2015). This exclusion criterion further ensured the review was relevant to the UK IAPT setting, where psychological interventions are the only available treatment.
Our outcomes of interest were (1) the proportion of participants who remitted from PEs (primary outcome) and (2) changes in depression, anxiety, functioning, distress, quality of life or positive/negative psychotic symptoms (secondary outcomes). We placed no restriction on which tools were used to measure any of these outcomes, so long as they were valid and reliable. We did not set an a priori inclusion criterion for how to define remission from PEs (we include in our results how each study defined/measured this outcome). In addition, we included studies that reported any of the following economic outcomes: resource use, cost, partial economic evaluations, and full economic evaluations (where full economic evaluations are those that consider both the cost and outcomes of two or more interventions in a comparative analysis and partial economic evaluations focus only on cost description, cost-outcome description or comparative cost, analysis [Drummond et al., 2015]). Outcomes did not need to be the primary outcome of a study to be included in our review.
We placed no restriction on study design or comparator. We chose not to limit our review to controlled trials in order to ensure that newer intervention frameworks (which may be at pilot or earlier stages) could be represented.
We reviewed studies published in any language provided they had an English abstract (no foreign language articles advanced past the title/abstract screening stage). We excluded reports published before 2000 (when the at-risk mental state became widely adopted), reports where only an abstract was available, and secondary analyses of data from the same trial (to avoid including the same data from one individual multiple times within our results).
Two reviewers (E.S. and D.R.) independently screened titles and abstracts and excluded obviously irrelevant titles. We then reviewed the full texts of potentially relevant citations against our inclusion and exclusion criteria. Disagreements were resolved by discussion, with input from a third reviewer (C.K., J.P.) as necessary.
Data extraction and quality appraisal
Three reviewers (E.S., D.R., and M.H.) designed and piloted data extraction sheets. We extracted information on study/sample characteristics, intervention components and descriptions, data for outcomes related to our primary or secondary outcomes, and data required for quality assessment (see protocol for more detail (Soneson et al., 2019). Where information was not available, we consulted study protocols and contacted study authors by email. Two reviewers independently extracted data from a subset of four papers (17%) and one reviewer extracted the rest. Both reviewers reviewed all quantitative data for each included study.
We assessed risk of bias using the Effective Public Health Practice Project’s (EPHPP) Quality Assessment Tool for Quantitative Studies (Armijo-Olivo et al., 2012) for all reports and additionally used the Drummond Critical Appraisal of Economic Evaluations Checklist (Drummond and Jefferson, 1996) for economic reports. Two raters (E.S. and D.R.; M.H. and S.B. for economic studies) independently assessed quality, compared ratings and resolved disagreements by discussion.
Data synthesis and analysis
Meta-analysis
We analysed controlled studies through random-effects meta-analysis of standardised mean differences (SMDs) for our secondary clinical and functional outcomes (we did not have sufficient reports to perform meta-analysis for our primary outcome; see below). To combine outcomes from multiple follow-up points within individual reports, we fitted meta-analytic multilevel random-effects models via functions in the metafor package (Viechtbauer, 2010). A relatively new methodology, multilevel meta-analysis is becoming popular in the literature (Fernández-Castilla et al., 2019). The model overcomes the possibility of bias of overall effect by acknowledging that different time-points are not independent and correcting for this.
Ultimately, we conducted seven separate meta-analyses (one for each secondary outcome), separating each by the framework of the psychological intervention being investigated (as per protocol; Soneson et al., 2019). As CBT was the only intervention to be represented in more than one study, we were not able to conduct meta-analyses for the other intervention frameworks included in the review.
We separated results by comparator framework (supportive treatments [STs] vs treatment as usual [TAU]). We classified the following interventions as ST: supportive therapy, supportive counselling, non-directive reflective listening, NBI, and needs-focused intervention. The decision to group these interventions was based on similarities in their purpose and provision. We considered these interventions to have a common aim, namely, to act as non-specific active comparison groups. They further share several characteristics (e.g. warm, empathic listening and absence of active therapeutic techniques). This classification also facilitates comparison with related reviews that used similar groupings (Davies et al., 2018a, 2018b). In reporting our results, we provide separate pooled estimates for each comparator framework (i.e. ST and TAU separately) as well as an estimate for both comparators combined (i.e. ST and TAU combined). There are clinical and statistical reasons for this decision. First, the difference between TAU and ST is not well-defined; for example, ‘TAU’ sometimes consisted of CBT for depression or anxiety. Second, we found no statistical evidence to indicate any meaningful difference between outcomes for these comparators. As both interpretations are valid, and to ensure our results can adequately inform clinical practice, we include both estimates.
Sensitivity and sub-group analyses
We also conducted sensitivity analyses by including only those reports that received a global rating of ‘strong’ on the EPHPP tool.
No controlled clinical trials (CCTs) met inclusion criteria, and so our planned sensitivity analysis on the impact of CCTs was not possible. We had also intended to conduct sub-group analyses based on population (clinical vs non-clinical), but no studies of non-clinical populations were eligible for inclusion in the meta-analyses. Finally, we had intended to use sub-group analyses to quantitatively assess four a priori components of interest for cognitive therapy as previously highlighted in the literature: assessment of problems and goals, formulation, homework, and active change strategies (Flach et al., 2015; Morrison and Barratt, 2009). However, included reports did not meet our pre-specified criteria for sub-group analyses (see protocol for more detail; Soneson et al., 2019).
Assessment of heterogeneity and meta-biases
Although we aimed to assess heterogeneity of the meta-analytic results, our estimates were unreliable due to low numbers of included reports in each meta-analysis (Deeks et al., 2018). We still report Cochran’s Q for each meta-analysis, but interpretation needs to be cautious. For the same reason, it was not possible to perform the assessments of bias (e.g. publication bias, citation bias) specified in our protocol.
Narrative synthesis
We use narrative synthesis (Popay et al., 2006) to synthesise effectiveness findings and pre–post changes in our outcomes of interest from (1) controlled studies not eligible for inclusion in the meta-analyses and (2) uncontrolled studies. We furthermore narratively describe findings relating to common components of effective therapies.
Economic analysis
We present economic studies in tables containing study characteristics and results and use a narrative approach to synthesise findings as a result of the very small number of identified studies meeting inclusion criteria for the economic component of the review. We further discuss reports in terms of quality, using the Drummond checklist (Drummond et al., 2015).
Results
Search results
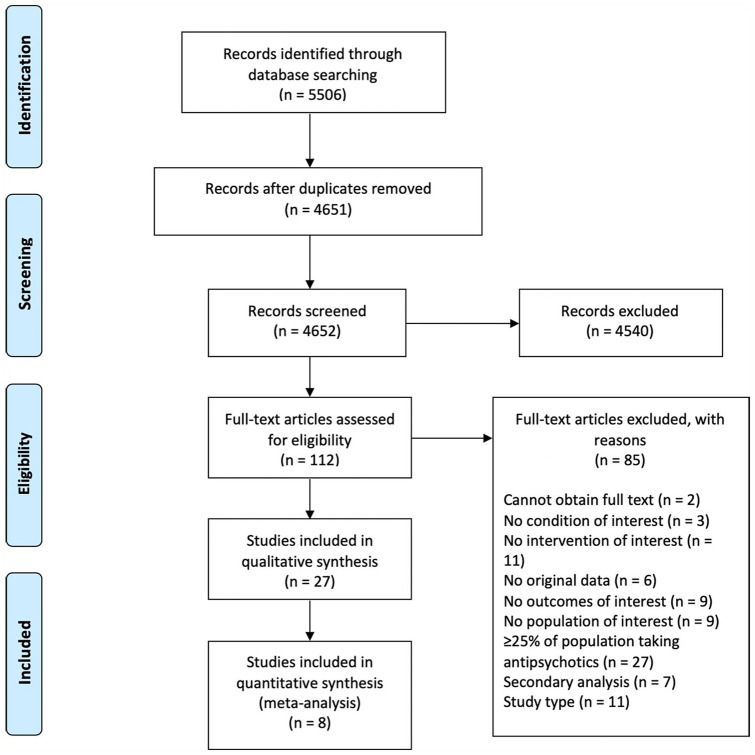
We identified 27 reports from 21 studies that met inclusion criteria (flowchart in Figure 1; summary of studies’ characteristics in Table 1; justifications for exclusion after full-text screening in Supplemental Appendix B; summary of baseline and outcome data in Supplemental Appendix C; intervention components in Supplemental Appendix D). Of these 27 reports, 4 reports using data from two randomised controlled trials (RCTs) included economic components that met our inclusion criteria. The interventions had diverse frameworks; while the vast majority of studies focused on variations on CBT or ST (with the latter always serving as the comparator), one study each represented strengths and mindfulness-based online social therapy, sleep CBT, mindfulness-based cognitive therapy, FFT, family psychoeducational intervention, cognitive remediation, and systemic therapy (each described below). The majority of these frameworks have been tested in the past 5 years, suggesting increased interest in new intervention frameworks for people with PEs.
Figure 1.
PRISMA flowchart (Liberati et al., 2009).
Table 1.
Summary of studies included in the clinical effectiveness component of the review.
