Abstract
Background
Hepatic hemangioma (HH) is the most common benign tumor of the liver. In special conditions such as rapidly growing tumors, persistent pain, hemorrhage and when pressure effect on adjacent organs exist treatment is indicated. Surgical management is the most common treatment for HH.
Case presentation
A 38-year-old male patient was diagnosed with HH for 7 years. The initial presentation of the mass was progressive abdominal distention causing early satiety, gastro-esophageal reflux disease, vomiting, dysphagia and weight loss. Later, the patient developed bilateral lower extremity edema. Imaging with computed tomography (CT scan) showed a large mass measuring 32.4*26*3.1 cm which was considered unresectable. The patient underwent a deceased donor liver transplantation. The excised mass was 9 kg. After nine days of hospitalization the patient was discharged in good condition. Three months later, the patient was admitted due to fever and cytomegalovirus infection for which he received intravenous ganciclovir and was discharged. In the latest follow-up the patient had no liver or kidney dysfunction eight months after the transplantation.
Conclusion
With appropriate patient selection, liver transplantation can be considered as a treatment option for patients with huge HHs which are life-threatening and surgically unresectable.
Keywords: Hepatic Hemangioma, Liver transplantation, Benign liver neoplasms, Cavernous Hemangioma
Background
Hepatic Hemangioma (HH) is the most common benign tumor of the liver. The tumor has a vascular nature and is usually solitary and small in size. The majority of HHs originate from the right hepatic lobe. Classically HHs are not clinically symptomatic and are incidental findings in imaging studies [1]. No definite genetic background has been suggested for the occurrence of HH; however few cases of familial hepatic hemangiomas have been described in literature [2]. A well-established gender disparity with female to male ration of 5:1 is reported for the tumor [3]. Estrogen therapy and pregnancy are the major causes promoting tumor growth in HHs, highlighting the role of female sex hormones in the pathogenesis of the tumor [4]. Adulthood is the usual period of presentation, with the average age of diagnosis varying from 30 to 50 years old [5]. HH tumors are mostly asymptomatic; however larger tumors present with abdominal discomfort and rarely cause jaundice, high cardiac output heart failure, hemorrhage and consumptive coagulopathy, a syndrome known as Kasabach-Merritt syndrome (KMS). Treatment of HH is only indicated in special cases [1, 6]. Herein, we present a case of a 9 kg giant HH that underwent liver transplantation and evaluate existing literature.
Case presentation
A 38-year-old patient was under conservative follow-up for a huge HH for 7 years. At the time of presentation, the patient had noticed gradual abdominal distention and epigastric discomfort for which he sought medical consultation. He was previously healthy and had no significant past medical or family history of any significant disease. Imaging with ultrasonography and abdominal 4-phase CT scan were in favor of a hemangioma probably originating from the fifth and eighth right liver lobe, initially measuring 12*10*1.5 cm.
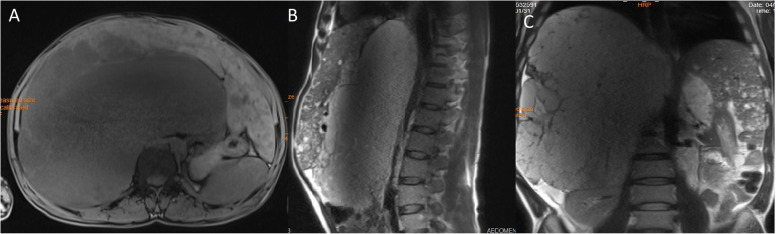
At the time of diagnosis, his management plan included a biannual follow-up of the mass via imaging studies including abdominal ultrasonography and magnetic resonance imaging (MRI). During the next 7 years the mass was growing in size and the patient complained of exacerbation of abdominal discomfort, early satiety, vomiting and bilateral lower extremity edema. Due to mass effect of the HH, he developed disturbing gastro-esophageal reflux disease causing significant weight loss and dysphagia to solid foods, for which an upper gastrointestinal tract endoscopy was performed. The study revealed severe esophagitis and stricture of the lower portion of the esophagus with hiatal hernia. The last abdominal MRI showed a heterogeneous solid mass measuring 32.4*26*3.1 cm in size originating from the right liver lobe. The mass had displaced the right kidney downwards and the right diaphragm upwards, however it had not caused any pressure effect on the intra or extra hepatic bile ducts. The IVC was also compressed, causing venous stasis in the lower extremities (Fig. 1).
Fig. 1.
