Abstract
Purpose of review:
Ionizing radiation is a highly effective treatment for a wide range of malignancies, yet the cardiovascular (CV) toxicity that can result from chest radiotherapy impairs the long-term health of cancer survivors and can be a limiting factor for its use. Despite over 100 years of successful clinical use, the mechanisms by which high-energy photons damage critical components within cells of the heart’s myocardium, pericardium, vasculature and valves remain unclear.
Recent findings:
Recent studies exploring the acute and chronic effects of radiation therapy on cardiac and vascular tissue have provided new insights into the development and progression of heart disease, including the identification and understanding of age- and complication-associated risk factors. However, key questions relating to the connection from upstream signaling to fibrotic changes remain. In addition, advances in the delivery of chest radiotherapy have helped to limit heart exposure and damage, but additional refinements to delivery techniques and cardioprotective therapeutics are absolutely necessary to reduce patient mortality and morbidity.
Summary:
Radiation therapy (RT)-driven CV toxicity remains a major issue for cancer survivors and more research is needed to define the precise mechanisms of toxicity. However, recent findings provide meaningful insights that may help improve patient outcomes.
Keywords: Cardiotoxicity, cardiovascular toxicity, heart disease, heart failure, radiation therapy, apoptosis
Introduction
The advancement of chest radiotherapy (RT) as an efficient treatment option for a wide range of cancers has generated an ever-growing cohort of cancer survivors who are at increased risk of developing cardiovascular disease [1–3]. Malignancies including Hodgkin’s and non-Hodgkin’s lymphomas, Wilms tumors, lung adenocarcinomas, breast cancers and mediastinal testicular cancers can all be treated with radiation therapy that results in the delivery of non-negligible doses of ionizing radiation to the susceptible cells and structures within the heart. The resulting cardiac disease, which varies in presentation and severity, may offset some of the clear benefits of RT to tumor control and reduce quality of life. In this review, we will introduce the mechanisms by which different radiation-induced heart diseases develop, and discuss recent developments in preventative and therapeutic measures to counter this toxicity.
Prevalence and Risk Factors
It has been clearly established that treatment-induced CV dysfunction is a major potential complication of chest RT. For instance, when comparing the incidences of fatal cardiac events in cancer survivors, Gernaat, et al. recently reported that 1.6–10% among over 1.2 million women with breast cancer subsequently died of cardiovascular diseases [4], and the risk is higher for women with left-sided breast cancer, as opposed to right-sided [5]. Furthermore, the risk of a fatal cardiac event in patients with any cancer form is 1.5 to 3 times higher in those who have been treated with thoracic RT than in those who were not treated with radiotherapy [6], and the risk is highest in young patients, with an emphasis on those treated for Hodgkin’s lymphoma and Wilms tumor [7–9].
The higher degree of cardiotoxicity in young individuals treated with RT [7,8] correlates with data from other cancer treatments, especially those that also induce genotoxic damage [10,11]. For example, patients treated with anthracyclines (e.g. doxorubicin, daunorubicin or epirubicin) are at risk of developing symptoms such as thinning of the cardiac ventricular walls and reduction of ventricular mass, eventually leading to heart failure, and the risk is higher the younger the patient is at the time of treatment [12]. Given the young age of these cancer patients, the survival cohort is especially vulnerable to disorders which have a long latency period before clinically observable symptoms manifest. Additionally, there may be other factors to consider when examining mechanisms of radiation-induced cardiotoxicity that are developmentally-related and will be discussed in more detail below.
Aside from clinical RT, humans are also exposed to ionizing radiation from a multitude of natural (e.g. cosmic irradiation, terrestrial radiation) and artificial sources (e.g. medical imaging, nuclear fallout) [13]. Although the discussion of these non-treatment-related sources is outside the scope of this review, it is pertinent to note that the risk of developing radiation-induced cardiovascular disease is significantly increased at absorbed doses as low as 0.5 Gy, and may exist at even lower doses [13–15].
