This cohort study examines the association of early-life cognitive enrichment with late-life Alzheimer disease and other common dementia-related pathological changes in an autopsy sample.
Key Points
Question
Is a higher level of early-life cognitive enrichment associated with lower levels of late-life Alzheimer disease and other common dementia-related pathological changes?
Findings
In this cohort study of 813 patients with postmortem data, a higher level of early-life cognitive enrichment was associated with a decreased Alzheimer disease pathology score but was not associated with any other dementia-related pathological changes.
Meaning
Early-life cognitive enrichment was associated with late-life cognitive health in part through an association with fewer Alzheimer disease-related pathological changes.
Abstract
Importance
Indicators of early-life cognitive enrichment (ELCE) have been associated with slower cognitive decline and decreased dementia in late life. However, the mechanisms underlying this association have not been elucidated.
Objective
To examine the association of ELCE with late-life Alzheimer disease (AD) and other common dementia-related pathological changes.
Design, Setting, and Participants
This clinical-pathological community-based cohort study, the Rush Memory and Aging Project, followed up participants before death for a mean (SD) of 7.0 (3.8) years with annual cognitive and clinical assessments. From January 1, 1997, through June 30, 2019, 2044 participants enrolled, of whom 1018 died. Postmortem data were leveraged from 813 participants. Data were analyzed from April 12, 2019, to February 20, 2020.
Exposures
Four indicators of ELCE (early-life socioeconomic status, availability of cognitive resources at 12 years of age, frequency of participation in cognitively stimulating activities, and early-life foreign language instruction) were obtained by self-report at the study baseline, from which a composite measure of ELCE was derived.
Main Outcomes and Measures
A continuous global AD pathology score derived from counts of diffuse plaques, neuritic plaques, and neurofibrillary tangles.
Results
The 813 participants included in the analysis had a mean (SD) age of 90.1 (6.3) years at the time of death, and 562 (69%) were women. In a linear regression model controlled for age at death, sex, and educational level, a higher level of ELCE was associated with a lower global AD pathology score (estimate, −0.057; standard error, 0.022; P = .01). However, ELCE was not associated with any other dementia-related pathological changes. In addition, a higher level of ELCE was associated with less cognitive decline (mean [SD], −0.13 [0.19] units per year; range, −1.74 to 0.85). An indirect effect through AD pathological changes constituted 20% of the association between ELCE and the rate of late-life cognitive decline, and 80% was a direct association.
Conclusions and Relevance
These findings suggest that ELCE was associated with better late-life cognitive health, in part through an association with fewer AD pathological changes.
Introduction
Indicators of early-life cognitive enrichment (ELCE), such as childhood socioeconomic status and school performance, are associated with higher levels of late-life cognition and decreased risk of Alzheimer disease (AD)–related dementia.1,2 However, few studies3,4,5,6 have examined the association of ELCE with dementia-related pathological changes as the outcome of interest.
Herein, we leverage data from a clinical-pathological study to test the hypothesis that higher levels of ELCE are associated with fewer AD pathological changes in the brain. We extended previous work7,8,9,10 in 4 important ways. First, we developed a composite measure from the 4 elements of ELCE7,8,9,10 and examined the association of the composite measure with postmortem AD pathology indices. Second, we included a far larger sample size, increasing power to detect an association. Third, we examined the association of the ELCE composite measure with 8 other common dementia-related pathological changes. Fourth, we assessed the association of the measure with cognitive decline over several years before death. Then, we estimated how much of the association between ELCE and cognitive decline was explained by the pathological changes.
Methods
Participants
The Rush Memory and Aging Project is an ongoing community-based cohort study of chronic conditions of aging whose participants agree to annual clinical assessments and organ donation at the time of death.11 The study was approved by the institutional review board of Rush University Medical Center, Chicago, Illinois, and participants provided written consent for the study and consented for the Uniform Anatomical Gift Act for organ donation.12 Participants were recruited from retirement facilities, subsidized housing, and individual homes across the Chicago metropolitan area. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
From January 1, 1997, to June 30, 2019, 1018 Rush Memory and Aging Project participants had died, of whom 838 had undergone a brain autopsy and neuropathological examination (82% autopsy rate). At the time of these analyses, the neuropathological examination had been completed in 824 decedents, of whom 813 had complete ELCE data (eFigure in the Supplement). Compared with participants without postmortem pathological examination, participants included in the present analyses were older at the time of death (mean [SD] age, 90.1 [6.3] vs 88.0 [6.6] years) and had higher levels of ELCE (mean [SD] score, 0.0 [0.7] vs −0.2 [0.8]) and educational attainment (mean [SD], 14.6 [2.9] vs 13.8 [3.4] years) (eTable 1 in the Supplement).
Assessment of ELCE
At the baseline, participants were asked questions about 4 indicators of ELCE: early-life socioeconomic status, based on maternal and paternal educational levels and number of children in the family10; availability of cognitive resources at 12 years of age, based on features of the home environment supporting cognitive activities, such as a newspaper subscription, encyclopedia, globe, or atlas7; frequency of participation in cognitively stimulating activities, with 3 activities at 6 years of age (eg, being read to) and 8 activities at 12 years of age (eg, reading books)9; and early-life foreign language instruction, based on self-report of years of foreign language instruction to 18 years of age.8 Because the 4 indicators were correlated (eTable 2 in the Supplement), we conducted principal component analysis. Using the Kaiser criterion,13 only 1 principal component was retained that explained 50% of the total variance of data. The 4 indicators had factor loadings greater than 0.4 (eTable 3 in the Supplement), indicating their association with the derived factor. For parsimony, we created a composite measure of ELCE by standardizing the indicators’ scores (mean [SD], 0 [1]). We then calculated the mean of the 4 standardized scores to measure ELCE. The measure had a mean (SD) of 0.0 (0.7) and ranged from −2.0 to 1.7, with higher scores indicating higher levels of ELCE.
