Abstract
Liver cirrhosis and other chronic liver diseases are usually compartmentalized into separate categories based on etiology (e.g., due to alcohol, virus infection, etc.), but it is important to study the intersection of, and possible interactions between, risk factors. The aim of this study is to summarize evidence on the association between alcohol use disorders (AUDs) and decompensated liver cirrhosis and other complications in patients with chronic Hepatitis C virus (HCV) infection. A systematic search of epidemiological studies was conducted using Ovid Medline databases in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria. Relative Risk estimates were combined using random-effects meta-analyses. The proportion of cases with liver disease progression that could be avoided if no person with a chronic HCV infection had an AUD was estimated using an attributable fraction methodology. A total of 11 studies fulfilled the inclusion criteria, providing data from 286,641 people with chronic HCV infections, of whom 63,931 (22.3%) qualified as having an AUD. Using decompensated liver cirrhosis as the outcome for the main meta-analysis (n = 7 unique studies), an AUD diagnosis was associated with a 3.3-fold risk for progression of liver disease among people with a chronic HCV infection (95% Confidence Interval (CI): 1.8–4.8). In terms of population-attributable fractions, slightly less than 4 out of 10 decompensated liver cirrhosis cases were attributable to an AUD: 35.2% (95% CI: 16.2–47.1%). For a secondary analyses, all outcomes related to liver disease progression were pooled (i.e., liver deaths or cirrhosis in addition to decompensated liver cirrhosis), which yielded a similar overall effect (n = 13 estimates; OR = 3.7; 95% CI: 2.2–5.3) and a similar attributable fraction (39.3%; 95% CI: 21.9–50.4%). In conclusion, AUDs were frequent in people with chronic HCV infections and contributed to worsening the course of liver disease. Alcohol use and AUDs should be assessed in patients who have liver disease of any etiology, and interventions should be implemented to achieve abstinence or to reduce consumption to the greatest possible extent.
Keywords: Alcohol, Alcohol-use-disorders, Hepatitis C virus infection, Liver-disease progression, Liver cirrhosis, Decompensated liver cirrhosis, Meta-analysis
Main text
The compartmentalization of liver cirrhosis
Both epidemiologically and clinically, liver cirrhosis and other chronic liver diseases are generally compartmentalized into separate categories based on their etiology. Thus, the Global Health Estimates [1] or the Global Burden of Disease Study [2] give prevalence, incidence, and mortality rates for them in different categories, separating cirrhosis and other chronic liver diseases by etiology: alcohol use, hepatitis B virus infection, hepatitis C virus (HCV) infection, non-alcoholic steatohepatitis, and other causes. Similar differentiation can be found clinically and in the International Classification of Diseases [3], even though there have been some calls to change this system [4, 5]. This contribution will not focus on the logic of current classifications, but will look at the intersection between two of these seemingly separate categories, i.e. liver diseases due to alcohol use and due to HCV infection.
Aims of the current contribution
Based on a systematic literature search, we examined the role of heavy alcohol use—as operationalized via alcohol use disorders (AUDs) [6, 7]—on the progression of liver disease in people with chronic HCV infection. We hypothesized, based on a recent large-scale retrospective cohort study on all hospitalizations in France [8], that a large proportion of complications arising over the course of liver disease in people with HCV infection is attributable to AUD. The main outcome was “decompensated liver cirrhosis”, defined as an acute deterioration in liver function in a patient with cirrhosis, and characterized by jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome and/or variceal hemorrhage [9, 10]. We will summarize the link between AUDs and decompensated liver cirrhosis and other complications of liver disease in people with HCV infection, by pooling relevant studies using meta-analytical techniques [11].
Methods
Systematic search and inclusion/exclusion
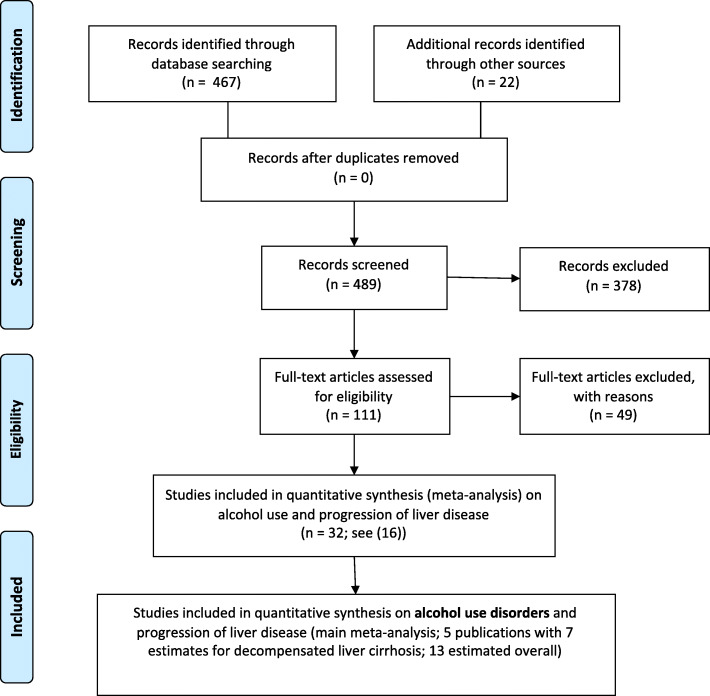
As a first step, we conducted a systematic search of epidemiological studies on the relationship between alcohol use and progression of liver disease due to HCV, using Ovid Medline databases, and applying the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [12]. The exact search terms can be found in the Supplementary Materials (Table S1), but we looked for cohort or case-control studies (for definition, see [13]) of people with chronic HCV infections; with at least two different forms of alcohol use (e.g., alcohol use yes/no; AUD yes/no); and a verified indicator of progression of liver disease (e.g., progression of fibrosis, progression of cirrhosis to decompensated cirrhosis; liver death). This search was initially conducted on July 28, 2019 and updated on December 22, 2019, with the updated search yielding 467 references (see Fig. 1).
