Abstract
The rapid emergence and subsequent global dissemination of SARS-CoV-2 disease (COVID-19) has resulted in over 4 million cases worldwide. The disease has a marked predilection for adults, and children are relatively spared. Understanding the age-based differences in pathophysiological pathways and processes relevant to the onset and progression of disease both in the clinical course and in experimental disease models may hold the key to the identification of therapeutic targets. The differences in the clinical course are highlighted by the lack of progression of the SARS-CoV-2 infection beyond mild symptoms in a majority of children, whereas in adults the disease progresses to acute lung injury and an acute respiratory distress syndrome (ARDS)-like phenotype with high mortality. The pathophysiological mechanisms leading to decreased lung injury in children may involve the decreased expression of the mediators necessary for viral entry into the respiratory epithelium and differences in the immune system responses in children. Specifically, decreased expression of proteins, including angiotensin-converting enzyme 2 (ACE2) and Transmembrane Serine Protease 2 (TMPRSS2) in the airway epithelium in children may prevent viral entry. The immune system differences may include a relative preponderance of CD4+ T cells, decreased neutrophil infiltration, decreased production of proinflammatory cytokines, and increased production of immunomodulatory cytokines in children compared with adults. Notably, the developing lung in children may have a greater capacity to recover and repair after viral infection. Understanding the relative contributions of the above processes to the protective phenotype in the developing lung can guide the trial of the appropriate therapies in adults.
Keywords: age-based susceptibility, children, coronavirus, COVID-19, pediatric lung, SARS-CoV-2
INTRODUCTION
SARS-CoV-2 is a newly identified member of the β-coronavirus family that emerged from Wuhan (Hubei Province, China) in December 2019 (13). The rapid emergence and subsequent global dissemination of this novel coronavirus (SARS-CoV-2) disease in 2019 (COVID-19) has resulted in over 4.7 million cases worldwide (https://coronavirus.jhu.edu/map.html; accessed May 18, 2020). Moreover, there have been more than 300,000 deaths, which calculates to an overall mortality rate of ~6.7%. Domestically in the United States, we have experienced over 1.4 million cases and over 89,000 deaths, for a mortality of ~6.0% (https://coronavirus.jhu.edu/us-map; accessed May 18, 2020). Although COVID-19 is mild in the majority of cases, a subset of patients rapidly develop acute respiratory distress syndrome (ARDS), a clinical presentation of acute lung injury (ALI), that leads to respiratory failure requiring mechanical ventilation (10).
DIFFERENCES IN CLINICAL PRESENTATION IN ADULTS VS. CHILDREN
One of the most intriguing observations is the significantly reduced prevalence, severity, and mortality among pediatric patients (3). Early reports from China and Italy noted low case numbers among children < 18 yr old (12, 21). These trends remained the same in the United States, and even more compelling data emerged. Among the first 149,082 US cases (through April 2, 2020), only 2,572 (~1.7%) were infants, children, and adolescents < 18 yr old (children < 18 yr old make up 22% of the US population) (4). A systematic review of literature showed that children accounted for 1–5% of diagnosed cases (23). Furthermore, children were less likely to have symptoms and had a lower rate of hospitalization. Strikingly, only three pediatric deaths were identified by the Centers for Disease Control and Prevention (CDC) at that time. These profoundly decreased rates of symptomatic infection, hospitalization, and death are well beyond statistical significance, require further examination, and may hold the key to identifying therapeutic targets.
In 2015 alone, 291.8 million episodes of lower respiratory tract infection (LRI) occurred worldwide, of which more than one-third occurred among children < 5 yr old (11). An estimated 704,000 deaths occurred among children < 5 yr old. Approximately 6.6% of these pediatric deaths (more than 46,000) were attributed to respiratory syncytial virus (RSV) or influenza. Although these viruses cause significant morbidity and mortality, other coronavirus outbreaks have led to a pattern curiously similar to SARS-CoV-2 (3) (Fig. 1).