Assessment of Postmortem Brain Pathological Changes
The mean (SD) postmortem interval was 9.1 (7.6) hours. During a uniform structured procedure, brains were removed, and 1 hemisphere was frozen and used to generate a multilevel, multiregion omic atlas.14 The other hemisphere was fixed and sectioned into 1-cm slabs, and tissue blocks were prepared from predetermined regions for more pathological evaluations.
Global AD Pathology Score
A modified Bielschowsky silver stain was used to visualize AD pathological hallmarks (diffuse plaques, neuritic plaques, and neurofibrillary tangles).14 Each of the 3 AD pathological hallmarks was counted in each brain region, and a summary measure was calculated for each of the AD pathology hallmarks by calculating the mean of its brain regions’ standard scores. Then, we constructed a global measure, the global AD pathology score, by calculating the mean of the 3 AD pathology hallmarks’ summary measures, as described previously.15
β-Amyloid and Tau Assessment
Quantification of β-amyloid and tau levels was performed through image analysis (β-amyloid) and stereology (tau) of immunohistochemically stained brain sections. Images were obtained from β-amyloid–stained sections by a computer-controlled motorized image-capturing system. Then, algorithms were used for calculation of the percentage area occupied by β-amyloid immunoreactive pixels. The means of the percentage areas were used for each brain region and across the brain regions to yield an overall level of β-amyloid. Tau levels were quantified using a microscope connected to a computer. An operator delimited a region of interest at low power. Then, software engaged by placing a grid over the region directed the motorized stage on the microscope to stop at each intersection point of the grid, where the operator counted the tau-labeled objects on the video monitor. The means of the counts were used for each brain region and across the brain regions to yield an overall level of tau. Details of the procedures are provided in the eMethods in the Supplement and elsewhere.16
Other Common Dementia-Related Pathological Changes
We assessed and quantified 8 other common dementia-related pathological changes. Details of the pathological assessment are provided in the eMethods in the Supplement and elsewhere.17
Cognition Assessment
At the baseline evaluation and annually thereafter, 19 neuropsychological tests were used to create a composite measure. The included tests were Word List Memory recall and recognition tasks, immediate and delayed recall of story A from the Wechsler Logical Memory Scale, the Story Recall Test of the East Boston Memory Test, Verbal Fluency Test, Boston Naming Test, National Adult Reading Test, Extended Range Vocabulary Test, Digits Forward and Digits Backward of the Memory for Digit Span Test, Digit Ordering Test, Alpha Span, Symbol Digit Modalities Test, Number Comparison Test, Judgement of Line Orientation Test, and Standard Progressive Matrices. Test scores were standardized and means were calculated to make a global cognition score, as previously described.18 We have developed the composite global cognition score to reduce measurement errors in longitudinal analyses, particularly floor and ceiling effects. In addition, making the global cognition score was supported by factor analysis.19
Assessment of Other Variables
At the baseline evaluation, sex and race were assessed by self-report because they are associated with late-life cognitive decline and its underlying pathological changes.17,20 Chronic health conditions included the sums of 3 self-reported vascular risk factors (hypertension, diabetes, and smoking) and 4 self-reported vascular diseases (myocardial infarction, congestive heart failure, stroke, and claudication). Self-report questionnaires were used to assess socioeconomic status and late-life cognitive activity level, as described previously21 and in the eMethods in the Supplement. Assessment of the presence of the allele ε4 of the APOE gene included sequencing rs429358 (codon 112) and rs7412 (codon 158) at exon 4 of the APOE gene, as described previously.22
Statistical Analyses
Data were analyzed from April 12, 2019, to February 20, 2020. Owing to normal distribution violation, we used square root transformation of the AD pathological indices in the analyses. We used Spearman correlation coefficients and unpaired t tests for examining the association of ELCE with continuous and binomial variables, respectively. Next, we used linear regression models to examine the association of ELCE with the AD pathology indices while controlling for demographics including educational attainment and other covariates. Then, we examined the association of ELCE with other common dementia-related pathological changes through use of logistic regressions controlled for age at death, sex, and educational attainment.
We used linear mixed-effects models to estimate person-specific cognitive change during follow-up that was used to test the association of ELCE with the annual rate of cognitive decline in a linear regression, controlled for age at death, sex, and educational attainment. Then, we added the global AD pathology score to the model and its interaction with ELCE. Finally, we used path analysis, controlled for age at death, sex, and educational attainment, to assess the direct and indirect (through the global AD pathology score) associations of ELCE with cognitive decline. The following 2 paths were simultaneously examined in the path analysis: (1) age at death, sex, educational attainment, ELCE, and global AD pathology score as the variables and cognitive decline rate as the outcome; and (2) age at death, sex, educational attainment, and ELCE as the variables and the global AD pathology score as the outcome. The statistical significance was determined at 2-sided P < .05, and the analyses were done using SAS, version 9.4 (SAS Institute, Inc).
