Abstract
Sepsis from Escherichia coli expressing the K1 antigen is a leading cause of death in neonates. In a murine model, E. coli K1 grew rapidly in the peritoneal cavity of neonatal mice, causing fatal disease. In contrast, adult mice cleared the infection. Neonatal mice mounted a rapid and equivalent antimicrobial immune response compared to adult mice. Interestingly, peritoneal fluid from neonatal mice contained significantly more total iron than that of adult mice, which was sufficient to support enhanced E. coli growth. Transient iron overload in adult mice infected with E. coli resulted in 100% mortality. Maternal diet–induced mild iron deficiency decreased offspring peritoneal iron, decreased bacterial growth, and conferred protection against sepsis. Taken together, neonatal susceptibility to E. coli K1 sepsis is enhanced by a localized excess of peritoneal iron that allows for unchecked bacterial growth. Targeting this excess iron may provide a new therapeutic target in human patients.
Keywords: sepsis, E. coli, neonatal, immunology, nutritional immunity, pediatrics
Sepsis is a leading cause of death in neonates. Neonatal mice have an iron rich niche in the peritoneal cavity that facilitates Escherichia coli K1 growth, and are protected by mild iron deficiency.
Early-onset sepsis, which occurs within the first 3–5 days after birth, afflicts 1 in 1000 live births in the United States and has a case fatality rate approaching 25% [1, 2]. Escherichia coli is a leading cause of early-onset sepsis in both preterm and term infants [2, 3]. Escherichia coli expressing the K1 antigen, which confers resistance to phagocytosis, is a neonatal-specific pathogen associated with neonatal sepsis and meningitis [4]. Infection occurs by translocation of E. coli K1 from the lumen of the gut and subsequent invasion into the bloodstream [5]. Antibiotic treatment must be initiated immediately to control disease and permit survival. This is challenging because sepsis often presents with nonspecific signs and symptoms without reliable biomarkers to assist in diagnosis. Antibiotic administration for all critically ill neonates is undesirable because unnecessary antibiotic exposure is associated with disruption in beneficial microbial colonization and increased autoimmunity and allergy later in life [3, 6]. It is therefore critical to identify additional therapeutic options in the treatment of neonatal sepsis.
Neonatal susceptibility to infections has been studied almost exclusively in the context of immune system immaturity [1, 3]. Previous research has shown that many aspects of the neonatal immune system are hyporesponsive when compared to that of adults. This includes decreased cytokine responses following Toll-like receptor (TLR) stimulation of leukocyte subsets and reduced ability to mobilize mature neutrophils during acute infection [7–10]. However, recent work suggests that neonatal immunity is not as suppressed as previously believed. Some studies report that neonatal monocytes and cord blood leukocytes produce equivalent, or enhanced, cytokine responses when stimulated with TLR agonists compared to adult cells [9, 11–13]. It is therefore important to consider both immune cell intrinsic and extrinsic factors when studying neonatal immunity.
Iron is a trace nutrient that virtually all organisms need for survival. Reduced iron acquisition cripples the virulence of many pathogens [14], and disrupted iron homeostasis renders hosts susceptible to infections [15–17]. In adult mammals at homeostasis, most iron is used for renewing erythrocytes with relatively low iron demand in other tissue types, which can be sustained with minimal dietary input [18]. In contrast, neonates have high iron demands to support blood expansion, tissue growth, and neurodevelopment [19, 20]. In the first 6 months of life, most iron needs can be met by mobilizing abundant stores of iron in the liver, which are present at birth. In the gut, the protein lactoferrin promotes high-efficiency iron absorption from breast milk [21]. In contrast to adults, who limit iron absorption if their internal stores are sufficient, iron absorption in the neonatal gut is more rapid and not downregulated by sufficient iron stores [22–24]. The developing neonate therefore must mobilize large amounts of iron to support growth, yet keep local levels low to prevent toxicity and infection.
In this study, we present evidence that neonates have an iron-rich niche in the peritoneal cavity that can permit outgrowth of pathogenic bacteria. This process occurs independently from immune cell function, and highlights a novel mechanism of neonatal susceptibility to a common pathogen.
METHODS
Animals
Animal protocols were approved by the University of Michigan Institutional Animal Care and Use Committee. C57BL/6 mice were bred and maintained at the university animal facility under standard specific-pathogen free conditions. A female mouse was placed in a cage with a male mouse for 5 days to generate timed pregnancies.
Bacterial Strain and Culture
Escherichia coli strain C5 was acquired from the American Type Culture Collection (ATCC 700973) and stored in glycerol. Bacteria were grown overnight in tryptic soy broth at 37°C, shaking at 200 rpm. The day of infection, bacteria were diluted 1:10 in tryptic soy broth and incubated for 1 hour at 37°C at 200 rpm. Bacterial concentration was determined by measuring culture optical density at 600 nm on a plate reader (Molecular Devices). Bacteria were washed in phosphate-buffered saline (PBS) and diluted for experiments.
Sepsis Model
Male and female neonatal (4–6 days old) and adult (2–4 months) mice were infected with 1 × 103 colony-forming units (CFU) of E. coli C5 in 50 μL PBS via intraperitoneal (IP) injection. Adult mice were infected with 1 × 104 CFU in 100 μL PBS. In select experiments, neonatal mice were infected by oropharyngeal gavage with 1 × 106 CFU in 10 μL PBS using a 24-gauge gavage needle (Prime Bioscience). Mice were euthanized if they reached humane endpoints. Neonatal mice were euthanized by decapitation. Blood was collected from the carcass into a plate treated with heparin (APP Pharmaceuticals). Adult mice were euthanized by sodium pentobarbital (Vortech Pharmaceuticals) and exsanguinated by cardiac puncture. Serum was collected after centrifugation at 3000 relative centrifugal force for 15 minutes. Peritoneal wash was performed in neonates by creating an incision in the abdomen, pipetting 300 μL PBS into the peritoneal cavity, and recovering the fluid via transfer pipette; in adult mice, peritoneal wash was collected in 3 mL through an 18-gauge needle. Bacterial burden was determined by serial dilutions, plating on tryptic soy agar, and colony enumeration. For select experiments, neonatal mice were injected with deferoxamine mesylate 100 mg/kg IP (Sigma-Aldrich) or deferiprone 50 mg/kg IP (Selleckchem) 4 hours prior to E. coli infection. For select experiments, adult mice were injected with 450 µg of sterile ammonium iron(III) citrate (FAC; Sigma-Aldrich).
