Abstract
mTOR is a serine-threonine kinase and participates in cell proliferation, cellular metabolism was found to be activated during Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral infection and replication. During viral replication mTOR, downstream target genes such as ribosomal protein S6 kinase beta 1 (S6K1) and Eukaryotic translational initiation factor 4E-binding protein1 (4-E-BP1) are activated result in ribosome biosynthesis and efficient protein synthesis. In plasmacytoid dendritic cells (pDCs), mTOR plays a key role in the association of adapter protein myeloid differentiation primary response gene 88 (MyD88), Toll-like receptor 9 (TLR9) and interferon regulatory factor (IRF-7) leading to the transcriptional activation of type-I interferon (IFN) genes. Viruses also inactivate the interferon α (IFN-α) pathway by impairing the IRF-7 mediated activation of IFN-α gene transcription. Thus, mammalian target of rapamycin (mTOR) inhibitors can help in suppressing the early stages of viral infection and replication. Interestingly, the key tumor-suppressor p53 protein will undergo degradation by virus-encoded E3 ubiquitin ligase Ring-finger and CHY zinc-finger domain-containing 1 (RCHY1) leading to an increased viral survival in host cells. Thus, the mTOR inhibitors and p53 activators or microRNAs that functions as p53 and can target 3′-UTR of mTOR and RPS6KB1 might effectively inhibit viral replication in the human respiratory tract and lung cells.
Keywords: mTOR, p53, Dicer, MicroRNA, nCOVID19 SARS-CoV, MERS
1. Introduction
Researchers are trying to understand the possible pathways in coronavirus infection, inflammation of lung, and mortality. The virus and host interaction occurs between spike glycoprotein and the cell receptor angiotensin-converting enzyme II (ACE2) (Li et al., 2020). Corona viruses (CoVs) belong to order Nidovirales, family Coronaviridae, sub-family ortho corona virinae and genus Betacoronavirus (King et al., 2011). The SARS-CoV Coronavirus is a positive single-stranded RNA virus that belongs to the family Corona Viridae and has a genome size of 26–31 kb in length. It spreads through Bat and yet the actual reservoir host is yet to be identified. The Coronaviridae family has 4 genera such as Alphacoronavirus (alpha CoV), Betacoronavirus (beta CoV), Deltacoronavirus (delta CoV), and Gammacoronavirus (gamma CoV). Furthermore, the beta CoV genus divides into five sub-genera or lineages (Chan et al., 2013). The phylogenetic analyses and whole-genome analysis have revealed that 2019-nCoV/SARS-CoV-2 shows sequence identity with SARS-CoV up to 80%) (Zhou et al., 2020).
2. Coronaviruses and disease
The human coronaviruses such as HCoVs include severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), caused high mortality. The SARS-CoV has infected 8098 individuals in 2002–2003 and whereas MERS caused 858 deaths, most of them are belong to Saudi Arabia. Recently, nCOVID 19 originated in China and has spread to almost all the countries affecting into locking down life into cocoons, a restrictive phase. Thus, to control the spread of disease, researchers are tirelessly working in designing various drugs that inhibit virus-host interactions, such as ACE2 inhibitors, antibody-based therapeutic molecules, Nanotechnology-based drug delivery systems into the lung cells such as nano-sprays, microRNAs, siRNA as therapeutic molecules, nucleoside analogs such as Azidothymidine, which contribute inhibition of reverse transcriptase action in human immune-deficiency virus (HIV), aciclovir, ganciclovir that can inhibit the infection of herpes simplex virus (HSV), Oseltamivir (Tamiflu), the drug that can inhibit the viral budding in influenza. Many groups are trying to understand the cytokine profiling and attempting to inhibit the inflammation in the lung during COVID 19 infection phase. Here, in this study, we propose mTOR inhibitors and p53 activators-based drugs are the potential therapeutic candidates or targets that could be employed for nCOVID-19 infection.
3. mTOR inhibitors therapy against COVID19 infection
PI3K/AKT/mTOR pathway regulates fundamental cellular processes such as transcription, protein synthesis, and cell metabolism (Magnuson et al., 2012). Epstein-Barr virus study has indicated that mTOR inhibitor drug, manassantinB, inhibits the phosphorylation of AKT (i. e. Akt Ser 473) and PKC at Ser-657 mediated by mTOR complex 2 (mTORC2) and thus overall inhibition of EBV lytic replication via mTORC2-PKC/AKT signaling pathway (Wang et al., 2020). Few others have indicated that 16 potential anti-HCoV repurposable drugs (e.g., melatonin, and sirolimus) from drug-gene signatures (Zhou et al., 2020). The recent study had indicated influenza infects human lung cells A549 and increases mTOR activity and is needed for activating host translation processes during the initial 24 h of its mTOR inhibitor Everolimus, was found to suppress the viral protein synthesis by inhibiting the miR-101, a p53 dependent microRNA that targets the mTOR gene infection (Sharma et al., 2020a, Sharma et al., 2020b).