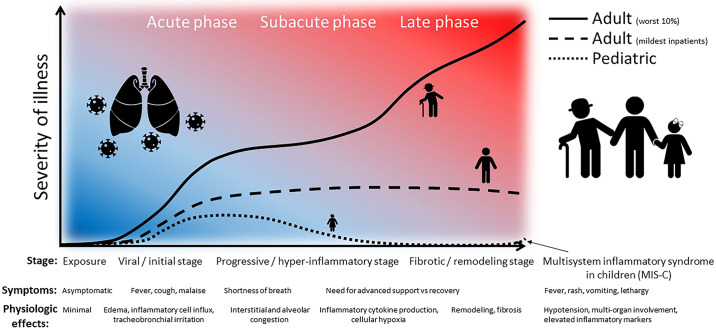
Fig. 1.
Differences in the clinical course in children and adults with SARS-CoV-2 infection: depiction of the general progression of disease and overarching severity of illness among symptomatic adult and pediatric patients. Although the most severe adults progress through the inflammatory phase to profound clinical severity, mild/moderate adults seem to stabilize and recover over a protracted course. Pediatric patients rarely require hospitalization for symptoms and, when more symptomatic early, generally recover quickly. Some children develop a multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. [Adapted in part from Siddiqui et al. (32a), with permission from Elsevier.]
Adults exposed to SARS-CoV-2 who are beginning to develop COVID-19 usually display fever, cough, or shortness of breath (93%) (4). Cough is the most common presenting symptom, occurring 80% of the time, whereas fever and shortness of breath occur among 71% and 43% of affected adults, respectively. Finally, myalgia (61%) and headache (58%) are also symptoms found in over half of infected adults. Furthermore, when it comes to adults, advanced age, obesity, male sex, and the presence of diabetes appear to confer an independent risk for mortality compared with healthy adults. Of note, what is most striking is that COVID-19 is not just a pulmonary disease but one with a pulmo-hematological-endothelial-inflammatory consequence, unlike any other viral pneumonia that has been reported thus far. COVID-19 patients with elevated D-dimer and ferritin, lower lymphocyte count, and elevated neutrophil-to-lymphocyte ratio probably have a proinflammatory microenvironment, which in turn portends a worse prognosis compared with those who do not.
Pediatric patients respond to SARS-CoV-2 exposure differently. Neonates, children, and adolescents < 18 yr old with SARS-CoV-2 are less likely to have any symptoms (4). Among children, fever is the most common presenting symptom, occurring 56% of the time, whereas cough and shortness of breath occur in only 54% and 13%, respectively. Myalgia (23%) and headache (28%) are relatively infrequent symptoms in children. In neonates and infants, the disease may have a nonspecific presentation with fever and lethargy (16, 27). Among 40 children admitted in North American pediatric intensive care units with COVID-19, an overwhelming majority (83%) had preexisting underlying medical conditions (32). These statistics may even overstate the frequencies, given that many children may have such mild symptoms that few seek medical care. Recently, a multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19) has been reported in children, leading to a CDC advisory. This Kawasaki disease-like syndrome presents with persistent fever and symptoms including hypotension, multiorgan involvement, and elevated inflammatory markers (29). Intriguingly, respiratory involvement was not seen in all cases (36). This syndrome is still rare among children, with a reported incidence of no more than 1 in 100 SARS-CoV-2-exposed children.
To further understand the difference between children and adults, we focused on the following mechanisms that have been reported or postulated to date: differences in 1) mediators necessary for viral entry and 2) immune system-mediated response.
