Abstract
The differential impact of preemptive therapy (PET) and antiviral prophylaxis (AP) on development of cytomegalovirus (CMV)–specific neutralizing antibody (nAb) and T-cell responses have not previously been directly compared in high-risk donor-seropositive/recipient-seronegative (D+R−) organ transplant recipients. We prospectively assessed T-cell and nAb responses 3 months after transplantation in cohorts of high-risk D+R− liver transplant recipients who received either PET (n = 15) or AP (n = 25) and a control group of CMV-seropositive transplant recipients (R+) (AP; n = 24). CMV phosphoprotein 65 (pp65)– and immediate early protein 1–specific multifunctional T-cell responses were determined by means of intracellular cytokine staining and nAbs against BADrUL131-Y4 CMV in adult retinal pigment epithelial cell line-19 human epithelial cells; nAbs were detected in 8 of 12 (67%) in the PET group, none of 17 in the AP group, and 20 of 22 (91%) in the R+ group. Multifunctional CD8 and CD4 T-cell responses to pp65 were generally similar between PET and R+ groups, and lower for the AP group; multifunctional CD4 responses were similar across all groups. Among D+R− liver transplant recipients, PET was associated with the development of greater nAb and multifunctional CD8 T-cell responses compared with AP, providing a potential mechanism to explain the relative protection against late-onset disease with PET. Future studies are needed to define specific immune parameters predictive of late-onset CMV disease with AP.
Keywords: Cytomegalovirus, transplant, immunity
Cytomegalovirus-specific T-cell responses and neutralizing antibody (nAb) responses were directly compared between high-risk donor-seropositive/recipient-seronegative liver transplant recipients receiving either preemptive therapy or antiviral prophylaxis. Preemptive therapy was associated with greater nAb and multifunctional CD8 T-cell responses compared with antiviral prophylaxis.
Cytomegalovirus (CMV) is a major viral pathogen in solid organ transplant (SOT) recipients, and CMV-specific immune responses, and especially multifunctional T cells, have been linked to control of CMV in this setting [1, 2]. The risk for CMV infection and disease in SOT recipients is highest in donor-seropositive/recipient-seronegative (D+R−) patients and is hypothesized to result from impaired ability to effectively control primary CMV infection. Prior studies have focused primarily on cellular (T-cell) immune responses as immune correlates of risk for CMV infection and/or disease in transplant recipients, but emerging data suggest that humoral immune responses (functionally neutralizing antibodies [nAbs]) might also be important in the control of primary CMV infection, which represents the situation in D+R− SOT recipients [3, 4]. CMV entry into epithelial and endothelial cells requires distinct viral proteins, including UL128, UL130, UL131A, glycoprotein B (gB) and glycoprotein H/glycoprotein L, that combine to form a pentameric complex, and antibodies to this complex are associated with control of viral dissemination [5, 6].
The original demonstration that antibody reduced CMV disease in SOT recipients was reported using a hyperimmune globulin preparation [7]. Recent data from trials of monoclonal antibody demonstrated a trend toward an effect on time to viremia [8]. gB–specific antibodies have also been shown to be neutralizing, and gB vaccination was shown to reduce the need for antiviral therapy in D+R− transplant recipients [3]. Recent studies have assessed humoral correlates of immune protection in various settings [9, 10]. In addition, a randomized controlled clinical trial published in 2017 showed that administration of human monoclonal nAbs to pentameric complex decreased the incidence of primary CMV infection and disease in D+R− SOT recipients [4].
Preemptive therapy (PET) and antiviral prophylaxis (AP) are the 2 major strategies for prevention of CMV disease in transplant recipients, and the relative superiority of one strategy versus another remains controversial [11]. A well-documented observation with AP, but less frequently seen after PET, is the development of postprophylaxis (late-onset) CMV disease, especially in D+R− patients [12]. It has been speculated that controlled viral replication inherent to the strategy of PET (compared with effective viral suppression with AP) might better facilitate the development of CMV-specific immune responses that are protective against late-onset CMV disease. However, no prior studies have directly compared the development of CMV-specific immune responses in D+R− transplant recipients who received either AP or PET.
We hypothesized that PET would be associated with the development of greater CMV-specific humoral and cellular immune responses compared with AP in high-risk D+R− patients, providing a potential mechanism to explain the relative protection against late-onset CMV disease seen among those who receive PET. We prospectively assessed cellular and humoral immune responses and clinical CMV disease in cohorts of high-risk D+R− liver transplant recipients who received either PET or AP.
METHODS
Study Participants and Study Design
This study was approved by the University of Washington Human Subjects Division and VA Pittsburgh Healthcare System, and each participant provided written informed consent. This was a prospective, 2-center observational study of consecutive D+R− liver transplant recipients who received either PET (University of Pittsburgh) or AP (University of Washington) as their standard CMV prevention strategy for 3 months after transplantation. An additional cohort of CMV-seropositive transplant recipients (R+) who received AP was included as a control group to assess the effects of immunosuppression on detection and quantitation of CMV-specific immune responses, in previously exposed and naturally immune CMV-seropositive patients. A cohort of 16 CMV-seropositive (n = 18) and CMV-seronegative (n = 22) healthy volunteers (aged >18 years; 32 female, 8 male) was included to establish the nAb assay cutoff level.