Results
The participants’ demographic and clinical characteristics and their association with the ELCE are shown in Table 1. Among the 813 participants with postmortem data available for analysis, 562 were women (69%) and 251 were men (31%); mean (SD) age at death was 90.1 (6.3) years.
Table 1. Demographic and Clinical Characteristics of Participants .
| Variable | Data (n = 813) | Association with ELCEa | P value |
|---|---|---|---|
| Age at death, mean (SD), y | 90.1 (6.3) | r = –0.02 | .52 |
| Women, No. (%) | 562 (69) | t684 = 0.45 | .65 |
| Educational level, mean (SD), y | 14.6 (2.9) | r = 0.45 | <.001 |
| White non-Hispanic, No. (%) | 782 (96) | t1003 = –5.41 | <.001 |
| APOE ε4, No. (%) | 193 (24) | t676 = –0.43 | .67 |
| Last visit | |||
| AD dementia, No. (%) | 323 (40) | t961 = 1.60 | .11 |
| MMSE score, mean (SD)b | 20.9 (9.0) | r = 0.12 | <.001 |
| Global cognitive function score, mean (SD)c | –0.9 (1.1) | r = 0.16 | <.001 |
| Vascular risk score, mean (SD)d | 1.3 (0.8) | r = –0.00 | .99 |
| Vascular diseases score, mean (SD)e | 0.8 (0.9) | r = –0.03 | .37 |
| Baseline cognitive activity level, mean (SD)f | 3.2 (0.8) | r = 0.32 | <.001 |
| Baseline self-perceived socioeconomic status, mean (SD)g | 6.6 (1.3) | r = 0.26 | <.001 |
Abbreviations: AD, Alzheimer disease; ELCE, early-life cognitive enrichment; MMSE, Mini-Mental State Examination.
Estimates are either correlation coefficients (derived from Spearman correlations) or the t values with df derived from independent t tests.
Scores range from 0 to 30, with higher scores indicating better cognition.
Calculated as the sum of 3 self-reported vascular risk factors. Scores range from −4.0 to 1.3, with higher scores indicating better cognition.
Calculated as the sum of 4 self-reported vascular diseases. Scores range from 0 to 3, with higher scores indicating higher burden of vascular risk factors.
Based on self-reported cognitive activity. Scores range from 0 to 4, with higher scores indicating higher burden of vascular diseases.
Based on self-reported socioeconomic status. Scores range from 1 to 5, with higher scores indicating higher levels of cognitive activity.
Available for 353 participants.
ELCE and AD Pathology Indices
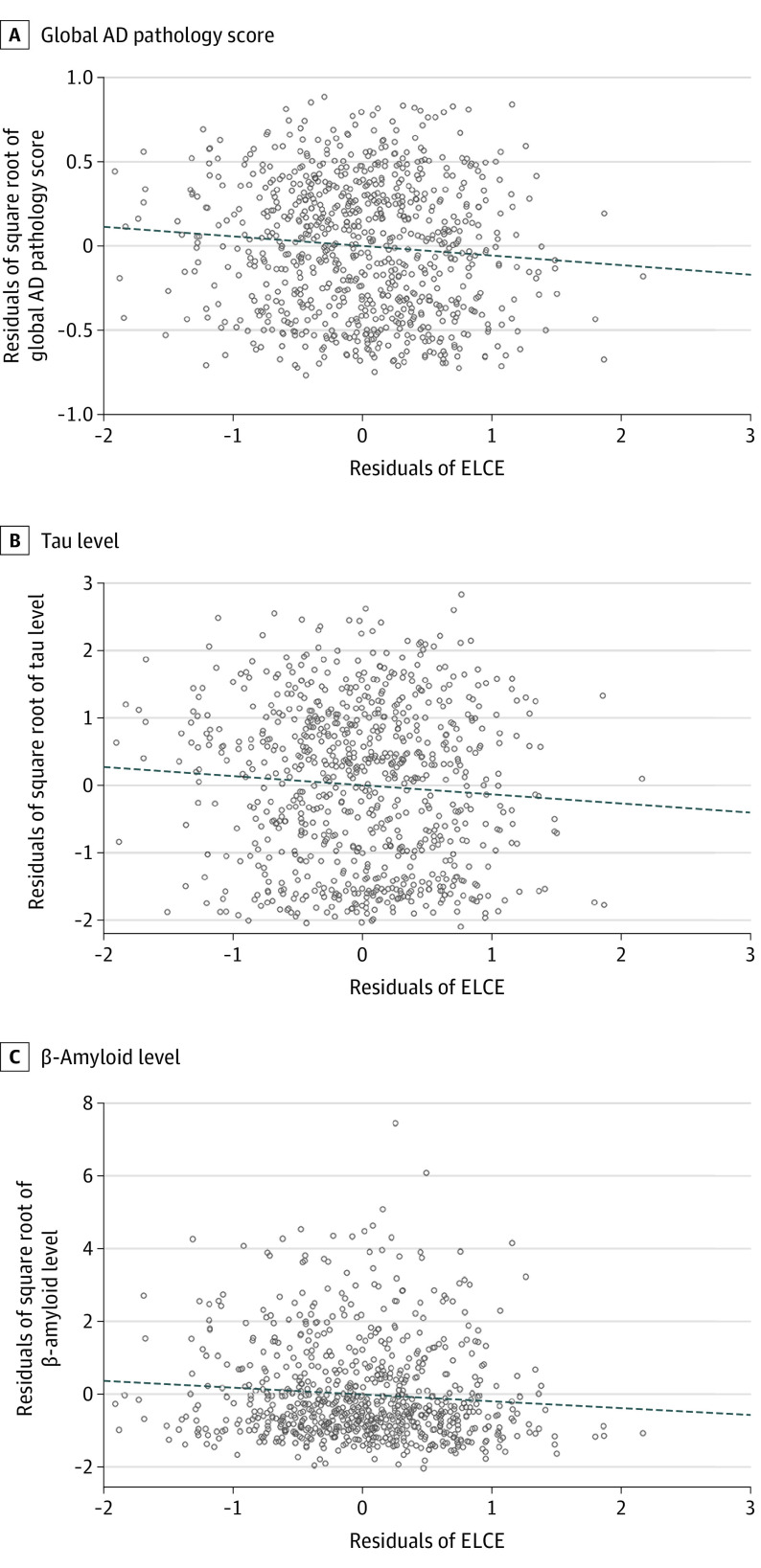
In a series of linear regression models, a higher level of ELCE was associated with lower global AD pathology score (estimate, −0.057; standard error [SE], 0.022; P = .01) and lower levels of tau (estimate, −0.188; SE, 0.076; P = .01) and β-amyloid (estimate, −0.136; SE, 0.066; P = .04) (Figure 1). Estimates represent the difference in burden of AD pathology indices with every additional 1-unit increase in the ELCE score. To contextualize the effect size, we compared the estimate of ELCE with the estimate of age at death (estimate, 0.007; SE, 0.002; P = .003) in their association with the global AD pathology score. The effect size associated with a 1-unit increase in the ELCE was equivalent to the effect size associated with being 8 years younger.