| 1st author (year)Country/study region | Outcomes of interest1 | Intervention frameworkComparator (if any)2 | Study designDuration of follow-up measurement | Sample size & characteristics | Consent rateDrop-out rate | Main findings (as relevant to review aims) |
|---|---|---|---|---|---|---|
| Addington (2011)36Canada | Primary: transition to psychosisSecondary: depression, anxiety, functioning | CBTSupportive therapy | Single-blind RCTPost-intervention, 6 & 12 mo. post-intervention (6, 12, 18 mo. post-baseline) | All: N=5170.1% maleCBT: N = 2766.7% male; mean age = 20.8 (SD 4.5) years; 48.1% WhiteST: N = 24 75.0% male; mean age = 21.1 (SD 3.7) years; 66.7% White | 50%45.1% (CBT: 44.4%; ST: 45.8%) | Sig. improvement in positive symptoms over time for both groups (p<0.001) but no between-group differences in change rate over time (p=0.44).No sig. between-group differences in depression; sig. improvement in ST group from baseline to 6 mo. post-baseline (p<0.05). No sig. between-group differences in anxiety, but both groups improved over time on SIAS (p<0.05). Significant improvement ST group on SAS between baseline and 18 mo. post-baseline (p<0.05). No sig. between group differences or within-group time effects in negative symptoms or social functioning. |
| Alvarez-Jimenez (2018)37Australia | Acceptability, safety, social functioning | Strengths and mindfulness-based online social therapyNA | Pre-post (pilot)2 mo. post-baseline | N = 1421.4% male; mean age = 20.3 (SD 3.4) years | ND7.1% | Trend toward improvement in all clinical measures at 2 mo. follow-up: 42% of participants improved reliably across clinical measures; 25% showed increased depressive symptoms at follow-up (no evidence for association between system use and increased depressive symptoms). Sig. large improvement in social functioning from baseline to follow-up (d=1.83, p<0.001) with all participants reliably improving. Sig. large increase in subjective wellbeing (d=0.75); 42% of participants improved reliably in subjective wellbeing and 33% declined in loneliness. |
| Bechdolf (2005)38Germany | Prodromal symptoms, prevention of social decline/stagnation, and prevention or delay of transition to psychosis | CBTNA | Prospective pre-post designPost-intervention only | N = 1275.0% male; mean age = 22.9 (SD 3.6) years | ND16.8% | Sig. pre-post improvements in global psychopathology (p=0.009), social adjustment (p=0.005), basic symptoms (p=0.008), depression (p=0.009), anxiety (p=0.013), and social adjustment (p=0.005) in group who completed intervention. Depression improved from medium-severe to no longer clinically relevant; social adjustment improved from serious to mild impairment. |
| Bechdolf (2007)39Germany | Social adjustment | CBTSupportive counselling (SC) | RCTPost-intervention only | N = 113CBT: N = 5464.8% male; mean age = 25.2 (SD 5.3) yearsSC: N = 5967.8% male; mean age = 26.4 (SD 5.7) years | ND40.7% (CBT: 46.3%; SC: 35.6%) | No sig. between-group differences in any dimension of social adjustment. Large pre-post improvements for both CBT and SC groups. Sig. improvements for CBT group in ‘work’ dimension (p=0.009) and global rating (p=0.010) but not other dimensions. Sig. improvements in SC group for ‘work’ dimension (p=0.003), ‘social activities’ dimension (p=0.03), and global rating (p<0.001). |
| Bradley (2017)40UK | DSM-IV sleep disorders, insomnia, sleep quality, attenuated psychotic experiences, negative affect, quality of life | Sleep CBTNA | A-BPost-intervention, 1 mo. post-intervention | N = 1241.7% male; mean age = 18.5 (SD 1.9) years; 83.3% White | 100%8.3% | Improvement across all outcome measures (depression d=0.5, stress d=0.8, anxiety d=0.2, wellbeing d=0.7, occupational and social functioning d=0.7, paranoia d=0.6, hallucinations d=0.3). Statistical significance not reported. |
| Evans (2017)41UK | Primary: depression, anxiety, distressSecondary: return to prison rates | CBTNA | Pre-postFollow-up not explicit (from paper: ‘The current study was run between the end of 2011 and mid-2014; follow-up data were collected in September 2015) | N = 52100% male; mean age = 23.9 (SD 5.2) years; 38.5% White | 100%28.8% | Reductions in depression (p<0.01), anxiety (p<0.001), and psychological distress (p<0.002). |
| Ising (2016)42Netherlands | Primary: transition to psychosisSecondary: depression, anxiety, positive and negative symptoms, distress, social functioning | CBTuhr + TAUTAU only | Single-blind RCT3.5 years post-intervention (48 mo. post-baseline) | N= 113CBT: N = 5650.5% male; mean age = 22.7 (SD 5.6) years; 49.5% ethnic minorityTAU: N = 5748.5% male; mean age = 22.6 (SD 5.4) years; 39.6% ethnic minority | ND42.3% | Proportion of participants remitting from subclinical psychotic symptoms:CBT: 45/59TAU: 37/63Remission proportion significantly higher in CBTuhr group (p=0.04).No sig. differences in psychopathology between groups (who didn’t transition to psychosis). Reduced depression and anxiety scores (statistical significance not reported). |
| Kommescher (2016)43Germany | Basic symptoms, depression, functioning | Integrated psychological intervention (including CBT) (IPI)Supportive counselling (SC) | RCTPost-intervention only (12 mo. post-baseline) | N = 91IPI: N = 4566.7% male; mean age = 24.1 (SD 4.3) yearsSC: N = 4658.7% male; mean age = 26.9 (SD 6.0) years | 91.1%28.9% | No sig. differences in improvement between IPI and SC in any outcome (PANSS total, basic symptoms, depression, functioning). For both groups combined, significant improvement in every measure (PANSS total d=1.12, basic symptoms: d=0.76, depression symptoms d=1.07, functioning d=-0.73). |
| Langer (2010)44Spain | Frequency & anxiety/distress associated with psychotic-like experiences | Mindfulness-based cognitive therapy (MBCT)Video-forum viewing | Quasi-RCTPost-intervention, 16 wk. post-intervention | N = 38MBCT: N = 1822.2% male; mean age = 21.6 (SD 3.3) yearsVideo-forum viewing: N = 2010.0% male; mean age = 21.1 (SD 1.8) years | NDPost-intervention: 0%; follow-up: 36.8% | Sig. difference between-group difference in anxiety at post-intervention (d=0.88) and 16 wk. follow-up (d=0.91); no other sig. differences, but ‘important changes’ in distress, intrusive thoughts, and auditory distortions at post-intervention. |
| Matsumoto (2018)45 Japan | Primary: total score on the PANSSSecondary: PANSS subscale scores, positive symptoms on the CAARMS, depression, anxiety, functioning, quality of life, client satisfaction | CBTNA | Pre-post (open-label pilot study)Post-intervention, 6 mo. post-intervention (6 & 12 mo. post-baseline) | N = 1338.5% male; mean age = 18.7 (SD 3.6) years | ND0% | 46.2% and 84.6% of participants had remitted from At-Risk Mental State at 6 and 12 mo. post-baseline, respectively. Sig. (p<0.05) pre-post differences at 6 and 12 mo. post-baseline for all outcomes except PANSS negative (i.e. PANSS total, PANSS positive, PANSS global psychopathology, CAARMS positive symptoms, CAARMS suicidality & self-harm, CAARMS aggression/dangerous behaviour, depression, anxiety, functioning, and quality of life (effect sizes d=0.75-2.20). No sig. improvement in PANSS negative scores at either timepoint. |
| McGorry (2017)46Multiple | Primary: transition to psychosisSecondary: general levels of psychopathology and functioning | CBT with case management (CBCM) + placeboCBCM + poly-unsaturated fatty acids (PUFAs) | Double-blind placebo-controlled RCTPost-intervention, 6 mo. post-intervention (6 & 12 mo. post-baseline) | N = 304CBCM + placebo: N = 15140.4% male; mean age = 18.9 (SD 4.3) years; 80.1% WhiteCBCM + PUFAs: N = 15351.0% male; mean age = 19.4 (SD 4.8) years; 80.4% White | 66.2%CBCM + placebo: 26.5%CBCM + PUFAS: 25.5% | Sig. improvement over time for CBCM participants for each measure (i.e. BPRS score, negative symptoms, mania, depression, social and occupational functioning, global functioning). |
| McGorry (2013)47Australia | Primary: transition to psychosisSecondary: psychiatric symptoms, social functioning, quality of life | Cognitive therapy plus low-dose risperidone (CT + R)Cognitive therapy plus placebo (CT + PL)Supportive therapy plus placebo (ST + PL)Monitoring only (not randomised) (MON GR) | Double-blind, placebo-controlled RCTPost-intervention only (12 mo. post-baseline) | N = 115CT + R: N = 4334.9% male; mean age = 17.6 (SD 3.0) years CT + PL: N = 4438.6% male; mean age = 18.0 (SD 2.7) yearsST + PL: N = 2846.4% male; mean age = 18.8 (SD 3.7) yearsMON GR: N = 7839.7% male; mean age = 17.8 (SD 2.6) yearsNB: Sample characteristics for the 62.7% of participants that remained in the study for the 12 month follow-up are not reported. | 24.8%12 mo. follow-up:CT + R: 37.2%CT + PL: 34.1% ST + PL: 32.1% | Improvement in all measures (i.e. BPRS scores, negative symptoms, depression, global functioning, quality of life; statistical significance not reported). No sig. difference in improvement between CBT + placebo, supportive counselling + placebo, CBT + risperidone, or monitoring groups on any outcome.Z |
| McGorry (2002)48Australia | Primary: transition to psychosisSecondary: depression, anxiety, psychiatric symptoms, negative psychotic symptoms, mania, quality of life, functioning | Psychological, needs-based intervention (NBI)Specific preventive intervention (SPI) (all elements of NBI plus CBT and risperidone) | RCTPost-intervention, 6 mo. post-intervention (6 & 12 mo. post-baseline) | N = 59NBI group = 2850.0% male; mean age = 20.0 (SD 3.0) yearsSPI group = 3164.5% male; mean age = 20.0 (SD 4.0) years | 68.1% for study participation; 43.7% for randomisationSPI: 0%NBI: ND | Levels of all symptoms improved in NBI (BPRS score/BPRS psychotic subscore, anxiety, depression, negative symptoms, mania; statistical significance not reported); functional levels (global functioning, quality of life) were more stable. |
| Morrison (2012)49UK | Primary: transition to psychosis, severity of psychotic symptoms, distress caused by psychotic symptomsSecondary: emotional dysfunction, quality of life | Cognitive therapy + mental state monitoringMental state monitoring only | Single-blind RCTPost-intervention, 6 & 18 mo. post-intervention (6, 12, 24 mo. post-baseline) | N = 28862.5% male; mean age = 20.7 (SD 4.3) yearsCT + mental state monitoring: N = 14461.8% male; mean age = 20.7 (SD 4.2) yearsMental state monitoring only: N = 14463.2% male; mean age = 20.8 (SD 4.5) years | NDCT: 6 mo. follow-up: 32.6%12 mo. follow-up: 34.0%24 mo. follow-up: 76.4%Monitoring:6 mo. follow-up: 31.3%12 mo. follow-up: 35.4%24 mo. follow-up: 78.5% | Sig. greater reduction in severity of psychotic experiences in CT group (effect size at 12 mo. post-baseline -3.67 95% CI: -6.71 to -0.64, p=0.018) but no sig. between-group difference in distress from psychotic experiences (estimated difference at 12 mo. post-baseline -3.00 95% CI: -6.95 to 0.94). No sig. between-group differences in levels of functioning, depression, social anxiety, or quality of life. |