Middle East respiratory syndrome coronavirus (MERS-CoV) belongs to betacoronavirus and MERS infection causes acute respiratory syndrome. The kinase inhibitors targeting the ERK/MAPK and PI3K/AKT/mTOR pathways, decreased MERS-CoV propagation in vitro, indicating that the mTOR pathway is a potential drug target (Kindrachuk et al., 2015). Studies in human papillomavirus (HPV) have shown that activated PI3K/AKT/mTOR signaling complex, resulted in the initiation of viral replication and is mediated by overexpression of virally encoded HPV E6 oncogene. Also, HPV E16 activates mTOR and its downstream target genes such as S6K1 and eukaryotic initiation factor binding protein 1 (4E-BP1) that are involved in the protein translation process (Spangle and Munger, 2010). During COVID-19 mediated inflammation of the lung, increased expression of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, IL-12, and IFNγ caused cytokine storm (Ye et al., 2020; Chen et al., 2006). Rapamycin, was found to inhibit the stimulatory effect of IL-6 (Conti et al., 2020; Ekshyyan et al., 2016; Zegeye et al., 2018). The recent study, in this direction, that highlighted the use of Azithromycin (AZM) in COVID-19 positive patients that target mTORC1 and control virus proliferation and pathological effects (Al-Kassar and Al-Afif, 2020).
Recent studies have indicated about several mTOR inhibitors that are possible COVID-19 inhibitors (Table 1 ). The mTOR pathway is regulated by interferons (IFNs) during viral infection as part of the anti-viral response. Type-I IFNs are cytokines that regulate antiviral immunity. During viral infection, various cellular pattern recognition receptors (PRRs) are expressed and their association with adopter protein may lead to the activation of interferon regulatory factors (IRFs) such as IRF-3 and IRF-7 and Nuclear factor-kappa B (NF-kB). These proteins transactivates the type-I-IFN gene in the virus affected cells (Takeuchi and Akira, 2009; Brubaker et al., 2015; Hoffmann et al., 2015; Levy et al., 2011). The IFNs that produced in the infected cells go and bind to IFN α receptors present on the neighbouring healthy cells. This would lead to the activation of cellular signaling pathway such as JAK-STAT leading to the expression of IFN-stimulated genes. The other signaling pathways, associated with activation of type-I-IFNs and expression of interferon-stimulated genes (ISGs) are the PI3K-AKT-mTOR pathway and mitogen activated protein kinase (MAPK) pathway. The PI3K-mTOR-p70S6 kinase pathway is required for toll-like receptor (TLR) dependent type-I-IFN production in plasmacytoid dendritic cells (Cao et al., 2008). Few others have indicated that type-I IFNs or IFN responses can activate mTOR downstream target S6K1 and inactivate 4E-BP1 and thereby regulate translation process and also involved in the stimulation of type-I-interferon IFN responses (Lekmine et al., 2003; Livingstone et al., 2015).
Table 1.
mTOR inhibitors with potential to inhibit COVID-19 infection and replication in human lung cells.
| S. no. | mTOR inhibitor | Biological action | Reference |
|---|---|---|---|
| 1. | Rapamycin (Sirolimus) | It targets mTORC1 complex (i.e. mTOR, Raptor, Deptor, mLST8, PRAS40, FKBP38) and inhibit PI3K/Akt/mTOR dependent signaling pathway as well as MERS-CoV activity. | Kindrachuk et al., 2015 |
| Rapamycin binds to immunophilin FK506-binding protein12A (FKBP12A) and inhibits the mTORC1 activity. It also disrupts the interaction between Raptor and mTOR. | Wullschleger et al., 2006 | ||
| Inhibits the interaction between mTOR translational repressor (LARP1) and inhibit MERS infection up to 60% | Gordon et al., 2020 | ||
| Rapamycin gives cross-strain protection against influenza infection. | Keating et al., 2013 | ||
| 2. | Metformin | Activates 5-AMP activated protein kinase (AMPK) via liver kinase B1 (LKB1) and inhibits the mTOR pathway. Also, metformin indirectly attenuates Akt activity through phosphorylation of insulin receptor substrate (IRS1). Thus, the possibility of its use as anti-COVID19. | Sharma et al., 2020a, Sharma et al., 2020b |