Figure 1. Association of Early-Life Cognitive Enrichment (ELCE) With Alzheimer Disease (AD) Pathology Indices.
Each partial residual plot is derived from linear regression models with 1 of the AD pathology indices as the separate outcome and ELCE as the variable, controlled for age at death, sex, and educational level. Higher levels of ELCE were associated with lower levels of AD pathology indices. Dotted line indicates the linear regression.
Next, we examined the association of ELCE with the AD pathology indices in more detail. Addition of a term for possession of an APOE ε4 allele did not affect results (eTable 4 in the Supplement), indicating that the association of ELCE with AD pathological changes was not confounded by APOE ε4 genotype. We also considered the possibility that age or sex might modify the association, and there was no evidence that age at death (eTable 5 in the Supplement) or sex (eTable 6 in the Supplement) interacted with ELCE. However, our sample size was limited in providing enough power for detection of the interaction effects. Finally, we examined the association of ELCE in the presence of terms for vascular risk factors and diseases, and participants’ self-perceived socioeconomic status and late-life cognitive activity level. The association of a higher level of ELCE with a lower level of AD pathology score persisted (estimate in the model including vascular risk factors, −0.052 [SE, 0.022; P = .02]; estimate in the model including vascular diseases, −0.052 [SE, 0.022; P = .02]; estimate in the model including socioeconomic status, −0.089 [SE, 0.035; P = .01]; estimate in the model including cognitive activity, −0.060 [SE, 0.022; P = .007]) (eTable 7 in the Supplement).
ELCE and Non-AD Pathological Changes
We next examined the association of ELCE with non-AD brain pathological changes (Table 2). Early-life cognitive enrichment was not associated with any of the 8 other pathological changes measured for this study. Power calculations showed that the minimum required sample sizes ranged from 2600 to 163 000 to detect the observed odds ratios with 80% power at α = .05.
Table 2. Association of Non-AD Dementia-Associated Brain Pathological Changes With ELCE.
| Pathological change | No. (%) of participants (n = 813)a | Association with ELCEb | |
|---|---|---|---|
| Odds ratio (95% CI) | P value | ||
| Cortical Lewy bodies | 109 (13) | 0.79 (0.57-1.09) | .17 |
| TDP-43 protein | 1.11 (0.90-1.36) | .35 | |
| Stage 0 | 359 (44) | ||
| Stage 1 | 156 (19) | ||
| Stage 2 | 145 (18) | ||
| Stage 3 | 124 (15) | ||
| Hippocampal sclerosis | 85 (10) | 1.17 (0.81-1.69) | .40 |
| Macroscopic infarcts | 255 (31) | 0.90 (0.70-1.16) | .43 |
| Cortical macroscopic infarcts | 114 (14) | 0.97 (0.70-1.33) | .83 |
| Subcortical macroscopic infarcts | 230 (28) | 0.87 (0.68-1.12) | .29 |
| Microinfarcts | 205 (25) | 0.96 (0.73-1.25) | .75 |
| Atherosclerosis | 1.08 (0.88-1.33) | .44 | |
| None | 170 (21) | ||
| Mild | 396 (49) | ||
| Moderate | 194 (24) | ||
| Severe | 52 (6) | ||
| Arteriolosclerosis | 1.12 (0.92-1.37) | .26 | |
| None | 243 (30) | ||
| Mild | 290 (36) | ||
| Moderate | 218 (27) | ||
| Severe | 60 (7) | ||
| Cerebral amyloid angiopathy | 0.84 (0.68-1.02) | .08 | |
| None | 164 (20) | ||
| Mild | 354 (44) | ||
| Moderate | 184 (23) | ||
| Severe | 107 (13) | ||
Abbreviations: AD, Alzheimer disease; ELCE, early-life cognitive enrichment.
Owing to missing data, numbers of participants do not total 813.