PET was administered (valganciclovir, 900 mg orally every12 hours; dose adjusted according to renal function per manufacturer recommendations) when CMV replication was detected at any level; CMV surveillance was performed every 2 weeks for 3 months after transplantation, as described elsewhere [13]. During the course of the study period, the laboratory assay for detection of CMV replication in blood changed from antigenemia (phosphoprotein 65 [pp65] antigen) to quantitative plasma CMV DNA. AP included valganciclovir (900 mg orally once daily; dose adjusted according to renal function per manufacturer recommendations) for 3 months after transplantation. After completion of either PET or AP, patients underwent testing for CMV only in the presence of signs or symptoms compatible with CMV disease (ie, no routine CMV surveillance testing was done after completion of PET or AP). Participants had blood samples collected 3 months after transplantation (at discontinuation of AP or PET) for immune analyses and were prospectively followed up for development of CMV disease until 12 months after transplantation (or earlier, if death occurred earlier). CMV disease was defined according to published consensus criteria [14].
Patient Specimens
Peripheral blood mononuclear cells (PBMCs) were isolated from sodium-heparinized blood by density centrifugation and cryopreserved in liquid nitrogen in 90% fetal bovine serum (HyClone) and 10% dimethyl sulfoxide (DMSO) (Sigma-Aldrich). Plasma was isolated from ethylenediaminetetraacetic acid–anticoagulated blood by centrifugation and stored at −80°C until use. When thawed, plasma was then treated with 20% calcium chloride (Fisher Bioreagents) 1:100 by volume and incubated at 4˚C for 1 hour to coagulate, after which the clot was removed and the remaining serum used. Blood was processed within 24 hours of collection. Not all participants had samples available for all immune assays because of missed study visits or insufficient samples; in particular, plasma collection did not occur for the first 6 months of the study. The actual number of patients and samples is specified for each analysis.
Cells and Virus
Human retinal pigmented epithelial (adult retinal pigment epithelial cell line-19 [ARPE-19]; American Type Culture Collection CRL-2302) cells were maintained in Dulbecco modified Eagle medium (DMEM; Gibco) supplemented with 10% NuSerum (Corning), 1% L-glutamine, 10 000 IU/L penicillin, and 10 mg/L streptomycin (all Gibco) at 37°C with 5% carbon dioxide (CO2). Cells were plated in 96-well flat-bottom plates 1 to 2 days before assay setup and used when confluent.
We used the BADrUL131-Y4 laboratory strain of human CMV, originally modified from the AD169 genome by Wang and Shenk [15]. This strain expresses a functional UL131 protein and green fluorescent protein cassette that allows for quantitation of viral entry and replication. Viral stocks were grown from infected human foreskin fibroblasts, suspended in DMEM and sterile skim milk, and stored at −80 °C.
Immune Assays
All immune assays were performed by personnel who were blinded to clinical parameters.
nAb Assay
A method adapted from Cui et al [16] was used. Briefly, 10 two-fold serial dilutions of patient plasma in DMEM were prepared in 96-well round-bottom plates from 1:8–1:4096 (total initial volume, 120 μL). One row of the dilution plate was reserved for plasma from a known CMV-seropositive donor. The first and last wells of each row were reserved for medium-only and medium plus virus-only negative and positive controls, respectively. The plasma dilutions were then mixed 1:1 with a BADrUL131-Y4 virus stock solution, diluted to yield a final concentration of 1000 plaque-forming units per well, incubated for 1 hour (all incubations were at 37°C with 5% CO2) and then 50 μL per well of plasma dilutions plus virus were used to inoculate wells of duplicate 96-well plates of ARPE-19 cells. After 1 hour of incubation, the medium was aspirated, 100 μL of fresh DMEM was added to each well, and the plates were incubated for 7 days. On day 7, fluorescence was measured using a fluorimeter (485-nm excitation and 527-nm emission; Fluoroskan Ascent, Thermo Lab Systems).
GraphPad Prism software v. 7 (GraphPad Software) was used to fit 4-parameter logistic virus neutralization curves to each sample’s fluorescence data and estimate the negative-log2 plasma concentration at which 50% neutralization was achieved (median inhibitory concentration [IC50]). IC50 values >5.0 were reliably quantifiable and were considered positive; this cutoff was corroborated by minimum levels measured in CMV-seropositive healthy donors, which were all >5. Projected IC50 values <5 that showed evidence of some neutralizing capacity only at the lowest plasma dilutions were considered to have a detectable but not quantifiable response and were scored as 2.5 for the purposes of analysis. If there was no evidence of any neutralization at the highest concentrations of plasma, the result was scored as 0 (no response).