T1-weighted abdominal MRI showing a huge heterogeneous mass originating from right liver lobe in axial, sagittal and coronal views (a, b and c, respectively) with nodular discontinuous enhancement after gadolinium injection (b, c)
Ultrasonography of the portal system showed pressure effect of the mass causing deviation of the portal vein and hepatic artery to the left sub-diaphragmatic aspect of the abdomen with mild portal hypertension and small amount of ascitic fluid. However, he had no esophageal varices reported in esophagoscopy. Despite the huge mass size, the patient did not develop KMS and had a relatively normal liver function test until the transplantation, which was as followed: AST = 45 IU/L (normal range: 5–42 IU/L), ALT = 34 IU/L (normal range: 5–37 IU/L), ALP = 256 IU/L (normal range: 50–275 IU/L) with total and direct bilirubin of 0.9 mg/DL (normal range 0–1 mg/DL) and 0.3 mg/DL (normal range: 0–0.35 mg/DL); respectively. He also had a platelet count of 256.000 (normal range: 141.000–356.000) and prothrombin time of 15.6 (normal range: 11–12.5) seconds. His echocardiography was also normal with an ejection fraction of 55%.
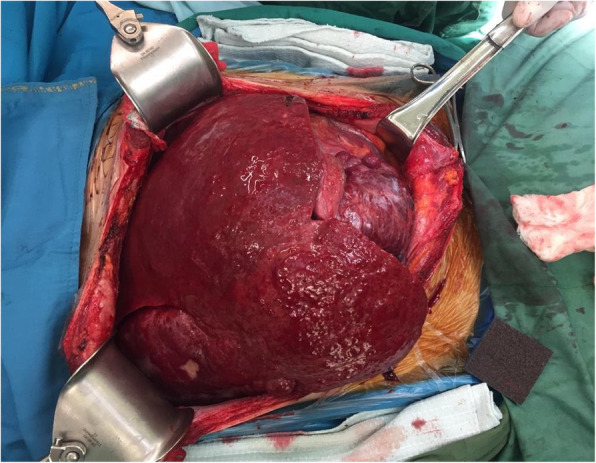
Later, due to patient’s extreme discomfort and risk of rupture and hemorrhage, a multi-disciplinary team decided to put the patient on the liver transplant waiting list. After 12 months, the patient underwent whole organ liver transplantation using a cadaver graft. During laparotomy, numerous collateral abdominal veins and approximately 400 cc of ascitic fluid were observed.
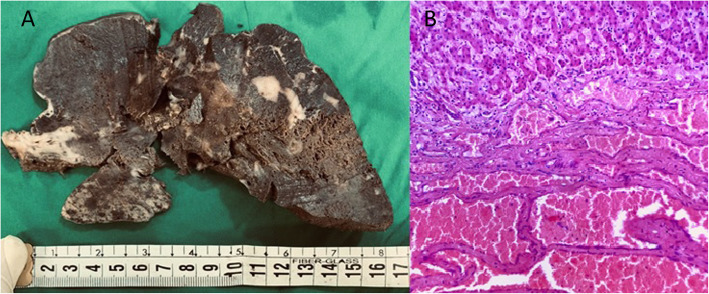
The native liver was dissected using a traditional hepatectomy technique and the estimated bleeding during hepatectomy was 1000 cc. Duct to duct anastomosis was done with 1000 cc bleeding after reperfusion. Fluid transfusion during surgery included 2500 cc of crystalloid fluid and 20 mg of albumin before and after declamping. He also received a transfusion of 4 units of packed red blood cells during the operation. The surgery lasted for 330 min and the patient was transferred to the intensive care unit with no acute complication. The explanted liver weighed 9 kg (Fig. 2).
Fig. 2.
Gross pathology of the native liver showing a huge inhomogeneous well-circumcised sub-capsular mass weighing 9 kgs which occupied the whole abdomen
During pathologic examination, serial sections showed multiple infiltrative masses with spongy microcystic surface occupying the whole liver (Fig. 3a).
Fig. 3.
Cut section of liver with multiple diffuse ill-defined spongy brown masses (a) and numerous dilated blood vessels adjacent to hepatocytes (H&E × 200) (b)
Microscopic examination revealed dilated vascular spaces, located between hepatocytes, lined by endothelial cells and containing red blood vessels. There was no significant atypia (Fig. 3b).