| 4. | Sapanisertib (INK0128; INK128) | Orally bioavailable mTOR inhibitor. It inhibits mTORC1 and mTORC2 | Fonseca et al., 2018 |
| 5. | PP-242 | During PRRSV infection PP-242 modulates the mTOR signaling cascade and repress the IFN production by inhibiting the transcriptional activation of IRF-3, NF-kB, etc. and suppress the production and activity of type-I interferons in macrophages and dendritic cells during early viral infection. It is a non-selective inhibitor that targets the ATP binding site of mTOR kinase and suppresses mTORC1 and mTORC2. It also suppresses Porcine reproductive respiratory syndrome virus (PRRSV) infection up to 90% |
Liu et al., 2017 |
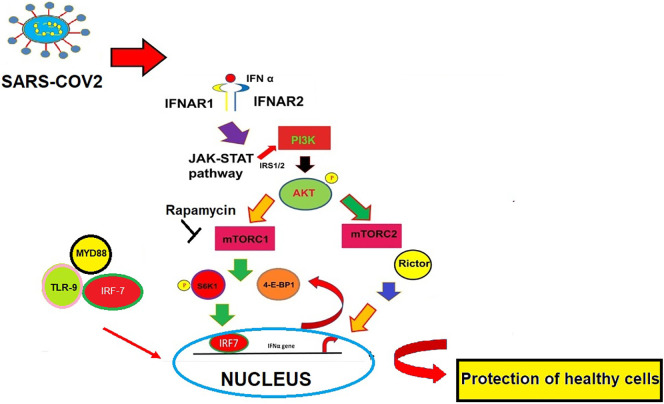
mTOR forms two complexes mTORC1 and mTORC2. It has been shown that both mTORC1- and mTORC2-signaling cascades regulate transcription and translation of interferon-stimulated genes (ISGs) and in the production of type-I-IFNs. (Livingstone et al., 2015). Some studies further demonstrated that an Unc51-like kinase (ULK1) interconnects the type-I-IFN response with the mTOR signaling pathway, and the mTOR-ULK1 pathway is critical for gene transcription mediated by the cis-elements (such as ISREs and GAS) in type-I-IFN genes (Saleiro et al., 2015). Studies have indicated that Rictor, the key subunit of mTORC2, was upregulated by IFN-α. IFN induced AKT phosphorylation on Ser-473 was absent in cells that are knockouts for the genes such as Rictor−/−, Sin1−/−, and mLST8−/−. This revealed the crucial role of mTORC2 for AKT engagement in response to type-I-IFNs. These results have indicated that mTORC2 may play a crucial role in macrophage polarization and antiviral regulation (Kaur et al., 2008). (Fig. 1 ).
Fig. 1.
Role of PI3K-Akt-mTOR pathway in interferon production. In plasmacytoid DCs (pDCs), the s6 kinase 1 (s6K1) phosphorylation by mTOR complex1 promotes the interaction between MYD88, TLR-9, and IRF7 leading to nuclear translocation of IRF7 leading to transcriptional activation of type I interferon (IFN) genes. The key component of mTORC2, Rictor was also found to regulate IFN α production. The produced IFNα go and bind to IFN α receptor (IFNAR1-IFNAR2) and result in the production of IFN.
The type I IFNs such as IFN α or IFNβ are produced by plasmacytoid dendritic cells, macrophages, fibroblasts, and form an effector innate immune response (Wang and Fish, 2012). Previous studies have indicated that IFN therapy was useful for the therapy against the respiratory syncytial virus (RSV), SARS-CoV, hepatitis C virus (HCV), hepatitis B virus (HBV), etc. Studies have reported that IFN-α and -β combinations are found to be effective against SARS-CoV in vitro and in SARS patients (Zheng et al., 2004; Zorzitto et al., 2006).
4. P53 dependent effects in inhibiting SARs-CoV virus replication
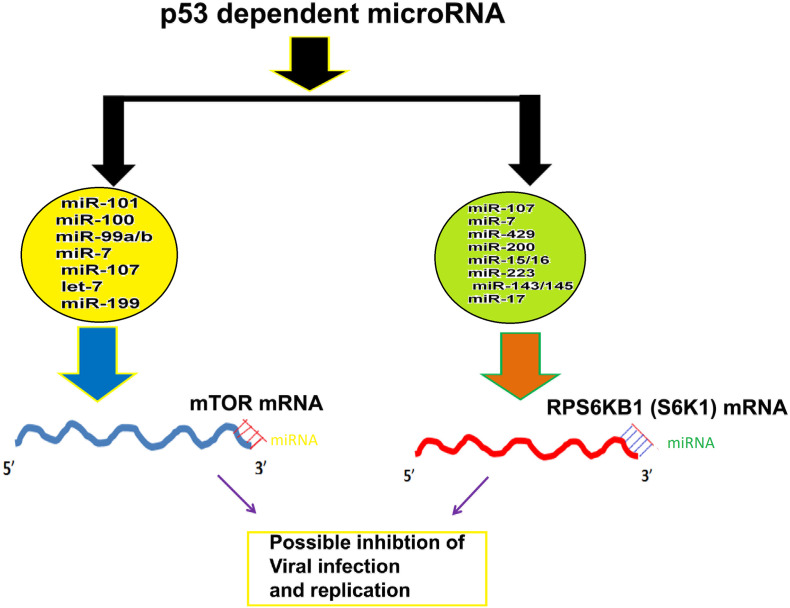
Infection by human coronaviruses is characterized by enhanced viral replication and immune suppression in host cells. In Severe acute respiratory syndrome coronavirus (SARS-CoV) p53 acts as an anti-viral factor and inhibits viral replication. During SARS virus infection non-structural protein of SARS-CoV interacts with E3 ubiquitin ligase ring-finger and CHY zinc-finger domain-containing 1 (RCHY1) leading to the degradation of p53 (Ma-Lauer et al., 2016). Von, Brunn, German Center for Infection Research (DZIF) found the rate of virus replication is enhanced several orders in cells that lack p53 than cells in which the p53 is expressed. Thus, p53 is involved in the host cell's non-specific antiviral defense system. On the other hand, studies have indicated that, the coronavirus papain-like proteases (PLPs) cause suppression of the innate immune response. The PLP2, a catalytic domain of the non-structural protein 3 