Fig. 1.
Systematic search results and selection process of studies for the meta-analyses
In addition, we conducted a search on systematic reviews and meta-analyses on this theme and searched for relevant articles this way (in particular [14–16], see the latter for further detail on the search strategy). As the second step, all articles which included AUDs as exposure were selected as the basis of the analyses of this paper.
For measurement of AUDs, we included the following: hospital record, other record in the healthcare or government database (e.g., registration), physician judgement, standardized measurement with a validated instrument such as the World Health Organization (WHO) Composite International Diagnostic Interview [17], self-report about major criteria of AUDs (for a general discussion of criteria, see [6, 18]). While chronic heavy drinking above 80 g pure alcohol per day before or at some point over the course of the disease could be part of the inclusion criteria (e.g., [19]), it alone did not suffice for inclusion in the study, as no generally accepted quantitative threshold for AUDs based on level of drinking has yet been established [7, 20]. We also excluded studies solely based on screening instruments, such as the CAGE [21] or the AUDIT [22].
All articles were screened by two of the authors (either LLF, MG, or JR). An overview of the selection process can be found in Fig. 1 [23]. All included studies were extracted by two authors (LLF and JR) for key information (study characteristics: title, authors, year published, country, study design, year of study; study population: total number of patients with HCV infection; participant details: mean age, sex, HIV coinfection, number of people with AUD; measurement of AUD; risk relations: relative risk indicator, confidence intervals, p value, adjustments (other covariates); and outcomes).
Statistical methods
Relative Risk estimates (either Odds Ratios, Relative Risks or Hazard Ratios [13, 24]) were taken directly from the respective article or calculated based on a 2*2 table [25] or, in one case, using the methodology specified by Hamling et al. [26].
In the main analysis, only decompensated liver cirrhosis or its main constituents (defined above [9, 10]) as confirmed from medical records, hospitalization or death was chosen as the endpoint (n = 7 estimates from 5 studies). A random effect meta-analysis [27], accounting for the hierarchical structure of the data (three estimates from one study) was conducted [28].
In a secondary analysis, we repeated the random-effect meta-analyses for all estimates of progression of liver disease, i.e. applied a looser inclusion criterion. Here, the following endpoints were including as well (see also Table 1: advanced fibrosis, liver cirrhosis, liver deaths). For both models, we tested if adjustment for important covariates has an impact on the overall effect. Cochran’s Q and the I-squared statistic were used as indicators for heterogeneity [38, 39].
Table 1.
Characteristics of studies included in the meta-analyses
| Reference | Country | Type of study | Years of Study | N (N of outcome) |
Measurement of AUD (N of people with AUD) |
Outcome | Risk Relationsa Adjusted yes/no |
|---|---|---|---|---|---|---|---|
| Alavi et al., 2018 [29] | Australia | Cohort study based on record linkage | 1995–2013 (1995–2012 HCV notifications) | 82,526 (2559) |
Non-liver-related hospitalization due to alcohol use disorders 2001–2013 (prior to outcome) (N = 14,797) |
First-time hospitalization (or death, if no prior hospitalization) due to decompensated cirrhosis |
HR: 3.68 (3.38–4.00) y |
| Alavi et al., 2018 [29] | Canada | Cohort study based on record linkage | 1995–2012 (1995–2011 HCV notifications) | 55,873 (2443) |
Non-liver-related hospitalization due to alcohol use disorders 2001–2012 (prior to outcome) (N = 11,078) |
First-time hospitalization (or death, if no prior hospitalization) due to decompensated cirrhosis |
HR: 1.92 (1.76–2.10) y |
| Alavi et al., 2018 [29]b | Scotland | Cohort study based on record linkage | 1995–2014 (1995–2013 HCV notifications) | 30,746 (1020) |
Non-liver-related hospitalization due to alcohol use disorders 2001–2014 (prior to outcome) (N = 8757) |
First-time hospitalization (or death, if no prior hospitalization) due to decompensated cirrhosis |
HR: 3.88 (3.42–4.40) y |
| Harris et al., 2001 [19] | USA | Retrospective cohort study. | 1968–1980 | 836 (142) |