Patient was discharged after 9 days with an immunosuppressive regimen of Tacrolimus (Prograf® 2 mg Q12H) and Mycophenolate (Myfortic® 360 mg Q12H). Three months post-surgery, the patient was admitted with fever and had a positive PCR for cytomegalovirus. During admission the patient was given intravenous gancyclovir (Valcyte® 350 mg Q24H, 10 days) and was discharged after a 7 day admission period with good conditions.
Discussion and conclusion
Hepatic hemangioma (HH) is a common benign liver neoplasm. Due to different prognosis and complications of liver masses, HH should be differentiated from other benign or malignant lesions using various imaging modalities and other diagnostic methods [7]. Most HHs remain asymptomatic during a persons’ lifetime and usually do not have the potential for malignant transformation. Considering these, most HHs do not require medical intervention and annual or biannual imaging follow-up is sufficient in the majority of cases. Treatment is only indicated for rapidly growing tumors, persistent pain, hemorrhage and when pressure effect on adjacent organs and vessels exists, which may results in symptoms such as Budd-Chiari syndrome, jaundice and lower extremity edema. KMS is also an indication to seek treatment, the syndrome is characterized by thrombocytopenia, coagulopathy and microangiopathic hemolytic anemia [1]. Spontaneous or trauma induced bleeding from the tumor, is a rare but potentially fatal complication of HH which needs emergent laparotomy [6].
Up to this date, no medication has been proposed as a definite choice for medical treatment of HH. Some previous studies have reported promising results with medical management of HHs using bevacizumab, sorafenib, interferon and combination of sirulimus with high dose propranolol, however more studies are needed to support these findings [8–11].
In rare cases, patients with huge HH undergo liver transplantation. Indications for liver transplantation include huge masses compromising liver function, KMS and inoperable life threatening huge masses [12]. Up to this date and to the best of the authors’ knowledge, 20 liver transplantation for huge HH have been reported in 15 studies, using both living and deceased donor liver transplantation [13–27]. The youngest patient was a 4 week old infant [15] and the oldest to have liver transplantation was 51 years old [27]. Most transplants have been done due to KMS (n = 9) [13–16, 20–22, 24, 25]. Other causes included diffuse mass or rapid growth with imminent rupture (n = 4) [19, 21, 23, 26], respiratory distress (n = 4) [14, 21, 25, 26], rupture (n = 2) [16, 24], pain/discomfort (n = 3) [16, 17] and bleeding (n = 1) [17]. A summary of existing literature is presented in Table 1.
Table 1.
Existing literature on liver transplantation in hepatic hemangioma
| Report no. | Author (year) | Age (yrs)/sex | Graft Type | Follow-up | Cause of Tx | Complications | Condition/cause of death |
|---|---|---|---|---|---|---|---|
| 1. | Klompmaker et al. (1989) [13] | 28/Male | Whole | 3 years | KMS | Uneventful | Alive |
| 2. | Mora, et al. (1995) [14] | 42/Female | Whole | 16 days postop | KMS, respiratory distress | NA | Alive |
| 3. | Tepetes et al. (1995) [15] | 4wks/Male | Whole | 8 days | KMS | Graft mal-function, intraventricular hemorrhage | Died, graft mal-function |
| 4. | Brouwers et al. (1997) [16] | NM | Whole |
1. 1 month 2. 1 year 3. 4 years 4. 9 years |
Pain (n = 2), rupture (n = 1), KMS (n = 1) | 1. Rejection, bile leakage & pleural effusion; 2. cytomegalovirus pneumonia, duodenal ulcer, steroid diabetes, peripheral nerve palsy & Strongyloides stercoralis infection; 3. uneventful | 1. died Others alive |
| 5. | Chui et al. (1996) [17] |
1. 33/Female 2. 43/Female |
Whole | 1. 18 months 2. 14 months |
1. Bleeding 2. Abdominal discomfort |
1. Massive hemorrhage during surgery, ischemic graft with malfunction, acute renal failure, second transplantation was done; 2. Uneventful | Both alive |
| 6. | Longeville et al. (1997) [18] | 47/Male | Whole | 12 months | KMS | Post-transplantation internal hemorrhage | Alive |
| 7. | Russo et al. (1997) [19] | 43/Female | Whole | 14 days postop | Huge mass | NA | Alive |
| 8. | Kumashiro, et al. (2002) [20] | 48/Female | Posterior lobe | 15 days postop | KMS, acute liver failure | Massive hemorrhage during operation due to KMS, uneventful post-operation course | Alive |