DIFFERENCES IN MEDIATORS NECESSARY FOR VIRAL ENTRY
SARS-CoV-2 enters host cells after the binding of the viral spike (S) protein to angiotensin-converting enzyme 2 (ACE2) and priming of the S protein by host proteases such as TMPRSS2 (14). ACE2 also converts angiotensin-2 to angiotensin(1–7) (8) and protects the lung from injury by keeping angiotensin-2 levels in check. Binding of SARS-CoV-2 to ACE2 can result in inhibition of the enzyme and even its internalization, tilting the balance from the generation of angiotensin(1–7), which are protective toward angiotensin-2 that is known to have detrimental effects (30). A preprint study with snRNA-seq data from the lung across the age span shows an increase in the proportion of alveolar epithelial cells expressing ACE2 and TMPRSS2 in adult compared with young lungs. This may suggest reduced viral entry and replication in the lung epithelial cells in children compared with adults (37). Another preprint study with an integrated analysis of a single-cell atlas to elucidate the cell-specific expression of viral entry mediators found that ACE2 and TMPRSS2 expression in airway epithelial and alveolar type 2 (AT2) cells increases with age, with very low expression in infants and young children (26). Recent Gene Set Enrichment Analysis (GSEA) analysis revealed that high expression of ACE2 was also related to the activation of neutrophils, NK cells, Th17 cells, Th2 cells, Th1 cells, dendritic cells, and TNF-α-secreting cells leading to a severe inflammatory response (20). However, a clinical study in ARDS patients showed that expression levels of ACE or ACE2 did not appear to differ in bronchoalveolar lavage in ARDS patients between children and adult populations (31). Thus, children may be protected from serious pulmonary consequences in part by the decreased expression of receptors and other proteins that are essential for viral entry into the respiratory epithelium.
DIFFERENCES IN IMMUNE SYSTEM
The immune system is considered immature or weakened in the newborn and the aged, respectively, with reduced antimicrobial activity by neutrophils and macrophages, reduced antigen presentation by dendritic cells, and decreased natural killer cell-mediated defense (32b). On the other hand, the heightened immune response to the virus in many adult patients can lead to the worsening of lung disease with SARS-CoV-2 infection (37). The extent of lung damage in adult patients due to virus replication and due to the contribution from the overactive immune system still needs to be elucidated. With the 2002 SARS-CoV infection and the relative sparing of children, one of the hypotheses was that the second phase of respiratory deterioration in the infected adults was immune mediated and not directly related to viral replication, and this phase was muted in children. By using in vivo-passaged SARS-CoV in BALB/c mice, Nagata et al. (25) showed lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. In nonhuman primates, SARS-CoV-infected aged macaques develop more severe pathology with more vigorous host response to virus infection, with NF-κB emerging as a central player (34).
The T-cell response plays an important protective role against respiratory viruses. In 21 adult patients with moderate or severe COVID-19, there was a marked reduction in both CD4+ and CD8+ T cell populations (5) in patients with severe disease compared with those with moderate disease. In a murine model of the SARS-CoV infection using the mouse-adapted SARS-CoV (MA15), Zhao et al. (42) showed that a virus-specific T-cell response, even in the absence of activation of the innate immune response, was sufficient to enhance survival and attenuate disease. In a murine study with senescent mice, CD4+ T cell (but not CD8+ T cell)-mediated immunity was crucial for controlling viral replication and disease severity in primary SARS-CoV (6). The profound lymphocytopenia seen in adults compounded by the effects of aging on the adaptive immune response may play a role in the increased virulence of SARS-CoV-2 in adults compared with children (9).
Imbalance in the production of pro- versus anti-inflammatory cytokines may also contribute to this process. One such example would be declining levels of IL-10 production with age. IL-10 has an anti-inflammatory role by decreasing macrophage activation as well as the release and activity of inflammatory cytokines, such as IL-6, TNF-α, and IL-1β (7, 24). Adult murine lungs had significantly lower IL-10 and IL-13 levels before infection than young murine lungs and produced high levels of proinflammatory mediators leading to macrophage and neutrophil infiltration and activation (25). Young mice, on the other hand, produced immunomodulatory cytokines like IL-10 and -13 in addition to the proinflammatory mediators. In a lung injury model with both LPS and mechanical ventilation, there was a synergistic increase in neutrophil infiltration and IL-1β levels in adult but not juvenile mice (33). Markers known to be involved in the neutrophil response (MPO, IL-6), are one of the hallmarks of ARDS (31). These markers were significantly lower in neonates and children compared with adults/older adults. This study found that the absolute number of neutrophils is significantly lower in juveniles and suggested that the extravasation of neutrophils was limited in juveniles compared with adult animals. This could be secondary to lower expression of adhesion molecules such as P-selectin, a result found in human studies as well (17, 19).