Clinical Presentation
Radiation-induced heart diseases comprise a spectrum of disorders, reflecting the differential radiation sensitivity of the various cell types and structures within the heart (Figure 1). Among the most prominent of these is coronary artery atherosclerosis, in which foam cells (lipid-laden macrophages), fibroblasts, and collagen accumulate in the tunica intima, forming plaques. This is followed by fibrotic build-up in the tunica media, and eventual plaque rupture which may lead to myocardial infarction [16]. Radiation associated valvular disease has been reported to occur in 81% of patients receiving more than 35 Gy to the heart [17]. These patients often develop focal leaflet fibrosis, valve calcification and stenosis, which may lead to reduced ejection fraction and valve regurgitation. Pericardial diseases have also been reported following RT, and may be existent in up to 90% of patients which received significant mediastinal RT [17]. This manifests clinically as pericarditis which causes reduced drainage of extracellular fluids and pericardial effusions, and the subsequent formation of fibrosis leads to a thickening of the pericardium which may cause cardiac tamponade (compression of the heart) [16]. Another pathology which may account for the higher mortality seen in RT patients is radiation-induced cardiomyopathy. This is caused by microvascular damage and lack of proper blood delivery to cardiomyocytes and is accompanied by myocardial fibrosis and leads to decreased elasticity and distensibility of the myocardium, resulting in reduced ejection fraction and perfusion defects, and may also give rise to arrhythmia [16,18]. Furthermore, cardiac symptoms of autonomic dysfunction, such as elevated resting heart rate and abnormal heart rate recovery, are prevalent in patients treated with RT, resulting in impaired exercise tolerance and increased overall mortality [19]. Although there are known acute effects of radiation that have been described and should be monitored, clinical symptoms of these disorders typically occur long after radiation exposure [1,2,9,20].
Figure 1:
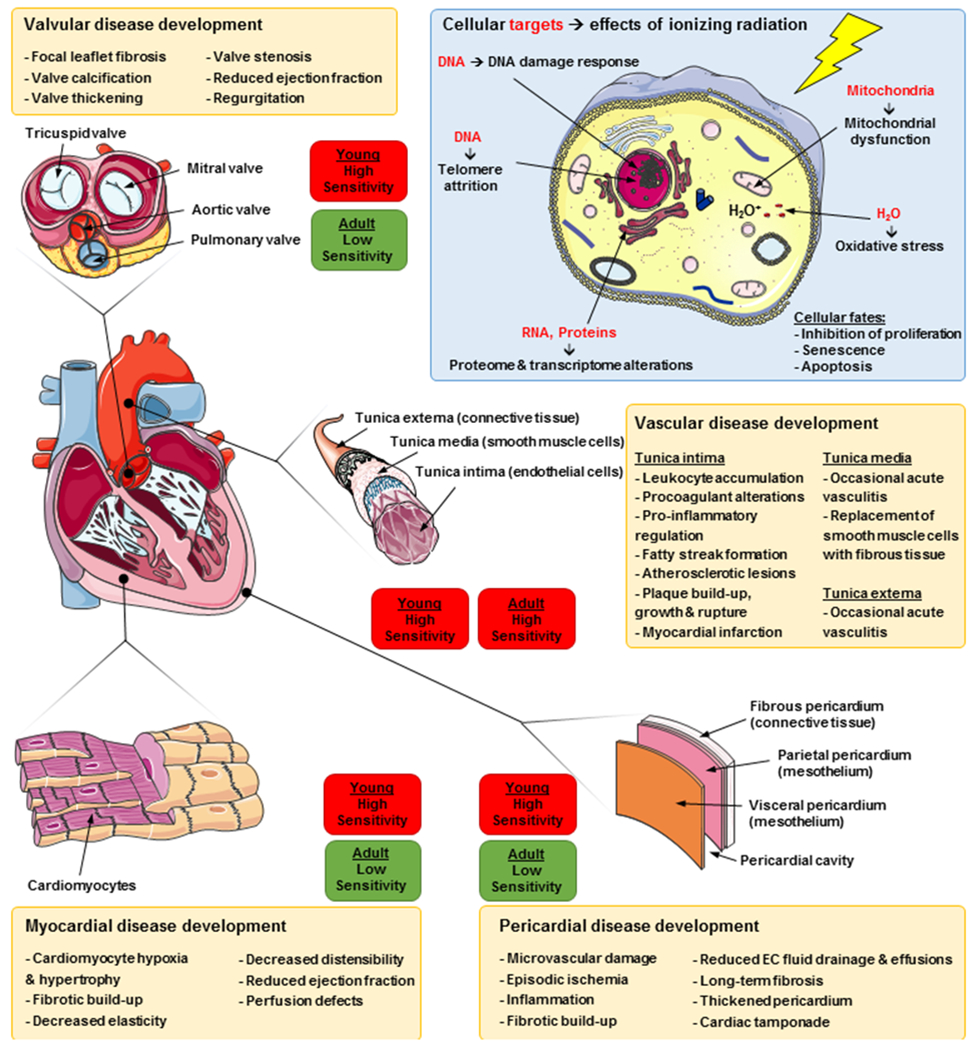
Mechanisms driving radiation-induced cardiotoxicity.