| 9. | Ferraz et al. (2004) [21] | 28/Female | Whole | 30 months | KMS, respiratory distress, huge mass size | One episode of acute rejection treated with corticosteroid pulse | Alive |
| 10. | Meguro et al. (2008) [22] | 45/Female | Left Lobe | 10 months | KMS | Massive hemorrhage during operation, acute rejection and small for size graft syndrome, sepsis | Alive |
| 11. | Zhong et al. (2014) [23] | 27/Female | Right lobe | 50 months | Huge mass | Two episodes of acute rejection | Alive |
| 12. | Vagefi et al. (2011) [24] | 39/Female | Whole | NM | Rupture, KMS | Uneventful | Alive |
| 13. | Yildiz et al. (2014) [25] | 44/Female | Whole | 1 month | KMS, respiratory distress | Uneventful | Alive |
| 14. | Lange et al. (2015) [26] | 46/Female | Whole | 7 wks | Huge mass causing portal vein thrombosis, ascites, DVT & PTE | Uneventful | Alive |
| 15. | Lee et al. (2017) [27] | 51/Female | Modified Right Lobe | 16 months | Rapid Growth | Uneventful | Alive |
KMS Kasabach-Merritt Syndrome; POSTOP Postoperative; TAE Transcatheter angiographic embolization; NM Not mentioned; NA Not accessible manuscript; DVT Deep vein thrombosis; PTE Pulmonary thromboembolism; TX transplantation
Currently the most common treatment approach, especially with huge HHs is surgical intervention. Treatment options for huge cavernous HHs include surgical resection, transcatheter angiographic embolization (TAE), radiofrequency ablation, radiotherapy and in some cases orthotopic liver transplantation, as discussed earlier [1]. Minimally invasive techniques have been more frequently applied in recent years. The TAE method is done via catheterization of femoral artery and getting access to the hepatic artery to discover the tumor’s feeding arteries. The feeding arteries are then embolized by using an embolic agent. Arterial embolization is usually left for tumors with a definite arterial supply and is usually indicated prior to surgical resection of inoperable lesions to reduce the tumor’s size, facilitating the surgery [28]. Successful treatment of HH with TAE without surgery has also been reported [29]. Radiofrequency ablation uses high frequency current passing through an electrode which creates a small area of heat targeting the lesion. This method is either applied percutaneously or via laparoscopy and laparotomy. For most cases, significant symptom relief is achieved by RF-ablation. Due to difficult application of radiofrequency ablation technique for lesions larger than 10 cm, patients with larger HH tumors do not efficiently benefit from this method [30, 31]. Radiotherapy is a less frequently applied method for management of HH [32].
Surgical treatment of HH is considered for patients with severe symptoms affecting lifestyle, those suspicious of malignancy and huge tumors as they have an increased risk of rupture and bleeding [6]. Yet the optimal surgical approach still remains to be controversial. Surgeons may consider either segmental resection or enucleation of the tumor based on the location of the lesion. One meta-analysis conducted in 2016 reported that tumor tissue removal by both techniques can be safe and efficient; however due to decreased amount of intraoperative bleeding and a better preservation of normal hepatic tissue the enucleation method is the preferred surgical procedure [33].
Conclusion
With appropriate patient selection, liver transplantation can be considered as a treatment option for patients with huge hemangiomas of the liver when other treatment options have failed or are not indicated.
Acknowledgements
Not applicable.
Abbreviations
- CT scan
Computed tomography scan
- HH
Hepatic hemangioma
- KMS
Kasabach-meritt syndrome
- MRI
Magnetic resonance imaging
- TAE
Transcatheter angiographic embolization
Authors’ contributions
HE was the lead surgeon in the transplantation process. NA conceived the study and wrote the manuscript. PA and HE assisted in the preparation of the manuscript. All authors critically reviewed the manuscript and approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
No funding was applied for this study.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
The study involving a human participant to report was approved by the ethics committee of the department of Transplantation at Shiraz University of Medical Sciences. Written consent was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hesameddin Eghlimi, Email: hessam_e62@yahoo.com.
Peyman Arasteh, Email: peyman.arasteh@yahoo.com.
Nazanin Azade, Email: nazaninazadeh91@gmail.com.