Transcriptomic analysis of the lung in aged mice with SARS-CoV infection showed upregulated immune response and cell-to-cell signaling genes (including TNF-α, IL-6, Ccl2, Ccl3, Cxcl10), which was sustained even after viral clearance, suggesting an exacerbated host response to virus (2). Wu et al. (38) reported high neutrophil counts and lymphocytopenia associated with the development of ARDS in COVID-19 patients. In 171 children with SARS-CoV-2 infection from China, only 3.5% or 6 patients have lymphocytopenia (22).
The immune system may not be impaired but more heavily regulated in the newborn and infancy period because of differences in the modulation of the Toll-like receptor (TLR) pathway or in the generation of regulatory cells (41). Newborns show decreased TLR-induced responses and reduced proinflammatory cytokine production compared with adults (18). Pediatric lung and intestinal tissues have a higher proportion of regulatory T cells, which in turn may suppress immune responses (35). In conclusion, differences in the immune system activation in children due to either a dampened immune response or activation of a different group of immune effectors could be protective in children against the development of severe life-threatening lung disease with SARS-CoV-2 infection.
OTHER FACTORS THAT MAY CONTRIBUTE TO AGE-BASED SUSCEPTIBILITY
Children who develop ARDS have lower mortality (28). On the other hand, adults experience more long-term impairment with permanent alveolar simplification and fibrosis (15). Alveologenesis and microvascular maturation is an ongoing process that continues in the pediatric lung up to 6–8 yr of life and by some reports even up to late adolescence. Biological mechanisms related to inflammation and repair in the injured lung are likely to be different in the adult and pediatric lung. One example is the NF-κB pathway. Differences in the activation of this pathway between adult and neonatal mice were found in both the hyperoxia- and LPS-induced lung injury models (1, 39). Endothelial cell apoptosis and dysfunction with the breakdown of the pulmonary endothelial cell barrier leads to pulmonary edema in ARDS. The pulmonary barrier function was better preserved in neonatal mice compared with adult mice. In response to LPS administration, neonatal pulmonary endothelial cells increased focal adhesion kinase 1 (FAK1) expression, leading to better preservation of the pulmonary barrier function (40).
The rapid emergence and universal geographic transmission of SARS-CoV-2, along with the selective, age-associated mortality, render COVID-19 a unique, infectious disease. Insights into age-related variability in pathophysiological processes (Fig. 2) may offer critical observations, revealing focused paths of therapeutic investigation. Multidisciplinary collaboration between physicians and scientists, engaged in both pediatric and adult pursuits, holds significant promise and should be encouraged.
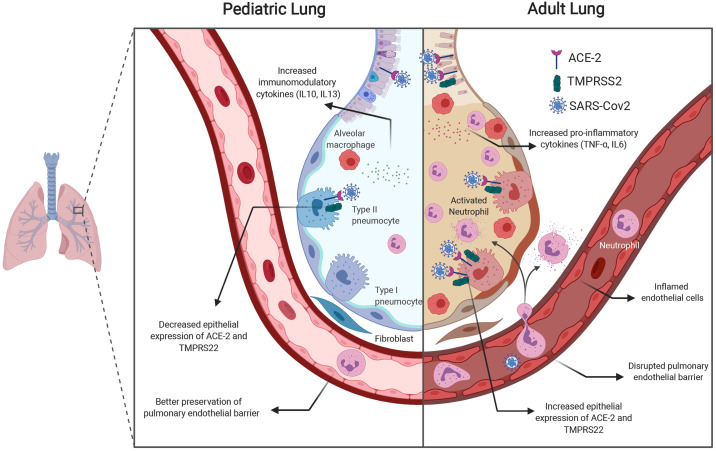
Fig. 2.
Mechanisms mediating differential susceptibility of adults and children to COVID-19. Increased expression of mediators essential for viral entry into airway epithelial cells (ACE-2 and TMPRSS2) in adults combined with the proinflammatory milieu in adults may predispose the adult lung to serious pulmonary injury and progression to acute respiratory distress syndrome (ARDS). The pediatric lung has greater expression of immunomodulatory cytokines and possibly a decreased expression of viral entry mediators.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants R01HL144775 and R01HL146395 to K.L.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.L., B.A., and M.T.H. prepared figures; K.L., H.K.-Q., B.A., and M.T.H. drafted manuscript; K.L., J.D., B.A., and M.T.H. edited and revised manuscript; K.L., H.K.-Q., J.D., B.A., and M.T.H. approved final version of manuscript.