For dietary iron manipulation, females were placed on the iron-sufficient diet for 2 weeks before mating (45 ppm iron, custom formulation of AIN-93, Envigo). At embryonic day 14, breeding females were either maintained on the iron-sufficient diet or were switched to the iron-deficient diet (2–4 ppm iron, AIN-93) for the remainder of pregnancy and lactation [25].
Adoptive Transfer
Spleens were collected from mice, disrupted into a cell suspension using a syringe, and separated using Ficoll-Isopaque centrifugation (GE Healthcare Biosciences); neutrophils and macrophages were collected from the pellet and buffy coat, respectively, and purified by CD11b+ magnetic separation (Miltenyi Biotec), and 1 × 105 neutrophils or macrophages from neonatal or adult mice were injected IP into neonatal mice immediately prior to E. coli K1 infection.
Peritoneal Wash In Vitro Experiments
Peritoneal wash fluid from naive neonatal and adult mice was centrifuged at 2500g for 5 minutes to generate cell-free fluid. Wash fluid was diluted to match protein content between samples. Escherichia coli K1 was seeded at 1 × 103 per 100 μL and incubated at 37°C with 5% carbon dioxide. At various timepoints, aliquots of the culture were removed for enumeration on tryptic soy agar plates. For select experiments, FAC 240 ng/well (Sigma-Aldrich) was added to wash fluid samples. Deferoxamine mesylate 1.5 µg/well (Sigma-Aldrich) or deferiprone 0.35 µg/well (Selleckchem) was added to neonatal wash fluid samples for select experiments.
Iron and Protein Measurement
Total iron was measured in peritoneal wash fluid and serum using the ferrozine assay from a commercial kit (Sigma-Aldrich). Protein was measured using the Bradford assay, and cytokines were measured by Bioplex (both from Bio-Rad).
In Vitro Phagocytosis and Killing Assays
Splenic macrophages and neutrophils were collected as previously described. Assays were conducted in complete RPMI (400 mM/L l-glutamine, 10% fetal calf serum, 1% sodium pyruvate, and 1% nonessential amino acids). Phagocytosis and intracellular killing were assessed using a gentamicin protection assay with 1 × 105 leukocytes and 1 × 107E. coli K1 CFU per well as previously described [26]. Opsonization-induced phagocytosis was measured by incubating E. coli with a 1:100 dilution of human immunoglobulin G (IgG) for 60 minutes (Sigma-Aldrich) followed by the addition of IgG-bound E. coli to neonatal or adult macrophages. Select experiments were performed after the addition of 50 µM FAC to cultures. Neutrophil total killing was measured both with and without the addition of 50 µM FAC to complete RPMI containing adult neutrophils at a concentration of 1 × 105 cells/well with an E. coli K1 concentration of 1 × 105 CFU/well.
Flow Cytometry
Peritoneal cells were collected from wash fluid by centrifugation and resuspended in PBS, 1% fetal calf serum, and 2 mM ethylenediaminetetraacetic acid. Cells were stained for 20 minutes with antimouse CD11b BV 785 clone M1/70, antimouse Ly6C PE clone HK1.4, antimouse Ly6G fluorescein isothiocyanate clone 1A8, antimouse F4/80 Pe-Cy7 clone BM8 antibodies at a 1:100 dilution (Biolegend), and purified human IgG (Sigma-Aldrich). Cells were enumerated by counting beads (Beckman Coulter). Cell populations were analyzed on a Novocyte with 405-, 488-, and 640-nm lasers (ACEA Biosciences).
Statistical Analysis
Data were analyzed using GraphPad Prism. Survival was assessed by Kaplan–Meier method and log-rank test. Bacterial burden loads were compared using the Mann–Whitney test. Iron, protein, and cytokine concentrations were compared by a 2-tailed t test, a one-way analysis of variance (ANOVA) with a Tukey post hoc test, or a 2-way ANOVA (multiple groups and multiple time points). A P value of .05 was considered significant.
RESULTS
Neonatal Mice Are Highly Susceptible to E. coli K1 Sepsis
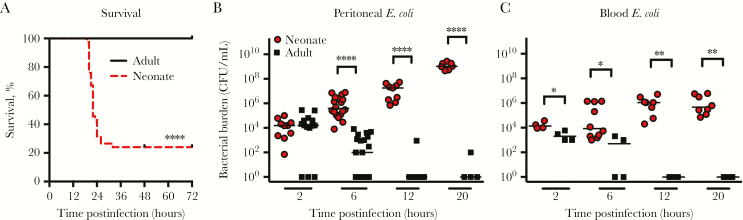
We generated a sepsis model by injecting 4- to 6-day-old neonatal mice IP with 1 × 103 CFU of E. coli K1 antigen C5. Adult mice were given 1 × 104 CFU IP to account for the approximately 10-fold difference in body size. IP injection has been used extensively by other groups to model both neonatal and adult sepsis in mice [27–30]. Treatment caused rapid death in neonatal mice, with 80% of all neonates dying within 30 hours of infection, but spared adult mice (Figure 1A). In neonates, E. coli K1 grew rapidly in the peritoneal cavity and blood between 2 and 20 hours postinfection, but was cleared by adults in 12 hours (Figure 1B and 1C).
Figure 1.
Neonatal mice are highly susceptible to Escherichia coli K1 sepsis. Neonatal 4- to 6-day-old mice and adult 2- to 4-month-old mice were infected intraperitoneally with 1 × 103 and 1 × 104 colony-forming units (CFU), respectively, of E. coli. A, Survival for 72 hours following infection (n = 18 adults, n = 79 neonates). ****P < .0001, log-rank test. Bacterial burden in the peritoneal wash (B) and blood (C) of infected animals. Results are combined for 2 independent experiments. Bars represent median. *P < .05, **P < .01, ****P < .0001, Mann–Whitney test.
