Dear Editor,
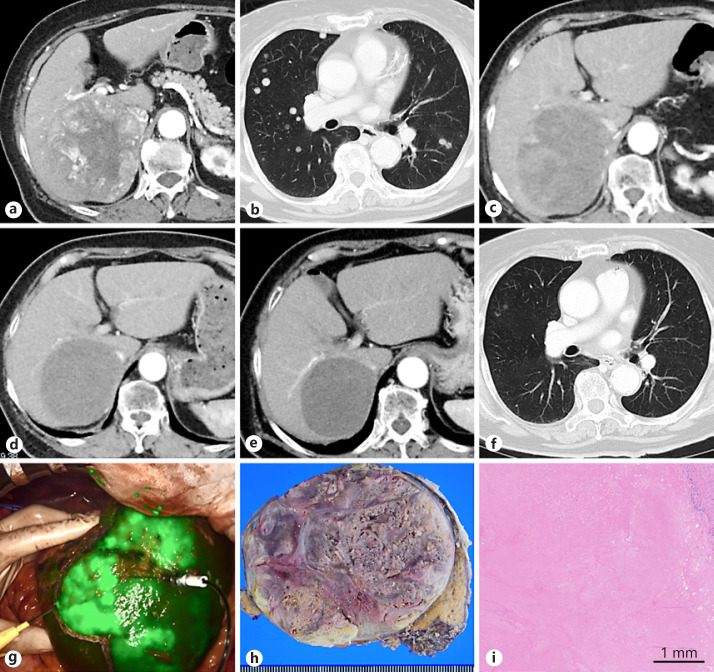
The patient was an 82-year-old woman without viral hepatitis. Dynamic computed tomography (CT) showed a hepatic mass in segment 7 of the liver, 10 cm in diameter, with strong enhancement in the arterial phase (Fig. 1a). There was no intrahepatic metastasis or vascular invasion, but multiple lung nodules were found (Fig. 1b). Tumor markers such as α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) were significantly increased to 220 (normal range: 0–20) ng/mL and 44,550 (normal range: 0–40) mAU/mL, respectively. On the diagnosis of unresectable HCC, lenvatinib was administered at an initial dose of 12 mg/day. After 1 week of lenvatinib, her general condition deteriorated with adverse events of continuous low-grade fever and appetite loss, and lenvatinib was interrupted. On CT, 3 months after the initial treatment, the intratumor vascularity of the liver lesion decreased (Fig. 1c); thus, the treatment was re-started with a reduced dose of 4 mg/day. Five months after initial administration, the intratumor vascularity of the liver lesion disappeared, and the mean CT value of the tumor in the arterial ph ase was decreased by 49% of the initial CT value (decreased from 75.4 to 37.3 HU; maximum CT value decreased from 288 to 70 HU) suggesting objective response of the tumor, with the tumor size decrease of 13% suggesting stable disease (SD) on the RECIST 1.1 (Fig. 1d) and partial response on the modified RECIST (mRECIST) [1]. Since bipedal edema remained, the lenvatinib dose was increased to 6 mg/day. Though her bipedal edema remained, the tumor shrank by 24% of the initial size (Fig. 1e), and the mean CT value decreased to 33.4 HU (Fig. 1f). Her serum AFP and DCP levels decreased to 1.9 ng/mL and 26 mAU/mL, respectively, both within normal ranges, and most bilateral lung nodules disappeared (Fig. 1f). Adjuvant hepatectomy to control the bipedal edema caused by IVC compression was conducted 13 months after administration of lenvatinib. Her liver function was Child-Pugh A (5 points), and the ICG-R15 value was 11.8%, suggesting minimal liver damage. An extended right posterior sectionectomy with right hepatic vein resection was performed using the counter-staining technique with indocyanine green fluorescence imaging (Fig. 1g). The operative time was 7 h 40 min, and the estimated blood loss was 990 mL. A brownish necrotic tumor, 8.5 cm in diameter, was seen macroscopically in a surgical specimen (Fig. 1h). Microscopically, the tumor was completely necrotic, with no viable tumor cells (Fig. 1i). Thus, the pathological diagnosis was total necrosis of the HCC. The patient's postoperative course was uneventful. She was discharged on postoperative day 14, and her bipedal edema gradually improved. There were no lung metastases on chest CT 5 months after liver resection with no further adjuvant lenvatinib.