| Morrison (2004)50UK | Primary: transition to psychosisSecondary: prescription of antipsychotic medication, probable DSM-IV diagnosis from blinded consultant psychiatrist, scores on PANSS | Cognitive therapy + monitoringTAU (monitoring only) | Single-blind RCTPost-intervention, 6 mo. post-intervention3 | All: N = 60 (N = 58 analysed; 2 cases of psychosis at baseline)66.7% male; mean age = 22.0 (SD 4.5) yearsCT: N = 37 (N = 35 analysed) 60.0% male; median age = 20.6 (IQR 4.9) yearsTAU: N = 2383.0% male; median age - 21.5 (IQR 5.2) years | 95%14% | Sig. greater reduction of frequency positive symptoms in CT group compared with TAU (p=0.049). No sig. between-group differences in functioning or distress (but many missing 12-mo. scores). |
| Nelson (2018)51Multiple | Primary: transition to psychosis, symptomatic & functional outcomeSecondary: clinical predictors of medium-term outcome | CBT with case management (CBCM) + placeboCBCM + poly-unsaturated fatty acids (PUFAs) | Double-blind placebo-controlled RCTMean = 3.4 (SD 0.9; range 1.5-5.7) years post-baseline | N = 270CBCM + placebo: N = 13740.4% male; mean age = 18.9 (SD 4.3) years; 80.1% WhiteCBCM + PUFAs: N = 13351.0% male; mean age = 19.4 (SD 4.8) years; 80.4% WhiteNB: characteristics of follow-up sample not given; characteristics reflect those of the original sample. | 66.2%11% (CBCM + placebo: 9%; CBCM + PUFAs: 13%) | Improvement in CBCM group for all symptom and functioning measures from baseline to medium-term follow-up (i.e. CAARMS subscales, BPRS total/psychotic subscale, anxiety, negative symptoms, mania, depression symptoms, social & occupational functioning, global functioning). Most improvement in symptom & functioning outcomes achieved by end of the intervention period (12 mo.) with only minimal improvement afterwards. |
| O’Brien (2015)52North America | Primary: perceived criticism (mother and child)Secondary: positive psychotic symptoms | Family focused therapy (FFT)Family psychoeducational intervention (EC) | RCTPost-intervention, 6 mo. post-intervention (6 & 12 mo. post-baseline) | N = 90FFT: N = 4658.7% male; mean age = 16.7 (SD 3.3) yearsEC: N = 4459.1% male; mean age = 17.0 (SD 3.1) years | NDPost-intervention: 52% 6 mo. follow-up: 54% | Changes in mothers’ criticism predicted youths’ positive symptoms at 12 mo. post-baseline. Regression model including positive symptoms at baseline/6 mo. post-baseline, changes in maternal criticism, treatment condition, interaction of change in criticism & treatment condition, and use of antipsychotics explained 42% of variance in positive symptoms at 12 mo. post-baseline but treatment not a statistically sig. predictor in this model. |
| Phillips (2007)53Australia | Primary: transition to psychosisSecondary: levels of psychopathology, general functioning | Psychological, needs-based intervention (NBI)Specific preventive intervention (SPI) (all elements of NBI plus CBT and risperidone) | RCT2.5-3.5 years post-intervention (3-4 years post-recruitment) | N = 41NBI = 1750.0% male; mean age = 20.0 (SD 3.0) yearsSPI = 2465.0% male; mean age = 20.0 (SD 4.0) yearsNB: characteristics of follow-up sample not given; characteristics reflect those of the original sample. | 69.5% of original sample (NBI: 61%; SPI: 77%)0% | Sig. higher scores for mania and quality of life in NBI group as compared with baseline scores. No other sig. within-group differences between baseline and 3-4 year follow-up on any measure (i.e. anxiety, depression, BPRS, BPRS psychotic subscale, negative symptoms, functioning). |
| Piskulic (2015)54Canada | Primary: cognitive functionSecondary: social and role functioning | An auditory processing Cognitive Remediation Therapy (the Brain Fitness Program) (CRT)Control treatment of commercially available computer games | Single-blind, pilot RCTPost-intervention, 6 mo. post-intervention (3 & 9 mo. post-baseline) | N = 32CRT: N = 1861.1% male; mean age = 19.7 (SD 5.7) yearsControl: N = 1471.4% male; mean age = 17.5 (SD 3.5) years | NDCRT: 69.6%Control: 65% | Sig. improvements in global functioning (social scale) for CRT group from post-CRT to 9 mo. post-baseline (p<0.05) and ‘trend’ (p=0.06) from baseline to 9 mo. post-baseline. No sig. within-group changes in role functioning. |
| Ruhrmann (2007)55Germany | Prodromal symptoms, global functioning, extrapyramidal side effects | Needs-focused intervention(NFI)Needs-focused intervention + amisulpride (NFI + AMI) | Open-label, randomised parallel-group studyPost-intervention only (12 wk. post-baseline) | N = 124 (102 analysed)NFI: N = 59 (44 analysed)47.5% male; mean age = 25.1 (SD 6.6) yearsNFI + AMI: N = 65 (58 analysed)60.0% male; mean age = 26.1 (SD 6.1) years | 70.9%All: 38.7%NFI: 49.2%NFI + AMI: 29.2% | 20.5% (9 of 44) of participants in the NFI group remitted from psychotic experiencesSig. within-group improvement for NFI for basic & positive psychosis spectrum symptoms (ERI-BAPPSS p<0.01), positive psychosis spectrum (ERI-PPS, p<0.05), basic symptoms (ERI-BS, p<0.01), general psychopathology (PANSS-G, p<0.05), and depression symptoms (MADRS, p<0.05). |
| Shi (2017)56China | Positive and negative psychotic symptoms, depression, functioning, self-esteem, social support | Systemic Therapy Supportive Therapy (ST) | Single-blind RCTPost-intervention only (6 mo. post-baseline) | N = 26Systemic: N = 1335.8% male; mean age = 18.9 (SD 1.0) yearsST: N = 1361.5% male; mean age = 18.9 (SD 1.3) years | 81.3%0% | No sig. between-group differences on any outcome measure: 61.5% of participants in systemic therapy group remitted from attenuated psychotic symptoms as compared with 46.2% of participants in supportive therapy group. Sig. within-group improvements for systemic therapy group in positive symptoms (d=0.53, p=0.005), depressive symptoms (d=0.75, p=0.010), self-esteem (d=0.59, p=0.011), and social support (d=0.45, p=0.013). No sig. within-group improvements for supportive therapy group. |
| Stafford (2015)57Australia | Psychotic-like experiences, distress | CBT (online intervention) NA |
Pre-postPost-intervention only (3 mo. post-baseline) | N = 1225.0% male; mean age = 22.6 (SD 4.0) years | 71%0% | Sig. reduction in number (d=0.64, p<0.005) and frequency of psychotic-like experiences (d=0.89, p<0.005) and associated distress (d=0.53, p<0.005). No sig. pre-post difference in K10 score, but 25% reduction in number of participants who scored 17 or above on the K10 (100% to 75%). |
| Stain (2016)58Australia | Primary: transition to psychosisSecondary: severity of psychotic symptoms, distress associated with psychotic symptoms, depression, anxiety, social functioning, quality of life | CBTNon-Directive Reflective Listening (NDRL) | Single-blind RCTPost-intervention, 6 mo. post-intervention (6 & 12 mo. post-baseline) | N = 57CBT: N = 3033.3% male; mean age = 16.2 (SD 2.7) yearsNDRL: N = 2748.1% male; mean age = 16.5 (SD 3.2) years | 58.2%CBT: 60%NDLR: 44.4% | Sig. improvement in distress associated with subclinical psychotic symptoms in favour of NDRL (p=0.029). No other between-group differences in any measures (i.e. frequency/intensity of psychotic experiences, anxiety, depression, overall symptom severity, global/social/role functioning). |
| van der Gaag (2012)59Netherlands | Primary: transition to psychosisSecondary: depression, anxiety, quality of life, social functioning, personal beliefs about illness | CBT + TAUTAU | RCTPost-intervention, 6 & 12 mo. post-intervention (6, 12 & 18 mo. post-baseline) | N = 201CBTuhr + TAU: N = 9850.0% male; mean age = 22.9 (SD 5.6) yearsTAU: N = 10348.5% male; mean age = 22.6 (SD 5.5) years | 77.9%CBTuhr + TAU: 14.4%TAU: 12.5% | Across both groups, 35% of participants were in remission from ARMS at 6 mo. post-baseline, 48% at 12 mo. post-baseline, and 63% at 18 mo. post-baseline. The proportion of participants who remitted was higher in the CBTuhr group than in the TAU group (70.4% vs. 57.0% at end of study; p=0.039). No sig. between-group differences at 6, 12, or 18 mo. post-baseline amongst non-transitioning participants in frequency/intensity of subclinical psychotic symptoms, depression, anxiety, quality of life, or social functioning. Distress from subclinical psychotic symptoms was significantly lower in the CBTuhr group than in the TAU group at 6 mo. post-baseline (p=0.012). Across both groups, percentage of participants with clinical depression decreased from ~60% to <20% and clinical social phobia from ~40% to <20% from baseline to 18 mo. post-baseline. |
Abbreviations: ND (not described), NA (not applicable), RCT (randomised controlled trial); Treatments: CBCM (cognitive behavioural therapy with case management), CBT (cognitive behavioural therapy), CT (cognitive therapy), IPI (integrated psychological therapy), MBCT (mindfulness-based cognitive therapy), NBI (needs-based intervention), NFI (needs-focused intervention), NDRL (non-directive reflective listening), PUFAs (poly-unsaturated fatty acids), SPI (specific preventive intervention), ST (supportive therapy), SC (supportive counselling), TAU (treatment as usual). Measures: BPRS (Brief Psychiatric Rating Scale), CAARMS (Comprehensive Assessment of At-Risk Mental States), ERI BAPPSS/PPS/BS (Early Recognition Inventory Basic and Positive Psychotic Spectrum Symptoms/Psychotic Positive Symptoms/Basic Symptoms), K10 (10-item Kessler Psychological Distress Scale), MADRS (Montgomery-Åsberg Depression Rating Scale), PANSS (Positive and Negative Syndrome Scale), QLS (Quality of Life Scale), SAS (Social Anxiety Scale), SIAS (Social Interaction Anxiety Scales). Notes: 1If study authors have explicitly listed outcomes, these are reflected; if not, outcomes are inferred based on results and discussion. 2Psychopharmacological and dietary treatments (which meet our exclusion criteria) included for completeness only when they are compared with a psychological intervention. 3No explicit follow-up times are presented in the original paper; these were chosen by the reviewers (with full access to study data) to maximise comparison in meta-analysis
CBT
CBT for PEs (and other therapies where CBT is the key component, e.g. integrated psychological interventions) (Addington et al., 2011; Bechdolf et al., 2005, 2007; Evans et al., 2017; Ising et al., 2015, 2016, 2017; Kommescher et al., 2016; McGorry et al., 2013, 2017; Matsumoto et al., 2018; Morrison et al., 2004, 2012; Nelson et al., 2018b; Stafford et al., 2015; Stain et al., 2016; Van der Gaag et al., 2012) explores the links between thoughts, emotions and behaviour. The therapy is formulation-driven, problem-oriented, time-limited, and tailored to patients’ needs. The key components include patient engagement, creation of a mutually-agreed problem list, formulation, normalisation of PEs and patients’ interpretations of them, evaluation of alternative explanations, and behavioural experiments to challenge patients’ appraisals of PEs.