Loss of friends, family or job because of drinking; admitted to ever having a problem with alcoholism, medical records; sustained use of > 80 g/day (N = 149) |
Liver cirrhosis |
OR: 4.0 (2.1–7.7) y |
| Lim et al., 2014 [30] | USA | Case-control study | 2002–2010 | 997 (27) |
ICD-9 diagnosis for alcohol dependence/abuse recorded (N = 376) |
Medical record–confirmed decompensated cirrhosis |
OR: 2.46 (1.13–5.37) n |
| Marcellin et al., 2014 [31] | France | Case-control study | Not specified | 304 (77) |
Alcohol-related problems (physician’s report) (N = 41) |
Advanced fibrosis |
OR: 3.06 (1.42–6.60) y |
| Marcellin et al., 2015 [32] | France, Germany, Italy, Spain, UK | Case-control study | 2006 | 1333 (438) |
Chronic alcoholism (physician’s judgement) (N = 55) |
Advanced fibrosis |
OR: 2.51 (1.24–5.08) y |
| McDonald et al., 2010 [33]b | Scotland | Cohort study based on record linkage | 1996–2006 | 15,878 (481) |
Hospitalization due to alcohol use disorders or 100% alcohol-attributable disease 1996–2006 (prior to outcome) (N = 274) |
First-time hospitalizations (or death, if no prior hospitalization) due to decompensated cirrhosis |
HR: 5.50 (4.56–6.63) y |
| Nilsson et al., 2016 [34] e | Sweden | Case-control analysis at baseline of a cohort study | 2001–2010 | 284 (67 ascites, 15 variceal-bleeding, 9 encephalopathy) |
Alcoholism or overconsumption of alcohol as stated in the medical records (N = 114) |
Decompensated cirrhosis |
OR: 3.24 (1.77–8.99)e n |
| Nilsson et al., 2016 [34] c | Sweden | Cohort study | 2001–2010 (average follow-up 4.3 years) | 284 (174) |
Alcoholism or overconsumption of alcohol as stated in the medical records (N = 114) |
Death (majority due to liver disease) |
HR: 1.83 (1.34–2.51) yd |
| Schwarzinger et al., 2017 [8] | France | Retrospective cohort study based on record linkage | 2008–2013 | 97,347 (15,630) |
Hospitalization due to alcohol use disorders or 100% alcohol-attributable disease (N = 28,101) |
First record of decompensated cirrhosis hospitalization |
OR: 6.20 (5.85–6.58) y |
| Schwarzinger et al., 2017 [8] | France | Retrospective cohort study based on record linkage | 2008–2013 | 97,347 (6677) |
Hospitalization due to alcohol use disorders or 100% alcohol-attributable disease (N = 28,101) |
Liver death (without liver transplantation) |
OR: 7.63 (8.30–7.97) y |
| Sultanik et al., 2016 [35] | France | Retrospective cohort study | 2006–2015 | 341 (136) | Either ICD-10 codes describing mental and behavioural states due to alcohol use disorders or 100% alcohol attributable | Hepatocellular carcinoma (35%) and/or end-stage liver disease |
HR: 1.47 (1.02–2.13) y |
| Verbaan et al., 1998 [36] | Sweden | Case control | 1991–1997 | 99 (20) |
Use of > 80 g/day for at least 5 years; 92% of these were registered at Department of Alcohol Diseases, University Hospital, Malmö (N = 45) |
Cirrhosis |
OR: 11.8 (1.9–72.1) y |
| Wawrzynowicz-Syczewska et al., 2004 [37] | Poland | Cohort study | 1988–2001 | 77 (22) |
History of alcohol abuse (physician’s judgment) (N = 32) |
Advanced fibrosis |
OR: 10.00 (2.29–43.70) n |
Highlighted areas were included in the main outcome variable: decompensated liver cirrhosis
HR Hazards Ratio, OR Odds Ratio
a Risk relations are either Relative Risks, Hazard Ratios or Odds Ratios
b The samples of the two studies [19, 33] overlap, with the methodology being slightly different (see definition of AUD). Only Alavi et al., 2018 [29] was included in the main quantitative meta-analysis
c This outcome was not included into the second meta-analysis, as it was all-cause mortality, which is not a liver-specific outcome
d The HR was estimated based on the methodology of Hamling et al. [26]
e The combined OR was estimated by weighting the OR for ascites (OR: 4.39 (2.45–7.85)), variceal-bleeding (OR: 0.53(0.16–1.69)) and encephalopathy (OR: 5.50 (1.12–2.95)) by weighting the excess risks by the probability of risk occurrence
Lastly, we calculated the population-attributable fraction (PAF), i.e. the proportion of cases with liver disease progression that could have been avoided if no person with a chronic HCV infection had an AUD. The PAF was calculated using Formula 1 by combining data on the prevalence of AUD (P) with corresponding RRs [40]. All analyses were performed with R version 3.6.1 [41].
| 1 |
Results
Table 1 gives an overview of the studies and their characteristics. In total, studies including 286,641 people with chronic HCV infection fulfilled the inclusion criteria, of whom 63,931 (22.3%) qualified for an AUD.