Mechanisms of disease formation and progression
Research into the mechanisms of RT efficacy for cancer control may also be informative for the mechanisms responsible for CV toxicity [21]. Ionizing radiation is known to induce single-strand and double-strand breaks in cellular DNA, either directly via charged particles or indirectly via the production of free radicals (Figure 1) [22]. This DNA damage usually results in activation of the p53 signaling pathway in cells that contain the wild-type gene which, depending on the extent of DNA damage, then promotes cell survival (by cell cycle arrest and DNA damage repair), or activates cell death mechanisms such as apoptosis [21,23,24]. Beyond direct DNA damage, RT can also modulate intra- and intercellular signaling pathways by damaging RNA and proteins within the cell, further driving pro-death responses. The cellular mechanisms involved in radiation responses vary between different tissues, and depend on absorbed dose, dose rate, and type of radiation [15,25]. Classical radiobiology states that non-proliferating, highly differentiated tissues are resistant to ionizing radiation [26]. The adult heart was long considered to be unable to regenerate once fully developed, and as such has been viewed as a radioresistant organ. Despite this, in many cases the cardiovascular system is considered one of the most dose-limiting organs [27]. Recent studies have shown that adult cardiomyocytes do have a limited regenerative capacity (about 0.5% yearly turnover after 40 years of age), but the majority of lost or damaged cardiomyocytes will not be replenished after injury [28,29]. This suggests that not only cell death but also cell cycle arrest and senescence may contribute to the development of heart disease.
The major common denominator of radiation-induced heart diseases is the formation of fibrosis, in both acute and chronic settings [2,6,16]. This can affect all structures of the heart, and although clinical outcomes are well documented, the underlying biological mechanisms are not fully understood. The acute phase of the radiation response consists of vasodilation and vascular permeability, stemming from an acute inflammatory response which releases pro-fibrotic cytokines (e.g. PDGF, TGF-β, bFGF, IGF and CTGF) [30]. This is followed by initiation of the coagulation cascade and degradation of the endothelial basement membrane, allowing clearance of injured tissue and initiation of healing [16]. This acute phase spans from minutes to several days after the irradiation. Apart from these short-term responses, the radiation-induced up-regulation of c-Myc, c-Jun, TGF- β, and several interleukins may play a role in inducing later development of fibrosis [31]. In addition, radiation can induce premature differentiation of fibroblasts, resulting in post-mitotic fibroblasts within 3-4 cell cycles (as compared to the 25-35 cell cycles usually required). These fibrocytes (inactive counterparts to fibroblasts) have significantly shorter life-spans than unirradiated fibrocytes, and produce higher levels of collagens. This results in a chronic deposition of collagen leading to fibrotic scar tissue within the heart, eventually reducing its elasticity and, consequently, function [16]. This indicates that the development of radiation-induced heart disease is a slow but constantly progressing process, and the long latency period between irradiation and diagnosis of heart disease may be due to a lack of sensitivity in tools used for clinical screening and diagnosis.
Apart from the inflammatory pathway, DNA damage response, chronic oxidative stress, chronic hypoxia, epigenetic regulation and telomere extension have also been implicated in the formation of radiation-induced fibrosis and subsequent heart diseases [13,16,32]. The DNA-damaging capacity of ionizing radiation is the most important effect when it comes to radiation-induced cell stress. Radiation-induced DNA lesions can lead to cytotoxic, mutagenic and carcinogenic effects if they are not repaired. It has been reported that the repair of DNA double strand breaks in the heart is not efficient compared with other healthy organs [33], and irradiation can induce up-regulation of BAX and down-regulation of BCL2 in cardiomyocytes, leading to apoptosis and subsequent development of fibrosis [34,35]. Consequently, the status of the p53/p21 pathway in cardiomyocytes plays an important role in the protection against radiation-induced damage and development of disease [23,36].
As previously mentioned, young heart tissue is more sensitive to genotoxic damage and a patient’s young age at diagnosis is a known risk factor for long-term cardiac dysfunction post therapy [8]. Although there are clear differences in rates of proliferation, growth and metabolism in the hearts of young vs adult humans, there are also differences in the regulation of cell death that may contribute to the heightened sensitivity. Specifically, it was recently reported that cells within the young heart are hypersensitive to apoptosis-inducing signals, and that this sensitivity is reduced as the mammalian heart ages and matures [37]. This was found to be a major factor in determining whether cardiomyocytes undergo apoptotic cell death in response to genotoxic damage induced by ionizing radiation or doxorubicin. The dynamic regulation of apoptosis sensitivity in the heart during postnatal development may be a key determinant of radiation- and chemotherapy-induced cardiotoxicity.