Neonatal Mice Exhibit a Robust Antimicrobial Response to E. coli K1
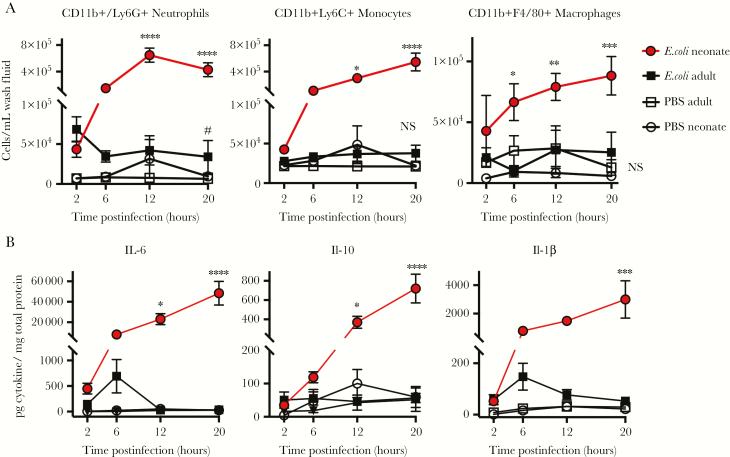
Neonatal immunity is considered immature, so we hypothesized that decreased immune activation contributed to E. coli K1 sepsis susceptibility. We assessed immune cell recruitment by flow cytometry in the first 20 hours post–E. coli infection (Supplementary Figure 1). Neonatal mice infected with E. coli exhibited an infiltration of neutrophils, monocytes, and macrophages shortly after infection. In contrast, there was minimal leukocyte recruitment in adult mice treated with E. coli (Figure 2A). Neonates expressed significantly elevated levels of the proinflammatory cytokines interleukin (IL) 6, IL-10, and IL-1β compared to adult mice (Figure 2B). The lack of proinflammatory cytokines in adult mice is consistent with the observation that adult mice rapidly cleared the bacteria and never became ill. These results show that neonatal mice generate a robust proinflammatory response to infection.
Figure 2.
Neonatal mice have an intact innate immune response to Escherichia coli K1 infection. Adult 2- to 4-month-old mice and neonatal 4- to 6-day-old mice were injected intraperitoneally with 104 or 103E. coli colony-forming units, respectively, or an equivalent volume of phosphate-buffered saline. Peritoneal wash was harvested from mice at indicated timepoints (300 μL from neonates, 3 mL from adults). Cell populations were analyzed for CD11b+/Ly6G+ Neutrophils, CD11b+Ly6C+ Monocytes, and CD11b+F4/80 Macrophages (A). #P < .05, indicating that E. coli adult is significantly different from PBS adult. *P < .05, **P < .01, ***P < .001, ****P < .0001, indicating that E. coli neonate is significantly different from all other groups, 2-way analysis of variance (ANOVA) Tukey multiple comparisons test. NS, not significant; n = 4–6, representative results of 2 independent experiments. Peritoneal wash fluid from infected neonates and adults was analyzed for interleukin (IL) 6, IL-10, and IL-1β (B). *P < .05, **P < .01, ***P < .001, ****P < .0001, 2-way ANOVA Tukey multiple comparisons test; n = 4–8 per group per time point, combined results from 2 separate experiments. Bars indicate ±1 standard error of the mean.
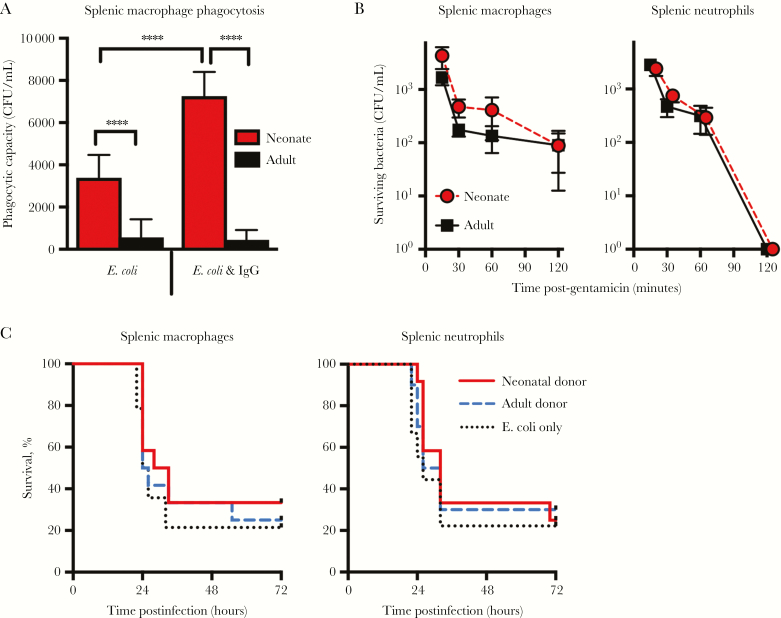
To further interrogate the neonatal immune response, we isolated splenic macrophages and neutrophils from naive neonatal and adult mice and assessed their phagocytosis and intracellular killing capacity. We could not harvest sufficient purified peritoneal leukocytes from naive mice to conduct functional assays. Splenic macrophages isolated from neonatal mice had an enhanced capacity to phagocytose E. coli K1 compared to those from adult mice, both with and without pretreatment with IgG (Figure 3A). Internalized bacteria were killed similarly by splenic macrophages and neutrophils from adult and neonatal mice (Figure 3B). We hypothesized that the in vitro assays may not recapitulate differences between neonatal and adult phagocytes present in vivo. We therefore harvested splenic macrophages from adult and neonatal mice, and infected neonatal mice with 1 × 103E. coli CFU alone, E. coli plus 1 × 105 macrophages from adult donors, or E. coli plus 1 × 105 macrophages from neonatal donors. There was no protective benefit from transferring adult macrophages at the time of infection (Figure 3C). We found similar results with splenic neutrophils (Figure 3C). Taken together, these results suggest that inherent defects in neonatal immune cell function do not fully account for the rapid outgrowth of bacteria in the peritoneal cavity and subsequent death of neonatal mice.