In the main analysis, 268,114 people with chronic HCV were included, of whom 21,882 had decompensated liver cirrhosis (8.2%). A total of 63,335 people, or 23.6% of this sample, were identified with AUD, a proportion much higher than seen in the general population [2, 42]. Using decompensated liver cirrhosis as the outcome for the main analysis (based on n = 7 estimates), an AUD diagnosis was associated with a 3.3-fold risk for progression of liver disease among people with a chronic HCV infection (95% Confidence Interval (CI): 1.8–4.8), see Fig. 2). There was no significant difference between studies that were adjusted for important covariates and those that were not (p-value = 0.878). In terms of population attributable fractions, slightly less than 4 out ten cases of decompensated liver cirrhosis cases were attributable to AUD: 35.2% (95% CI: 16.2–47.1%).
Fig. 2.
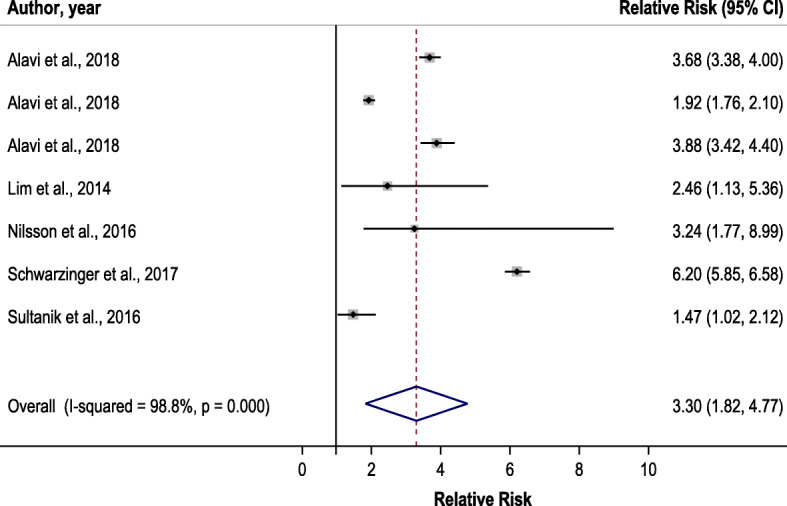
Forest plot for risk of decompensated liver cirrhosis associated with alcohol use disorder
As a secondary analysis, all estimates of liver disease progression were pooled, which yielded a similar overall effect of AUD as compared to the main analysis (n = 13 estimates; OR = 3.7; 95% CI: 2.2–5.3; see Supplementary Materials Figure S1). The risk difference between decompensated liver cirrhosis and the other indicators of liver disease progression was not significant (relative risk ratio: 0.6; 95% CI: 0.3–1.3). Again, adjustment for important covariates was not related to substantial reductions of the effect (p-value = 0.969). Again, this would be equivalent to about 40% of cases with liver progression being attributable to AUDs (attributable fraction: 39.3%; 95% CI: 21.9–50.4%).
In both analyses, substantial heterogeneity was identified using Cochran’s Q statistic ( [38]; main analysis for n = 7 estimates: Q (df = 6) = 507.3, p < .001; secondary analysis for n = 13 estimates: Q (df = 12) = 1184.5, p < .001), presumably associated with the large variation in sample sizes in the included studies (minimum = 77, maximum = 97,347). The I-square statistics also indicated substantial heterogeneity.
Discussion
Before discussing the results and implications of our findings further, we would like to highlight the potential limitations.
Limitations
One limitation to this review and meta-analysis is the reliance on aggregate data, which relies on the qualities of the underlying published studies, based on heterogeneous populations, different study designs and different statistical models, and in different historical periods of time [11]. Even though the populations were heterogeneous, almost all of the studies included are from high-income countries. Given the global load of alcohol-attributable liver cirrhosis burden [43, 44], we urgently need data from other regions of the world, especially from regions with a high prevalence of HCV infections such as Africa and Central Asia [2, 45], but also from countries in Eastern Europe where prevalence of HCV infections and of AUDs are high (e.g., Moldova, Georgia [46]).
Also, the largest studies [8, 29] relied on medical hospital records of AUD, which likely underestimated the true prevalence, as this disorder is highly stigmatized [47] and neither necessarily disclosed nor recorded in hospitals or healthcare settings (for a wider discussion, see [48, 49]), even for 100% alcohol-attributable disorders [50, 51]. However, the bias introduced by underestimating the prevalence of AUDs is conservative; the attributable fractions would likely be higher with higher prevalence (for formulas, see [40]). Additionally, relying on hospital records for the largest studies removes potential biases due to self-report of AUDs [52].
Another potential limitation involves the exclusion of studies where AUDs could only have been inferred by a mention of chronic heavy drinking or other drinking behaviours closely related to AUDs. On the one hand, heavy drinking is a key characteristic of AUDs [20]. To give one example, there is a high likelihood that lifetime drinkers with more than 175 g pure alcohol consumed daily—such as in the study of Corrao and colleagues [53]—would qualify for AUDs had this condition been measured with validated instruments. One the other hand, it is hard to draw a threshold. In the same study by Corrao and colleagues, the following thresholds were used to indicate the drinking level: 50 g, 75 g, 100 g, 125 g and 150 g pure alcohol per day. It is not clear which of these drinking-level categories would indicate AUD. Thus, while AUDs constitute a common medical diagnosis, the use of this diagnosis—which is clinically relevant and can be used in health services research—in epidemiological research may lead to biases, as the active ingredient in disease progression—ethanol—is only indirectly assessed (see also [7, 54]). Another aspect of patterns of drinking deserve mentioning. These patterns – especially the prevalence of heavy episodic drinking – differ vastly between the countries examined here [55]. It has been shown that a pattern of daily heavy drinking is most detrimental for worsening of liver disease [56, 57], for daily drinking is less prevalent among heavy drinkers in countries like Poland or Scotland, compared countries like France, to mention just three of the countries in our sample. Without measuring patterns of drinking at the individual level, variation is introduced into our results. Future research should not only rely on wide categories such as AUD [6], but should measure drinking level and patterns.
