Abstract
Background:
Heartmate III (HM3) Left Ventricular Assist Device (LVAD) (Abbott Inc, Chicago IL) is a fully magnetically levitated centrifugal implantable pump used to treat chronic heart failure patients. The MOMENTUM trial demonstrated that patients treated with the HM3 experienced reduced need for reoperation for LVAD replacement compared to a control group receiving an axial flow design, Heartmate II (HMII, Abbott Inc. Chicago IL). However, there are few reports of using HM3 as the replacement pump in patients, who already are supported by a durable LVAD and experienced a device related complication, necessitating replacement.
Methods:
An institutional LVAD database was used to identify, 19 consecutive patients who underwent pump replacement to HM3 (group 1), versus 85 consecutive control patients who underwent pump replacement to either HMII or HVAD (Medtronic Inc.) (group2), at a single institution from January 2010 to August 2018. Patient baseline characteristic and outcomes were obtained from a prospectively maintained database. The primary endpoint was a composite of freedom from death or need for another replacement surgery.
Results:
There was no difference between the groups in HF etiology, indication for replacement as well as the average days on the previous pump or the type of previous pump. The HM3 group did have a significantly higher body mass index (37 vs 31.6 p=0.01), a higher number of previous LVAD implants (36.8% vs 5.9%, had two previous LVADs, p<0.001) and a higher number of previous sternotomies (31.6% vs 7.1%, had three previous sternotomies, p=0.001). No difference was found between the groups in terms of post-operative adverse event rates. With regards to the primary endpoint, the patients with HM3 replacements (group 1) versus group 2, experienced significantly greater freedom from either death or need for another replacement during the follow up period (P=0.039). During follow up, there were no thrombosis events for the patients who received replacement with HM3.
Conclusion:
Therefore, we conclude that LVAD replacement with HM3 can be performed safely and may be considered as the pump of choice in patients requiring LVAD replacement.
Keywords: HMIII, Pump Thrombosis, circulatory assist devices
Graphical Abstract:
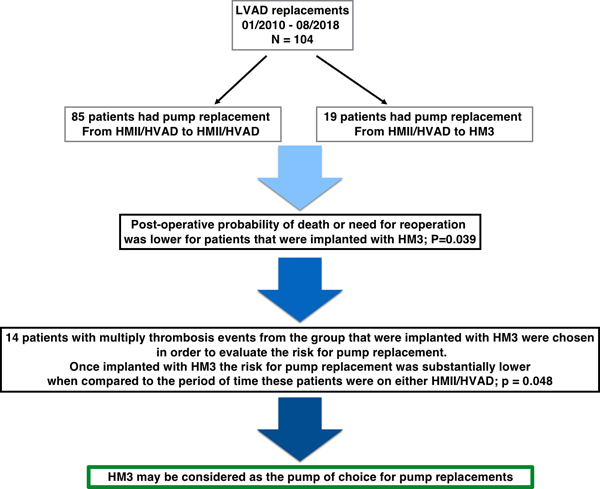
Study outline describing the study cohort, purpose, results and conclusion.
Introduction
Rotary flow, durable LVADs are increasingly utilized for patients with chronic, end stage heart failure. While newer rotary flow designs have significantly improved pump durability, LVAD replacement procedures are still required in some instances. Potential indications for rotary LVAD replacement fall into three broad categories: pump thrombosis/hemolysis, device malfunction, and device related infections. Following replacement, Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) data demonstrate that need for another replacement procedure and overall mortality is increased relative to rates after primary implants (1). Thus, these patients who require replacement represent a group that is at high risk for future adverse events and should be studied independent of primary implants.