Figure 3.
Neonatal neutrophils and macrophages do not demonstrate defects in Escherichia coli K1 phagocytosis and killing. A, Phagocytic capacity of neonatal or adult CD11b+ splenic macrophages plated at a multiplicity of infection of 100, treated with or without human immunoglobulin G (IgG) followed by a gentamicin protection assay to determine how many bacteria were engulfed; n = 6 per group and results combined from 2 separate experiments. ****P < .0001, one-way analysis of variance, multiple comparisons test (F value = 87.94, degrees of freedom = 35). Escherichia coli intracellular killing capacity determined by gentamicin protection assay using splenic CD11b+ macrophages and splenic CD11b+ neutrophils collected from neonatal and adult mice (B); n = 6 per group per time point and results are combined from 2 independent experiments. Bars represent ± standard error of the mean. C, 1 × 105 splenic macrophages (n = 12–14 per group) or 1 × 105 splenic neutrophils from adult (2–4 months old) or neonatal (4–6 days old) mice (n = 10–12 per group) were adoptively transferred intraperitoneally (IP) into neonatal mice immediately prior to IP E. coli infection with 103 colony-forming units (CFU). Recipients were monitored for survival using the log-rank test.
Neonatal Mice Have Excess Iron in the Peritoneal Cavity
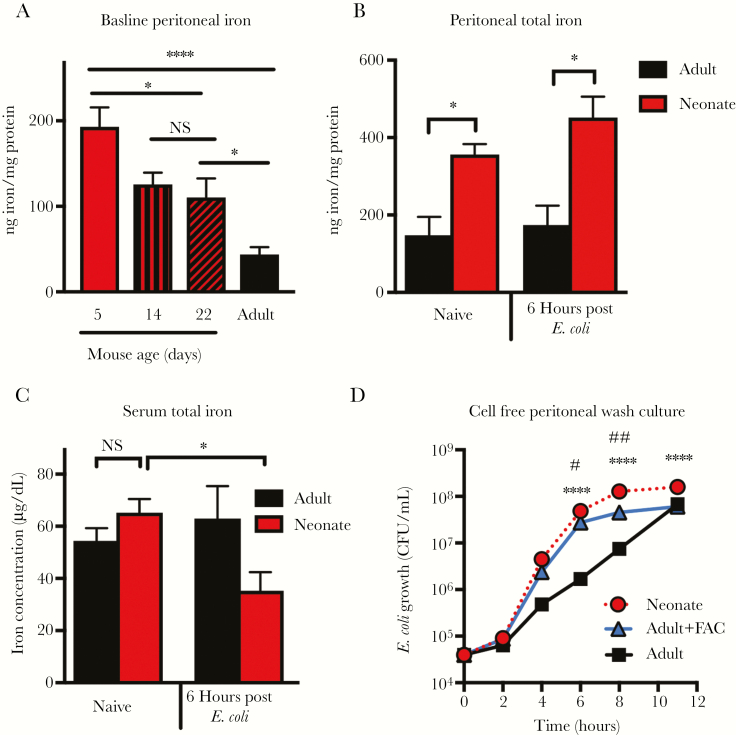
We investigated other factors that might influence bacterial growth. As iron is known to influence bacterial growth during infections, we measured iron levels in adult and neonate peritoneal wash fluid and found elevated iron levels in neonates compared to adults, which gradually decreased with age (Figure 4A and 4B). Iron concentration was higher in neonates when normalized to either body size or protein content (Supplementary Figure 2A and 2B). We found that naive neonatal and adult mice had similar serum iron levels, and that these iron levels dropped in the neonates but not in the adults following infection (Figure 4C). Thus the neonates exhibited a well-described defensive action against infection [17]. Neonatal and adult mice had similar iron content in the lung, a relevant target in sepsis (Supplementary Figure 2C). As adults were able to rapidly clear bacteria with minimal inflammation, they did not develop hypoferremia.
Figure 4.
Neonatal mice have increased total iron in their peritoneal cavity allowing for rapid growth of Escherichia coli K1. Total iron content was measured in peritoneal wash fluid (n = 6–12 per group; F value of 11.03, degrees of freedom [df] = 30) (A and B) and serum from neonatal (4–6 days old) and adult (2–4 months old) mice (n = 28, n = 11, n = 5, and n = 13 for control neonates, infected neonates, control adults, and infected adults, respectively; F value = 2.64, df = 56) (C). Data were pooled from 2 independent experiments. NS, not significant; *P < .05, one-way analysis of variance (ANOVA), Tukey post hoc test. Error bars indicate ± standard error of the mean. D, Growth of E. coli K1 (colony-forming units [CFU]) in cell-free peritoneal wash fluid of neonatal and adult mice was compared to adult wash fluid supplemented with ammonium iron(III) citrate (FAC) 240 ng/well. n = 3 per time point, representative results of 3 experiments. ****P < .0001, #P < .05, ##P < .01, 2-way ANOVA Tukey multiple comparisons test.
We next removed cells from the peritoneal wash fluid of experimental mice by centrifugation. We seeded wells with E. coli K1, added the cell free wash fluid, and assessed bacterial growth in wells containing wash fluid from neonates, adults, or adult wash fluid supplemented with sufficient FAC to normalize iron levels between adults and neonates. Escherichia coli K1 grew more slowly in adult peritoneal wash fluid compared to neonatal peritoneal wash fluid, but had equivalent growth with the addition of FAC (Figure 4C). These results suggest that the difference in iron availability between the neonatal and adult environment is sufficient to enhance bacterial growth. Because neonatal sepsis is caused by translocation of bacteria from the lumen of the gut, we investigated whether bacteria could gain access to the peritoneal cavity and bloodstream by the oral route. We gavaged neonatal mice with E. coli K1, and observed that 47% of neonates had detectable bacteria in the peritoneal cavity and 53% had detectable bacteria in the blood 24 hours after treatment, suggesting translocation can occur during orally acquired infection (Supplementary Figure 3). These results show that neonatal mice have localized excess iron in the peritoneal cavity that might contribute to their enhanced susceptibility to infection.