Taken together, these mechanisms are involved in developing the phenotypic pathology of radiation-induced heart disease. However, the mechanisms which drive the pathogenesis, and lay the foundation for later development of fibrosis and heart diseases, remain largely unknown. More research is needed to determine the role of factors that can affect the fate of cells after irradiation including cardiomyocyte proliferation and baseline sensitivity to cytotoxic stress.
Preventative measures
Dosimetric considerations in RT:
The risk of developing radiation-induced heart disease after RT has been shown to increase linearly with the mean absorbed dose to the heart by about 7% per Gy, without an apparent threshold at the low-dose end [38]. Consequently, no clear dose-volume constraint can be defined, and current RT planning recommendations advise that the dose to the heart should be kept as low as possible [39,40]. The advancement of target-specific dose-delivery in clinical RT has progressed during the last century, from 2D beam filters and compensators to computer-controlled intensity-modulated beams to achieve 3D dose conformity. This, along with the development of 3D treatment planning software using dose calculation algorithms has led to a decrease in absorbed dose to healthy tissues, including the cardiovascular system, and has lowered but not eliminated the risks for radiation-induced heart disease [8]. For conventional RT, various heart-sparing techniques have been developed to reduce unwanted absorbed dose to the heart, including prone position RT, deep inspiration breath hold, and dynamic breathing guidance [41,42]. These techniques, however, require extra resources and workload, and methods to evaluate their benefit on an individual patient basis as well as simplify the treatment planning are being developed [43,44]. Efforts are also being made to further improve the accuracy in dosimetry and treatment planning [44,45].
The increased use of hypofractionated RT, such as stereotactic body RT (SBRT) to deliver high doses to small target volumes, suggests that heart exposure and subsequent development of dysfunctions need to be closely monitored when irradiating target lesions in close proximity to the heart [1]. Recently, owing to encouraging results in local control and a favorable toxicity profile, the use of ablative SBRT has been spreading. Despite the favorable spatial dose-distribution achieved with this technique, the high dose-per-fraction to small tissue volumes means that dose-constraints from conventional RT settings must be re-evaluated [1]. Proton and charged particle therapy has also gained momentum recently, and offers attractive spatial dose-distributions using the dose-painting intensity-modulated approach. This technique has been shown to effectively reduce the dose to the heart and other cardiovascular structures, compared with conventional photon RT, but also demands re-evaluated dose-constraints, for the same reasons as SBRT [46–49].
Cardioprotective measures:
There has been an ongoing interest in biomedical substances that may counteract the effects of ionizing radiation. Unfortunately, at present, established radioprotectors such as amifostine (an organic thiophosphate that acts, at least in part, by free radical scavenging) have severe side effects (e.g. vomiting, diarrhea), and are seldom used clinically [9,50]. Various other anti-oxidants (e.g. black grape juice, water saturated with molecular hydrogen, L-carnitine, sodium tanshinone IIA sulfonate, tetrahydrobiopterin, melatonin, and β-blockers) have shown reduced adverse cardiac effects when administered prior to irradiation in preclinical settings [18,51–57]. Further research is needed to determine the feasibility and efficacy of these substances in patients. Due to the multi-faceted developmental path to radiation-induced heart disease, several therapeutic targets are currently being explored, including anti-inflammatory, anti-fibrotic, and anti-apoptotic mediators [58–61].
Screening echocardiograms:
According to guidelines published by the Children’s Oncology Group, screening echocardiograms for childhood cancer survivors exposed to anthracyclines and/or cardiotoxic radiation are recommended every year, every two years, or every five years, depending on age at treatment and cumulative treatment dose to the heart [62]. However, these guidelines especially pertain to patients who received concomitant anthracyclines. In addition, recent studies report poor reproducibility in echocardiogram detection of heart disease development, suggesting the guidelines should be re-evaluated, especially in lower risk patients [63]. In addition, while echocardiograms may detect myocardial toxicity from radiation therapy, they fail to provide proper screening for vascular toxicity. In contrast, recent years have seen several new developments in echocardiography, including stress and contrast echocardiography, three-dimensional echocardiography, diastolic dysfunction, tissue Doppler imaging, and strain parameters, which may enhance the prognostic value of the screenings [64,65]. Regarding coronary artery disease, current guidelines recommend stress testing for patients which received 40 Gy or more to the mediastinum and are asymptomatic 10 years after treatment. However, false positives as well as false negatives from stress echocardiograms are frequent in this population, warranting a need for caution in the interpretation of results [62,66].