Recently, the MOMENTUM trial has demonstrated reduced rates of thrombosis in primary implants with a fully magnetically levitated centrifugal LVAD (HeartMate III, HM3, Abbott Inc.) versus rates with an earlier axial flow design (HeartMate II, HMII, Abbott Inc.) (2). Outcomes after replacement with the HM3 have not been extensively reported and the MOMENTUM trial excluded replacement cases. Patients who have developed need for replacement, may be at increased risk for adverse events which may lead to the need for a second replacement (1,3,4). In this report, we compare early outcomes following pump replacement with HM3 versus replacements performed with either HMII or HVAD devices. In addition to mortality, need for another replacement is captured in a composite outcome.
Methods
Patients Study protocol
The Duke University LVAD database was reviewed, and all consecutive adult patients undergoing a durable, rotary flow, LVAD replacement procedure between January 2010 to August 2018 were included in the analysis. Pediatric cases, and those involving extracorporeal or pulsatile LVAD systems were excluded. LVAD replacement procedure was defined as removal of an implanted LVAD system and insertion of another implanted system. The indication for device replacement was determined from the operative note and divided into three categories: 1) pump dysfunction - mechanical/electrical failure, 2) pump thrombosis/hemolysis and 3) device infection. IRB approval for the analysis was obtained and need for individual patient consent was waived. All baseline and outcomes data were extracted from the pre-existing database or from electronic medical record related to the surgical encounter and all subsequent encounters. RV dysfunction was evaluated by ECHO according to the INTERMACS definitions. The primary endpoint was a composite consisting of freedom from death or need for another replacement surgery.
A total of 104 patients underwent 125 of durable LVAD replacement surgeries. Replacements were conducted with three different durable devices: HVAD (Medtronic Inc. Minneapolis MN), HMII and HM3 (Abbott Inc. Chicago Il.) In order to examine the utility of replacement with a fully magnetically levitated centrifugal durable device (HM3), we compared early outcomes following HM3 replacements (n=19 patients, Group 1) versus replacement cases with either HMII or HVAD (n = 85 patients, Group 2). Notably for the primary composite end point, the groups are distinct from each other and for patients with multiple replacement procedures, the most recent replacement was included in the analysis. For example, a patient that was first replaced with a HMII, but subsequently required a second replacement in which HM3 was used, was included only in the HM3 group (group 1).
Statistics:
Baseline characteristic and in hospital morbidities are presented for the most recent pump replacement and were compared between HM3 patients and HMII/HVAD patients. All comparisons were two-sided and a p-value of 0.05 was considered significant. The primary endpoint for the analysis was freedom from death or the need for reoperation for another replacement, comparing group 1 patients (HM3 devices) to group 2 patients (HMII/HVAD) device. All continuous variables were checked for normality using Shapiro-Wilk test. (Only age and BMI were normally distributed, the remaining variables were skewed). All normal data are shown as mean +/−SD, non-normal data are shown as median with minimum and maximum values reported. Discrete variables are reported as a percentage. Continuous variables were compared between the groups with a Wilcoxon-rank sum non-parametric test. Categorical variables were compared across the groups with Fisher exact test. Analyses were performed in R 2.14.2 software using the ‘survival’ package.
In a separate analysis, outcomes for all procedures were presented only for Group 1 patients that had pump thrombosis as their reason for replacement (n=14). These 14 patients had a total of 39 LVAD implant procedures (14 primary and 25 replacements). HM3 was the 4th device for 2 patients, the 3rd device for 7 patients, and the 2nd device for 5 patients. Time to replacement for thrombosis was plotted for initial procedures including the primary (n=25) which did not use the HM3, to the course following HM3 replacement (n= 14). All serum LDH values before and after the HM3 replacement were obtained from the EMR and plotted, a LOESS curve was used to describe the average weighted LDH values of the population.
Results:
Baseline characteristics
During the study period, 104 patients underwent 125 durable LVAD pump replacements; 30 pump replacements were performed in 19 patients that eventually were implanted with HM3 and these patients comprised group 1. 95 pump replacements were performed in 85 patients that were eventually either implanted with HMII or HVAD, these patients comprised group 2. The mean follow-up for group 1 was of 221 days (range: 34–711 days) and the mean follow-up for group 2 was 565 days (range: 4–2709 days). There was no difference between the groups in terms of age, gender, pre-op conditions or laboratory values. Furthermore, no difference was found between the groups in the HF etiology, indication for replacement as well as the average days on the previous pump or the type of previous pumps. However, the HM3 group had significantly greater BMI, number of previous LVADs and number of previous sternotomies (Table 1). The distribution of the type of pump explanted as a percentage of the pump implanted is described in Table 2 and Figure 1.