Cognitive remediation
Cognitive remediation refers to behavioural training aimed at improving cognitive processes (e.g. attention, memory and executive function) (Barlati et al., 2013). The cognitive remediation intervention included in this review focuses on improving auditory processing in people with PEs (Piskulic et al., 2015). It is computer-based and includes several different exercises to improve the diverse aspects of auditory processing.
Family-focused therapy
This therapy (O’Brien et al., 2015) treats people with PEs in the context of the family. The key components include psychoeducation around topics such as symptoms, daily stressors, coping strategies, the vulnerability–stress perspective, family support, and prevention action plans. Family members learn a structured approach to defining problems, breaking down complex problems, brainstorming solutions, analysing pros and cons of possible solutions, and selecting and implementing action plans.
Family psychoeducational intervention
The included family psychoeducational intervention (O’Brien et al., 2015) was a brief, three-session process of providing education and information. The content mirrored that of the psychoeducation aspect of the FFT described above.
Mindfulness-based cognitive therapy
Mindfulness-based cognitive therapy (MBCT; Langer et al., 2010) includes psychoeducation and exercises to demonstrate the links between thinking and feeling. Specific techniques include ‘Body Scan’ training, mindful breathing, breathing space, yoga, and sitting meditation. The intervention uses a group-based format.
Sleep CBT
The sleep CBT included this review (Bradley et al., 2017) used the ‘SleepWell’ treatment package, which utilises CBT techniques to address insomnia and circadian rhythm disruption to reduce sleep disturbances. Therapists use individualised formulation of sleep problems to identify treatment targets and actigraphy data to monitor changes in sleep patterns and highlight potential areas for change.
Strengths and mindfulness-based online social therapy
This intervention, set within a social media context, takes a strengths and mindfulness-based focus, and uses a self-determination theory of motivation to foster self-efficacy and increase positive emotions (Alvarez-Jimenez et al., 2018). The intervention provides social ‘online’ support moderated by expert and peer moderators. Modules addressed personal strengths, mindfulness, connecting with others, and group problem-solving to promote self-efficacy and interpersonal problem-solving.
Systemic therapy
Systemic therapy (Shi et al., 2017) is centred around systemic-constructivist and psychosocial resilience theories. The therapy focuses on solutions and resources, and encourages patients to reframe their problems and better understand their available resources in order to solve these problems.
Supportive treatments
As stated above, the category of STs includes supportive therapy, supportive counselling, non-directive reflective listening, NBI, and needs-focused intervention (Addington et al., 2011; Bechdolf et al., 2007; Kommescher et al., 2016; McGorry et al., 2013; Phillips et al., 2007; Ruhrmann et al., 2007; Shi et al., 2017; Stain et al., 2016). In general, these interventions use general counselling techniques, including warm, empathic, and non-judgmental face-to-face contact and supportive listening. They do not include active therapeutic techniques.
The quality of included studies was mixed (Table 2); 21 of the 27 reports used an RCT design, of which only 4 received a global rating of ‘strong’, 10 received a global rating of ‘moderate’, and 7 received a global rating of ‘weak’. Selection bias, confounding, and drop-out were the categories that most limited the global ratings (it should be noted that a rating of ‘strong’ in the selection bias category is not achievable when only help-seeking patients are included. Importantly, no study was excluded in the sensitivity analyses based solely on studying a help-seeking population). The remaining four studies used a pre–post design – relatively, a much weaker study design – but none of these received a ‘weak’ rating in any of the applicable categories.
Table 2.
Quality of included studies (EPHPP rating tool).
| First author (year); study design | Selection bias | Study design | Confounders | Blinding | Data collection | Drop-out | Global ratinga |
|---|---|---|---|---|---|---|---|
| Addington et al. (2011), RCT | Weak | Strong | Strong | Moderate | Strong | Weak | Weak |
| Alvarez-Jimenez et al. (2018), pre–post | Moderate | Moderate | NA | NA | Strong | Strong | NA |
| Bechdolf et al. (2007), RCT | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| Bechdolf et al. (2005), pre–post | Moderate | Moderate | NA | NA | Strong | Strong | NA |
| Bradley et al. (2017), A-B | Moderate | Moderate | NA | NA | Strong | Strong | NA |
| Evans et al. (2017), pre–post | Moderate | Moderate | NA | NA | Strong | Moderate | NA |
| Ising et al. (2017), economic evaluation of RCT | Weak | Strong | Strong | Moderate | Strong | Weak | Weak |
| Ising et al. (2016), RCT | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| Ising et al. (2015), economic evaluation of RCT | Weak | Strong | Strong | Moderate | Strong | Strong | Moderate |
| Kommescher et al. (2016), RCT | Moderate | Strong | Strong | Moderate | Strong | Moderate | Strong |
| Langer et al. (2010), quasi-RCT | Weak | Strong | Weak | Moderate | Strong | Weak | Weak |
| Matsumoto et al. (2018), pre–post | Moderate | Moderate | NA | NA | Strong | Strong | NA |
| McGorry et al. (2017), RCT | Moderate | Strong | Weak | Strong | Strong | Moderate | Moderate |
| McGorry et al. (2013), RCT | Weak | Strong | Strong | Strong | Strong | Moderate | Moderate |
| McGorry et al. (2002), RCT | Weak | Strong | Strong | Weak | Strong | Strong | Weak |
| Morrison et al. (2012), RCT | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| Morrison et al. (2004), RCT | Moderate | Strong | Strong | Moderate | Strong | Moderate | Strong |
| Nelson et al. (2018), RCT | Moderate | Strong | Weak | Strong | Strong | Strong | Moderate |
| O’Brien et al. (2015), RCT | Moderate | Strong | Strong | Moderate | Strong | Weak | Moderate |
| Phillips et al. (2009), economic evaluation of RCT | Weak | Strong | Strong | Strong | Strong | Moderate | Moderate |
| Phillips et al. (2007), RCT | Moderate | Strong | Strong | Weak | Strong | Moderate | Moderate |
| Piskulic et al. (2015), RCT | Moderate | Strong | Weak | Moderate | Strong | Weak | Weak |
| Ruhrmann et al. (2007), RCT | Moderate | Strong | Strong | Weak | Strong | Weak | Weak |
| Shi et al. (2017), RCT | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
| Stafford et al. (2015), pre–post | Moderate | Moderate | NA | NA | Strong | Strong | NA |
| Stain et al. (2016), RCT | Moderate | Strong | Weak | Moderate | Strong | Weak | Weak |
| Van der Gaag et al. (2012), RCT | Moderate | Strong | Strong | Moderate | Strong | Strong | Strong |
EPHPP: Effective Public Health Practice Project; RCT: randomised controlled trial; NA: not applicable.
Global ratings are not provided for studies with NA ratings in any category to ensure comparability of results (personal communication with EPHPP team, 19 September 2017).
Primary outcome
Five reports from four studies provided the proportion of participants that remitted from PEs following psychological intervention (Ising et al., 2016; Matsumoto et al., 2018; Ruhrmann et al., 2007; Shi et al., 2017; Van der Gaag et al., 2012). Meta-analysis was not possible for the primary outcome: only two reports had the same intervention framework and comparator category, and the more recent was a follow-up of the first (Ising et al., 2016; Van der Gaag et al., 2012).
CBT
Both studies of CBT used the Comprehensive Assessment of At-Risk Mental States (CAARMS; Yung et al., 2005) to determine remission status. In an RCT examining differences between CBT + TAU versus TAU, 70.4% of participants receiving CBT + TAU had remitted from at-risk mental state (ARMS) status by 12 months post-intervention, as compared with 57.0% of participants receiving TAU only (p = 0.039) (Van der Gaag et al., 2012). The difference remained significant at medium-term follow-up (approximately 3.5 years post-therapy), with 76.3% of the CBT + TAU group versus 58.7% of the TAU only group in remission (p = 0.04) (Ising et al., 2016). A pre–post study of CBT found ARMS remission rates of 46.2% at post-intervention and 84.6% 6 months post-intervention (Matsumoto et al., 2018).
Other frameworks
An RCT comparing systemic therapy with supportive therapy found greater remission from clinical high-risk status (measured using the Scale of Prodromal Symptoms; Miller et al., 2003) among those receiving systemic therapy (61.5% versus 46.2%), but the difference was not significant (p = 0.431) (Shi et al., 2017). Finally, a trial of a needs-focused intervention found a 20.5% remission rate from all psychotic symptoms (assessed with the Early Recognition Inventory – Positive Psychosis Spectrum [ERI-PPS]; Klosterkötter et al., 2001) at post-therapy (Ruhrmann et al., 2007).
Secondary outcomes
As mentioned above, we were only able to include studies of CBT in our meta-analyses, as CBT was the only framework examined in two or more studies.