Treatment
In patients who develop cardiac disease following exposure to ionizing radiation, several clinical steps are necessary to define the specific toxicity and as to whether this is due to vascular toxicity (for example, arteriosclerosis), or a myocardial one (for example, valvular or pericardial). Standard heart failure regimens may have some efficacy [18,67], but surgery is often the most effective treatment [2]. However, survival rates post-surgery for patients who had previously undergone RT are worse than in patients who did not undergo radiation [68], suggesting that other sub-clinical damage may exist within irradiated heart tissue. Updated guidelines for the management of patients with valvular heart disease have recently been released by the American Heart Association and the American College of Cardiology [69]. Patients with radiation-induced constrictive pericarditis can benefit short-term from decongestion with diuretics, but pericardiectomy is usually the only definitive treatment [70–72].
Conclusions
Radiation-induced heart disease constitutes a growing clinical issue, mainly in cancer survivors treated with chest RT and/or genotoxic chemotherapies. The diseases comprise a wide spectrum of disorders, often with long latency periods between exposure and clinical presentation, requiring consistent follow up and screening [7]. However, multifactorial disease initiation and progression as well as largely unknown driving mechanisms currently limit the utility of early detection and treatment strategies. Consequently, there is a need for a continued development of prognostic screening techniques and new treatment options.
Footnotes
Disclosure: The authors declare that they have no conflict of interest
Human and Animal Rights: All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References and Recommended Reading
• Of importance
•• Of major importance
- 1.De Rose F, Franceschini D, Reggiori G, Stravato A, Navarria P, Ascolese AM, et al. Organs at risk in lung SBRT. Phys. Medica 2017; [DOI] [PubMed] [Google Scholar]
- 2.Gujral DM, Lloyd G, Bhattacharyya S. Radiation-induced valvular heart disease. Heart. 2016;102:269–76. [DOI] [PubMed] [Google Scholar]
- 3.Zheng HC, Onderko L, Francis SA. Cardiovascular Risk in Survivors of Cancer. Curr. Cardiol. Rep 2017. [DOI] [PubMed] [Google Scholar]
- 4.Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res. Treat 2017;164:537–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGale P, Darby SC, Hall P, Adolfsson J, Bengtsson NO, Bennet AM, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother. Oncol 2011;100:167–75. [DOI] [PubMed] [Google Scholar]
- 6.Raghunathan D, Khilji MI, Hassan SA, Yusuf SW. Radiation-Induced Cardiovascular Disease. Curr. Atheroscler. Rep. Current Atherosclerosis Reports; 2017;19. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA. 2013;309:2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidler MM, Reulen RC, Henson K, Kelly J, Cutter D, Levitt GA, et al. Population-Based Long-Term Cardiac-Specific Mortality Among 34,489 Five-Year Survivors of Childhood Cancer in Great Britain. Circulation. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Highlights the importance of monitoring cardiac diseases in survivors of childhood cancer, and suggests that recent initiatives to reduce cardiotoxicity may have a measurable impact.
- 9.Boerma M, Sridharan V, Mao X-W, Nelson GA, Cheema AK, Koturbash I, et al. Effects of ionizing radiation on the heart. Mutat. Res. Mutat. Res 2016;770:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes acute and late radiation-induced cardiovascular toxicities, as well as epidemiology for clinical and non-clinical radiation exposure situations.