Iron Supplementation Sensitizes Adult Mice to E. coli K1 Sepsis
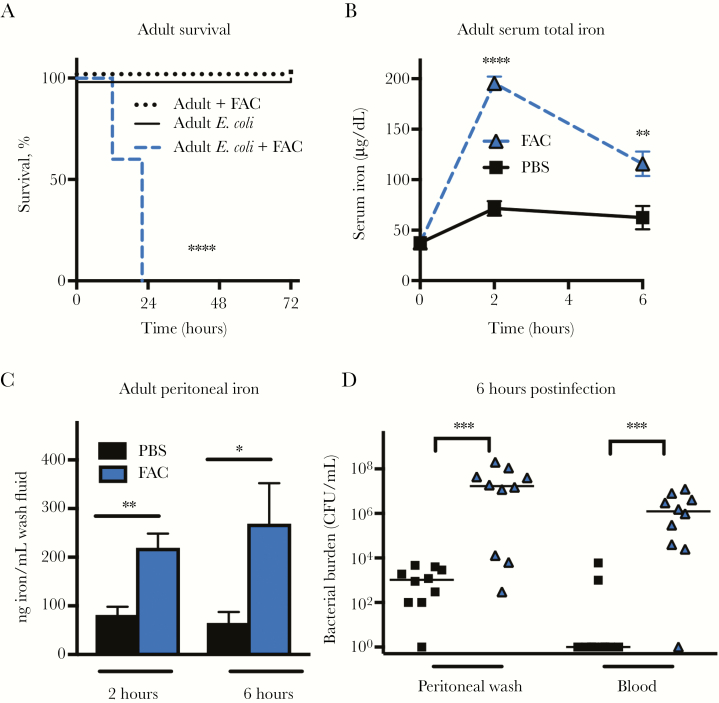
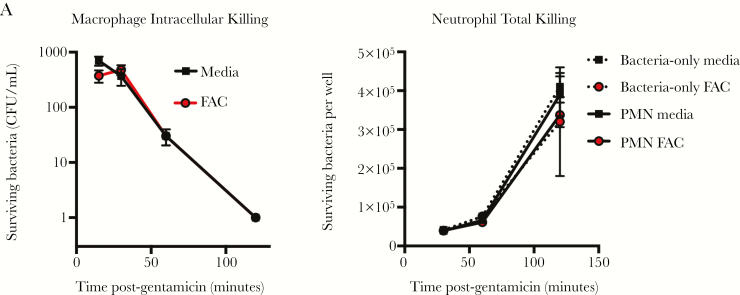
Next, we sought to determine whether elevated iron levels could sensitize adult mice to E. coli K1 infection. We injected adult mice with either PBS or with 450 μg of FAC IP at the time of E. coli injection. This amount of FAC is 1000-fold lower than the dose required to induce clinically relevant iron overload [15, 31]. We observed that adult mice injected with E. coli and PBS or FAC alone all survived, but adult mice injected with FAC and E. coli all died (Figure 5A). Treatment resulted in increased serum iron and peritoneal iron 2 and 6 hours postinjection (Figure 5B and 5C). Mice injected with FAC displayed a rapid outgrowth of bacteria in the peritoneal cavity and blood compared to PBS-treated mice (Figure 5D). In vitro, the addition 50 μM FAC (a concentration 40% higher than peak iron levels detected in the serum of FAC-treated mice) had no effect on the bactericidal capacity of adult splenic macrophages or neutrophils (Figure 6A and 6B). These results suggest that modestly elevated iron levels at the site of infection are sufficient to allow bacterial outgrowth without significantly impacting immune function.
Figure 5.
Supplemental iron sensitizes adult mice to Escherichia coli K1 sepsis and allows for rapid bacterial growth. Adult mice (2–4 months old) were injected intraperitoneally (IP) with 450 μg of ammonium iron(III) citrate (FAC) or an equivalent volume of phosphate-buffered saline (PBS) IP immediately prior to injection with 104 colony-forming units (CFU) of E. coli K1 IP. A, Survival of adult mice injected with FAC alone (n = 6), E. coli alone (n = 10), or E. coli with FAC (n = 10). ****P < .0001 using the log-rank test. B, Total iron in serum of adult mice injected with FAC or PBS at the time of infection. n = 4–12 per time point. Results are the combination of 2 experiments. **P < .01, ****P < .0001 using the 2-way analysis of variance Tukey multiple comparisons test. C, Peritoneal total iron levels 6 hours post–E. coli infection in FAC- and PBS-treated adult mice (n = 4–6 per group, t = 1.98, degrees of freedom = 9). Iron content is presented as ng/mL rather than ng iron/mg protein because total peritoneal protein content was significantly increased in FAC-treated mice. Error bars indicate ±1 standard error of the mean. *P < .05, **P < .01, 2-tailed t test. D, Bacterial burden in the peritoneal wash and blood of adult mice pretreated with FAC or PBS prior to E. coli infection. Bars indicate median CFU. *P < .05, ***P < .001, Mann–Whitney test. Data are combined results of 2 independent experiments.
Figure 6.
Iron supplementation does not adversely affect macrophage or neutrophil bacterial killing. Effect of 50 μM ammonium iron(III) citrate supplementation on in vitro splenic CD11b+ macrophage intracellular killing (A) and splenic CD11b+ neutrophil total killing (B) in 2- to 4-month-old adult mice using the gentamicin protection assay. Error bars indicate ±1 standard error of the mean, n = 4 per group, Two-way analysis of variance Tukey multiple comparisons test. Results represent 2 independent experiments. Abbreviations: CFU, colony-forming units; FAC, ammonium iron(III) citrate; PMN, polymorphonuclear cells.