CBT
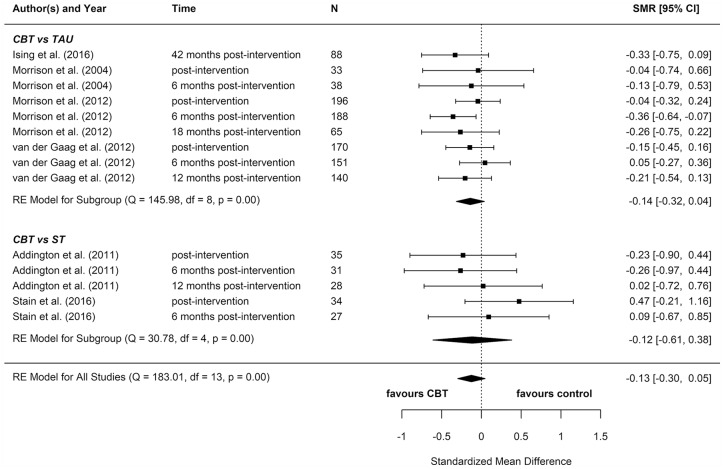
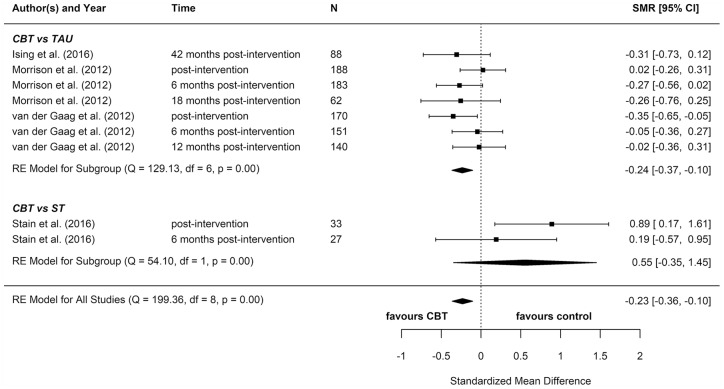
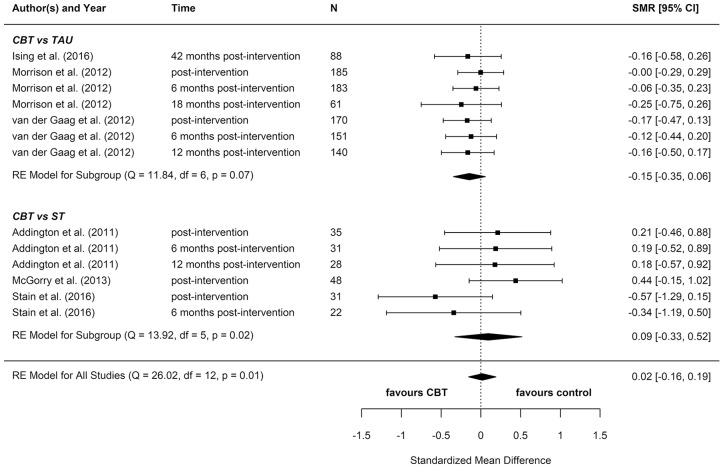
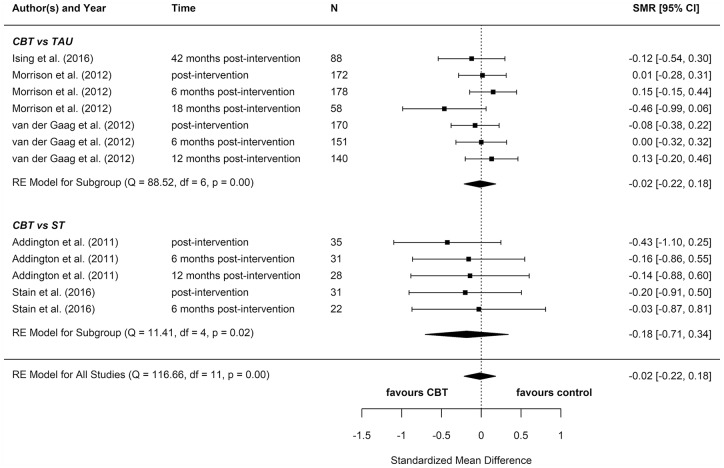
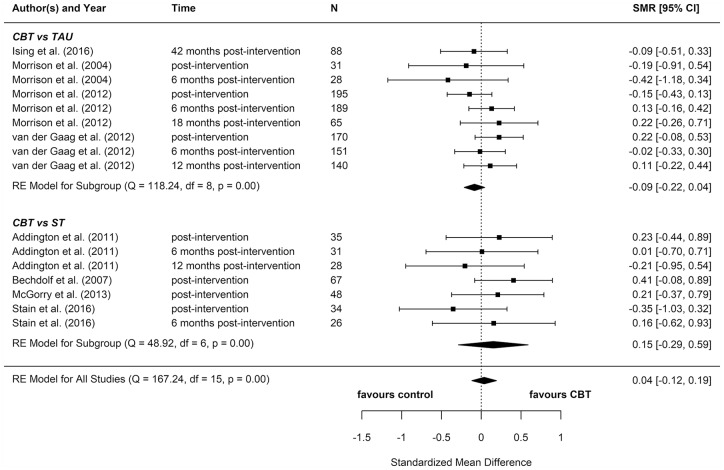
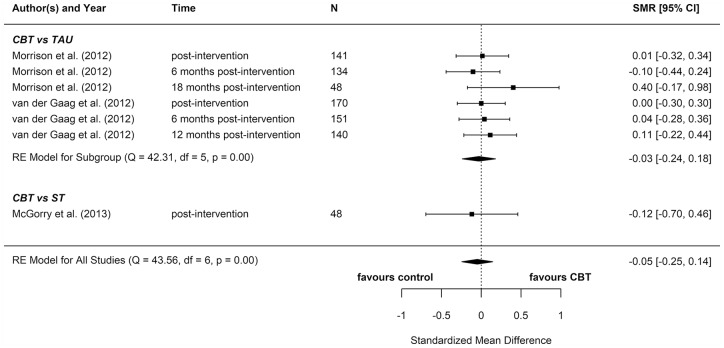
We included eight reports from seven studies in our meta-analyses (Figures 2–8), four of which compared CBT (with or without TAU) with TAU only. CBT was superior to TAU in reducing distress (pooled SMD = −0.24 favouring CBT; 95% CI = [−0.37, −0.10]). No other statistically significant differences were found for positive psychotic symptoms (pooled SMD = −0.14 favouring CBT; 95% CI = [−0.32, 0.04]), depression (pooled SMD = −0.15 favouring CBT; 95% CI = [−0.35, 0.06]), anxiety (pooled SMD = −0.02 favouring CBT; 95% CI = [−0.22, 0.18]), functioning (pooled SMD = −0.09 favouring TAU; 95% CI = [−0.22, 0.04]), or quality of life (pooled SMD = −0.03 favouring TAU; 95% CI = [−0.24, 0.18]).
Figure 2.
Positive psychotic symptoms: meta-analysis summary plot (NB: follow-up times are measured from the end of the intervention).
CBT: cognitive behavioural therapy; TAU: treatment as usual; ST: supportive treatments.
Figure 3.
Negative psychotic symptoms: meta-analysis summary plot (NB: follow-up times are measured from the end of the intervention).
CBT: cognitive behavioural therapy; TAU: treatment as usual; ST: supportive treatments.
Figure 4.
Distress: meta-analysis summary plot (NB: follow-up times are measured from the end of the intervention).
CBT: cognitive behavioural therapy; TAU: treatment as usual; ST: supportive treatments.
Figure 5.
Depression: meta-analysis summary plot (NB: follow-up times are measured from the end of the intervention).
CBT: cognitive behavioural therapy; TAU: treatment as usual; ST: supportive treatments.
Figure 6.
Anxiety: meta-analysis summary plot (NB: follow-up times are measured from the end of the intervention).
CBT: cognitive behavioural therapy; TAU: treatment as usual; ST: supportive treatments.
Figure 7.
Functioning: meta-analysis summary plot (NB: follow-up times are measured from the end of the intervention).
CBT: cognitive behavioural therapy; TAU: treatment as usual; ST: supportive treatments.
Figure 8.
Quality of life: meta-analysis summary plot (NB: follow-up times are measured from the end of the intervention).
CBT: cognitive behavioural therapy; TAU: treatment as usual; ST: supportive treatments.
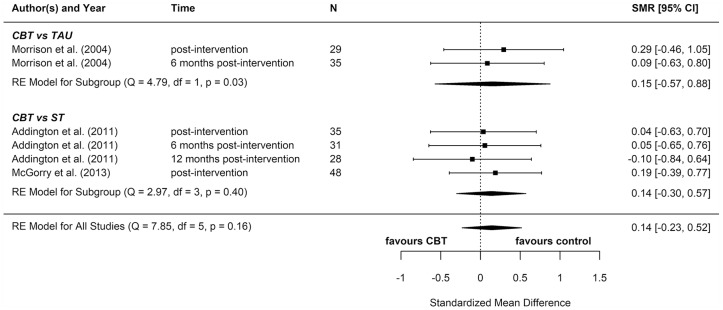
Four additional reports compared CBT with an ST (e.g. supportive therapy, supportive counselling or non-directive reflective listening). No statistically significant differences were found for positive psychotic symptoms (pooled SMD = −0.12 favouring CBT; 95% CI = [−0.61, 0.38]), negative psychotic symptoms (pooled SMD = 0.14 favouring ST; 95% CI = [−0.30, 0.57]), depression (pooled SMD = 0.09 favouring ST; 95% CI = [−0.33, 0.52]), anxiety (pooled SMD = −0.18 favouring CBT; 95% CI = [−0.71, 0.34]), or functioning (pooled SMD = −0.15 favouring CBT; 95% CI = [−0.29, 0.59]).
To determine whether there was a difference between the two different control groups, we included TAU and ST as predictors of SMD in a meta-regression model. Because there was not a statistically significant difference between the different control groups for any outcome, we also computed a pooled estimate for all reports regardless of comparator. When TAU and ST were collapsed into a single comparator group, CBT remained more effective than the combined TAU/ST comparison groups at reducing distress (pooled SMD: −0.23 favouring CBT; 95% CI = [−0.36, −0.10]). There were no other statistically significant differences between CBT and controls.
Most of these meta-analyses suffered from high heterogeneity (Cochran’s Q, p < 0.05). However, this measure is unreliable when the number of studies included is very low, so although heterogeneity cannot be ruled out, it is hard to ascertain its extent.
Two reports found significant between-group differences in severity of psychotic symptoms in two distinct trials, in each instance favouring cognitive therapy (p = 0.049 and p = 0.018, respectively) (Morrison et al., 2004, 2012). A further two reports found significant between-group differences in distress, but in opposite directions: while one found lower distress among participants in the CBT group (p = 0.012) (Van der Gaag et al., 2012), the other found lower distress among participants in the non-directive reflective listening (ST) group (p = 0.029) (Stain et al., 2016). No RCT found any statistically significant between-group differences for depression, anxiety, functioning, or quality of life.
In addition, reports from three controlled (Addington et al., 2011; Bechdolf et al., 2007; McGorry et al., 2017) and four uncontrolled studies (Bechdolf et al., 2005; Evans et al., 2017; Matsumoto et al., 2018; Stafford et al., 2015) provided results of significance tests for within-group pre–post changes for individuals receiving CBT (several more noted symptom improvement, but did not provide formal significance testing results). The three reports providing data on positive psychotic symptoms (Addington et al., 2011; Matsumoto et al., 2018; Stafford et al., 2015), and one of the three providing data on negative psychotic symptoms (McGorry et al., 2017) found significant improvement. Significant improvement was also noted in four of the five reports providing data on depression (Bechdolf et al., 2005; Evans et al., 2017; McGorry et al., 2017; Matsumoto et al., 2018) and functioning (Bechdolf et al., 2005, 2007; McGorry et al., 2017; Matsumoto et al., 2018), all four reports providing data on anxiety (Addington et al., 2011; Bechdolf et al., 2005; Evans et al., 2017; Matsumoto et al., 2018), one of the two providing data on distress (Evans et al., 2017), and in the one report that provided data on quality of life (Matsumoto et al., 2018). No study found statistically significant decline in any domain.
Supportive treatments
Reports from five controlled studies (Addington et al., 2011; Bechdolf et al., 2007; Phillips et al., 2007; Ruhrmann et al., 2007; Shi et al., 2017) provided results from significance testing for within-group pre–post changes for individuals receiving supportive or needs-focused treatments. Two of four reports providing data on positive psychotic symptoms (Addington et al., 2011; Ruhrmann et al., 2007), but none of the four providing data on negative psychotic symptoms, found significant improvement. Significant improvement was noted in two of four reports providing data on depression (Addington et al., 2011; Ruhrmann et al., 2007), one of two providing data on anxiety (Addington et al., 2011), one of five providing data on functioning (Bechdolf et al., 2007), and in the one report providing data on in quality of life (Phillips et al., 2007). No study found statistically significant decline in any domain.