- 10.Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. Cardiovascular Disease in Adult Survivors of Childhood Cancer. Annu. Rev. Med 2015;66:161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat. Rev. Clin. Oncol. Nature Publishing Group; 2013;10:697–710. [DOI] [PubMed] [Google Scholar]
- 12.Hutchins KK, Siddeek H, Franco VI, Lipshultz SE. Prevention of cardiotoxicity among survivors of childhood cancer. Br. J. Clin. Pharmacol 2017. p. 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Asaithamby A. Ionizing radiation and heart risks. Semin. Cell Dev. Biol 2016. p. 14–25. [DOI] [PubMed] [Google Scholar]
- 14.Tapio S Pathology and biology of radiation-induced cardiac disease. J. Radiat. Res 2016;57:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughson RL, Helm A, Durante M. Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol 2017; [DOI] [PubMed] [Google Scholar]
- 16.Taunk NK, Haffty BG, Kostis JB, Goyal S. Radiation-Induced Heart Disease: Pathologic Abnormalities and Putative Mechanisms. Front. Oncol 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brosius FC, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am. J. Med 1981;70:519–30. [DOI] [PubMed] [Google Scholar]
- 18.Finet JE. Management of Heart Failure in Cancer Patients and Cancer Survivors. Heart Fail. Clin 2017. p. 253–88. [DOI] [PubMed] [Google Scholar]
- 19.Groarke JD, Tanguturi VK, Hainer J, Klein J, Moslehi JJ, Ng A, et al. Abnormal exercise response in long-term survivors of Hodgkin lymphoma treated with thoracic irradiation: Evidence of cardiac autonomic dysfunction and impact on outcomes. J. Am. Coll. Cardiol 2015;65:573–83. [DOI] [PubMed] [Google Scholar]
- 20.Nielsen KM, Offersen BV, Nielsen HM, Vaage-Nilsen M, Yusuf SW. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin. Cardiol 2017. p. 255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksson D, Stigbrand T. Radiation-induced cell death mechanisms. Tumour Biol. 2010;31:363–72. [DOI] [PubMed] [Google Scholar]
- 22.Kaina B DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem. Pharmacol 2003;66:1547–54. [DOI] [PubMed] [Google Scholar]
- 23.Lee C-L, Moding EJ, Cuneo KC, Li Y, Sullivan JM, Mao L, et al. p53 Functions in Endothelial Cells to Prevent Radiation-Induced Myocardial Injury in Mice. Sci. Signal 2012;5:ra52–ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarosiek K a, Ni Chonghaile T, Letai A. Mitochondria: gatekeepers of response to chemotherapy Trends Cell Biol. Elsevier Ltd; 2013;23:612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willers H, Held KD. Introduction to clinical radiation biology. Hematol. Oncol. Clin. North Am 2006. p. 1–24. [DOI] [PubMed] [Google Scholar]
- 26.Bergonié J, Tribondeau L. De Quelques Résultats de la Radiotherapie et Essai de Fixation d’une Technique Rationnelle. Comptes Rendus des Séances l’Académie des Sci. 1906;143:983–5. [Google Scholar]
- 27.Jaworski C, Mariani JA, Wheeler G, Kaye DM. Cardiac complications of thoracic irradiation. J. Am. Coll. Cardiol 2013;61:2319–28. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for Cardiomyocyte Renewal in Humans. Science (80-. ). 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzahor E, Poss KD. Cardiac regeneration strategies: Staying young at heart. Science (80-. ). 2017;356:1035 LP–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yarnold J, Vozenin Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol 2010. p. 149–61. [DOI] [PubMed] [Google Scholar]
- 31.Sherman ML, Datta R, Hallahan DE, Weichselbaum RR, Kufe DW. Ionizing radiation regulates expression of the c-jun protooncogene. Proc. Natl. Acad. Sci. U. S. A 1990;87:5663–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kara M, Özçağlı E, Jannuzzi AT, Alpertunga B. Oxidative stress mediated cardiac apoptosis. J. Fac. Pharm. Istanbul Univ 2015;45:217–32. [Google Scholar]
- 33.Firsanov D, Vasilishina A, Kropotov A, Mikhailov V. Dynamics of γh2AX formation and elimination in mammalian cells after X-irradiation. Biochimie. 2012;94:2416–22. [DOI] [PubMed] [Google Scholar]
- 34.Salata C, Ferreira-Machado SC, De Andrade CBV, Mencalha AL, Mandarim-De-Lacerda CA, de Almeida CE. Apoptosis induction of cardiomyocytes and subsequent fibrosis after irradiation and neoadjuvant chemotherapy. Int. J. Radiat. Biol 2014;90:284–90. [DOI] [PubMed] [Google Scholar]
- 35.Sarosiek KA, Chi X, Bachman JA, Sims JJ, Montero J, Patel L, et al. BID Preferentially Activates BAK while BIM Preferentially Activates BAX, Affecting Chemotherapy Response. Mol. Cell 2013;51:751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchel REJ, Hasu M, Bugden M, Wyatt H, Hildebrandt G, Chen Y-X, et al. Low-dose radiation exposure and protection against atherosclerosis in ApoE(−/−) mice: the influence of P53 heterozygosity. Radiat. Res 2013;179:190–9. [DOI] [PubMed] [Google Scholar]
- 37.Sarosiek KA, Fraser C, Muthalagu N, Bhola PD, Chang W, McBrayer SK, et al. Developmental Regulation of Mitochondrial Apoptosis by c-Myc Governs Age- and Tissue-Specific Sensitivity to Cancer Therapeutics. Cancer Cell. Elsevier Inc; 2017;31:142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Introduces the concept of developmental regulation of apoptosis as a component of the treatment-associated toxicities observed in pediatric patients.