As we wanted to conduct a meta-analysis with a narrow outcome – decompensated liver cirrhosis, we defined our search terms excluding wider definitions such as hepatocellular carcinoma. Indeed, we achieved this goal, and only in one study [35], a minority of cases included hepatocellular carcinoma. This does not mean, however, that AUDs do not causally impact on hepatocellular carcinoma in people with chronic HCV infections. As Schwarzinger and colleagues demonstrated in the national French hospital cohort comprising 6404 patients with hepatocellular carcinoma [8], AUDs were associated with a fourfold-increased risk (4.23; 95% CI: 3.99–4.49).
Alcohol use disorders as a key determinant of liver disease progression
Our results show that AUDs are quite common among people with chronic HCV infections, and that they are a key determinant for worsening of liver disease. Our design did not allow us to answer the question of whether alcohol use or AUDs were the only factor in disease progression (see [15]); however, other research seems to indicate that HCV in people with AUDs also showed increased disease progression (e.g., [6, 58, 59]) and, thus, there seems to be an interaction effect of alcohol use and HCV infection. There are also plausible biological pathways, such as increased viral replication and altered immune response [60].
Our design also does not answer the question regarding a dose-response relationship for alcohol use, i.e., whether all levels of alcohol use are detrimental for liver disease progression (see [16, 61]). However, we can clearly state that AUDs, with their high levels of alcohol consumption, produce a markedly worsened progression for liver diseases, and were responsible for about 40% of all these complications in the large cohorts underlying our study (see [62, 63], for further discussion). Such a high attributable fraction also calls into question the compartmentalization of liver cirrhosis into subtypes/categories [4, 5].
Conclusions
AUDs are relatively frequent in people with chronic HCV infections and contributed markedly to progression of liver disease. Two main conclusions result: despite the current clinical compartmentalization, alcohol use and AUDs should be assessed in patients with liver cirrhosis of any etiology (see also [64] for the dose-response relationship for alcohol use on any kind of liver cirrhosis), and irrespective, if the HCV infection has been treated successfully of not. This is even true for so-called “non-alcoholic” liver disease categories, where AUDs do not play a role by definition, but alcohol use still may [65]. But assessment is not sufficient, AUDs need to be treated by either achieving full abstinence (the best outcome of alcohol interventions for any kind of liver disease [66]) or, if this is not possible, reducing consumption to the highest degree possible [67–69].
Supplementary information
Additional file 1. Supplementary materials.
Additional file 2: Figure S1. Forest plot for risk of negative course of liver disease associated with alcohol use disorder.
Acknowledgements
The authors would like to thank Ms. Astrid Otto for copy-editing and referencing assistance.
Abbreviations
- AUD/AUDs
Alcohol Use Disorder(s)
- CI
Confidence Interval
- HCV
Hepatitis C Virus
- PAF
Population-attributable fraction
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RE model
Random-effects model
- WHO
World Health Organization
Authors’ contributions
JR conceptualized the study and JR and LLF wrote a first draft. MG did the systematic searches. LLF, MG and JR chose the studies to be included, and LLF and JR extracted information. JM and KS did the main statistical analysis. All authors reviewed various versions of the text and approved the final version.
Funding
This contribution was supported by the WHO Collaborating Centre for Addiction and Mental Health at the Centre for Addiction and Mental Health, Toronto, Canada. JR acknowledges funding from the Canadian Institutes of Health Research, Institute of Neurosciences, and Mental Health and Addiction (CRISM Ontario Node grant no. SMN-13950). Research reported in this publication was also supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (NIAAA) [Award Number R01AA024443]. This research was conducted as part of the Calibrated Agent Simulations for Combined Analysis of Drinking Etiologies (CASCADE) project and we would like to thank the whole CASCADE team for their input to wider discussions in generating the research reported in this paper. Content is the responsibility of the authors and does not reflect official positions of NIAAA or the National Institutes of health. In addition, JR was supported for the general systematic search on alcohol use, HCV infection and progression of liver disease by a consultant contract of the Pan American Health Organization (NMH/NV/IPC/18/05).
Availability of data and materials
All data has been derived from published studies.
Ethics approval and consent to participate
Not applicable, as the analyses were all based on published anonymized data.
Consent for publication
Not applicable in a secondary data analysis.
Competing interests
None declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13011-020-00287-1.
References
- 1.World Health Organization. Global Health Estimates (GHE).2019 Accessed: 10/02/2020; Available from: http://www.who.int/healthinfo/global_burden_disease/en/..