REFERENCES
- 1.Alvira CM, Abate A, Yang G, Dennery PA, Rabinovitch M. Nuclear factor-kappaB activation in neonatal mouse lung protects against lipopolysaccharide-induced inflammation. Am J Respir Crit Care Med 175: 805–815, 2007. doi: 10.1164/rccm.200608-1162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baas T, Roberts A, Teal TH, Vogel L, Chen J, Tumpey TM, Katze MG, Subbarao K. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. J Virol 82: 9465–9476, 2008. doi: 10.1128/JVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. In press. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 4.Bialek S, Gierke R, Hughes M, McNamara LA, Pilishvili T, Skoff T; CDC COVID-19 Response Team . Coronavirus Disease 2019 in Children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep 69: 422–426, 2020. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130: 2620–2629, 2020. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol 84: 1289–1301, 2010. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagdeviren S, Jung DY, Friedline RH, Noh HL, Kim JH, Patel PR, Tsitsilianos N, Inashima K, Tran DA, Hu X, Loubato MM, Craige SM, Kwon JY, Lee KW, Kim JK. IL-10 prevents aging-associated inflammation and insulin resistance in skeletal muscle. FASEB J 31: 701–710, 2017. doi: 10.1096/fj.201600832R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 87: E1–E9, 2000. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 9.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 8: 1960, 2018. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung SY, Yuen KS, Ye ZW, Chan CP, Jin D. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect 9: 558–570, 2020. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2015 LRI Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 17: 1133–1161, 2017. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A, Nailescu A, Corona A, Zangrillo A, Protti A, Albertin A, Forastieri Molinari A, Lombardo A, Pezzi A, Benini A, Scandroglio AM, Malara A, Castelli A, Coluccello A, Micucci A, Pesenti A; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323: 1574, 2020. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo ZD, Wang ZY, Zhang SF, Li X, Li L, Li C, Cui Y, Fu R-, Dong YZ, Chi XY, Zhang MY, Liu K, Cao C, Liu B, Zhang K, Gao YW, Lu B, Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. In press. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im D, Shi W, Driscoll B. Pediatric acute respiratory distress syndrome: fibrosis versus repair. Front Pediatr 4: 28, 2016. doi: 10.3389/fped.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15-day-old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond) 52: 427–429, 2020. doi: 10.1080/23744235.2020.1747634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kling KM, Lopez-Rodriguez E, Pfarrer C, Mühlfeld C, Brandenberger C. Aging exacerbates acute lung injury-induced changes of the air-blood barrier, lung function, and inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol 312: L1–L12, 2017. doi: 10.1152/ajplung.00347.2016. [DOI] [PubMed] [Google Scholar]
- 18.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37: 771–783, 2012. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence SM, Corriden R, Nizet V. age-appropriate functions and dysfunctions of the neonatal neutrophil. Front Pediatr 5: 23, 2017. doi: 10.3389/fped.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li G, He X, Zhang L, Ran Q, Wang J, Xiong A, Wu D, Chen F, Sun J, Chang C. Assessing ACE2 expression patterns in lung tissues in the pathogenesis of COVID-19. J Autoimmun. In press. doi: 10.1016/j.jaut.2020.102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KS, Lau EH, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TT, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 382: 1199–1207, 2020. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GW; Chinese Pediatric Novel Coronavirus Study Team . SARS-CoV-2 infection in children. N Engl J Med 382: 1663–1665, 2020. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109: 1088–1095, 2020. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19: 683–765, 2001. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Nagata N, Iwata N, Hasegawa H, Fukushi S, Harashima A, Sato Y, Saijo M, Taguchi F, Morikawa S, Sata T. Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am J Pathol 172: 1625–1637, 2008. doi: 10.2353/ajpath.2008.071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NHLBI LungMAP Consortium, Human Cell Atlas Lung Biological Network . Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type- specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells (Preprint). bioRxiv 2020.04.19.049254, 2020. doi: 10.1101/2020.04.19.049254 [DOI]