Table 1:
Pre-operative Patient Characteristics
| Group 1 (HM3) (n=19) |
Group 2 (n=85) |
p-value | |
|---|---|---|---|
| CHF Etiology | |||
| ICMP | 9 (47.4%) | 42 (49.4%) | 0.99 |
| NICMP | 10 (52.6%) | 43 (50.6%) | |
| Age | |||
| Mean ± (SD) | 56.8 (13.1) | 59.6 (12.4) | 0.34 |
| Female | 8 (42.1%) | 23 (27.1%) | 0.27 |
| BMIa | |||
| Mean ± (SD) | 37.0 (8.9) | 31.6 (7.34) | 0.01 |
| Chronic Lung Disease | 7 (36.8%) | 17 (20.0%) | 0.14 |
| CVA History | 4 (21.1%) | 21 (24.7%) | 0.99 |
| Ventilator | 1 (5.3%) | 15 (17.6%) | 0.29 |
| IV Inotropes | 2 (14.3%) | 13 (30.2%) | 0.31 |
| Creatinine | |||
| Median [Min, Max] | 1.20 [0.700, 2.40] | 1.40 [0.600, 6.30] | 0.09 |
| Bilirubin | |||
| Median [Min, Max] | 1.40 [0.500, 5.30] | 1.40 [0.400, 19.5] | 0.56 |
| Plasma Free Hbb | |||
| Median [Min, Max] | 26 [8.00, 517] | 38.0 [5.00, 917] | 0.95 |
| LDHb | |||
| Median [Min, Max] | 920 [214, 2580] | 781 [173, 11200] | 0.93 |
| Number of Previous LVAD | <0.001 | ||
| 1 | 10 (52.6%) | 77 (91.8%) | |
| 2 | 7 (36.8%) | 6 (5.9%) | |
| 3 | 2 (10.5%) | 2 (2.4%) | |
| Number of Previous Sternotomies | 0.001 | ||
| 1 | 5 (26.3%) | 56 (65.9%) | |
| 2 | 8 (42.1%) | 23 (27.1%) | |
| 3 | 6 (31.6%) | 6 (7.1%) | |
| Indication for replacement | 0.21 | ||
| Malfunction | 3 (15.8%) | 19 (22.4%) | |
| Infection | 2 (10.5%) | 23 (27.1%) | |
| Thrombosis | 14 (73.7%) | 43 (50.6%) | |
| Duration of Explanted LVAD Supportc | |||
| Median [Min, Max] | 456 [39.0, 2400] | 456 [2.00, 1930] | 0.66 |
BMI, body mass index; CHF, congestive heart failure; CVA, cerebrovascular accident; Hb, hemoglobin; ICMP, ischemic cardiomyopathy; LDH, lactate dehydrogenase; LVAD, left ventricular assist device; NICMP, non-ischemic cardiomyopathy; NYHA, New York Heart Association; RV, right ventricle; SD, standard deviation;
kg/m2;
mg/dL;
days
Table 2:
The distribution of the type of pump explanted as a percentage of the pump implanted.