Intracellular Cytokine Staining
All reagents were purchased from BD Biosciences, unless otherwise specified. Cryopreserved PBMCs were thawed in enriched medium (Roswell Park Memorial Institute–HEPES; Gibco) with 10% human AB serum (Sigma-Aldrich), 2 mmol/L L-glutamine (Gibco), and 1% antibiotic/antimycotic (Sigma-Aldrich) and rested overnight at 1 × 106 cells per well in 96-well round-bottom plates. The next day, PBMCs were incubated with anti-CD107a phycoerythrin-cyanine 5 (PECy5) antibody for 10 minutes, followed by the addition of the costimulatory antibodies anti-CD28 and anti-CD49d (both 1 μg/mL). Cells were then stimulated with CMV immediate early protein 1 (IE-1) or pp65 overlapping peptide libraries (2 μg/mL; JPT Peptide Technologies), Staphylococcal enterotoxin B (0.05 μg/mL; Sigma-Aldrich), or DMSO (used to reconstitute the peptide libraries) for 6 hours at 37°C and 5% CO2. Brefeldin A (10 μg/mL; Sigma-Aldrich)/GolgiStop protein transport inhibitor (BD Biosciences) was added during the last 4 hours of incubation. Samples were incubated overnight at 4°C and then stained for flow cytometric analysis the next day.
After incubation with EDTA (20 mmol/L), samples were incubated first with violet fixable viability dye (Invitrogen/Thermo Fisher) for 30 minutes in 1× phosphate-buffered saline, washed, successively incubated with 1× FACSlyse and 1× FACSperm solutions, washed with fluorescence-activated cell sorter (FACS) wash buffer (0.5% bovine serum albumin in 1× phosphate-buffered saline), and then incubated for 30 minutes in the dark at room temperature with a cocktail of fluorescently labeled antibodies against CD3 electron coupled dye (Beckman Coulter), CD4 fluorescein isothiocyanate (FITC), CD8 Peridinin-chlorophyll-cyanine 5.5 (PerCPCy5.5), interferon γ allophycocyanin (APC), interleukin 2 phycoerythrin (PE) and tumor necrosis factor α Alexa Fluor 700. Finally, samples were fixed with 1% paraformaldehyde (Sigma-Aldrich) and cell acquisition (100 000–400 000 events) was done on an LSRII flow cytometer (BD Biosciences) within 24 hours. All antibodies were titrated for optimum performance, and appropriate single-color compensation and fluorescence-minus-one controls were run. Data were analyzed with FlowJo software, version 9 (FlowJo). The gating strategy is shown in Supplementary Figure 1. Absolute lymphocyte counts obtained from clinical records were used to calculate CMV-specific cell counts. Multifunctional T cells were defined as those expressing >1 cytokine or degranulation marker after background subtraction of the DMSO control.
Statistical Analyses
Descriptive analyses (mean, median, range) were used for comparisons of immunologic parameters (nAb titers and T-cell responses) among cohorts. Time-to-event curves (for CMV disease) were obtained using the cumulative incidence method, with death as a competing event.
RESULTS
Study Population
The characteristics of each of the cohorts is shown in Table 1. The number of patient samples available for nAb and T-cell assays are summarized in Supplementary Table 1.
Table 1.
Characteristics of the Clinical Cohorts
| Characteristic | D+R− Transplant Recipients, No. (%)a | R+ Transplant Controls, No. (%)a (AP) (n = 24) |
|
|---|---|---|---|
| AP (n = 25) | PET (n = 15) | ||
| Age, mean (SD), y | 50 (9) | 57 (5) | 55 (8) |
| Sex | |||
| Male | 20 (80) | 15 (100) | 17 (71) |
| Female | 5 (20) | 0 (0) | 7 (29) |
| MELD, mean (SD) | 18.0 (8) | 23.2 (4) | 19.5 (9) |
| Indication for transplant | |||
| Hepatitis Cb | 13 (52) | 7 (47) | 10 (42) |
| Other | 12 (48) | 8 (53) | 14 (58) |
| Immunosuppression | |||
| ATG induction therapy | 2 (8) | 0 (0) | 0 (0) |
| Maintenance | |||
| Tacrolimus | 24 (96) | 14 (93) | 24 (100) |
| Prednisone | 3 (12) | 15 (100) | 6 (25) |
| MMF | 10 (40) | 5 (33) | 9 (38) |
| AP | |||
| Valganciclovir | 20 (80) | NA | 20 (83) |
| Oral ganciclovir | 5 (20) | NA | 4 (17) |
| Rejection within 3 mo | 3 (12) | 2 (13) | 4 (17) |
| CMV disease by 1 y after transplantation | 10 (40) | 0 (0) | 1 (4) |
| Tissue invasive | 6 (24) | 0 (0) | 0 (0) |
| Hepatitis | 4 (16) | 0 (0) | 0 (0) |
| Gastroenteritis | 1 (4) | 0 (0) | 0 (0) |
| Duodenitis | 1 (4) | 0 (0) | 0 (0) |
| Gastritis | 0 (0) | 0 (0) | 1 (4) |
| Syndrome | 4 (16) | 0 (0) | 0 (0) |
| Death by 1 y | 0 (0) | 0 (0) | 2 (8) |
Abbreviations: AP, antiviral prophylaxis; ATG, anti-thymocyte globulin; CMV, cytomegalovirus; D+R−, donor-seropositive/recipient-seronegative; MELD, model for end-stage liver disease; MMF, mycophenolate mofetil; NA, not applicable; PET, preemptive therapy; R+, recipient-seropositive; SD, standard deviation.
aData represent no. (%) of patients or controls, unless otherwise specified.
bPatients with hepatitis C and another disease were classified as hepatitis C.