- 38.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 39.De Ruysscher D, Faivre-Finn C, Moeller D, Nestle U, Hurkmans CW, Le Péchoux C, et al. European Organization for Research and Treatment of Cancer (EORTC) recommendations for planning and delivery of high-dose, high precision radiotherapy for lung cancer. Radiother. Oncol 2017;124:1–10. [DOI] [PubMed] [Google Scholar]; • Comprehensive guidelines for the planning of lung cancer radiotherapy, in terms of both target volume and organs at risk.
- 40.Gagliardi G, Lax I, Ottolenghi A, Rutqvist LE. Long-term cardiac mortality after radiotherapy of breast cancer - Application of the relative seriality model. Br. J. Radiol 1996;69:839–46. [DOI] [PubMed] [Google Scholar]
- 41.Pollock S, Keall R, Keall P. Breathing guidance in radiation oncology and radiology: A systematic review of patient and healthy volunteer studies. Med. Phys 2015;42:5490–509. [DOI] [PubMed] [Google Scholar]
- 42.Lymberis SC, De Wyngaert JK, Parhar P, Chhabra AM, Fenton-Kerimian M, Chang J, et al. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: Volumetric and dosimetric correlations in 100 patients. Int. J. Radiat. Oncol. Biol. Phys 2012;84:902–9. [DOI] [PubMed] [Google Scholar]
- 43.Sung KH, Choi YE, Lee KC. Cardiac risk index as a simple geometric indicator to select patients for the heart-sparing radiotherapy of left-sided breast cancer. J. Med. Imaging Radiat. Oncol 2017;61:410–7. [DOI] [PubMed] [Google Scholar]
- 44.Nona Duma M, Herr A-C, Borm KJ, Trott KR, Molls M, Oechsner M, et al. Tangential Field Radiotherapy for Breast Cancer—The Dose to the Heart and Heart Subvolumes: What Structures Must Be Contoured in Future Clinical Trials? Front. Oncol 2017;7:130. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights the importance of heart substructure-specific contouring in RT planning, especially considering late toxicities.
- 45.Hedin E, Bäck A, Chakarova R. Impact of lung density on the lung dose estimation for radiotherapy of breast cancer. Phys. Imaging Radiat. Oncol 2017;3:5–10. [Google Scholar]
- 46.Hoppe BS, Flampouri S, Su Z, Latif N, Dang NH, Lynch J, et al. Effective dose reduction to cardiac structures using protons compared with 3DCRT and IMRT in mediastinal Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys 2012;84:449–55. [DOI] [PubMed] [Google Scholar]
- 47.Vogel J, Lin L, Simone CB, Berman AT. Risk of major cardiac events following adjuvant proton versus photon radiation therapy for patients with thymic malignancies. Acta Oncol. (Madr). 2017;56:1060–4. [DOI] [PubMed] [Google Scholar]
- 48.Amino M, Yoshioka K, Shima M, Okada T, Nakajima M, Furusawa Y, et al. Changes in arrhythmogenic properties and five-year prognosis after carbon-ion radiotherapy in patients with mediastinum cancer. Ann. Noninvasive Electrocardiol 2017;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stick LB, Yu J, Maraldo MV, Aznar MC, Pedersen AN, Bentzen SM, et al. Joint Estimation of Cardiac Toxicity and Recurrence Risks After Comprehensive Nodal Photon Versus Proton Therapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. Elsevier Inc; 2017;97:754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu S, Tao L, Wang J, Xu Z, Wang J, Xue Y, et al. Amifostine Pretreatment Attenuates Myocardial Ischemia/Reperfusion Injury by Inhibiting Apoptosis and Oxidative Stress. Oxid. Med. Cell. Longev 2017;2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Freitas RB, Boligon AA, Rovani BT, Piana M, De Brum TF, Da Silva Jesus R, et al. Effect of black grape juice against heart damage from acute gamma TBI in rats. Molecules. 2013;18:12154–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian L, Cao F, Cui J, Wang Y, Huang Y, Chuai Y, et al. The potential cardioprotective effects of hydrogen in irradiated mice. J. Radiat. Res 2010;51:741–7. [DOI] [PubMed] [Google Scholar]