of human coronavirus NL63 (HCoV-NL63) causes deubiquitination and stabilization of the cellular oncoprotein Mdm2 and thus help in proteasomal degradation of p53 (Yuan et al., 2014). Although vaccines against COVID-19 is the best option. Till that time to control mortality and save the life the use of microRNA that are non-coding RNAs of 21–22 bp in length and bind to 3′-UTR of mTOR mRNA i.e. miR-101, miR-100, miR-99a/b, miR-7, miR-107, let-7 and miR-199 that target mTOR (Zhang et al., 2017; Rapa et al., 2018; Hu et al., 2014; Blume et al., 2015; Feng et al., 2011a; Marcais et al., 2014; Wang et al., 2017). and its downstream target S6K1 (i.e. miR-107, miR-7, miR-429, miR-200, miR-15/16, miR-223, miR-143/145 and miR-17) (Feng et al., 2011b; Blume et al., 2015; Kim et al., 2011; Calin et al., 2008, Bonci et al., 2008, Ramaiah et al., 2014; Fang et al., 2012; Zhang et al., 2013; Chen et al., 2009; Yan et al., 2009) involved in ribosomal biogenesis and viral protein translation are highly important for the possible future therapy against COVID 19 control (Fig. 2 ). In conclusion, p53 activators such as 4,5-dihydro-imidazoline (nutlin-3a) (Vassilev et al., 2004) and inhibitors of p53-Hdm2 interactions such as AMG232 (Sun et al., 2014), idasanutlin (Ding et al., 2013) and overexpression of microRNA that functions like p53 such as miR-15/16 or adenoviral vector expressing p53 in lung cells that are infected by coronavirus are effective drug targets in therapy against deadly COVID-19 virus.
Fig. 2.
MicroRNAs regulate mTOR and RPS6KB1. The p53 dependent microRNAs bind to 3′-UTR of mTOR and RPS6KB1 genes and possibly generate IFN α response.
5. RNA interference during viral infection and p53 role
RNA interference (RNAi) is a process of post-transcriptional gene silencing (PTGS) observed in plants, animals, insects, and nematodes. Recent studies have shown the crucial role of RNAi in the anti-viral mechanism. In SARS-CoV, the nucleocapsid protein (N-protein) suppresses the RNAi by binding to small interfering RNA (siRNA) and short hairpin RNA (shRNA). The knockdown of key genes such as Dicer and Ago2 enhanced the viral replication in the mouse hepatitis virus which is highly similar to SARS-CoV (Cui et al., 2015). Interestingly, bronchoalveolar stem cells (BASCs) — “microbiome” that reveal the interaction between microRNAs in stem cells and viral genome has indicated that the miR-223 was inhibited by nucleocapsid and spike protein by SARS-CoV. This indicates the functional interplay between SARS virus-RNAi and p53 (Mallick et al., 2009).
6. P53 activation and immune responses
Some studies have identified, another human coronavirus HCoV-NL63 that causes respiratory tract infection, bronchiolitis, conjunctivitis in both children and adults (Chen et al., 2007). The combination of Actinomycin D and nutlin-3a activates p53 via phosphorylation of p53 at Ser 392 in A549 lung cancer cell lines and induce apoptosis that looks like inflammation-inducing pyroptosis and cell death induced by caspase-1. These drugs also induce the expression of innate immunity genes such as Nlrx1, String (stimulator of interferon genes), and two antiviral proteins, IFIT1 and IFIT3. P53 activation induces interferons (through upregulation of STING). Pyroptosis converts inactivated caspases to active caspase-1 that activates Interleukin-1-β and interleukin-18 from their inactive state that results in activation of neutrophils, NK cells, and T cells and interferon-γ-response (Krzesniak et al., 2020). RITA (reactivation of p53 and induction of tumor cell apoptosis) is another agent that restores p53 expression in cells and induces apoptosis. The reactivation of p53 following RITA treatment is critically dependent on eIF2α phosphorylation. Thus, p53 restoration is interlinked with the translation machinery (Ristau et al., 2019). Thus, small molecules that activate p53 may be of potential use in mediating host immune response.