CMV Infection and Disease
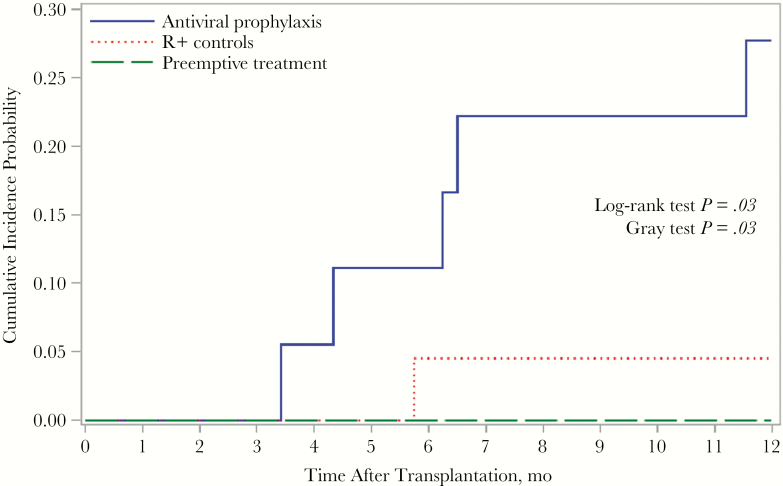
Among the 15 patients in the D+R− PET cohort, 10 (67%) had evidence of CMV replication in blood by antigenemia or DNAemia assay during the monitoring period; the mean, median, and range of time to onset of CMV replication at any level were 29.4, 28, and 14–56 days, respectively. The cumulative incidence of CMV disease, stratified by cohort, is shown in Figure 1.
Figure 1.
Cumulative incidence of cytomegalovirus (CMV) disease in first year after transplantation, stratified by serogroup. Abbreviation: R+, CMV-seropositive transplant recipients (control group).
nAb Activity
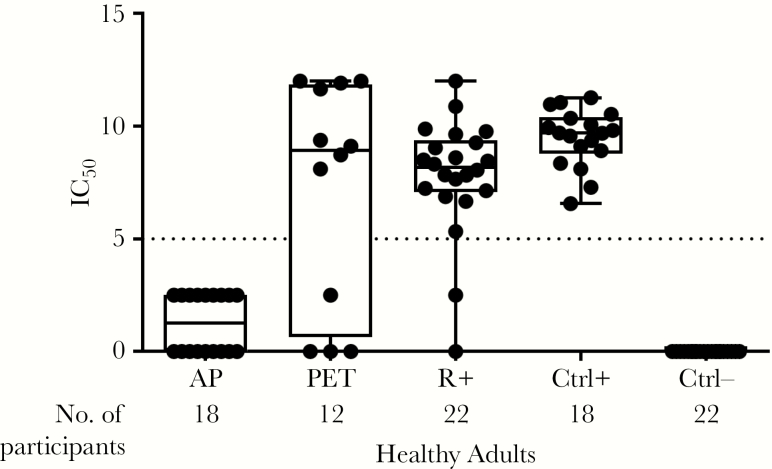
In Figure 2, nAb titers to virus expressing the pentameric complex among D+R− patients who received either PET or AP, R+ transplant recipients who received AP (all 3 months after transplantation), and seronegative and seropositive healthy controls are shown in a box-and-whiskers plot, with each dot representing data from a single patient. The nAb activity after PET was similar to that seen in R+ SOT recipients and healthy seropositive controls, and higher than among those who received AP. In the AP group, 9 of 18 (50%) had low levels of nAb, below the threshold for quantitation (IC50 = 5). Healthy CMV-seronegative controls all had nAb activity values of 0. Among the 12 (of 15 total) patients in the PET group who had available samples, nAb was detected in all 8 patients with preceding CMV viremia versus 1 of 4 without preceding viremia, consistent with the hypothesis that CMV replication facilitated development of a nAb immune response.
Figure 2.
Neutralizing antibody titer to a virus containing a reconstructed pentameric complex, stratified by cohort. Each black dot indicates the result for an individual patient. Boxes represent first to third quartiles; lines within boxes, medians; whiskers, minimum and maximum values; and dotted line, cutoff for quantitation. Projected median inhibitory concentration (IC50) values <5 that showed evidence of some neutralizing capacity at only the highest plasma dilutions were considered to have a detectable but not quantifiable response and were scored as 2.5. If there was no evidence of any neutralization even at the highest concentrations of plasma, the result was shown as 0. Abbreviations: AP, antiviral prophylaxis; Ctrl+, cytomegalovirus (CMV)–seropositive healthy controls; Ctrl−, CMV-seronegative healthy controls; PET, preemptive therapy; R+, CMV-seropositive transplant recipients who received AP as controls.