- 53.Fan Z, Han Y, Ye Y, Liu C, Cai H. L-carnitine preserves cardiac function by activating p38 MAPK/Nrf2 signalling in hearts exposed to irradiation. Eur. J. Pharmacol 2017;804:7–12. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Li Y, Li R, Wang Y, Zhu M, Wang B, et al. Sodium Tanshinone IIA Sulfonate Prevents Radiation-Induced Toxicity in H9c2 Cardiomyocytes. Evidence-based Complement. Altern. Med 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang ZY, Li Y, Li R, Zhang AA, Shang B, Yu J, et al. Tetrahydrobiopterin protects against radiation-induced growth inhibition in H9c2 cardiomyocytes. Chin. Med. J. (Engl). 2016;129:2733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res 2011. p. 1–16. [DOI] [PubMed] [Google Scholar]
- 57.Elitok A, Oz F, Ahmet Y, Kilic L, Ciftci R, Sen F, et al. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: A prospective randomized controlled study with six-month follow-up. Cardiol. J 2014;21:509–15. [DOI] [PubMed] [Google Scholar]
- 58.Panel M, Ghaleh B, Morin D. Targeting mitochondrial permeability as a pharmacological cardioprotective strategy . Med. Res. Arch 2017;5. [Google Scholar]
- 59.Frankenreiter S, Bednarczyk P, Kniess A, Bork N, Straubinger J, Koprowski P, et al. cGMP-Elevating Compounds and Ischemic Conditioning Provide Cardioprotection Against Ischemia and Reperfusion Injury via Cardiomyocyte-Specific BK Channels. Circulation. 2017; [DOI] [PubMed] [Google Scholar]
- 60.Guo X, Yin H, Li L, Chen Y, Li J, Doan J, et al. Cardioprotective Role of TRAF2 by Suppressing Apoptosis and Necroptosis. Circulation. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kura B, Babal P, Slezak J. Implication of microRNAs in the development and potential treatment of radiation-induced heart disease. Can. J. Physiol. Pharmacol 2017;95:1236–44. [DOI] [PubMed] [Google Scholar]
- 62.Children’s Oncology Group. Guidelines for Survivors of Childhood , Adolescent , and Young Adult Cancer Long-Term Follow-Up Guidelines, Version 4.0. 2013;1–241. [Google Scholar]
- 63.Spewak MB, Williamson RS, Mertens AC, Border WL, Meacham LR, Wasilewski-Masker KJ. Yield of screening echocardiograms during pediatric follow-up in survivors treated with anthracyclines and cardiotoxic radiation. Pediatr. Blood Cancer. 2017;64. [DOI] [PubMed] [Google Scholar]
- 64.Sritharan HP, Delaney GP, Lo Q, Batumalai V, Xuan W, Thomas L. Evaluation of traditional and novel echocardiographic methods of cardiac diastolic dysfunction post radiotherapy in breast cancer. Int. J. Cardiol 2017;243:204–8. [DOI] [PubMed] [Google Scholar]; • Describes the current status of echocardiographic diagnostics, and the need for novel methods in future guidelines.
- 65.Patel AA, Labovitz AJ. Advanced Echocardiographic Techniques in Detection of Cardiotoxicity. Curr. Treat. Options Cardiovasc. Med 2016. p. 1–13. [DOI] [PubMed] [Google Scholar]
- 66.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation. 2013;128:1927–55. [DOI] [PubMed] [Google Scholar]
- 67.Yusuf SW, Sami S, Daher IN. Radiation-Induced Heart Disease: A Clinical Update. Cardiol. Res. Pract 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu W, Masri A, Popovic ZB, Smedira NG, Lytle BW, Marwick TH, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: A cohort study. Circulation. 2013;127:1476–84. [DOI] [PubMed] [Google Scholar]
- 69.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease. J. Am. Coll. Cardiol 2017;70:252–89. [DOI] [PubMed] [Google Scholar]; •• Comprehensive clinical guidelines applicable to patients with or at risk of developing valvular heart disease
- 70.Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J 2015. p. 2921–64.26320112 [Google Scholar]
- 71.Johnston DR. Surgical Management of Pericardial Diseases. Prog. Cardiovasc. Dis 2017. p. 407–16. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y, Naruse Y, Tanaka K. Effectiveness and long-term outcomes of surgical intervention for constrictive epicardium in constrictive pericarditis. Gen. Thorac. Cardiovasc. Surg. Springer Japan; 2017;0:0. [DOI] [PubMed] [Google Scholar]