Dietary Iron Restriction During Pregnancy Provides a Survival Advantage in Neonatal E. coli K1 Sepsis
To determine whether elevated peritoneal iron contributed to neonatal sepsis, we manipulated iron levels in the neonates. Treatment with the iron chelators deferiprone and deferoxamine did not inhibit bacterial growth in vitro (Supplementary Figure 4A and 4B), lower peritoneal iron content in the neonates (Supplementary Figure 4C), or protect against infection (Supplementary Figure 4D and 4E), and showed evidence of toxicity. These results are consistent with reports that clinical iron chelators do not directly inhibit iron acquisition in Enterobacteriaceae, suggesting that iron chelation therapy does not target the peritoneally localized excess of iron in neonatal mice [32]. We then developed a model of mild maternal dietary iron deficiency during pregnancy and lactation to evaluate its impact on iron status and response to E. coli K1 sepsis in offspring [25]. We placed breeding females on an iron-sufficient diet (45 ppm) for 2 weeks before mating. On embryonic day 14, females were either switched to a diet containing 2–4 ppm iron or were maintained on their current diet of 45 ppm iron.
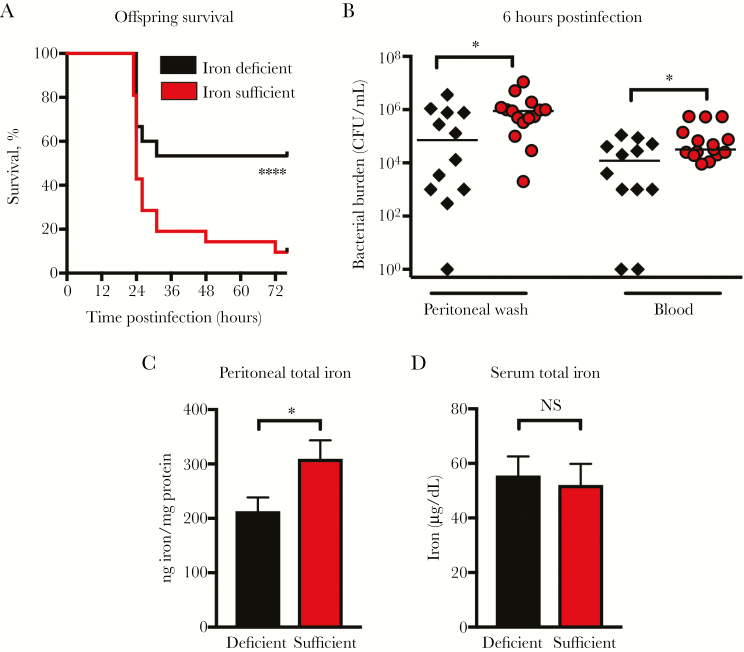
Offspring from mothers placed on an iron-deficient diet during late gestation had a marked survival advantage during neonatal sepsis compared to offspring of mothers on an iron-sufficient diet (Figure 7A). Increased survival was accompanied by decreased bacterial burden in the peritoneal cavity and blood 6 hours postinfection (Figure 7B). Consistent with increased protection, offspring from the iron-deficient mothers had significantly lower iron content in the peritoneal wash fluid compared to offspring from iron-sufficient mothers (Figure 7C). Offspring from iron-deficient and iron-sufficient mothers had similar serum iron levels (Figure 7D).
Figure 7.
Maternal diet–induced mild iron deficiency decreased offspring peritoneal iron and provided a survival advantage in neonatal Escherichia coli K1 sepsis. Breeding female mice were placed on an iron-sufficient diet (45 ppm iron) for 14 days prior to mating, and at day E14 were switched to an iron-deficient diet (2–4 ppm iron) or were maintained on the iron-sufficient diet (45 ppm iron). Offspring were infected with 103 colony-forming units (CFU) of E. coli K1 IP when 4–6 days old. A, Survival of offspring infected with E. coli. Iron-deficient, n = 15; iron-sufficient, n = 21. Results are a combination of 2 independent experiments. ****P < .0001, log-rank test. B, Bacterial burden in the peritoneal wash and blood of infected offspring. Iron-deficient, n = 12; iron-sufficient, n = 15. Results are a combination of 2 independent experiments. *P < .05, Mann–Whitney test. Iron levels in the peritoneal wash fluid (n = 12–23 per group, t = 2.093, degrees of freedom [df] = 37) (C) and serum (n = 6–12 per group, t = 0.323, df = 19) (D) of offspring 6 hours after infection. Results are a combination of 2 independent experiments. *P < .05, Mann–Whitney test. NS = not significant. Error bars indicate ±1 standard error of the mean.
DISCUSSION
This study demonstrates that neonatal mice have increased iron levels in the peritoneal cavity compared to adult mice, which is sufficient to promote the rapid outgrowth of E. coli K1. Host sequestration of trace minerals is known to play a pivotal role in resistance to infectious disease [14, 28, 33]. The clinical relevance of our findings is supported by reports that parenteral iron supplementation in healthy term infants has been associated with increased rates of E. coli sepsis and meningitis [34, 35]. Taken together, these studies and our own data identify iron availability as an important factor in determining neonatal susceptibility to infection.
To our knowledge, we are the first to demonstrate that neonatal mice have an iron-rich niche in the peritoneal cavity that permits bacterial growth. It is unclear how this occurs. During the neonatal period, breast milk is the sole source of iron intake. While breast milk is low in iron content, it is well absorbed by the neonatal intestine [21]. Both rodent and human neonatal intestines have enhanced iron absorption activity compared to those of adults [22, 23, 36, 37]. The mechanisms of increased absorption are incompletely understood, but there is evidence that iron is retained in the intestinal wall [22, 37]. Decreased intestinal barrier function may also influence the increased neonatal intestinal iron absorption [22, 37]. Further investigation is needed to understand the mechanisms of neonatal intestinal iron absorption and how these contribute to excess peritoneal iron levels.