- 27.Paret M, Lighter J, Pellett Madan R, Raabe VN, Shust GF, Ratner AJ. SARS-CoV-2 infection (COVID-19) in febrile infants without respiratory distress. Clin Infect Dis. In press. doi: 10.1093/cid/ciaa452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quasney MW, López-Fernández YM, Santschi M, Watson RS; Pediatric Acute Lung Injury Consensus Conference Group . The outcomes of children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 16, Suppl 1: S118–S131, 2015. doi: 10.1097/PCC.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 29.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395: 1607–1608, 2020. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues Prestes TR, Rocha NP, Miranda AS, Teixeira AL, Simoes-E-Silva AC. The anti-inflammatory potential of ACE2/Angiotensin-(1-7)/Mas receptor axis: evidence from basic and clinical research. Curr Drug Targets 18: 1301–1313, 2017. doi: 10.2174/1389450117666160727142401. [DOI] [PubMed] [Google Scholar]
- 31.Schouten LR, van Kaam AH, Kohse F, Veltkamp F, Bos LD, de Beer FM, van Hooijdonk RT, Horn J, Straat M, Witteveen E, Glas GJ, Wieske L, van Vught LA, Wiewel MA, Ingelse SA, Cortjens B, van Woensel JB, Bos AP, Walther T, Schultz MJ, Wösten-van Asperen RM; MARS consortium . Age-dependent differences in pulmonary host responses in ARDS: a prospective observational cohort study. Ann Intensive Care 9: 55, 2019. doi: 10.1186/s13613-019-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, Heidemann SM, Kleinman LC, Sen AI, Hall MW, Priestley MA, McGuire JK, Boukas K, Sharron MP, Burns JP; International COVID-19 PICU Collaborative . Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. In press. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 39: 405–407, 2020. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32b.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282: 20143085, 2015. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LS, Gharib SA, Frevert CW, Martin TR. Effects of age on the synergistic interactions between lipopolysaccharide and mechanical ventilation in mice. Am J Respir Cell Mol Biol 43: 475–486, 2010. doi: 10.1165/rcmb.2009-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smits SL, de Lang A, van den Brand JM, Leijten LM, van IJcken WF, Eijkemans MJ, van Amerongen G, Kuiken T, Andeweg AC, Osterhaus AD, Haagmans BL. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog 6: e1000756, 2010. doi: 10.1371/journal.ppat.1000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, Granot T, Griesemer A, Lerner H, Kato T, Farber DL. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med 22: 72–77, 2016. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395: 1771–1778, 2020. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang A, Chiou J, Poirion OB, Buchanan J, Valdez MJ, Verheyden JM, Hou X, Guo M, Newsome JM, Kudtarkar P, Faddah DA, Zhang K, Young RE, Barr J, Misra R, Huyck H, Rogers L, Poole C, Whitsett JA, Pryhuber G, Xu Y, Gaulton KJ, Preissl S, Sun X; NHLBI LungMap Consortium. Single nucleus multiomic profiling reveals age-dynamic regulation of host genes associated with SARS-CoV-2 infection (Preprint). bioRxiv 2020.04.12.-37580, 2020. doi: 10.1101/2020.04.12.037580. [DOI]
- 38.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. In press. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang G, Abate A, George AG, Weng YH, Dennery PA. Maturational differences in lung NF-kappaB activation and their role in tolerance to hyperoxia. J Clin Invest 114: 669–678, 2004. doi: 10.1172/JCI200419300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ying L, Alvira CM, Cornfield DN. Developmental differences in focal adhesion kinase expression modulate pulmonary endothelial barrier function in response to inflammation. Am J Physiol Lung Cell Mol Physiol 315: L66–L77, 2018. doi: 10.1152/ajplung.00363.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol 17: 495–507, 2017. doi: 10.1038/nri.2017.54. [DOI] [PubMed] [Google Scholar]
- 42.Zhao J, Zhao J, Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol 84: 9318–9325, 2010. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]