CD8 and CD4 Multifunctional T-Cell Responses to pp65 and IE-1
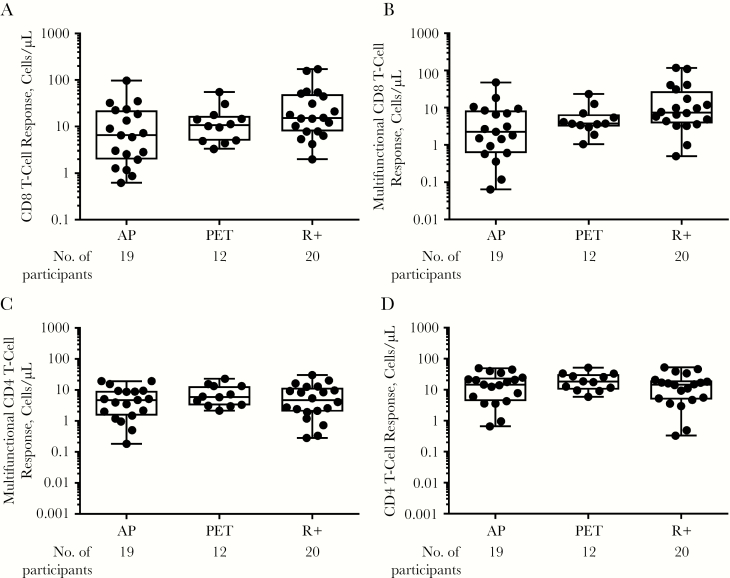
T-cell responses were assessed 3 months after transplantation, at the completion of either AP or PET. To assess for possible immunosuppression-related impacts on measurement of T-cell immune parameters, and specifically to assess for differential impacts of immunosuppression between PET and AP groups that could confound interpretation of CMV-specific immune responses, we first measured SEB-induced cytokine T-cell responses [Figure 3]. As shown in Figure 3A–3D, multifunctional CD4 and CD8 T-cell responses to SEB were comparable across all groups, providing evidence that any observed CMV-specific T-cell responses were affected by the antiviral prevention strategy rather than confounded by other transplant-related factors (ie, differential immunosuppression).
Figure 3.
CD8 and CD4 T-cell responses to Staphylococcal enterotoxin B months after transplantation, expressed in cells per microliter, collectively for any cytokine or degranulation marker for CD8 (A), CD4 (D), and multifunctional CD8 (B), and CD4 (C) T cells, defined as those expressing ≥2 markers. Each black dot indicates results from an individual patient, and whiskers represent minimum and maximum values. Abbreviations: AP, antiviral prophylaxis; PET, preemptive therapy; R+, cytomegalovirus-seropositive transplant recipients who received AP.
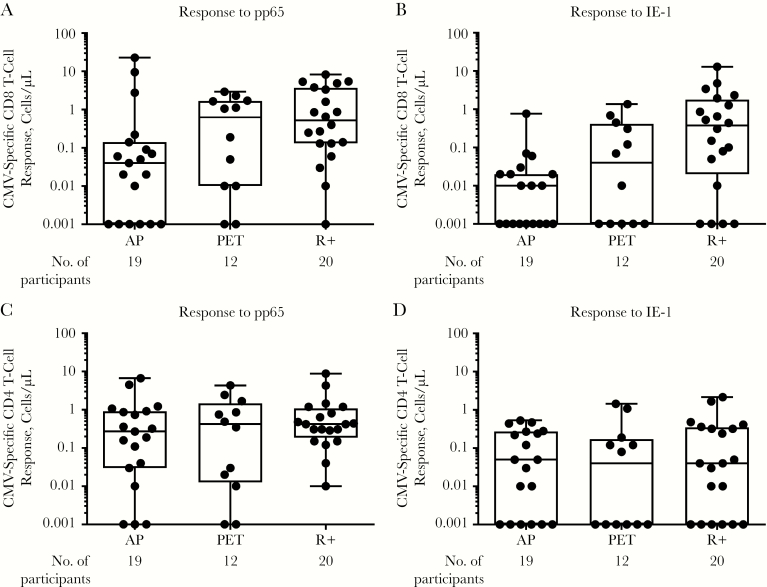
Multifunctional T-cell responses to pp65 and IE-1, expressed as cells/µL, are shown in Figure 4A–4D. Overall, multifunctional CD8 T-cell responses to both pp65 and IE-1 were similar between the R+ transplant control and PET groups, and lower for the AP group, and these differences were most pronounced for multifunctional CD8 T-cell responses to pp65. In contrast, multifunctional CD4 T-cell responses to both antigens were similar across all groups. In the PET group, multifunctional T-cell responses were detected even among some patients in whom CMV replication (either pp65 or DNAemia) was not detected during the monitoring period, suggesting the possibility that local replication within the allograft without concomitant detectable viremia might also lead to immune recognition and response in the recipient. The largest differences were seen in CMV-specific CD8 T-cell responses between the AP and R+ transplant control groups, providing support for the role of immune priming in the development of CMV-specific T-cell responses.
Figure 4.
Multifunctional CD8 (A, B) and CD4 (C, D) cytomegalovirus (CMV)–specific T-cell responses to phosphoprotein 65 (pp65) (A, C) and immediate early protein 1 (IE-1) (B, D) 3 months after transplantation. Each black dot represents results from an individual patient, and whiskers represent minimum and maximum values. A multifunctional T-cell response was defined as expressing ≥2 cytokines or degranulation markers. Abbreviations: AP, antiviral prophylaxis; PET, preemptive therapy; R+, CMV-seropositive transplant recipients who received prophylaxis.