References
- 1.Bajenaru N, Balaban V, Săvulescu F, Campeanu I, Patrascu T. Hepatic hemangioma –review. J Med Life. 2015;8(Spec Issue):4–11. [PMC free article] [PubMed] [Google Scholar]
- 2.Moser C, Hany A, Spiegel R. Familial giant hemangiomas of the liver. Study of a family and review of the literature. Praxis. 1998;87(14):461–468. [PubMed] [Google Scholar]
- 3.Henson SW, Jr, Gray HK, Dockety MB. Benign tumors of the liver. Surg Gynecol Obstet. 1956;103:327–331. [PubMed] [Google Scholar]
- 4.Van Malenstein H, Maleux G, Monbaliu D, Verslype C, Komuta M, Roskams T, et al. Giant liver hemangioma: the role of female sex hormones and treatment. Eur J Gastroenterol Hepatol. 2011;23:438–443. doi: 10.1097/MEG.0b013e328345c87d. [DOI] [PubMed] [Google Scholar]
- 5.Gandolfi L, Leo P, Solmi L, Vitelli E, Verros G, Colecchia A. Natural history of hepatic haemangiomas: clinical and ultra-sound study. Gut. 1991;32(6):677–680. doi: 10.1136/gut.32.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toro A, Mahfouz AE, Ardiri A, Malaguarnera M, Malaguarnera G, Loria F, et al. What is changing in indications and treatment of hepatic hemangiomas? A review. Ann Hepatol. 2014;13(4):327–339. doi: 10.1016/S1665-2681(19)30839-7. [DOI] [PubMed] [Google Scholar]
- 7.Bahirwani R, Reddy KR. Review article: the evaluation of solitary liver masses. Aliment Pharmacol Ther. 2008;28(8):953–965. doi: 10.1111/j.1365-2036.2008.03805.x. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan D, Miller C, Hirose K, McCullough A, Yerian L. Incidental reduction in the size of liver hemangioma following use of VEGF inhibitor bevacizumab. J Hepatol. 2008;49(5):867–870. doi: 10.1016/j.jhep.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita S, Okita K, Harada K, Hirano A, Kimura T, Kato A, Okita K. Giant cavernous hepatic hemangioma shrunk by use of sorafenib. Clin J Gastroenterol. 2013;6(1):55–62. doi: 10.1007/s12328-012-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezekowitz RAB, Mulliken JB, Folkman J. Interferon α2A therapy for 'life-threatening' hemangiomas in infancy. N Engl J Med. 1992;326(22):1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- 11.Warren D, Diaz L, Levy M. Diffuse hepatic hemangiomas successfully treated using sirolimus and high-dose propranolol. Pediatr Dermatol. 2017;34:e286–e287. doi: 10.1111/pde.13219. [DOI] [PubMed] [Google Scholar]
- 12.Stringer MD. The role of liver transplantation in the management of pediatric liver tumours. Ann R Coll Surg Engl. 2007;89(1):12–21. doi: 10.1308/003588407X155527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klompmaker IJ, Sloof MJ, van der Meer J, de Jong GM, de Bruijn KM, Bams JL. Orthotopic liver transplantation in a patient with a giant cavernous hemangioma of the liver and Kasabach-Merritt syndrome. Transplantation. 1989;48(1):149–151. doi: 10.1097/00007890-198907000-00035. [DOI] [PubMed] [Google Scholar]
- 14.Mora A, Cortés C, Roigé J, Noguer M, Camps MA, Margarit C. Orthotopic liver transplant for giant cavernous hemangioma and Kasabach-Merritt syndrome. Rev Esp Anestesiol Reanim. 1995;42(2):71–74. [PubMed] [Google Scholar]
- 15.Tepetes K, Selby R, Webb M, Madariaga JR, Iwatsuki S, Starzl TE. Orthotopic liver transplantation for benign hepatic neoplasms. Arch Surg. 1995;130(2):153–156. doi: 10.1001/archsurg.1995.01430020043005. [DOI] [PubMed] [Google Scholar]
- 16.Brouwers MA, Peeters PM, de Jong KP, Haagsma EB, Klompmaker IJ, Bijleveld CM, et al. Surgical treatment of giant haemangioma of the liver. Br J Surg. 1997;84(3):314–316. doi: 10.1002/bjs.1800840310. [DOI] [PubMed] [Google Scholar]
- 17.Chui AK, Vass J, McCaughan GW, Sheil AG. Giant cavernous haemangioma: a rare indication for liver transplantation. Aust N Z J Surg. 1996;66(2):122–124. doi: 10.1111/j.1445-2197.1996.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 18.Longeville JH, de la Hall P, Dolan P, Holt AW, Lillie PE, Williams JA, et al. Treatment of a giant haemangioma of the liver with Kasabach-Merritt syndrome by orthotopic liver transplant. HPB Surg. 1997;10(3):159–162. doi: 10.1155/1997/10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo MW, Johnson MW, Fair JH, Brown RS. Jr Orthotopic liver transplantation for giant hepatic hemangioma. Am J Gastroenterol. 1997;92(10):1940–1941. [PubMed] [Google Scholar]