Neonatal immunology has mostly focused on cell-intrinsic deficiencies and hematopoietic system immaturity [13]. While these observations are undeniably important, we have identified iron availability as a contributor that warrants further investigation. Consistent with other reports [30, 38], we found that neonates were profoundly susceptible to E. coli K1 sepsis, whereas adult mice easily cleared the infection. In contrast to a previous report that neonatal rat neutrophils exhibited decreased killing capacity against E. coli K1 compared to adult neutrophils [39], we found that adult neutrophils and macrophages were able to kill E. coli K1 equivalently. We attribute this discrepancy to more general observations that the neonatal immune response displays effective antimicrobial responses against some pathogens but attenuated responses against others [9, 12, 13]. Our data indicate the importance of cell-extrinsic factors in neonatal sepsis, particularly the role of iron.
There are limitations that need discussion. We cannot completely exclude the possibility that neonatal immune deficiencies contribute to E. coli K1 sepsis. Although our results suggest that increased iron levels cause bacterial outgrowth rather than impairment of the immune response, it has been reported that iron overload can alter immune function by enhancing proinflammatory responses [40–42]. However, these studies utilized severe iron overloading protocols and reported larger perturbations in iron homeostasis than what we observed. Sepsis pathology occurs as a complex interaction between unchecked bacterial growth and the host’s immune response, making it difficult to separate the effects of bacterial growth from immune-mediated pathology [43]. We acknowledge that the iron-enhanced growth may not be applicable to all neonatal infections. Furthermore, as we found excess extracellular iron levels to be site-specific, E. coli may need to penetrate the peritoneal space to access iron. It is unclear if bacteria readily access the peritoneal cavity during human infection, but our data demonstrate that bacteria in the lumen of the gut can readily reach the peritoneal cavity in neonatal mice [44, 45].
These findings suggest that iron metabolism is a component in neonatal vulnerability to certain infections and is a possible therapeutic target. While we found that marginal iron deficiency during pregnancy protected against neonatal sepsis, this is not a reasonable treatment strategy due to the importance of iron in neurodevelopment [46]. Our results highlight the importance of understanding more about neonatal iron metabolism so that an alternative treatment for neonatal E. coli sepsis can be identified. Clinical trials have shown that supplementation of very low birth weight neonates with bovine lactoferrin, an iron transporter that regulates iron availability in the intestine and serum, protects against the development of sepsis through unclear mechanisms [47–50]. Our studies may provide insights into why lactoferrin supplementation trials have been successful in other countries, and provide scientific justification to implement more of these studies in the United States. In conclusion, we believe our innovative studies on the role of peritoneal iron levels in neonatal sepsis open up a previously underinvestigated avenue of research on the role of cell extrinsic factors in neonatal immunology.
SUPPLEMENTARY DATA
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the National Institutes of Health Child Health Research Center (grant number 5K12HD028820-22); the University of Michigan Institute for Clinical and Health Research (grant number UL1TR000433); the Ament-Heller Award from the Department of Pediatrics at the University of Michigan; and private funds from the Korneffel family.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Society for Leukocyte Biology Meeting, Chandler, Arizona, October 2018; and American Society for Investigative Pathology Meeting, Ann Arbor, Michigan, October 2018
References
- 1. Kan B, Razzaghian HR, Lavoie PM. An immunological perspective on neonatal sepsis. Trends Mol Med 2016; 22:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weston EJ, Pondo T, Lewis MM, et al. . The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J 2011; 30:937–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camacho-Gonzalez A, Spearman PW, Stoll BJ. Neonatal infectious diseases: evaluation of neonatal sepsis. Pediatr Clin North Am 2013; 60:367–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cross AS, Gemski P, Sadoff JC, Orskov F, Orskov I. The importance of the K1 capsule in invasive infections caused by Escherichia coli. J Infect Dis 1984; 149:184–93. [DOI] [PubMed] [Google Scholar]
- 5. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev 2014; 27:21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arboleya S, Sánchez B, Milani C, et al. . Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr 2015; 166:538–44. [DOI] [PubMed] [Google Scholar]
- 7. Bermick JR, Lambrecht NJ, denDekker AD, et al. . Neonatal monocytes exhibit a unique histone modification landscape. Clin Epigenetics 2016; 8:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol 2014; 35:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kollmann TR, Crabtree J, Rein-Weston A, et al. . Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009; 183:7150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yost CC, Cody MJ, Harris ES, et al. . Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood 2009; 113:6419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nohmi K, Tokuhara D, Tachibana D, et al. . Zymosan induces immune responses comparable with those of adults in monocytes, dendritic cells, and monocyte-derived dendritic cells from cord blood. J Pediatr 2015; 167:155–62.e1–2. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen M, Leuridan E, Zhang T, et al. . Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS One 2010; 5:e10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harbeson D, Ben-Othman R, Amenyogbe N, Kollmann TR. Outgrowing the immaturity myth: the cost of defending from neonatal infectious disease. Front Immunol 2018; 9:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hennigar SR, McClung JP. Nutritional immunity: starving pathogens of trace minerals. Am J Lifestyle Med 2016; 10:170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michels KR, Zhang Z, Bettina AM, et al. . Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight 2017; 2:e92002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michels K, Nemeth E, Ganz T, Mehrad B. Hepcidin and host defense against infectious diseases. PLoS Pathog 2015; 11:e1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armitage AE, Eddowes LA, Gileadi U, et al. . Hepcidin regulation by innate immune and infectious stimuli. Blood 2011; 118:4129–39. [DOI] [PubMed] [Google Scholar]