Other intervention frameworks
Four additional RCTs focused on systemic therapy (Shi et al., 2017), MBCT (Langer et al., 2010), FFT (O’Brien et al., 2015), and cognitive remediation therapy (CRT) (Piskulic et al., 2015). Only the MBCT trial showed any between-group differences in our outcomes of interest. In this study, MBCT was more effective than the control condition (a video viewing forum) at reducing anxiety from baseline to post-therapy (d = 0.88, p = 0.012) as well as baseline to 12-week follow-up (d = 0.91, p = 0.048). However, they found no other significant between-group differences for psychotic symptoms or distress (Langer et al., 2010).
Systemic therapy, CRT and FFT were no more effective than their control treatments (supportive therapy, computer games, and family psychoeducation, respectively) (O’Brien et al., 2015; Piskulic et al., 2015; Shi et al., 2017). Although neither systemic therapy nor CRT was more effective than its control treatment, each showed within-group pre–post effects. Individuals who received systemic therapy showed significant reductions in positive symptoms (d = 0.53, p = 0.005) and depressive symptoms (d = 0.75, p = 0.010) from baseline to post-therapy, while no such changes were found for the supportive therapy group (Shi et al., 2017). Similarly, individuals assigned to CRT had significant improvements in social functioning (p < 0.05) from baseline to 6 months post-intervention, while those assigned to the computer games condition had no significant improvements (Piskulic et al., 2015).
A further two uncontrolled studies examined within-group pre–post effects of a strengths and mindfulness-based online social therapy (Alvarez-Jimenez et al., 2018) and a CBT intervention for sleep problems (Bradley et al., 2017). The former found significant improvements in social functioning (d = 1.83, p < 0.001) from baseline to post-intervention (Alvarez-Jimenez et al., 2018), and the latter found significant improvements in depression and quality of life (p < 0.05; exact values not given). These improvements were maintained at 1 month post-therapy, at which time improvement in paranoia and hallucinations also reached significance (p < 0.05; exact values not given) (Bradley et al., 2017).
Sub-group analyses
For sub-group analyses by quality, we were only able to perform two meta-analyses (for functioning and positive symptoms) due to the fact that in all other meta-analyses there was only one study without a high risk of bias in at least one category. We found no statistically significant difference between CBT and TAU in either sub-group analysis (see Supplemental Appendix E).
Components of effective interventions
We focused our components analysis on the five interventions that showed effectiveness for at least one outcome in controlled trials: three CBT (Morrison et al., 2004, 2012; Van der Gaag et al., 2012), one mindfulness-based cognitive therapy (Langer et al., 2010), and one non-directive reflective listening intervention (Stain et al., 2016) (intervention components in Supplemental Appendix D). Qualitative examination of the components of these five therapies revealed high heterogeneity: very few components were shared across the effective therapies, which is unsurprising given their differing frameworks. Furthermore, there were no ‘key ingredients’ that were particular to these five therapies: although there were some common components across the effective therapies (e.g. mode of delivery), these were also shared by therapies that did not demonstrate effectiveness.
Economic studies
Four reports met inclusion criteria for the economic component of the review (summary of studies’ characteristics in Supplemental Appendix F; quality assessment in Supplemental Appendix G; full economic analysis in Supplemental Appendix H). Two focused on CBT (Ising et al., 2015, 2017) and two on ST (Phillips et al., 2007, 2009).
CBT
Ising et al. (2015, 2017) reported the results of full economic evaluations in two reports, which were based on 18-month and 4-year post-baseline data, respectively, from a study conducted in the Netherlands between 2008 and 2010 comparing routine care plus CBT for the prevention of psychosis with routine care alone for individuals at ultra-high risk aged 14 to 35 years (Rietdijk et al., 2010). At 18 months post-baseline, the authors concluded that CBT proved to be cost-saving; however, differences in costs between groups were not tested statistically. When combined with outcome data, there was some evidence to suggest that CBT plus routine care may be cost-effective compared to routine care alone, but differences were small and no assessment of uncertainty was carried out. Results were clearer at 4 years post-baseline, with evidence to suggest a high probability (>80%) of the CBT group being cost-effective compared with routine care alone.
Supportive treatments
Phillips et al. (2007, 2009) explored resource use and cost-savings in two reports, both based on data from an RCT conducted in Australia between 1996 and 1999, which compared a NBI with NBI plus a specific preventive intervention (SPI) including psychotherapy and neuroleptic medication for individuals aged 14 to 30 years at ultra-high risk (McGorry et al., 2002). Phillips et al. (2007) explored resource use from a mental health service perspective between 12 and 36 months post-randomisation. Resource use was reported by group for some resource items and by those who did or did not develop psychosis for others. There was little difference in resource use with the exception of significantly higher mental health service use for those who did not develop psychosis in the control arm. However, sample sizes were small (total n = 41) and cost differences were not tested statistically. In Phillips et al. (2009), a cost-savings analysis was undertaken for the full 36-month post-baseline follow-up period. There were no significant differences in total cost between the groups over the full follow-up. In terms of outcomes (Phillips et al., 2007), no differences in the rate of transition to psychotic disorder, level of symptomatology, or functioning between the groups were identified, therefore indicating there may be no cost-effectiveness advantage of the intervention.
Discussion
This systematic review and meta-analysis included 27 reports concerning 21 studies of psychological interventions for PEs and aimed to determine their effectiveness and cost-effectiveness for improving a range of clinical and functional outcomes. In terms of the proportion of participants remitting from PEs, we found preliminary evidence from one RCT and one uncontrolled study for the potential effectiveness of CBT. We did not find meta-analytic evidence that CBT improved PEs on a continuous scale, though it is likely that our analyses were underpowered to detect small effects. While two individual RCTs favoured CBT over TAU for reducing the severity of psychotic symptoms, this effect was not consistent across all controlled studies. CBT, sleep CBT, and systemic therapy – but not ST – also showed promise in terms of within-group pre–post improvements in psychotic symptoms.
For our other non-psychotic secondary outcomes (depression, anxiety, functioning, distress and quality of life), only the meta-analysis of distress outcomes revealed evidence of comparative effectiveness, by which CBT was more effective than comparators. However, a high degree of heterogeneity cannot be ruled out in this meta-analysis, meaning that CBT may not reduce distress in all implementation scenarios in this patient population. Two individual trials showed a significant effect on distress, but in opposite directions. The only other RCT evidence of effectiveness was for mindfulness CBT, which significantly reduced participants’ anxiety symptoms. Low-quality evidence from uncontrolled studies showed that a number of therapies were effective for at least one non-psychotic clinical or functional outcome, including CBT, sleep CBT, systemic therapy, CRT, and mindfulness online social therapy. Supportive treatments were fairly effective at improving anxiety and depression, but not other outcomes.
The overall quality of studies included in the effectiveness component of the review was variable. While most reports (21 of the 27) focused on data from RCTs (the gold standard study design for investigating intervention effect), all but four of these received a rating indicating high risk of bias in at least one of the rating categories. High rates of attrition were the predominant reason for lower ratings, followed by high chance of selection bias. The six non-randomised, uncontrolled studies, although prone to the significant biases associated with low-quality study design, did not receive any rating indicating high risk of bias in any other applicable category (these were not rated in terms of blinding or confounders).
Economic data meeting the inclusion criteria were only available in four publications, which used data from two RCTs, one focusing on CBT and the other focusing on an intervention that included psychotherapy and antipsychotic medication. Both interventions were targeted at young adults at ultra-high risk of psychosis. The included economic studies were methodologically strong, meeting most of the Drummond checklist quality assessment criteria (Drummond and Jefferson, 1996). The economic studies focusing on CBT indicate that the addition of CBT to routine care has a high probability of being cost-effective compared to routine care alone in this ultra-high risk group.
Several previous systematic reviews and meta-analyses have examined the effectiveness of psychological, pharmacological, and nutritional interventions for people with PEs. Although most reviews focused primarily on transition (and four focused exclusively on transition), seven (Davies et al., 2018b; Devoe et al., 2019; Hutton and Taylor, 2014; Marshall and Rathbone, 2011; Okuzawa et al., 2014; Stafford et al., 2013; Van der Gaag et al., 2013) also reported selected secondary outcomes that do correspond with the current review’s focus, specifically psychotic symptoms (Davies et al., 2018b; Devoe et al., 2019; Marshall and Rathbone, 2011; Okuzawa et al., 2014; Stafford et al., 2013), distress (Hutton and Taylor, 2014; Okuzawa et al., 2014), depression (Marshall and Rathbone, 2011; Okuzawa et al., 2014; Stafford et al., 2013), anxiety (Marshall and Rathbone, 2011; Okuzawa et al., 2014), functioning (Hutton and Taylor, 2014; Marshall and Rathbone, 2011; Okuzawa et al., 2014; Van der Gaag et al., 2013), and quality of life (Hutton and Taylor, 2014; Marshall and Rathbone, 2011; Okuzawa et al., 2014; Stafford et al., 2013). Importantly, no prior review has included a consideration of remission from PEs. None of these reviews (including the review upon which current UK clinical guidelines are based) has found strong evidence to support the effectiveness of any particular psychological intervention for improving our outcomes of interest within this population. In general, these reviews reflect our own results. However, departing from previous findings, we found meta-analytic evidence that distress was significantly reduced after CBT as compared with control treatments (TAU/ST). It is possible that distress is a significant, under-measured, and under-reported outcome in the literature; indeed, only two previous reviews have reported distress as an outcome. Distress is an important factor to individuals with PEs as reductions can be interpreted as improvement, despite residual symptoms (Byrne and Morrison, 2014; Fowler et al., 2018; Law and Morrison, 2014); consequently, a broader consideration of this outcome is warranted.
Major treatment guidelines currently recommend CBT for the treatment of people at-risk for developing psychosis (Addington et al., 2017; Early Psychosis Guidelines Writing Group and EPPIC National Support Program, 2016; NICE, 2014; Schmidt et al., 2015). In the United Kingdom, NICE (2014) highlights the value of CBT for preventing transition to frank psychotic disorder. However, recent meta-analytic evidence published since the creation of these guidelines suggests that CBT for populations at-risk for developing psychosis may not be superior to other inventions in preventing transition (Davies et al., 2018a), although it is important to note that concerns have been raised about both the methodology and interpretation of results in this review (Nelson et al., 2018a). Our findings provide initial evidence that, while doubts remain about its effectiveness in terms of preventing transition to psychosis, CBT may nevertheless be more effective than other approaches at promoting remission from PEs and reduction of associated distress, and thus may still be considered as a potentially useful intervention for treating people with PEs. Conversely, when the aim of psychological intervention is to reduce other clinical symptoms (e.g. depression and anxiety) or functional impairment associated with PEs, CBT falls short in demonstrating effectiveness as compared with other treatments. This is an important shortcoming, as poor clinical and functional outcomes may serve to perpetuate mental ill health that may still require more than just monitoring for changes in post-CBT persistent symptoms, as currently recommended by NICE (2014).