7. P53, mTOR, and RNAi pathway interlink
P53 interacts with Drosha, the key component of the interference pathway via DEAD-box RNA helicase p68, and participates in the microRNA processing (i. e. from primary microRNA to pre-microRNA). The deletion of regulatory associated protein of mTOR (Raptor) and mouse embryonic fibroblasts with a double knock out for TSC (i.e. TSC−/−) have indicated that mTOR activity is related to microRNA processing (Suzuki et al., 2009; Ye et al., 2015).
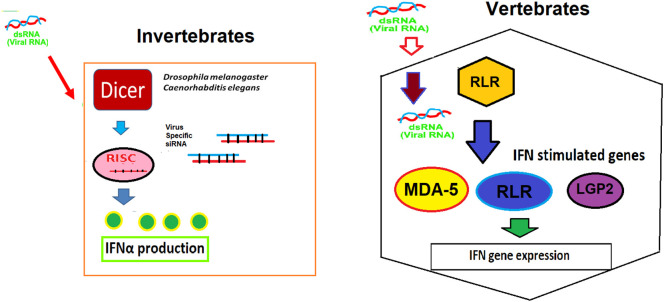
In invertebrates, the RNAi-mediated immunity the key pathway that operates during initial stages of viral infection (Nayak et al., 2013; Karlikow et al., 2014), Loss of Dicer-2 (Dcr-2) have shown increase infection by RNA viruses such as flock house virus. Drosophila C virus, and Sindbis virus (Galiana-Arnouse et al., 2006). Interestingly, Dicer mutants have shown reduced insulin activity and enhanced mortality in the Zika virus-infected in Drosophila (Harsh et al., 2018). Thus, Dicer plays a crucial regulatory role in immunity against viral infection. In vertebrates, RNAi-mediated immunity is connected with various pathways such as interferon (IFN) pathway (i.e. RLR mediated), dsRNA-inducible protein kinase R (PKR) pathway, and oligoadenylate synthetase/RNase L pathway (Fig. 3 ). Interestingly, Dicer ablation caused Naïve CD8 T cell activation (Zhang and Bevan, 2011; Trifari et al., 2013) and Dicer/miRNA balance regulates the fate of long-lived memory and short-lived effector cells, and cytotoxic T-lymphocyte (CTL) that play a pivotal role in immunity against viruses and cancer (Williams and Bevan, 2007).
Fig. 3.
Viral RNA sensing mechanisms and interferon production. In invertebrates Dicer alone sense the viral dsRNA and induce the production of siRNA. But in vertebrates, the viral dsRNA is sensed by RIG-I like receptor (RLR) that induce the interferon-stimulated genes (ISGs) such as RLR and melanoma differentiation-associated gene 5 (MDA-5) and laboratory genetics and physiology 2 (LGP-2) leading to the production of interferons.
8. Hypotheses
During viral infection, the virus activates mTOR and its downstream targets such as RPS6KB1 (S6K1) and 4-EBP1 and rapid activation of translation machinery for viral protein synthesis. Since SARS-CoV is a positive sense-strand RNA virus, there is a possibility of a faster translation process, where activation of mTOR was observed. During SARS virus infection, the non-structural protein of SARS-CoV interacts with E3 ubiquitin (E3U) ligase ring-finger and CHY zinc-finger domain-containing 1 (RCHY1) and stabilizes it leading to the degradation of p53. This indicates mTOR activation and loss-of-functional p53 could facilitate virus replication. Thus, inhibitors of mTOR and activators of p53 or microRNAs that can mimic-like p53 and could bind to 3′-untranslated regions (UTRs) of mTOR and RPS6KB1 are the potential target molecules for the therapy against nCOVID19.
9. Conclusions
mTOR is a serine-threonine kinase family protein, a key regulator in protein synthesis, and cellular metabolism that forms two major complexes, mTORC1 with Raptor and mTORC2 with Rictor play a central pivotal role in cell proliferation and cellular metabolism. mTOR is involved in cell proliferation, while p53 is involved in apoptosis. Increased mTOR expression levels and decreased p53 expression profiles is possibly facilitated virus replication in the case of coronavirus infection. The established drug target molecules, such as, Rapamycin and Everolimus function as mTOR inhibitors or p53 activators such as nutlin 3a can stabilize p53 by degrading Mdm2 could help to inhibit virus replication. Also, p53 activators were found to inhibit mTOR signaling via the AMPK pathway in mantle cell lymphoma. Towards the goal of rapid development of vaccine technology, it is advisable that the scientific community should give more thrust on uncovering potential of the mTOR inhibitors and p53 activators and various microRNAs that mimic-like p53 for combating COVID-19 infections.