- 20.Kumashiro Y, Kasahara M, Nomoto K, Kawai M, Sasaki K, Kiuchi T, et al. Living donor liver transplantation for giant hepatic hemangioma with Kasabach-Merritt syndrome with a posterior segment graft. Liver Transpl. 2002;8(8):721–724. doi: 10.1053/jlts.2002.33689. [DOI] [PubMed] [Google Scholar]
- 21.Ferraz AA, Sette MJ, Maia M, Lopes EP, Godoy MM, Petribú AT, et al. Liver transplant for the treatment of giant hepatic hemangioma. Liver Transpl. 2004;10(11):1436–1437. doi: 10.1002/lt.20250. [DOI] [PubMed] [Google Scholar]
- 22.Meguro M, Soejima Y, Taketomi A, Ikegami T, Yamashita Y, Harada N, et al. Living donor liver transplantation in a patient with giant hepatic hemangioma complicated by Kasabach-Merritt syndrome: report of a case. Surg Today. 2008;38(5):463–468. doi: 10.1007/s00595-007-3623-4. [DOI] [PubMed] [Google Scholar]
- 23.Zhong L, Men TY, Yang GD, Gu Y, Chen G, Xing TH, et al. Case report: living donor liver transplantation for giant hepatic hemangioma using a right lobe graft without the middle hepatic vein. World J Surg Oncol. 2014;12(1):83. doi: 10.1186/1477-7819-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vagefi PA, Klein I, Gelb B, Hameed B, Moff SL, Simko JP, et al. Emergent orthotopic liver transplantation for hemorrhage from a giant cavernous hepatic hemangioma: case report and review. J Gastrointest Surg. 2011;15(1):209–214. doi: 10.1007/s11605-010-1248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yildiz S, Kantarci M, Kizrak Y. Cadaveric liver transplantation for a giant mass. Gastroenterology. 2014;146:e10–e11. doi: 10.1053/j.gastro.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Lange UG, Bucher JN, Schoenberg MB, Benzing C, Schmelzle M, Gradistanac T, et al. Orthotopic liver transplantation for giant liver haemangioma: a case report. World J Transplant. 2015;5(4):354–359. doi: 10.5500/wjt.v5.i4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Yoon CJ, Kim YH, Han H-S, Cho JY, Kim H, et al. Living-donor liver transplantation for giant hepatic hemangioma with diffuse hemangiomatosis in an adult: a case report. Clin Mol Hepatol. 2018;24(2):163. doi: 10.3350/cmh.2017.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupinacci RM, Szejnfedld D, Farah JF. Spontaneous rupture of a giant hepatic hemangioma. Sequential treatment with preoperative transcatheter arterial embolization and conservative hepatectomy. Il giornale di chirurgia. 2011;32(11/12):469–472. [PubMed] [Google Scholar]
- 29.Sun JH, Nie CH, Zhang YL, et al. Transcatheter arterial embolization alone for giant hepatic hemangioma. PLoS One. 2015;10:e0135158. doi: 10.1371/journal.pone.0135158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GAO J, Ke S, Ding XM, Zhou YM, Qian XJ, Sun WB. Radiofrequency ablation for large hepatic hemangiomas. Initial experience and lessons. Surgery. 2012;153(1):78–85. doi: 10.1016/j.surg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Ji J, Gao J, Zhao L, Tu J, Song J, Sun W. Computed tomography-guided radiofrequency ablation following transcatheter arterial embolization in treatment of large hepatic hemangiomas. Medicine (Baltimore) 2016;95:e3402. doi: 10.1097/MD.0000000000003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaspar L, Mascarenhas F, da Costa MS, Dias JS, Afonso JG, Silvestre ME. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol. 1993;29(1):45–50. doi: 10.1016/0167-8140(93)90172-5. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Wei X, Wang K, Shan Q, Dai H, Xie H, Zhou L, Xu X, Zheng S. Enucleation versus anatomic resection for Giant hepatic Hemangioma: a meta-analysis. Gastrointest Tumors. 2017;3(3–4):153–162. doi: 10.1159/000455846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.