Strengths and limitations
This review has a number of important strengths and addresses key gaps in the literature concerning psychological interventions for people with PEs. First, to our knowledge, we were the first to meta-analyse studies across such a broad range of clinical and functional outcomes. Second, we focus on remission from PEs, a new and important outcome that was developed in collaboration with our lived experience advisory panel. Third, we include economic outcomes, which again have not been reviewed previously. Fourth, we review a large number of studies not included in any other review, including, importantly, studies of newer, non-CBT frameworks.
These strengths notwithstanding, our review, and in particular our meta-analyses, has a number of limitations. First, each meta-analysis included a small number of reports, each of which had a limited number of participants (sometimes short of the recruitment target). This will have reduced our power to detect small, but potentially clinically meaningful, treatment effects. We aimed to increase power by including multiple study follow-up points within each meta-analysis. Although we could also have combined outcomes to reduce the total number of meta-analyses (and also the probability of type I error), we chose not to do this as (1) sometimes outcomes changed in different directions following intervention (e.g. see Langer et al., 2010), and (2) Cochrane warns against combining heterogeneous outcomes (see section 9.1.4) (Higgins and Green, 2011). Second, the high number of meta-analyses performed will have increased the probability of false positive results, which is particularly important in our analyses due to the fact that we found only one significant effect. Third, we could not rule out high heterogeneity within our meta-analyses. Fourth, our decision to group several therapy types under ‘supportive therapy’ was not without limitations; for example, patients under TAU conditions may well receive CBT for other mental health problems outside of PEs (e.g. depression or anxiety). Fifth, our exclusion criteria regarding age range and antipsychotic use may limit the generalisability of our findings to younger populations or patients prescribed antipsychotic medication as part of their treatment plan. Sixth, in terms of the studies themselves, while many utilised randomised controlled designs, the overall methodological quality was not high; only four studies received a global rating of ‘high’ on the quality rating tool. Finally, we acknowledge that we were not able to fulfil all a priori review aims. While the review was ambitious, we contend that it was not possible to predict which aims could and could not be accomplished. Furthermore, we believe that highlighting gaps in the literature is an important step in moving the field forward.
Conclusion
This review has clear clinical relevance and will be central in the development of a new therapeutic framework for IAPT, as well as for other programmes aiming to address PEs in primary mental healthcare settings internationally. The broad aims, comprehensive outcomes, and specific selection criteria all reflect this purpose. The review will ensure any decisions concerning treatment development and treatment selection for people with PEs within primary care are supported by the most recent and high-quality evidence. Overall, our findings indicate that clinicians must consider a wider range of clinical and functional outcomes as well as interventions for people with PEs that go beyond strategies for preventing transition to psychotic disorders. Our systematic review and meta-analysis suggest that, despite its limited effectiveness in preventing transitions, CBT may be useful to reduce the distress associated with PEs and cost-effective in comparison with treatment as usual. However, the scarcity of studies focusing on remission from PEs and improvement of other non-psychotic clinical and functional outcomes suggests a need for further research into psychological treatments for this population.
Supplemental Material
Supplemental material, Soneson_Russo_et_al_appendices_R1 for Psychological interventions for people with psychotic experiences: A systematic review and meta-analysis of controlled and uncontrolled effectiveness and economic studies by Emma Soneson, Debra Russo, Jan Stochl, Margaret Heslin, Julieta Galante, Clare Knight, Nick Grey, Joanne Hodgekins, Paul French, David Fowler, Louise Lafortune, Sarah Byford, Peter B Jones and Jesus Perez in Australian & New Zealand Journal of Psychiatry
Acknowledgments
The authors are very grateful to medical librarians Veronica Phillips and Isla Kuhn at the University of Cambridge Clinical School, who helped design the search strategies for this review. The authors also thank the members of our Lived Experience Advisory Panel for their contributions to the design of this research.
Footnotes
Author Contributions: J.P., P.B.J., L.L., D.R., J.G., J.S. and E.S. conceived the review design. J.P. is the guarantor of the review. E.S., D.R., C.K., M.H., J.S., N.G., J.H., P.F., D.F., S.B., P.B.J and J.P. contributed to the design of the search strategy. E.S., D.R., J.S., M.H., S.B. and J.P. drafted the original manuscript draft. C.K., L.L., J.G., J.H., P.F., D.F., N.G., S.B. and P.B.J. contributed to the review of manuscript drafts. All authors approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.B., D.F., P.F., J.G., J.H., C.K., L.L., J.P., D.R. and E.S. declare no conflicts of interest. N.G. reports grants from Wellcome Trust and personal fees from book publishers and training events (outside the submitted work). P.B.J. reports personal fees from Lundbeck, Ricordati, and Janssen scientific advisory boards (outside the submitted work). J.S. reports personal fees from IESO Digital Health (outside the submitted work).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grant for Applied Research Programme (reference number RP-PG-0616-20003). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The funding body had no role in study design; collection, analysis, or interpretation of data; the writing of the report; or decision to submit the paper.
ORCID iD: Emma Soneson  https://orcid.org/0000-0003-1666-3012
https://orcid.org/0000-0003-1666-3012
Supplemental Material: Supplemental material for this article is available online.
References
- Addington J, Addington D, Abidi S, et al. (2017) Canadian treatment guidelines for individuals at clinical high risk of psychosis. The Canadian Journal of Psychiatry 62: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addington J, Epstein I, Liu L, et al. (2011) A randomized controlled trial of cognitive behavioral therapy for individuals at clinical high risk of psychosis. Schizophrenia Research 125: 54–61. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jimenez M, Gleeson JF, Bendall S, et al. (2018) Enhancing social functioning in young people at Ultra High Risk (UHR) for psychosis: A pilot study of a novel strengths and mindfulness-based online social therapy. Schizophrenia Research 202: 369–377. [DOI] [PubMed] [Google Scholar]
- Armijo-Olivo S, Stiles CR, Hagen NA, et al. (2012) Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. Journal of Evaluation in Clinical Practice 18: 12–18. [DOI] [PubMed] [Google Scholar]
- Barlati S, Deste G, De Peri L, et al. (2013) Cognitive remediation in schizophrenia: Current status and future perspectives. Schizophrenia Research and Treatment 2013: 156084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechdolf A, Veith V, Schwarzer D, et al. (2005) Cognitive-behavioral therapy in the pre-psychotic phase: An exploratory study. Psychiatry Research 136: 251–255. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Wagner M, Veith V, et al. (2007) Randomized controlled multicentre trial of cognitive behaviour therapy in the early initial prodromal state: Effects on social adjustment post treatment. Early Intervention in Psychiatry 1: 71–78. [DOI] [PubMed] [Google Scholar]
- Bradley J, Freeman D, Chadwick E, et al. (2017) Treating sleep problems in young people at ultra-high risk of psychosis: A feasibility case series. Behavioural and Cognitive Psychotherapy 46: 276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome MR, Woolley JB, Johns LC, et al. (2005) Outreach and support in south London (OASIS): Implementation of a clinical service for prodromal psychosis and the at risk mental state. European Psychiatry 20: 372–378. [DOI] [PubMed] [Google Scholar]
- Byrne RE, Morrison AP. (2014) Young people at risk of psychosis: Their subjective experiences of monitoring and cognitive behaviour therapy in the early detection and intervention evaluation 2 trial. Psychology and Psychotherapy: Theory, Research and Practice 87: 357–371. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, et al. (2008) Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry 65: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt B, Lencz T, Obuchowski M. (2002) The schizophrenia prodrome: Treatment and high-risk perspectives. Schizophrenia Research 54: 177–186. [DOI] [PubMed] [Google Scholar]
- Davies C, Cipriani A, Ioannidis JPA, et al. (2018. a) Lack of evidence to favor specific preventive interventions in psychosis: A network meta-analysis. World Psychiatry 17: 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C, Radua J, Cipriani A, et al. (2018. b) Efficacy and acceptability of interventions for attenuated positive psychotic symptoms in individuals at clinical high risk of psychosis: A network meta-analysis. Frontiers in Psychiatry 9: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG, et al. (2018) Analysing data and undertaking meta-analyses (Draft version 13 September 2018). In: Higgins J, Thomas J, Chandler J, et al. (eds) Cochrane Handbook for Systematic Reviews of Interventions. London: Cochrane, pp. 243–296. [Google Scholar]
- Devoe DJ, Farris MS, Townes P, et al. (2019) Attenuated psychotic symptom interventions in youth at risk of psychosis: A systematic review and meta-analysis. Early Intervention in Psychiatry 13: 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MF, Jefferson TO. (1996) Guidelines for authors and peer reviewers of economic submissions to the BMJ: The BMJ Economic Evaluation Working Party. BMJ 313: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MF, Sculpher MJ, Claxton K, et al. (2015) Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford university press. [Google Scholar]
- Early Psychosis Guidelines Writing Group and EPPIC National Support Program (2016) Australian Clinical Guidelines for Early Psychosis, 2nd Edition. Parkville, VIC, Australia: Orygen – The National Centre of Excellence in Youth Mental Health. [Google Scholar]
- Evans C, Forrester A, Jarrett M, et al. (2017) Early detection and early intervention in prison: Improving outcomes and reducing prison returns. The Journal of Forensic Psychiatry & Psychology 28: 91–107. [Google Scholar]
- Fernández-Castilla B, Beretvas SN, Onghena P, et al. (2019) Multilevel Models in Meta-Analysis: A Systematic Review of Their Application and Suggestions. Trier: ZPID; (Leibniz Institute for Psychology Information). [Google Scholar]
- Flach C, French P, Dunn G, et al. (2015) Components of therapy as mechanisms of change in cognitive therapy for people at risk of psychosis: Analysis of the EDIE-2 trial. The British Journal of Psychiatry 207: 123–129. [DOI] [PubMed] [Google Scholar]
- Fowler D, Hodgekins J, French P, et al. (2018) Social recovery therapy in combination with early intervention services for enhancement of social recovery in patients with first-episode psychosis (SUPEREDEN3): A single-blind, randomised controlled trial. The Lancet Psychiatry 5: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Nelson B, Valmaggia L, et al. (2012) Comorbid depressive and anxiety disorders in 509 individuals with an at-risk mental state: Impact on psychopathology and transition to psychosis. Schizophrenia Bulletin 40: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granö N, Karjalainen M, Suominen K, et al. (2011) Poor functioning ability is associated with high risk of developing psychosis in adolescents. Nordic Journal of Psychiatry 65: 16–21. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. (2011) Cochrane Handbook for Systematic Reviews of Interventions. New York: John Wiley & Sons. [Google Scholar]