Derived from logistic regression models controlled for age at death, sex, and educational level. In these analyses, ELCE was not associated with any of the examined pathological changes.
ELCE, AD Pathology Score, and Cognitive Decline
Because ELCE has been associated with better cognitive functioning in old age,21 we conducted additional analyses to test whether AD pathology score contributed to the association between ELCE and cognitive decline. Before death, the participants were followed up annually for a mean (SD) of 7.0 (3.8) years. We used linear mixed-effects models to estimate the person-specific slopes of cognitive change. Participants’ cognition declined a mean (SD) of −0.13 (0.19) units per year, with a range −1.74 to 0.85. Estimated cognitive change was zero or greater in approximately 16% of participants, indicating that some participants’ cognition did not decline during the study. Next, we examined the association of ELCE and the slope of cognitive decline (model 1 in Table 3). A 1-unit increase in ELCE was associated with a 25% slower rate of cognitive decline.
Table 3. Association of ELCE and the Slope of Cognitive Declinea.
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | |
| Adjusted R2 | 0.012 | NA | 0.088 | NA | 0.087 | NA |
| Intercept | –0.141 (0.008) | <.001 | –0.033 (0.016) | .04 | –0.032 (0.016) | .049 |
| Age at death | –0.000 (0.001) | .94 | 0.001 (0.001) | .52 | 0.001 (0.001) | .52 |
| Women | –0.027 (0.015) | .07 | –0.015 (0.015) | .30 | –0.015 (0.015) | .32 |
| Educational level | –0.004 (0.003) | .13 | –0.005 (0.003) | .08 | –0.005 (0.003) | .07 |
| ELCE | 0.035 (0.011) | .001 | 0.028 (0.011) | .007 | 0.017 (0.021) | .44 |
| Global AD score | NA | NA | –0.135 (0.017) | <.001 | –0.136 (0.017) | <.001 |
| ELCE × global AD score interaction | NA | NA | NA | NA | 0.015 (0.024) | .53 |
Abbreviations: AD, Alzheimer disease; ELCE, early-life cognitive enrichment.
Derived from linear regression models having slope of cognition change as the outcome. Model 1 is the reference model adjusted only for demographics, and the result shows that ELCE explained only 1% of the variance in the cognitive decline rate. AD pathology explained an additional 8% of the variance (model 2). However, an interaction term between ELCE and the global AD pathology score did not reach statistical significance (model 3), suggesting that ELCE did not modify the association of AD pathology score with cognitive decline. In addition, comparison of regression coefficients between model 1 and model 2 shows that the association of the ELCE is attenuated after AD pathology is included in the model. This finding suggests that part of the association between ELCE and cognitive decline rate might be explained by lower AD pathology score, and the other part was independent of it.
Third, we reexamined the last model by addition of the global AD pathology score to the variables to examine any change in the association of ELCE with the cognitive decline. Results (model 2 in Table 3) showed AD pathological change to have a strong association with cognitive decline (estimate, −0.135 [SE, 0.017]; P < .001). With the global AD pathology score included in the model, the variance explained increased from 1% (model 1 in Table 3) to 9% (model 2 Table 3). The results also showed that although AD pathological change attenuated the association between ELCE with the cognitive decline, the latter association persisted. This finding suggested that part of the association between higher levels of ELCE and slower cognitive decline was independent of AD pathological change. In further analyses, we examined whether ELCE modified the association of the global AD pathology score with the rate of cognitive decline by adding an interaction term between ELCE and the AD pathology score (model 3 in Table 3). The interaction term was not significant.
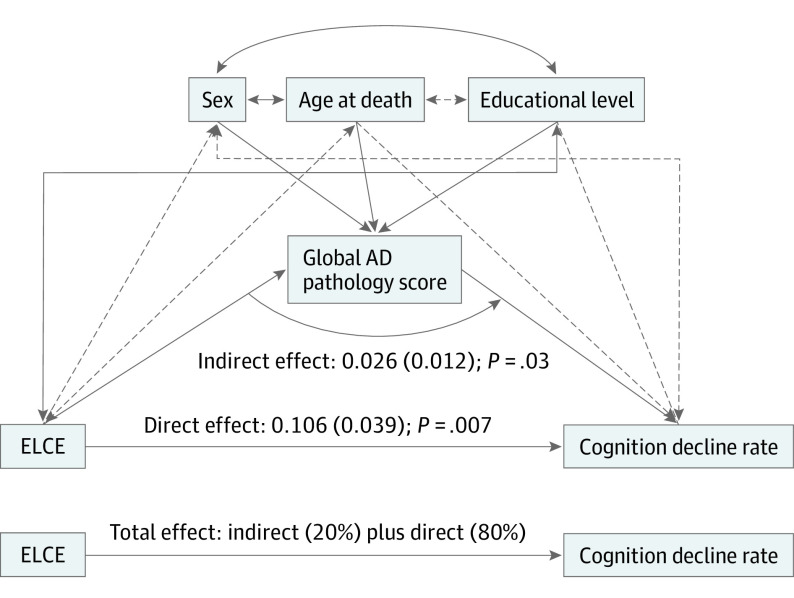
Because ELCE was associated with the rate of cognitive decline without and with the presence of the global AD pathology score in the regression model, we conducted mediation analysis to estimate direct and indirect associations of ELCE with late-life cognitive decline. We used path analysis for this purpose. Figure 2 shows the model with its standardized coefficients and their P values. Direct association of ELCE with the rate of cognition decline constituted 80%, and the indirect association through AD pathology score constituted 20%, of the total association of higher levels of ELCE with slower rate of late-life cognitive decline.