- 2.Global Health Data Exchange (GHDx). GBD Results Tool. . Seattle, Washington: Institute for Health Metrics and Evaluation; 2019. Available from: http://ghdx.healthdata.org/gbd-results-tool. Accessed: 10/02/2020.
- 3.World Health Organization. ICD-11 for Mortality and Morbidity Statistics (ICD-11 MMS): 2018 version.2018 Accessed: 08/02/2020; Available from: https://icd.who.int/browse11/l-m/en.
- 4.Eslam M, Sanyal AJ, George J. Toward more accurate nomenclature for fatty liver diseases. Gastroenterology. 2019;157(3):590–593. doi: 10.1053/j.gastro.2019.05.064. [DOI] [PubMed] [Google Scholar]
- 5.Lange S, Roerecke M, Rehm J. For most fully alcohol-attributable diagnoses in the ICD, the etiological specification should be removed. Adicciones. 2020;32(2):90–93. doi: 10.20882/adicciones.1376. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394(10200):781–792. doi: 10.1016/S0140-6736(19)31775-1. [DOI] [PubMed] [Google Scholar]
- 7.Rehm J, Marmet S, Anderson P, Gual A, Kraus L, Nutt DJ, et al. Defining substance use disorders: do we really need more than heavy use? Alcohol Alcohol. 2013;48(6):633–640. doi: 10.1093/alcalc/agt127. [DOI] [PubMed] [Google Scholar]
- 8.Schwarzinger M, Baillot S, Yazdanpanah Y, Rehm J, Mallet V. Contribution of alcohol use disorders on the burden of chronic hepatitis C in France, 2008-2013: a nationwide retrospective cohort study. J Hepatol. 2017;67(3):454–461. doi: 10.1016/j.jhep.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Mansour D, McPherson S. Management of decompensated cirrhosis. Clin Med (London, England) 2018;18(Suppl 2):s60–ss5. doi: 10.7861/clinmedicine.18-2s-s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blettner M, Sauerbrei W, Schlehofer B, Scheuchenpflug T, Friedenreich C. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol. 1999;28(1):1–9. doi: 10.1093/ije/28.1.1. [DOI] [PubMed] [Google Scholar]
- 12.PRISMA. The PRISMA Statement. PRISMA; 2015. Available from: http://www.prisma-statement.org/PRISMAStatement/PRISMAStatement. Accessed: 23/12/2019.
- 13.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Third ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 14.Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3(11):1150–1159. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 15.Vento S, Cainelli F. Does hepatitis C virus cause severe liver disease only in people who drink alcohol? Lancet Infect Dis. 2002;2(5):303–309. doi: 10.1016/s1473-3099(02)00271-2. [DOI] [PubMed] [Google Scholar]
- 16.Llamosas-Falcón L, Shield KD, Gelovany M, Manthey J, Rehm J. Alcohol-attributable hepatitis C burden. Toronto: Centre for Addiction and Mental Health; 2020. [Google Scholar]
- 17.Harvard College. The World Health Organization World Mental Health Composite International Diagnostic Interview (WHO WMH-CIDI). 2020. Available from: https://www.hcp.med.harvard.edu/wmhcidi/about-the-who-wmh-cidi/. Accessed: 10/02/2020.
- 18.Rehm J, Heilig M, Gual A. ICD-11 for alcohol use disorders: not a convincing answer to the challenges. Alcohol Clin Exp Res. 2019;43(11):2296–2300. doi: 10.1111/acer.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris DR, Gonin R, Alter HJ, Wright EC, Buskell ZJ, Hollinger FB, et al. The relationship of acute transfusion-associated hepatitis to the development of cirrhosis in the presence of alcohol abuse. AnnInternMed. 2001;134(2):120–124. doi: 10.7326/0003-4819-134-2-200101160-00012. [DOI] [PubMed] [Google Scholar]
- 20.Rehm J, Anderson P, Gual A, Kraus L, Marmet S, Nutt DJ, et al. The tangible common denominator of substance use disorders: a reply to commentaries to Rehm et al. (2013a) Alcohol Alcoholism (Oxford, Oxfordshire) 2014;49(1):118–122. doi: 10.1093/alcalc/agt171. [DOI] [PubMed] [Google Scholar]
- 21.Dhalla S, Kopec JA. The CAGE questionnaire for alcohol misuse: a review of reliability and validity studies. Clin Invest Med. 2007;30(1):33–41. doi: 10.25011/cim.v30i1.447. [DOI] [PubMed] [Google Scholar]
- 22.Lange S, Shield K, Monteiro M, Rehm J. Facilitating screening and brief interventions in primary care: a systematic review and meta-analysis of the AUDIT as an Indicator of alcohol use disorders. Alcohol Clin Exp Res. 2019;43(10):2028–2037. doi: 10.1111/acer.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PRISMA. Prism Flow Diagram. 2015. Available from: http://prisma-statement.org/prismastatement/flowdiagram.aspx. Accessed: 10/02/2020.