Author’s contributions
M. Janaki Ramaiah conceived the idea, drafted the manuscript, and prepared the figures and tables, references.
Edupalli V Subbaiah has corrected the grammar of the manuscript.
M. Rajasekhar Reddy has carried out thorough checking of the manuscript and helped in drafting.
Declaration of competing interest
The authors declare that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-Kassar W.Y., Al-Afif . 2020. Azithromycin Promising Medicine for COVID 19 in Early Stage by Effecting on mTOR and Immune System. [Google Scholar]
- Blume C.J., Hotz-Wagenblatt A., Hullein J., Sellner L., et al. p53-dependent non-coding RNA networks in chronic lymphocytic leukemia. Leukemia. 2015;29(10):2015–2023. doi: 10.1038/leu.2015.119. [DOI] [PubMed] [Google Scholar]
- Bonci D., Coppola V., Musumeci M., Addario A., et al. The miR-15a–miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat. Med. 2008;14:1271–1277. doi: 10.1038/nm.1880. [DOI] [PubMed] [Google Scholar]
- Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin G.A., Cimmino A., Fabbri M., Ferracin M., et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. 2008;105(13):5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Manicassamy S., Tang H., Kasturi S.P., Pirani A., et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI (3)K-mTOR-p70S6K pathway. Nat. Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., To K.K., Tse H., Jin D.Y., Yuen K.Y. Interspecies transmission and emergence of novel viruses: lessons from bats and birds. Trends Microbiol. 2013;21(10):544–555. doi: 10.1016/j.tim.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Guo X., Zhang H., Xiang Y., et al. Role of miR-143 targeting KRAS in colorectal tumorigenesis. Oncogene. 2009;28:1385–1392. doi: 10.1038/onc.2008.474. [DOI] [PubMed] [Google Scholar]
- Chen Z., Wang Y., Ratia K., Mesecar A.D., Wilkinson K.D., Baker S.C. Proteolytic processing and deubiquitinating activity of papain-like proteases of human coronavirus NL63. J. Virol. 2007;81:6007–6018. doi: 10.1128/JVI.02747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Y., Hsueh P.R., Cheng W.C., Yu C.J., Yang P.-C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11(6):715–722. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/CONTI-E. pii: 1. [DOI] [PubMed] [Google Scholar]
- Cui L., Wang H., Ji Y., Yang J., et al. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells. J. Virol. 2015;89(17):9029–9043. doi: 10.1128/JVI.01331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q., Zhang Z., Liu J.J., Jiang N., et al. Discovery of RG7388, a potent and selective p53–MDM2 inhibitor in clinical development. J. Med. Chem. 2013;56(14):5979–5983. doi: 10.1021/jm400487c. [DOI] [PubMed] [Google Scholar]
- Ekshyyan O., Khandelwal A.R., Rong X., Moore-Medlin T., Ma X., et al. Rapamycin targets Interleukin 6 (IL-6) expression and suppresses endothelial cell invasion stimulated by tumor cells. Am. J. Transl. Res. 2016;8(11):4822–4830. [PMC free article] [PubMed] [Google Scholar]
- Fang R., Xiao T., Fang Z., Sun Y., et al. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J. Biol. Chem. 2012;287(27):23227–23235. doi: 10.1074/jbc.M112.373084. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Zhang C., Hu W. Tumor suppressor p53 meets microRNAs. J. Mol. Cell Biol. 2011;3(1):44–50. doi: 10.1093/jmcb/mjq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Zhang C., Hu W. Tumor suppressor p53 meets microRNAs. J. Mol. Cell Biol. 2011;3(1):44–50. doi: 10.1093/jmcb/mjq040. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca B.D., Jia J.J., Hollensen A.K., Poinet R. LARP1 is major phosphorylation substrate of mTORC1. bioRxiv. 2018 doi: 10.1101/491274. [DOI] [Google Scholar]
- Galiana-Arnouse D., Dostert C., Schneemann A., Hoffmann J.A., Lmler J.L. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Mol. Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- Gordon D.E., Gwendolyn M., Jang G.M., Bouhaddou M., Xu J. 2020. A SARS-CoV-2-Human Protein-Protein Interaction Map Reveals Drug Targets and Potential Drug Repurposing. (doi) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsh S., Ozakman Y., Kitchen S.M., Paquin-proul X.D., et al. Dicer-2 regulates resistance and maintains homeostasis against Zika virus infection in Drosophila. J. Immunol. 2018;201(10):3058–3072. doi: 10.4049/jimmunol.1800597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H.H., Schneider W.M., Rice C.M. Interferons and viruses: an evolutionary arms race of molecular interactions. Trends Immunol. 2015;36:124–138. doi: 10.1016/j.it.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhu Q., Tang L. MiR-99a anti-tumor activity in human breast cancer cells through targeting of mTOR expression. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlikow M., Goic B., Saleh M.C. RNAi and antiviral defense in Drosophila: setting up a systemic immune response. Dev. Comp. Immunol. 