Relationship of Quantitative Levels of nAb and Multifunctional T-Cell Responses to CMV Prevention Strategy
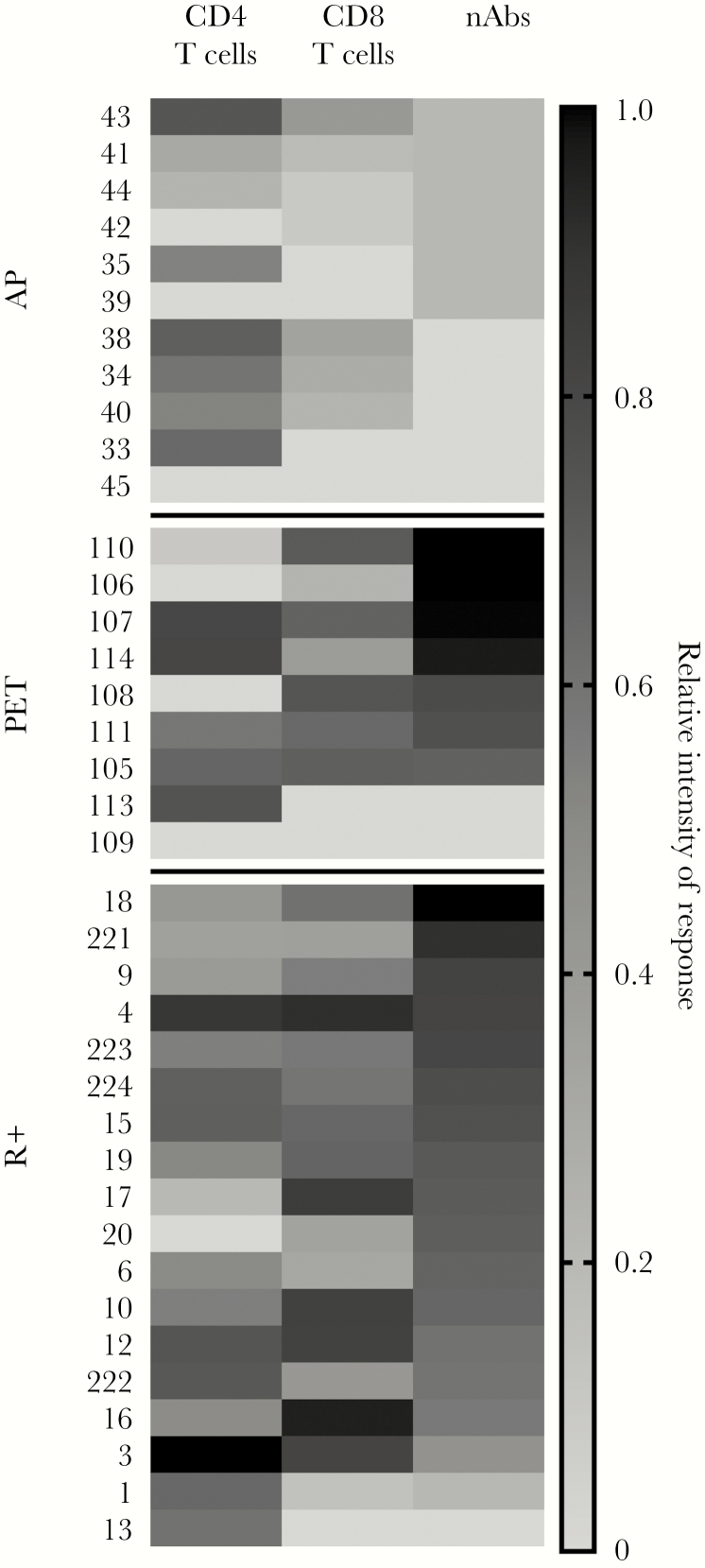
In Figure 5, nAb titer and multifunctional T-cell responses are depicted semiquantitatively in a heat map, according to CMV prevention strategy. The overall patterns of percentage of responders and quantitative responses were similar between the R+ and PET groups, and higher than for the AP group, and there was a concordance between the development and titer of nAb and the development and quantity of multifunctional T-cell responses.
Figure 5.
Summary of quantitative neutralizing antibody (nAb) and multifunctional cytomegalovirus (CMV)–specific T-cell responses according to CMV prevention strategy and the seropositive-recipient (R+) control group. Data were normalized for display, with orange representing the maximum response and blue, no response. CD4 and CD8 multifunctional CMV-specific T-cell responses were log-transformed before normalization. Abbreviations: AP, antiviral prophylaxis; PET, preemptive therapy; R+, CMV seropositive control transplant patients.
DISCUSSION
In this prospective pilot study that compared CMV-specific immune responses 3 months after transplantation between those who received PET and those who received AP as the CMV prevention strategy, PET was associated with greater ability to neutralize CMV entry via the pentameric complex and with multifunctional CD8 T-cell responses. These preliminary data provide insight into potential mechanisms to explain the unique risk for post-prophylaxis (late-onset) CMV disease associated with AP relative to PET.