Figure 2. Mediation Analysis of the Association Among Early-Life Cognitive Enrichment (ELCE), Global Alzheimer Disease (AD) Pathology Score, and the Cognition Decline Rate .
Early-life cognitive enrichment constitutes the causal variable; global AD pathology score, the intervening variable; and rate of cognitive decline, the outcome. The estimates are given as standardized coefficients (standard error), with P values derived from a path analysis including age at death, sex, and educational level as the covariates. The line’s thickness is proportional to the relative effect sizes of the estimates, and dashed lines indicate insignificant associations. The result shows that direct relation of ELCE with the rate of cognition decline constituted 80% and the indirect relation through AD pathology score constituted 20% of the total association of higher levels of ELCE with slower rates of late-life cognitive decline.
Discussion
In a community-based longitudinal clinical-pathological study, we found that a higher level of ELCE was associated with lower levels of AD pathology indices in late life. In addition, ELCE was associated with slower late-life cognitive decline, with AD pathological change accounting for approximately 20% of the association. The results suggest that the ELCE was associated with late-life cognitive health in part through an association with less AD pathological change. Future studies are needed to uncover molecular mechanisms underlying association of ELCE with late-life cognitive decline.
Prior research has established that level of cognitive function in childhood,23 adolescence,24,25 and young adulthood26 is associated with late-life cognitive outcomes. Early-life cognitive enrichment, which is hypothesized to support early-life cognitive development,27 has been consistently associated with late-life level of cognitive function1,2,7,8,9,10,28,29; it has been associated with late-life cognitive change in some studies,9,30 consistent with the present results, but not in others.8,31 Likewise, imaging studies have been inconsistent in clarifying the association of ELCE with AD-related pathological changes, with some studies reporting decreased β-amyloid levels with higher educational levels,32 whereas other studies did not find any association.6,33 Differences in the design of the studies, in measurement tools for the assessment of ELCE and cognitive function in late life, and in timing of late-life cognitive assessment can explain the discrepancies. We are aware of 1 previous study, the Nun Study,3,4 that found an association of linguistic ability in young adulthood with postmortem evidence of AD pathological change, but this study was based on findings of 25 autopsies. The present findings build on prior work in several ways. First, we focused on ELCE, which is likely to be more modifiable than early-life cognitive function, which is 50% to 70% heritable.34 Second, we found that ELCE was associated with AD pathological change but not with other postmortem neurodegenerative or cerebrovascular markers. Third, we showed that the association of ELCE with late-life cognitive function was partly owing to its association with less AD pathological change.
Studies that evaluated the association of middle- or late-life cognitive activities with cognitive decline and AD pathology indices have reported less cognitive decline and AD dementia in participants who had higher levels of cognitive activities, but the association was unrelated to AD pathology indices.9,28 One possible hypothesis for the discrepancy between early- vs late-life cognitive enrichment and activity in their association with AD pathological change is the amount of AD pathology indices already present in the brain. We hypothesized that many individuals have high AD pathology indices in brains in late life, and lifestyle factors, such as cognitive activity, can have little if any effect on further accumulation of AD pathological change. In contrast, very little if any AD pathological change is present in brains during early life. Therefore, early life shows better than late life any possible association between lifestyle factors, such as cognitive enrichment activities, and AD pathological change. Animal models showed that environmental enrichment can downregulate glycogen synthase kinase-3-beta, which is implicated in the formation of neurofibrillary tangles, a hallmark of AD pathological change.35 This hypothesis needs to be tested in future prospective studies.
These findings suggest that cognitive health in old age depends in part on cognitive development in early life. In fact, our study extends the findings of the Nun Study to earlier periods of life. In the Nun Study, a higher level of cognitive ability at a mean age of 22 years was associated with higher levels of cognition and less AD pathological change in late life in a sample size of 25 decedents.3 Findings of our study show that the association between early-life neurodevelopment and late-life neurodegeneration starts from the first decade of life in a sample size of 813 decedents. Therefore, our findings postulate possible brain susceptibility for accumulation of AD pathological changes even during early life. Indeed, the presence of abnormally phosphorylated tau has been reported even in individuals younger than 10 years.36 It has been difficult to establish that cognitive training programs can enhance underlying cognitive abilities in old persons.37 By contrast, cognitive growth is normative in early life, and this growth is stimulated by formal education38 and cognitively demanding experience.39 The present results suggest that intervention programs, such as Head Start,40 that target socially disadvantaged youths might not only boost early-life school performance41 but also enhance late-life cognitive resilience.
Strengths and Limitations
The large sample size enhanced statistical power in this study. The long duration of follow-up made it possible to calculate a reliable rate of cognitive decline. The follow-up and autopsy rates were high, minimizing selection bias due to attrition. However, the participants selected included more women and white participants and those with high levels of education, making the results not directly generalizable to the population. Our childhood socioeconomic measure is unlikely to have fully captured the variability in early life socioeconomic circumstances. Our sample was not rich in neurodegenerative dementia–related pathological changes other than AD, and power calculation showed that we might have had inadequate power to detect possible association of ELCE with these pathological changes. In addition, we did not have autopsy variables corresponding to magnetic resonance imaging–detected vascular pathological changes, such as white matter hyperintensities. Therefore, lack of association between ELCE and pathologic changes other than AD should be taken into account with caution. Finally, we captured ELCE data in a retrospective way, bringing forward the possibility of recall bias.