2014;42:85–92. doi: 10.1016/j.dci.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Kaur S., Sassano A., Dolniak B., Joshi S., Majchrzak-kita B., et al. Role of the Akt pathway in mRNA translation of interferonstimulated genes. Proc. Natl. Acad. Sci. 2008;105:4808–4813. doi: 10.1073/pnas.0710907105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating R., Hertz T., Wehenkel M., Harris T.L., Edwards B.A., McClaren J.L., Brown S.A., Surman S., Wilson Z.S., Bradley P., et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat. Immunol. 2013;14:1266–1276. doi: 10.1038/ni.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Veronese A., Pichiorru F., Jin Lee T., et al. P53 regulates epithelial-mesenchymal transition through microRNA targeting ZEB1 and ZEB2. J. Exp. Med. 2011;201, 208(5):875–883. doi: 10.1084/jem.20110235. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindrachuk J., Ork B., Hart B.J., Mazur S., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 2015;59(2):1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A.M., Adams M.J., Lefkowitz E.J., Carstens E.B. IXth Report of the International Committee on Taxonomy of Viruses. 2011. Virus taxonomy: classification and nomenclature of viruses. Access Online via Elsevier. [Google Scholar]
- Krzesniak M., Zajkowicz A., Gdowicz-kiosok A., et al. Synergistic activation of p53 by actinomycin D and nutlin-3a is associated with the upregulation of crucial regulators and effectors of innate immunity. Cell. Signal. 2020;69 doi: 10.1016/j.cellsig.2020.109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekmine F., Uddin S., Sassano A., Parmar S., Brachmann S.M., et al. Activation of the p70 S6 kinase and phosphorylation of the 4EBP1 repressor of mRNA translation by type I interferons. J. Biol. Chem. 2003;278:27772–27780. doi: 10.1074/jbc.M301364200. [DOI] [PubMed] [Google Scholar]
- Levy D.E., Marie I.J., Durbin J.E. Induction and function of type I and III interferon in response to viral infection. Curr. Opin. Virol. 2011;1:476–486. doi: 10.1016/j.coviro.2011.11.001. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li H., Zhou L. EZH2-mediated H3K27me3 inhibits ACE2 expression. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.04.010. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Millet L.C., Blecha F., Sang Y. Reduction of infection by inhibiting mTOR pathway is associated with reversed repression of type-I interferon by Porcine reproductive respiratory syndrome virus. J. Gen. Virol. 2017;98:1316–1328. doi: 10.1099/jgv.0.000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M., Sikström K., Robert P.A., Uze G., Larsson O., et al. Assessment of mTOR-dependent translational regulation of interferon stimulated genes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson B., Ekim B., Fingar D.C. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012;441(1):1–2. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Ma-Lauer Y., Carbajo-Lozoya, Hein M.Y., Muller M.A., et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc. Natl. Acad. Sci. 2016;113(35):E5192–E5201. doi: 10.1073/pnas.1603435113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick B., Ghosh Z., Chakrabarthi J. MicroRNome analysis unravels the molecular basis of SARS infection in bronchoalveolar stem cells. PLoS One. 2009;4(11):e7837. doi: 10.1371/journal.pone.0007837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcais A., Blevins R., Graumann J., Feytout A., et al. microRNA-mediated regulation of mTOR complex components facilitates discrimination between activation and energy in CD4 T cells. J. Exp. Med. 2014;211(11):2281–2295. doi: 10.1084/jem.20132059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak A., Tassetto M., Kunitomi M., Andino R. RNA interference-mediated intrinsic antiviral immunity in invertebrates. Curr. Top. Microbiol. Immunol. 2013;371:183–200. doi: 10.1007/978-3-642-37765-5_7. [DOI] [PubMed] [Google Scholar]
- Ramaiah M.J., Lavanya A., Honarpisheh M., Zarea M., Bhadra U., Bhadra M.P. MiR-15/16 complex targets p70S6 kinase 1 and controls cell proliferation in MDA-MB-231 breast cancer cells. Gene. 2014;552(2):255–264. doi: 10.1016/j.gene.2014.09.052. [DOI] [PubMed] [Google Scholar]