To date, most studies have focused on CMV-specific cellular (T-cell) immune responses rather than humoral immune responses as predictors of CMV disease risk in SOT recipients. More recently, several lines of evidence suggest that CMV-specific antibody responses might also be important in predicting risk in the transplant setting. In a trial of pretransplantation vaccination that elicited gB-specific antibodies, there was a reduction in the incidence of CMV viremia and/or need for antiviral therapy among vaccinated D+R− transplant recipients [3].
In addition, a randomized controlled trial of a combination of CMV-specific nAbs led to a reduction in CMV infection and disease in a cohort of high-risk D+R− kidney transplant recipients [4]. We found that nAb titers were higher in those who received PET versus AP, consistent with the hypothesis that controlled viral replication with PET might have allowed for more efficient development of antibody responses than a strategy of complete antiviral suppression with AP. Among the 12 patients receiving PET who were assessed, nAbs developed in all 8 (100%) with preceding viremia, compared with only 1 of 4 (25%) without preceding viremia. In the patients in whom CMV infection was not detected, it is unknown whether the graft did not contain or transmit CMV, the viremia period was too short or too low to be detected, or localized CMV replication occurred within the graft but was not detected by standardized measurements of viremia. This is an important area for future study. A 2018 study suggested that CMV-infected donor cells within the renal allograft present CMV peptides, likely because of frequent CMV replication within the allograft [17].
Specific T-cell responses that could be useful as immunologic correlates of protection or risk for CMV disease in high-risk D+R− transplant recipients remain to be identified. Unfortunately, the number of patients in this study who had available samples for both nAb and T-cell assays and in whom CMV disease developed was too small for meaningful assessment. Although a general association between both CD4 and CD8 CMV-specific cytokine-producing T cells and relative protection against CMV disease has been reported, variability in assay methods and quantitation has made it difficult to directly compare results across studies. In the present study, the number of patients with CMV disease was too small to assess associations among the specific CMV immune parameters measured and CMV disease risk, but as reported previously, postprophylaxis (late-onset) CMV disease was seen only among patients who received AP, and not PET.
Significantly larger studies with adequate CMV disease end points are needed to dissect the relative contribution of T-cell versus nAb-mediated immunity and to identify specific immune correlates of risk/protection from CMV disease; one such study (comparison of antiviral prevention strategies in liver transplant; NTC 01552369) is currently underway. Several commercial platforms that measure CMV-specific T-cell responses under standardized conditions have been developed and are being studied for their clinical utility in guiding CMV prevention and/or treatment [18, 19]. Importantly, none of these platforms incorporate assessment of nAbs, which have been shown to be important for control of primary CMV infection. If nAbs are shown in future studies to be important for predicting late-onset CMV disease risk, future immune assay platforms might need to include assessment of humoral CMV responses to pentameric complex or other targets to most optimally predict disease risk among D+R− patients who receive AP.
The current study has several strengths, including the use of sensitive and specific methods for detecting and quantitating CMV-specific humoral and cellular immune responses that have previously been associated with protection against CMV disease in transplant recipients, direct comparison of these immune markers in patients receiving the 2 major CMV prevention strategies, and a prospective and blinded study design. We also acknowledge limitations, including the relatively small sample size and the fact that not all immune assays were performed in all participants. The small number of CMV disease cases precluded the ability to assess for specific immune correlates of protection against or risk for CMV disease. In addition, since all participants who received a specific CMV prevention strategy were from a single institution, transplant-specific management practices (eg, immunosuppression levels) or other institution-specific practices may have affected the results, but the results from Staphylococcal enterotoxin B stimulation provide some reassurance that the observed differences in CMV-specific immune responses probably resulted from the CMV prevention strategy rather than confounding by other factors.
Assessment of nAb was determined only in a single laboratory-adapted strain and cell line, and the results and interpretation might be altered if other strains and/or cell lines were used. However, we chose the combination of ARPE-19 cells and the BADrUL131-Y4 virus based on prior work using this combination to quantify nAb responses in the settings of allogeneic hematopoietic stem cell transplantation and vaccine development [16, 20, 21]. Only a single time point (at the end of the CMV prevention strategy) 3 months after transplantation was assessed, to determine the differential impact on CMV-specific immune responses. Whether CMV-specific immune responses in the AP group ultimately increased over time to become comparable to responses in those who received PET requires further study.
In summary, in this pilot study, PET was associated with greater development of CMV-specific humoral and cellular immune responses than AP, and these results provide a potential mechanism to explain the higher incidence of postprophylaxis (late-onset) CMV disease seen with AP compared with PET in D+R− SOT recipients. Future larger prospective studies are necessary to confirm these findings and to identify specific immune correlates of protection against postprophylaxis (late-onset) CMV disease in high-risk D+R− SOT recipients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Annual meeting of the American Transplant Congress, San Francisco, California, May 30-June 3 2009; abstract 852.
Acknowledgments. Thanks to Sarah Johnson and Cherry Thuntarug for study coordination and Jeremy Smith, Tera Matson, and Jessica Yi for laboratory work. We thank Tom Shenk (Princeton University) for providing BADrUL131-Y4.