- 18. Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe 2013; 13:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Domellöf M. Iron requirements in infancy. Ann Nutr Metab 2011; 59:59–63. [DOI] [PubMed] [Google Scholar]
- 20. Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early-life iron deficiency: outcomes at 25 years. J Pediatr 2013; 163:1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shashiraj, Faridi MM, Singh O, Rusia U. Mother’s iron status, breastmilk iron and lactoferrin—are they related? Eur J Clin Nutr 2006; 60:903–8. [DOI] [PubMed] [Google Scholar]
- 22. Gallagher ND, Mason R, Foley KE. Mechanisms of iron absorption and transport in neonatal rat intestine. Gastroenterology 1973; 64:438–44. [PubMed] [Google Scholar]
- 23. Darshan D, Wilkins SJ, Frazer DM, Anderson GJ. Reduced expression of ferroportin-1 mediates hyporesponsiveness of suckling rats to stimuli that reduce iron absorption. Gastroenterology 2011; 141:300–9. [DOI] [PubMed] [Google Scholar]
- 24. Frazer DM, Wilkins SJ, Darshan D, Mirciov CSG, Dunn LA, Anderson GJ. Ferroportin is essential for iron absorption during suckling, but is hyporesponsive to the regulatory hormone hepcidin. Cell Mol Gastroenterol Hepatol 2017; 3:410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hubbard AC, Bandyopadhyay S, Wojczyk BS, Spitalnik SL, Hod EA, Prestia KA. Effect of dietary iron on fetal growth in pregnant mice. Comp Med 2013; 63:127–35. [PMC free article] [PubMed] [Google Scholar]
- 26. Subashchandrabose S, Smith SN, Spurbeck RR, Kole MM, Mobley HL. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog 2013; 9:e1003788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deshmukh HS, Liu Y, Menkiti OR, et al. . The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 2014; 20:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stefanova D, Raychev A, Deville J, et al. . Hepcidin protects against lethal Escherichia coli sepsis in mice inoculated with isolates from septic patients. Infect Immun 2018; 86. doi: 10.1128/IAI.00253-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elahi S, Ertelt JM, Kinder JM, et al. . Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013; 504:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cuenca AG, Joiner DN, Gentile LF, et al. . TRIF-dependent innate immune activation is critical for survival to neonatal gram-negative sepsis. J Immunol 2015; 194:1169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duong-Nu TM, Jeong K, Hong SH, et al. . All three TonB systems are required for Vibrio vulnificus CMCP6 tissue invasiveness by controlling flagellum expression. Infect Immun 2016; 84:254–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan GC, Chan S, Ho PL, Ha SY. Effects of chelators (deferoxamine, deferiprone and deferasirox) on the growth of Klebsiella pneumoniae and Aeromonas hydrophila isolated from transfusion-dependent thalassemia patients. Hemoglobin 2009; 33:352–60. [DOI] [PubMed] [Google Scholar]
- 33. Ganz T. Iron and infection. Int J Hematol 2018; 107:7–15. [DOI] [PubMed] [Google Scholar]
- 34. Barry DM, Reeve AW. Increased incidence of gram-negative neonatal sepsis with intramuscular iron administration. Pediatrics 1977; 60:908–12. [PubMed] [Google Scholar]
- 35. Farmer K. Letter: iron injections and the newborn. N Z Med J 1973; 78:500–1. [PubMed] [Google Scholar]
- 36. Collard KJ. Iron homeostasis in the neonate. Pediatrics 2009; 123:1208–16. [DOI] [PubMed] [Google Scholar]
- 37. Arévalo Sureda E, Weström B, Pierzynowski SG, Prykhodko O. Maturation of the intestinal epithelial barrier in neonatal rats coincides with decreased FcRn expression, replacement of vacuolated enterocytes and changed Blimp-1 expression. PLoS One 2016; 11:e0164775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He X, Zeng Q, Puthiyakunnon S, et al. . Lactobacillus rhamnosus GG supernatant enhance neonatal resistance to systemic Escherichia coli K1 infection by accelerating development of intestinal defense. Sci Rep 2017; 7:43305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lassiter HA, Christensen RD, Parker C, Rothstein G. Neutrophil-mediated killing, opsonization, and serum-mediated killing of Escherichia coli K1 by neonatal rats. Biol Neonate 1988; 53:156–62. [DOI] [PubMed] [Google Scholar]
- 40. Kao JK, Wang SC, Ho LW, et al. . Chronic iron overload results in impaired bacterial killing of THP-1 derived macrophage through the inhibition of lysosomal acidification. PLoS One 2016; 11:e0156713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sindrilaru A, Peters T, Wieschalka S, et al. . An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 2011; 121:985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ward RJ, Wilmet S, Legssyer R, et al. . Effects of marginal iron overload on iron homeostasis and immune function in alveolar macrophages isolated from pregnant and normal rats. Biometals 2009; 22:211–23. [DOI] [PubMed] [Google Scholar]
- 43. Dellinger RP, Levy MM, Rhodes A, et al. . Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Obata-Yasuoka M, Ba-Thein W, Tsukamoto T, Yoshikawa H, Hayashi H. Vaginal Escherichia coli share common virulence factor profiles, serotypes and phylogeny with other extraintestinal E. coli. Microbiology 2002; 148:2745–52. [DOI] [PubMed] [Google Scholar]
- 45. McCarthy AJ, Negus D, Martin P, et al. . Pathoadaptive mutations of Escherichia coli K1 in experimental neonatal systemic infection. PLoS One 2016; 11:e0166793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Radlowski EC, Johnson RW. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci 2013; 7:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He Y, Cao L, Yu J. Prophylactic lactoferrin for preventing late-onset sepsis and necrotizing enterocolitis in preterm infants: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2018; 97:e11976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaur G, Gathwala G. Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neonates: a randomized placebo-controlled clinical trial. J Trop Pediatr 2015; 61:370–6. [DOI] [PubMed] [Google Scholar]
- 49. Manzoni P, Rinaldi M, Cattani S, et al. . Italian Task Force for the Study and Prevention of Neonatal Fungal Infections, Italian Society of Neonatology Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA 2009; 302:1421–8. [DOI] [PubMed] [Google Scholar]
- 50. Sharma D, Shastri S. Lactoferrin and neonatology—role in neonatal sepsis and necrotizing enterocolitis: present, past and future. J Matern Fetal Neonatal Med 2016; 29:763–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.