- Rapa I., Votta A., Gatti G., Izzo S., et al. High miR-100 expression is associated with aggressive features and modulates TORC1 complex activation in lung carcinoids. Oncogene. 2018;9(44):27535–27546. doi: 10.18632/oncotarget.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristau J., van Hoef V., Peuget S., Zhu J., et al. RITA requires eIF2α-dependent modulation of mRNA translation for its anti-cancer activity. Cell Death Dis. 2019;10(11):845. doi: 10.1038/s41419-019-2074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleiro D., Mehrotra S., Kroczynska B., Beauchamp E.M., Lisowski P., et al. Central role of ULK1 in type I interferon signaling. Cell Rep. 2015;11:605–661. doi: 10.1016/j.celrep.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Chatterjee A., Kumar P., Lal S., Kondabagil K. Upregulation of miR-101 during influenza A virus infection abrogates viral life cycle by targeting mTOR pathway. Viruses. 2020;12(4) doi: 10.3390/v12040444. pii: E444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S., Ray A., Sadasivam B. Metformin in COVID19: a possible role beyond diabetes. 2020;164 doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangle J.M., Munger K. The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein synthesis. J. Virol. 2010;84(18):9398–9940. doi: 10.1128/JVI.00974-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Li Z., Rew Y., Gribble M., et al. Discovery of AMG 232, a potent, selective, and orally bioavailable MDM2-p53 inhibitor in clinical development. J. Med. Chem. 2014;57(4):1454–1472. doi: 10.1021/jm401753e. [DOI] [PubMed] [Google Scholar]
- Suzuki H.I., Yamagata K., Sugimoto K., Iwamoto T., et al. Modulation of microRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S., Pipkin M.E., Bandukwala H.S., et al. MicroRNA directed program of cytotoxic CD8+ T cell differentiation. Proc. Natl. Acad. Sci. 2013;110(46):18608–18612. doi: 10.1073/pnas.1317191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev L., Vu B.T., Graves, Carvaja D., et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Wang B.X., Fish E.N. The Ying and Yang of viruses and interferons. Trend Immunol. 2012;33(4):190–197. doi: 10.1016/j.it.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhu N., Hu J., Wang Y., Xu J., et al. The mTOR inhibitor Manassantin B reveals a crucial role of mTORC2 signaling in Epstein-Barr virus reactivation. J. Biol. Chem. 2020 doi: 10.1074/jbc.RA120.012645. pii: jbc.RA120.012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Luo J., Wang X., Yang B., Cui L. MicroRNA-199a-5p induced autophagand inhibits the pathogenesis of ankylosing spondylitis by modulating the mTOR signaling via directly targeting Ras homolog enriched in brain (Rheb) J. Cell. Physiol. 2017;42:2481–2491. doi: 10.1159/000480211. [DOI] [PubMed] [Google Scholar]
- Williams M.A., Bevan M.J. Effector and memory CTL differentiation. Annu. Rev. Immunol. 2007;5:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Wullschleger S., Loewith R., Hall M.N. TOT signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yan Hl, Xue G., Mei Q., Wang Y.Z., et al. Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J. 2009;28(18):2719–2732. doi: 10.1038/emboj.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P., Liu Y., Chen C., Tang F., et al. An mTORC1-Mdm2-Drosha axis for miRNA biogenesis in response to glucose-and amino acid-deprivation. Mol. Cell. 2015;57(4):708–720. doi: 10.1016/j.molcel.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J. Inf. Secur. 2020;80:607–630. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Chen Z., Song S., Wang S., et al. p53 degradation by a coronavirus papain-like protease suppresses type I interferon signaling. J. Biol. Chem. 2014;290:3172–3182. doi: 10.1074/jbc.M114.619890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegeye M.M., Lindkyist M., Falker K., Kumawat A.K., et al. Activation of the JAK/STAT3 and PI3K/AKT pathways are crucial for IL-6 trans-signaling-mediated pro-inflammatory response in human vascular endothelial cells. Cell Commun. Signal. 2018;16:55. doi: 10.1186/s12964-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Sun Q., Zhang Z., Ge S., Han Z.G., Chen W.T. Loss of microRNA-143/145 disturbs cellular growth and apoptosis of human epithelial cancers by impairing the MDM2-p53 feedback loop. Oncogene. 2013;32(1):61–69. doi: 10.1038/onc.2012.28. [DOI] [PubMed] [Google Scholar]
- Zhang N., Bevan M.J. CD 8+ T cells; foot soldiers of the immune system. Immunity. 2011;35(2):161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang M., Li Q., Zhu P. MiR-101 reduces cell proliferation and invasion and enhances apoptosis in endometrial cancer via regulating PI3K/Akt/mTOR. Cancer Biomark. 2017;21(1):179–186. doi: 10.3233/CBM-170620. [DOI] [PubMed] [Google Scholar]
- Zheng B., He M.L., Wong K.L. Potent inhibition of SARS associated corona virus (SCOV) infection and replication by type I interferons (IFN-alpha/beta) but not by type II interferon (IFN gamma) J. Interf. Cytokine Res. 2004;24:388–390. doi: 10.1089/1079990041535610. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Hou Y., Shen J., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6(14) doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzitto J., Galligan C.L., Ueng J.J.M., Fish E.N. Characterization of anti-viral effects of interferon-α against a SARS-like corona virus infection in vitro. Cell Res. 2006;16:220–229. doi: 10.1038/sj.cr.7310030. [DOI] [PMC free article] [PubMed] [Google Scholar]