Conclusions
The findings of this cohort study suggest that ELCE is associated with better late-life cognitive health. In part this association is accounted for by fewer AD pathological changes.
eFigure. Flowchart of Participants Enrolled at the Rush Memory and Aging Project (MAP) From 1997 to June 2019
eTable 1. Comparison of the Included Analytic Sample With Participants Excluded Due to Missing at Postmortem Pathology Indices
eTable 2. Spearman Correlation Coefficients of Bivariate Associations Among the 4 Indicators of Early-Life Cognitive Enrichment
eTable 3. Factor Loadings of the 4 Indicators of Early-Life Cognitive Enrichment
eTable 4. The Association Between Early-Life Cognitive Enrichment and AD Pathology Indices Adjusted by Demographics and APOE ε4 Genotype
eTable 5. Modification of the Association Between Early-Life Cognitive Enrichment and AD Pathological Indices by Age at Death
eTable 6. Modification of the Association Between Early-Life Cognitive Enrichment and AD Pathological Indices by Sex
eTable 7. Association of Early-Life Cognitive Enrichment With AD Pathological Indices Controlled for Vascular Risk Factors and Diseases, Participants’ Self-Perceived Socioeconomic Status (SES), or Late-Life Cognitive Activity Level
eMethods. Assessment of Dementia-Related Pathologies, Socioeconomic Status, and Late-Life Cognitive Activity Level
eReferences.
References
- 1.Bezerra AB, Coutinho ES, Barca ML, Engedal K, Engelhardt E, Laks J. School attainment in childhood is an independent risk factor of dementia in late life: results from a Brazilian sample. Int Psychogeriatr. 2012;24(1):55-61. doi: 10.1017/S1041610211001554 [DOI] [PubMed] [Google Scholar]
- 2.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30(2):256-263. doi: 10.1093/ije/30.2.256 [DOI] [PubMed] [Google Scholar]
- 3.Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life: findings from the Nun Study. JAMA. 1996;275(7):528-532. doi: 10.1001/jama.1996.03530310034029 [DOI] [PubMed] [Google Scholar]
- 4.Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR. Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiol Aging. 2005;26(3):341-347. doi: 10.1016/j.neurobiolaging.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 5.Field TS, Doubal FN, Johnson W, et al. . Early life characteristics and late life burden of cerebral small vessel disease in the Lothian Birth Cohort 1936. Aging (Albany NY). 2016;8(9):2039-2061. doi: 10.18632/aging.101043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ko K, Byun MS, Yi D, Lee JH, Kim CH, Lee DY. Early-life cognitive activity is related to reduced neurodegeneration in Alzheimer signature regions in late life. Front Aging Neurosci. 2018;10:70. doi: 10.3389/fnagi.2018.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes LL, Wilson RS, de Leon CF, Bennett DA. The relation of lifetime cognitive activity and lifetime access to resources to late-life cognitive function in older African Americans. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13(3-4):516-528. doi: 10.1080/138255890969519 [DOI] [PubMed] [Google Scholar]
- 8.Wilson RS, Boyle PA, Yang J, James BD, Bennett DA. Early life instruction in foreign language and music and incidence of mild cognitive impairment. Neuropsychology. 2015;29(2):292-302. doi: 10.1037/neu0000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81(4):314-321. doi: 10.1212/WNL.0b013e31829c5e8a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25(1):8-14. doi: 10.1159/000085307 [DOI] [PubMed] [Google Scholar]
- 11.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018;64(s1):S161-S189. doi: 10.3233/JAD-179939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uniform Law Commission National Conference of Commissioners on Uniform State Laws. Uniform Anatomical Gift Act (2006). Published 2017. Accessed May 21, 2020. https://www.uniformlaws.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=0e0c3a55-52cf-2082-704f-6185187a21fc&forceDialog=0
- 13.Coste J, Bouée S, Ecosse E, Leplège A, Pouchot J. Methodological issues in determining the dimensionality of composite health measures using principal component analysis: case illustration and suggestions for practice. Qual Life Res. 2005;14(3):641-654. doi: 10.1007/s11136-004-1260-6 [DOI] [PubMed] [Google Scholar]
- 14.De Jager PL, Ma Y, McCabe C, et al. . A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research. Sci Data. 2018;5(1):180142. doi: 10.1038/sdata.2018.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DA, Wilson RS, Schneider JA, et al. . Apolipoprotein E ε4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003;60(2):246-252. doi: 10.1212/01.WNL.0000042478.08543.F7 [DOI] [PubMed] [Google Scholar]
- 16.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61(3):378-384. doi: 10.1001/archneur.61.3.378 [DOI] [PubMed] [Google Scholar]
- 17.Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 2018;136(6):887-900. doi: 10.1007/s00401-018-1920-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson RS, Beckett LA, Barnes LL, et al. . Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179-193. doi: 10.1037/0882-7974.17.2.179 [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Bienias JL, Berry-Kravis E, Evans DA, Bennett DA. The apolipoprotein E ε 2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry. 2002;73(6):672-677. doi: 10.1136/jnnp.73.6.672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes LL, Leurgans S, Aggarwal NT, et al. . Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology. 2015;85(6):528-534. doi: 10.1212/WNL.0000000000001834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69(20):1911-1920. doi: 10.1212/01.wnl.0000271087.67782.cb [DOI] [PubMed] [Google Scholar]