- Hui C, Morcillo C, Russo DA, et al. (2013) Psychiatric morbidity, functioning and quality of life in young people at clinical high risk for psychosis. Schizophrenia Research 148: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton P, Taylor PJ. (2014) Cognitive behavioural therapy for psychosis prevention: A systematic review and meta-analysis. Psychological Medicine 44: 449–468. [DOI] [PubMed] [Google Scholar]
- Hutton P, Bowe S, Parker S, et al. (2011) Prevalence of suicide risk factors in people at ultra-high risk of developing psychosis: A service audit. Early Intervention in Psychiatry 5: 375–380. [DOI] [PubMed] [Google Scholar]
- Ising HK, Kraan TC, Rietdijk J, et al. (2016) Four-year follow-up of cognitive behavioral therapy in persons at ultra-high risk for developing psychosis: The Dutch Early Detection Intervention Evaluation (EDIE-NL) Trial. Schizophrenia Bulletin 42: 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising HK, Lokkerbol J, Rietdijk J, et al. (2017) Four-year cost-effectiveness of cognitive behavior therapy for preventing first-episode psychosis: The Dutch Early Detection Intervention Evaluation (EDIE-NL) Trial. Schizophrenia Bulletin 43: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising HK, Smit F, Veling W, et al. (2015) Cost-effectiveness of preventing first-episode psychosis in ultra-high-risk subjects: Multi-centre randomized controlled trial. Psychological Medicine 45: 1435–1446. [DOI] [PubMed] [Google Scholar]
- Kelleher I, Lynch F, Harley M, et al. (2012) Psychotic symptoms in adolescence index risk for suicidal behavior: Findings from 2 population-based case-control clinical interview studies. Archives of General Psychiatry 69: 1277–1283. [DOI] [PubMed] [Google Scholar]
- Klosterkötter J, Hellmich M, Steinmeyer EM, et al. (2001) Diagnosing schizophrenia in the initial prodromal phase. Archives of General Psychiatry 58: 158–164. [DOI] [PubMed] [Google Scholar]
- Kommescher M, Wagner M, Pützfeld V, et al. (2016) Coping as a predictor of treatment outcome in people at clinical high risk of psychosis. Early Intervention in Psychiatry 10: 17–27. [DOI] [PubMed] [Google Scholar]
- Langer ÁI, Cangas AJ, Gallego J. (2010) Mindfulness-based intervention on distressing hallucination-like experiences in a nonclinical sample. Behaviour Change 27: 176–183. [Google Scholar]
- Law H, Morrison AP. (2014) Recovery in psychosis: A Delphi study with experts by experience. Schizophrenia Bulletin 40: 1347–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. PLoS Medicine 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Hartmann JA, Spooner R, et al. (2018) Beyond the ‘at risk mental state’ concept: Transitioning to transdiagnostic psychiatry. World Psychiatry 17: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGorry PD, Nelson B, Markulev C, et al. (2017) Effect of ω-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: The NEURAPRO randomized clinical trial. JAMA Psychiatry 74: 19–27. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Nelson B, Phillips LJ, et al. (2013) Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: Twelve-month outcome. The Journal of Clinical Psychiatry 74: 349–356. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ, et al. (2002) Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Archives of General Psychiatry 59: 921–928. [DOI] [PubMed] [Google Scholar]
- Marshall M, Rathbone J. (2011) Early intervention for psychosis. Cochrane Database of Systematic Reviews 6: CD004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Ohmuro N, Tsujino N, et al. (2018) Open-label study of cognitive behavioural therapy for individuals with at-risk mental state: Feasibility in the Japanese clinical setting. Early Intervention in Psychiatry 13: 137–141. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, et al. (2002) Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: Preliminary evidence of interrater reliability and predictive validity. American Journal of Psychiatry 159: 863–865. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, et al. (2003) Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin 29: 703–715. [DOI] [PubMed] [Google Scholar]
- Morrison AP, Barratt S. (2009) What are the components of CBT for psychosis? A Delphi study. Schizophrenia Bulletin 36: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AP, French P, Stewart SLK, et al. (2012) Early detection and intervention evaluation for people at risk of psychosis: Multisite randomised controlled trial. BMJ 344: e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AP, French P, Walford L, et al. (2004) Cognitive therapy for the prevention of psychosis in people at ultra-high risk: Randomised controlled trial. The British Journal of Psychiatry 185: 291–297. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence (NICE) (2014) Psychosis and schizophrenia in adults: prevention and management: Clinical guideline (CG178). Available at: www.nice.org.uk/guidance/cg178/chapter/recommendations#ftn.footnote_2 (accessed 3 September 2019)
- Nelson B, Amminger GP, McGorry PD. (2018. a) Recent meta-analyses in the clinical high risk for psychosis population: Clinical interpretation of findings and suggestions for future research. Frontiers in Psychiatry 9: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Amminger GP, Yuen HP, et al. (2018. b) NEURAPRO: A multi-centre RCT of omega-3 polyunsaturated fatty acids versus placebo in young people at ultra-high risk of psychotic disorders – Medium-term follow-up and clinical course. NPJ Schizophrenia 4: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B, Sass LA, Škodlar B. (2009) The phenomenological model of psychotic vulnerability and its possible implications for psychological interventions in the ultra-high risk (‘prodromal’) population. Psychopathology 42: 283–292. [DOI] [PubMed] [Google Scholar]
- O’Brien MP, Miklowitz DJ, Cannon TD. (2015) Decreases in perceived maternal criticism predict improvement in subthreshold psychotic symptoms in a randomized trial of family-focused therapy for individuals at clinical high risk for psychosis. Journal of Family Psychology 29: 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuzawa N, Kline E, Fuertes J, et al. (2014) Psychotherapy for adolescents and young adults at high risk for psychosis: A systematic review. Early Intervention in Psychiatry 8: 307–322. [DOI] [PubMed] [Google Scholar]
- Perez J, Russo DA, Stochl J, et al. (2018) Common mental disorder including psychotic experiences: Trailblazing a new recovery pathway within the Improving Access to Psychological Therapies programme in England. Early Intervention in Psychiatry 12: 497–504. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Uher R, Ostacher M, et al. (2011) Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Archives of General Psychiatry 68: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LJ, Cotton S, Mihalopoulos C, et al. (2009) Cost implications of specific and non-specific treatment for young persons at ultra high risk of developing a first episode of psychosis. Early Intervention in Psychiatry 3: 28–34. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, McGorry PD, Yuen HP, et al. (2007) Medium term follow-up of a randomized controlled trial of interventions for young people at ultra high risk of psychosis. Schizophrenia Research 96: 25–33. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Barbato M, Liu L, et al. (2015) Pilot study of cognitive remediation therapy on cognition in young people at clinical high risk of psychosis. Psychiatry Research 225: 93–98. [DOI] [PubMed] [Google Scholar]
- Popay J, Roberts H, Sowden A, et al. (2006) Guidance on the conduct of narrative synthesis in systematic reviews. A Product from the ESRC Methods Programme Version 1: b92. [Google Scholar]
- Rietdijk J, Dragt S, Klaassen R, et al. (2010) A single blind randomized controlled trial of cognitive behavioural therapy in a help-seeking population with an at risk mental state for psychosis: The Dutch Early Detection and Intervention Evaluation (EDIE-NL) trial. Trials 11: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrmann S, Bechdolf A, Kühn K-U, et al. (2007) Acute effects of treatment for prodromal symptoms for people putatively in a late initial prodromal state of psychosis. The British Journal of Psychiatry 191: s88–s95. [DOI] [PubMed] [Google Scholar]
- Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, et al. (2015) EPA guidance on the early intervention in clinical high risk states of psychoses. European Psychiatry 30: 388–404. [DOI] [PubMed] [Google Scholar]
- Shi J, Wang L, Yao Y, et al. (2017) Systemic therapy for youth at clinical high risk for psychosis: A pilot study. Frontiers in Psychiatry 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneson E, Russo D, Knight C, et al. (2019) Psychological interventions for people with psychotic experiences: Protocol for a systematic review and meta-analysis. Systematic Reviews 8: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford E, Hides L, Kavanagh DJ. (2015) The acceptability, usability and short-term outcomes of get real: A web-based program for psychotic-like experiences (PLEs). Internet Interventions 2: 266–271. [Google Scholar]
- Stafford MR, Jackson H, Mayo-Wilson E, et al. (2013) Early interventions to prevent psychosis: Systematic review and meta-analysis. BMJ 346: f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stain HJ, Bucci S, Baker AL, et al. (2016) A randomised controlled trial of cognitive behaviour therapy versus non-directive reflective listening for young people at ultra high risk of developing psychosis: The detection and evaluation of psychological therapy (DEPTh) trial. Schizophrenia Research 176: 212–219. [DOI] [PubMed] [Google Scholar]
- Valiji Bharmal A, Goodyer I, Wilkinson P. (2015) Are psychotic features in adolescents with depression a predictor of severity? Paper presented at the congress of psychiatry medforum, Wisla. [Google Scholar]
- van der Gaag M, Nieman DH, Rietdijk J, et al. (2012) Cognitive behavioral therapy for subjects at ultrahigh risk for developing psychosis: A randomized controlled clinical trial. Schizophrenia Bulletin 38: 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag M, Smit F, Bechdolf A, et al. (2013) Preventing a first episode of psychosis: Meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophrenia Research 149: 56–62. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36: 1–48. [Google Scholar]
- Wigman JTW, Van Os J, Abidi L, et al. (2014) Subclinical psychotic experiences and bipolar spectrum features in depression: Association with outcome of psychotherapy. Psychological Medicine 44: 325–336. [DOI] [PubMed] [Google Scholar]
- Yates K, Lång U, Cederlöf M, et al. (2019) Association of psychotic experiences with subsequent risk of suicidal ideation, suicide attempts, and suicide deaths: A systematic review and meta-analysis of longitudinal population studies. JAMA Psychiatry 76: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, McGorry PD, McFarlane CA, et al. (1996) Monitoring and care of young people at incipient risk of psychosis. Schizophrenia Bulletin 22: 283–303. [DOI] [PubMed] [Google Scholar]
- Yung AR, Pan Yuen H, McGorry PD, et al. (2005) Mapping the onset of psychosis: The comprehensive assessment of at-risk mental states. Australian and New Zealand Journal of Psychiatry 39: 964–971. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, et al. (2003) Psychosis prediction: 12-month follow up of a high-risk (‘prodromal’) group. Schizophrenia Research 60: 21–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Soneson_Russo_et_al_appendices_R1 for Psychological interventions for people with psychotic experiences: A systematic review and meta-analysis of controlled and uncontrolled effectiveness and economic studies by Emma Soneson, Debra Russo, Jan Stochl, Margaret Heslin, Julieta Galante, Clare Knight, Nick Grey, Joanne Hodgekins, Paul French, David Fowler, Louise Lafortune, Sarah Byford, Peter B Jones and Jesus Perez in Australian & New Zealand Journal of Psychiatry