- 24.Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. 1987;125(5):761–768. doi: 10.1093/oxfordjournals.aje.a114593. [DOI] [PubMed] [Google Scholar]
- 25.Fleiss J, Levin B, Cho PM. Statistical Methods for Rates and Proportions. Third Edition ed. Hoboken: John Wiley & Sons; 2003. p. 2003. [Google Scholar]
- 26.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 27.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. New York: Springer-Verlag; 2015. [Google Scholar]
- 29.Alavi M, Janjua NZ, Chong M, Grebely J, Aspinall EJ, Innes H, et al. The contribution of alcohol use disorder to decompensated cirrhosis among people with hepatitis C: an international study. J Hepatol. 2018;68(3):393–401. doi: 10.1016/j.jhep.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Lim JK, Tate JP, Fultz SL, Goulet JL, Conigliaro J, Bryant KJ, et al. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus–infected, and uninfected patients. Clin Infect Dis. 2014;58(10):1449–1458. doi: 10.1093/cid/ciu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcellin F, Roux P, Loko MA, Lions C, Caumont-Prim A, Dabis F, et al. High levels of alcohol consumption increase the risk of advanced hepatic fibrosis in HIV/hepatitis C virus-coinfected patients: a sex-based analysis using transient elastography at enrollment in the HEPAVIH ANRS CO13 cohort. Clin Infect Dis. 2014;59(8):1190–1192. doi: 10.1093/cid/ciu525. [DOI] [PubMed] [Google Scholar]
- 32.Marcellin P, Grotzinger K, Theodore D, Demuth D, Manns M, Banares Canizares R, et al. Severity of liver disease among chronic hepatitis C patients: an observational study of 4594 patients in five European countries. J Gastroenterol Hepatol. 2015;30(2):364–371. doi: 10.1111/jgh.12698. [DOI] [PubMed] [Google Scholar]
- 33.McDonald SA, Hutchinson SJ, Bird SM, Mills PR, Robertson C, Dillon JF, et al. Hospitalization of hepatitis C-diagnosed individuals in Scotland for decompensated cirrhosis: a population-based record-linkage study. Eur J Gastroenterol Hepatol. 2010;22(1):49–57. doi: 10.1097/MEG.0b013e32832ff35d. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson E, Anderson H, Sargenti K, Lindgren S, Prytz H. Incidence, clinical presentation and mortality of liver cirrhosis in southern Sweden: a 10-year population-based study. Aliment Pharmacol Ther. 2016;43(12):1330–1339. doi: 10.1111/apt.13635. [DOI] [PubMed] [Google Scholar]
- 35.Sultanik P, Kramer L, Soudan D, Bouam S, Meritet JF, Vallet-Pichard A, et al. The relationship between liver stiffness measurement and outcome in patients with chronic hepatitis C and cirrhosis: a retrospective longitudinal hospital study. Aliment Pharmacol Ther. 2016;44(5):505–513. doi: 10.1111/apt.13722. [DOI] [PubMed] [Google Scholar]
- 36.Verbaan H, Widell A, Bondeson L, Andersson K, Eriksson S. Factors associated with cirrhosis development in chronic hepatitis C patients from an area of low prevalence. J Viral Hepat. 1998;5(1):43–51. doi: 10.1046/j.1365-2893.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- 37.Wawrzynowicz-Syczewska M, Kubicka J, Lewandowski Z, Boron-Kaczmarska A, Radkowski M. Natural history of acute symptomatic hepatitis type C. Infection. 2004;32(3):138–143. doi: 10.1007/s15010-004-3062-8. [DOI] [PubMed] [Google Scholar]
- 38.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 39.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 40.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9(3):531–541. [PubMed] [Google Scholar]
- 41.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing,; 2019. Available from: www.R-project.org/. Accessed: 10/02/2020.
- 42.World Health Organization. Global status report on alcohol and health 2018.2018 Accessed: 05/20/2019; Available from: https://www.who.int/substance_abuse/publications/global_alcohol_report/en/.