Fig. 1.
a Dynamic CT at the time of diagnosis of hepatocellular carcinoma. A tumor is located at the posterior section, which shows strong enhancement in the arterial phase. b The radiographic findings of lung lesions at the time of diagnosis of hepatocellular carcinoma. Multiple lung metastases are found in bilateral lung fields. c The radiographic findings of the liver lesion 3 months after lenvatinib administration. d The radiographic findings of the liver lesion 5 months after lenvatinib administration. e The radiographic findings of the liver lesion 12 months after lenvatinib administration (before adjuvant surgery). The tumor shows no vascularity in the arterial phase on dynamic CT. f The radiographic findings of lung lesions 12 months after lenvatinib administration. Most bilateral lung nodules have disappeared. g Counter-staining technique using indocyanine green fluorescence imaging. h Macroscopic findings of a surgical specimen. i Microscopic findings of a surgical specimen. The tumor is completely necrotic with hemorrhage. Foamy histiocytic infiltrates are found on the periphery of the tumor. No cancer cells remain alive in the tumor.
The REFLECT trial reported that the complete response (CR) rate of lenvatinib therapy was 2%, but no description of conversion surgery has been found, and pathological CR was not proven [2]. On the other hand, sorafenib therapy was reported to have achieved a CR of 1%, and pathological CR has been reported in a few cases [3]. To the best of our knowledge, this is the first case of pathological CR in conversion hepatectomy induced by lenvatinib for advanced HCC.
In the present case, the size of the hepatic tumor decreased by 24% after a 12-month treatment with lenvatinib, and this could be classified as SD according to RECIST1.1. However, the tumor showed objective change without any vascularity in the arterial phase on enhanced CT, and it showed pathological CR after hepatectomy. The radiographically objective changes were considered to correlate with the anti-angiogenic activity of lenvatinib. Shindoh et al. [4]reported that morphologic response is strongly correlated with overall survival of surgical patients with colorectal liver metastases, surrogating the therapeutic effects after chemotherapy using bevacizumab. Lenvatinib inhibits VEGF receptors 1–3, FGF receptors 1–4, PDGF receptor α, RET, and KIT, and it might show similar objective changes on CT as bevacizumab. Previously, objective response by mRECIST in advanced HCC was reported to predict OS [5], and it might be considered a candidate surrogate end-point after lenvatinib treatment. Thus, based on the objective response on mRECIST, one might predict the response of HCC after lenvatinib treatment. In ESMO-Asia 2019 Congress, a combination therapy of atezolizumab and bevacizumab was reported to show significantly better overall survival and progression-free survival than sorafenib for unresectable HCC. A clinical trial is currently underway in Japan regarding the possibility of conversion surgery during lenvatinib administration for unresectable HCC. Systemic therapy for HCC is progressing, and cases of conversion surgery and pathological CR will likely increase in the future.
Disclosure Statement
The authors declare that they have no conflicts of interest or sources of funding for this work.
Author Contributions
Pretreatment diagnosis: N.K., S.S. and T.H. Systemic lenvatinib therapy: K.K, N.O., D.N. and J.F. Operation and perioperative management: R.M., Y.S., M.K., T.N., T.M., and Y. Sakamoto. Pathological diagnosis: Y.O., H.K., and J.S. Manuscript writing: R.M. All authors have seen and approved the final version of the manuscript being submitted, and all authors fulfill the COPE (Committee on Publication Ethics) requirements for authorship.
References
- 1.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010 Feb;30((1)):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 3.Irtan S, Chopin-Laly X, Ronot M, Faivre S, Paradis V, Belghiti J. Complete regression of locally advanced hepatocellular carcinoma induced by sorafenib allowing curative resection. Liver Int. 2011 May;31((5)):740–3. doi: 10.1111/j.1478-3231.2010.02441.x. [DOI] [PubMed] [Google Scholar]
- 4.Shindoh J, Loyer EM, Kopetz S, Boonsirikamchai P, Maru DM, Chun YS, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012 Dec;30((36)):4566–72. doi: 10.1200/JCO.2012.45.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lencioni R, Montal R, Torres F, Park JW, Decaens T, Raoul JL, et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J Hepatol. 2017 Jun;66((6)):1166–72. doi: 10.1016/j.jhep.2017.01.012. [DOI] [PubMed] [Google Scholar]