Data statement. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available owing to privacy or ethical restrictions.
Financial support. This work was supported by grants K23 AI097234, K24 HL093294, CA15704 and 272201600019C-3-0-1 NIAID.
Potential conflicts of interest. A. P. L. is a consultant for and has received grant support from Merck; is a site investigator for Merck, Roche, Astellas, and Oxford Immunotec; is a DSMB member for Novartis; and has received placebo/ganciclovir for a clinical trial from Genentech. M. B is a consultant for and has received research support from Astellas, Chimerix GSK, Merck, Oxford Immunotec, and Shire; is a consultant for Helocyte; and has received research support from Lophius Biosciences and Qiagen. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mattes FM, Vargas A, Kopycinski J, et al. Functional impairment of cytomegalovirus specific CD8 T cells predicts high-level replication after renal transplantation. Am J Transplant 2008; 8:990–9. [DOI] [PubMed] [Google Scholar]
- 2. Nebbia G, Mattes FM, Smith C, et al. Polyfunctional cytomegalovirus-specific CD4+ and pp65 CD8+ T cells protect against high-level replication after liver transplantation. Am J Transplant 2008; 8:2590–9. [DOI] [PubMed] [Google Scholar]
- 3. Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377:1256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ishida JH, Patel A, Mehta AK, et al. Phase 2 randomized, double-blind, placebo-controlled trial of RG7667, a combination monoclonal antibody, for prevention of cytomegalovirus infection in high-risk kidney transplant recipients. Antimicrob Agents Chemother 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerna G, Revello MG, Baldanti F, Percivalle E, Lilleri D. The pentameric complex of human cytomegalovirus: cell tropism, virus dissemination, immune response and vaccine development. J Gen Virol 2017; 98:2215–34. [DOI] [PubMed] [Google Scholar]
- 6. Lilleri D, Kabanova A, Lanzavecchia A, Gerna G. Antibodies against neutralization epitopes of human cytomegalovirus gH/gL/pUL128-130-131 complex and virus spreading may correlate with virus control in vivo. J Clin Immunol 2012; 32:1324–31. [DOI] [PubMed] [Google Scholar]
- 7. Snydman DR, Werner BG, Heinze-Lacey B, et al. Use of cytomegalovirus immune globulin to prevent cytomegalovirus disease in renal-transplant recipients. N Engl J Med 1987; 317:1049–54. [DOI] [PubMed] [Google Scholar]
- 8. McBride JM, Sheinson D, Jiang J, et al. Correlation of cytomegalovirus (CMV) disease severity and mortality with CMV viral burden in CMV-seropositive donor and CMV-seronegative solid organ transplant recipients. Open Forum Infect Dis 2019; 6:ofz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanarsdall AL, Chin AL, Liu J, et al. HCMV trimer- and pentamer-specific antibodies synergize for virus neutralization but do not correlate with congenital transmission. Proc Natl Acad Sci USA 2019; 116:3728–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baraniak I, Kropff B, Ambrose L, et al. Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc Natl Acad Sci USA 2018; 115:6273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh N. Preemptive therapy versus universal prophylaxis with ganciclovir for cytomegalovirus in solid organ transplant recipients. Clin Infect Dis 2001; 32:742–51. [DOI] [PubMed] [Google Scholar]
- 12. Singh N. Late-onset cytomegalovirus disease as a significant complication in solid organ transplant recipients receiving antiviral prophylaxis: a call to heed the mounting evidence. Clin Infect Dis 2005; 40:704–8. [DOI] [PubMed] [Google Scholar]
- 13. Singh N, Wannstedt C, Keyes L, et al. Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts. Liver Transpl 2008; 14:240–4. [DOI] [PubMed] [Google Scholar]
- 14. Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 15. Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 2005; 79:10330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine 2008; 26:5760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gatault P, Al-Hajj S, Noble J, et al. CMV-infected kidney grafts drive the expansion of blood-borne CMV-specific T cells restricted by shared class I HLA molecules via presentation on donor cells. Am J Transplant 2018; 18:1904–13. [DOI] [PubMed] [Google Scholar]
- 18. Gliga S, Korth J, Krawczyk A, et al. T-Track-CMV and QuantiFERON-CMV assays for prediction of protection from CMV reactivation in kidney transplant recipients. J Clin Virol 2018; 105:91–6. [DOI] [PubMed] [Google Scholar]
- 19. El Haddad L, Ariza-Heredia E, Shah DP, et al. The ability of a cytomegalovirus ELISPOT assay to predict outcome of low-level CMV reactivation in hematopoietic cell transplant recipients. J Infect Dis 2019; 219:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giménez E, Blanco-Lobo P, Muñoz-Cobo B, et al. Role of cytomegalovirus (CMV)-specific polyfunctional CD8+ T-cells and antibodies neutralizing virus epithelial infection in the control of CMV infection in an allogeneic stem-cell transplantation setting. J Gen Virol 2015; 96:2822–31. [DOI] [PubMed] [Google Scholar]
- 21. Saccoccio FM, Gallagher MK, Adler SP, McVoy MA. Neutralizing activity of saliva against cytomegalovirus. Clin Vaccine Immunol 2011; 18:1536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]