- 22.Oveisgharan S, Buchman AS, Yu L, et al. . APOE ε2ε4 genotype, incident AD and MCI, cognitive decline, and AD pathology in older adults. Neurology. 2018;90(24):e2127-e2134. doi: 10.1212/WNL.0000000000005677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards M, James S-N, Sizer A, et al. . Identifying the lifetime cognitive and socioeconomic antecedents of cognitive state: seven decades of follow-up in a British birth cohort study. BMJ Open. 2019;9(4):e024404. doi: 10.1136/bmjopen-2018-024404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dekhtyar S, Wang HX, Fratiglioni L, Herlitz A. Childhood school performance, education and occupational complexity: a life-course study of dementia in the Kungsholmen Project. Int J Epidemiol. 2016;45(4):1207-1215. doi: 10.1093/ije/dyw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russ TC, Hannah J, Batty GD, Booth CC, Deary IJ, Starr JM Childhood cognitive ability and incident dementia: the 1932 Scottish Mental Survey cohort into their 10th decade. Epidemiology. 2017;28(3):361-364. doi: 10.1097/EDE.0000000000000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osler M, Christensen GT, Garde E, Mortensen EL, Christensen K. Cognitive ability in young adulthood and risk of dementia in a cohort of Danish men, brothers, and twins. Alzheimers Dement. 2017;13(12):1355-1363. doi: 10.1016/j.jalz.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 27.Morrissey TW, Hutchison L, Winsler A. Family income, school attendance, and academic achievement in elementary school. Dev Psychol. 2014;50(3):741-753. doi: 10.1037/a0033848 [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Yang R, Qi X, et al. . Association of lifespan cognitive reserve indicator with dementia risk in the presence of brain pathologies. JAMA Neurol. 2019;(July). doi: 10.1001/jamaneurol.2019.2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158(11):1083-1089. doi: 10.1093/aje/kwg263 [DOI] [PubMed] [Google Scholar]
- 30.Staff RT, Hogan MJ, Whalley LJ. The influence of childhood intelligence, social class, education and social mobility on memory and memory decline in late life. Age Ageing. 2018;47(6):847-852. doi: 10.1093/ageing/afy111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gow AJ, Johnson W, Mishra G, Richards M, Kuh D, Deary IJ; HALCyon Study Team . Is age kinder to the initially more able? yes, and no. Intelligence. 2012;40(1):49-59. doi: 10.1016/j.intell.2011.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arenaza-Urquijo EM, Bejanin A, Gonneaud J, et al. . Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging. 2017;59:72-79. doi: 10.1016/j.neurobiolaging.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 33.Lu K, Nicholas JM, Collins JD, et al. . Cognition at age 70: life course predictors and associations with brain pathologies. Neurology. 2019;93(23):e2144-e2156. doi: 10.1212/WNL.0000000000008534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchard TJ Jr, McGue M. Familial studies of intelligence: a review. Science. 1981;212(4498):1055-1059. doi: 10.1126/science.7195071 [DOI] [PubMed] [Google Scholar]
- 35.Hu Y-S, Long N, Pigino G, Brady ST, Lazarov O. Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3β, neurotrophin-3 and CREB signaling. PLoS One. 2013;8(5):e64460. doi: 10.1371/journal.pone.0064460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138(pt 10):2814-2833. doi: 10.1093/brain/awv236 [DOI] [PubMed] [Google Scholar]
- 37.Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11(11):e1001756. doi: 10.1371/journal.pmed.1001756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie SJ, Tucker-Drob EM. How much does education improve intelligence? a meta-analysis. Psychol Sci. 2018;29(8):1358-1369. doi: 10.1177/0956797618774253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaschke AC, Honing H, Scherder EJA. Longitudinal analysis of music education on executive functions in primary school children. Front Neurosci. 2018;12:103. doi: 10.3389/fnins.2018.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daley M. Head start: program and legislative update. J Pediatr Nurs. 1999;14(3):186-188. doi: 10.1016/S0882-5963(99)80007-4 [DOI] [PubMed] [Google Scholar]
- 41.Phillips D, Gormley W, Anderson S. The effects of Tulsa’s CAP Head Start program on middle-school academic outcomes and progress. Dev Psychol. 2016;52(8):1247-1261. doi: 10.1037/dev0000151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of Participants Enrolled at the Rush Memory and Aging Project (MAP) From 1997 to June 2019
eTable 1. Comparison of the Included Analytic Sample With Participants Excluded Due to Missing at Postmortem Pathology Indices
eTable 2. Spearman Correlation Coefficients of Bivariate Associations Among the 4 Indicators of Early-Life Cognitive Enrichment
eTable 3. Factor Loadings of the 4 Indicators of Early-Life Cognitive Enrichment
eTable 4. The Association Between Early-Life Cognitive Enrichment and AD Pathology Indices Adjusted by Demographics and APOE ε4 Genotype
eTable 5. Modification of the Association Between Early-Life Cognitive Enrichment and AD Pathological Indices by Age at Death
eTable 6. Modification of the Association Between Early-Life Cognitive Enrichment and AD Pathological Indices by Sex
eTable 7. Association of Early-Life Cognitive Enrichment With AD Pathological Indices Controlled for Vascular Risk Factors and Diseases, Participants’ Self-Perceived Socioeconomic Status (SES), or Late-Life Cognitive Activity Level
eMethods. Assessment of Dementia-Related Pathologies, Socioeconomic Status, and Late-Life Cognitive Activity Level
eReferences.