- 43.Rehm J, Shield KD. Global burden of alcohol use disorders and alcohol liver disease. Biomedicines. 2019;7(4):99. doi: 10.3390/biomedicines7040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shield K, Manthey J, Rylett M, Probst C, Wettlaufer A, Parry CDH, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health. 2020;5(1):E51–E61. doi: 10.1016/S2468-2667(19)30231-2. [DOI] [PubMed] [Google Scholar]
- 45.Thrift AP, El-Serag HB, Kanwal F. Global epidemiology and burden of HCV infection and HCV-related disease. Nat Rev Gastroenterol Hepatol. 2017;14(2):122–132. doi: 10.1038/nrgastro.2016.176. [DOI] [PubMed] [Google Scholar]
- 46.Maistat L, Kravchenko N, Reddy A. Hepatitis C in Eastern Europe and Central Asia: a survey of epidemiology, treatment access and civil society activity in eleven countries. Hepatol Med Policy. 2017;2(1):9. doi: 10.1186/s41124-017-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schomerus G, Lucht M, Holzinger A, Matschinger H, Carta MG, Angermeyer MC. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcoholism (Oxford, Oxfordshire) 2011;46(2):105–112. doi: 10.1093/alcalc/agq089. [DOI] [PubMed] [Google Scholar]
- 48.Hanschmidt F, Manthey J, Kraus L, Scafato E, Gual A, Grimm C, et al. Barriers to Alcohol Screening Among Hypertensive Patients and the Role of Stigma: Lessons for the Implementation of Screening and Brief Interventions in European Primary Care Settings. Alcohol Alcoholism (Oxford, Oxfordshire) 2017;52(5):572–579. doi: 10.1093/alcalc/agx032. [DOI] [PubMed] [Google Scholar]
- 49.Chen CH, Chen WJ, Cheng AT. Prevalence and identification of alcohol use disorders among nonpsychiatric inpatients in one general hospital. Gen Hosp Psychiatry. 2004;26(3):219–225. doi: 10.1016/j.genhosppsych.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Rehm J, Hasan OSM, Imtiaz S, Neufeld M. Quantifying the contribution of alcohol to cardiomyopathy: A systematic review. Alcohol (Fayetteville, NY) 2017;61:9–15. doi: 10.1016/j.alcohol.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Puffer RR, Griffith GW. Patterns of urban mortality: report of the inter-American investigation of mortality. Washington, D.C: Pan American Health Organization; 1967. [Google Scholar]
- 52.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction (Abingdon, England) 2003;98 Suppl 2:1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- 53.Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology (Baltimore, Md) 1998;27(4):914–919. doi: 10.1002/hep.510270404. [DOI] [PubMed] [Google Scholar]
- 54.Rehm J. How should prevalence of alcohol use disorders be assessed globally? Int J Methods Psychiatr Res. 2016;25(2):79–85. doi: 10.1002/mpr.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393(10190):2493–2502. doi: 10.1016/S0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- 56.Askgaard G, Gronbaek M, Kjaer MS, Tjonneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J Hepatol. 2015;62(5):1061–1067. doi: 10.1016/j.jhep.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 57.Rehm J, Roerecke M. Patterns of drinking and liver cirrhosis - what do we know and where do we go? J Hepatol. 2015;62(5):1061–1067. doi: 10.1016/j.jhep.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 58.Sanvisens A, Munoz A, Bolao F, Zuluaga P, Farre M, Jarrin I, et al. Do serum markers of liver fibrosis vary by HCV infection in patients with alcohol use disorder? Drug Alcohol Depend. 2018;188:180–186. doi: 10.1016/j.drugalcdep.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Muga R, Sanvisens A, Jarrin I, Fuster D, Bolao F, Tor J, et al. Hepatitis C infection substantially reduces survival of alcohol-dependent patients. Clin Epidemiol. 2018;10:897–905. doi: 10.2147/CLEP.S162308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novo-Veleiro I, Alvela-Suárez L, Chamorro A-J, González-Sarmiento R, Laso F-J, Marcos M. Alcoholic liver disease and hepatitis C virus infection. World J Gastroenterol. 2016;22(4):1411–1420. doi: 10.3748/wjg.v22.i4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peters MG, Terrault NA. Alcohol use and hepatitis C. Hepatology (Baltimore, Md) 2002;36(5 Suppl 1):S220–S2S5. doi: 10.1053/jhep.2002.36811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alavi M, Law MG, Dore GJ. Reply to: "'Who killed JR': chronic hepatitis C or alcohol use disorders?". J Hepatol. 2018;68(5):1099–1100. doi: 10.1016/j.jhep.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 63.Schwarzinger M, Rehm J, Mallet V. "who killed JR": chronic hepatitis C or alcohol use disorders? J Hepatol. 2018;68(5):1098–1099. doi: 10.1016/j.jhep.2017.11.042. [DOI] [PubMed] [Google Scholar]
- 64.Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis. Am J Gastroenterol. 2019;114(10):1574–1586. doi: 10.14309/ajg.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roerecke M, Nanau R, Rehm J, Neuman M. Ethnicity matters: a systematic review and meta-analysis of the non-linear relationship between alcohol consumption and prevalence and incidence of hepatic Steatosis. EBioMedicine. 2016;8:317–330. doi: 10.1016/j.ebiom.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altamirano J, Michelena J. Alcohol consumption as a cofactor for other liver diseases. Clini Liver Dis. 2013;2(2):72–75. doi: 10.1002/cld.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rehm J, Roerecke M. Reduction of drinking in problem drinkers and all-cause mortality. Alcohol Alcoholism (Oxford, Oxfordshire) 2013;48(4):509–513. doi: 10.1093/alcalc/agt021. [DOI] [PubMed] [Google Scholar]
- 68.Lieber CS, Weiss DG, Groszmann R, Paronetto F, Schenker S., II Veterans affairs cooperative study of polyenylphosphatidylcholine in alcoholic liver disease. Alcohol Clin Exp Res. 2003;27(11):1765–1772. doi: 10.1097/01.ALC.0000093743.03049.80. [DOI] [PubMed] [Google Scholar]
- 69.Charlet K, Heinz A. Harm reduction-a systematic review on effects of alcohol reduction on physical and mental symptoms. Addict Biol. 2017;22(5):1119–1159. doi: 10.1111/adb.12414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary materials.
Additional file 2: Figure S1. Forest plot for risk of negative course of liver disease associated with alcohol use disorder.
Data Availability Statement
All data has been derived from published studies.