| Explanted Device/Implanted Device | Heartware | HMII | HM3 |
|---|---|---|---|
| Heartware | 17 (77%) | 1 (1.5%) | 5 (26%) |
| HMII | 5 (23%) | 62 (98.5%) | 14 (74%) |
| HM3 | - | - | - |
Figure 1:
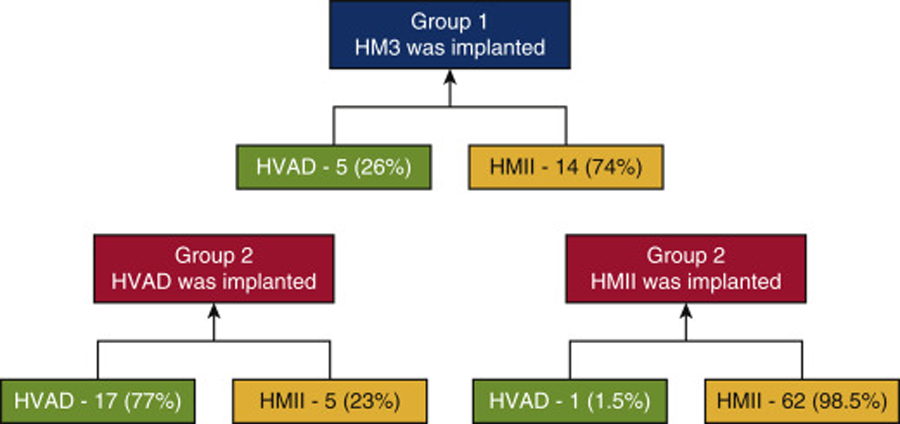
A schematic representation of the type of LVAD implanted and explanted. In group 1, 26% of the replacements to HM3 were from HVAD and 74% were from HMII. In group 2, 23% of the replacements to HVAD were from HMII and 77% were from HVAD. In group 2, 1.5% of the replacements to HMII were from HVAD and 98.5% were from HMII. HM3, HeartMate 3; HVAD, HeartWare Ventricular Assist Device; HMII, HeartMate II.
Primary outcome and adverse events
The two groups had similar rates of post-operative complications, hospital length of stay, RV dysfunction, as well as the rate of readmissions. Furthermore, there was no significant difference in the New York Heart Association functional classification between the groups at follow-up clinic visits (Table 3). However, post-operative probability of death or need for reoperation (for another replacement) was greater for group 2 versus group 1 (HM3), P=0.039 (Figure 2). When compared with any previous pump replacement and not only to the last one, replacing to HM3 was still advantageous (Supplemental Figure, P = .018).
Table 3:
Post-operative Patient Outcomes
| Group 1(HM3) (n=19) |
Group 2 (n=85) |
p-value | |
|---|---|---|---|
| 30 Day Mortality | 0 (0%) | 9 (10.6%) | 0.21 |
| 90 Day Mortality | 0 (0%) | 15 (17.6%) | 0.07 |
| Dialysis Post-op | 1 (5.3%) | 16 (18.8%) | 0.19 |
| RV Dysfunction Post-op | 0.47 | ||
| None | 2 (10.5%) | 14 (16.5%) | |
| Mild | 3 (15.8%) | 23 (27.1%) | |
| Moderate | 8 (42.1%) | 33 (38.8%) | |
| Severe | 6 (31.6%) | 15 (17.6%) | |
| Hospital LOSa | |||
| Median [Min, Max] | 24.0 [12.0, 226] | 27.0 [7.00, 165 | 0.51 |
| ICU LOSq | |||
| Median [Min, Max] | 7.00 [3.00, 223] | 13.5 [2.00, 165] | 0.32 |
| Number of Readmissions per 365 Days of VAD Support | |||
| Median [Min, Max] | 1.85 [0.00, 12.4] | 2.03 [0.00, 23.4] | 0.28 |
| NYHA Postop | 0.67 | ||
| NYHA I | 2 (10.5%) | 16 (18.8%) | |
| NYHA II | 10 (52.6%) | 33 (38.8%) | |
| NYHA III | 4 (26.3%) | 15 (17.6%) | |
| NYHA IV | 1 (10.5%) | 4 (4.7%) | |
| Missing | 0 (0%) | 17 (20%) | |
LOS, length of stay; RV, right ventricular; NYHA, New York Heart Association; ICU, intensive care unit; VAD, ventricular assist device;
days
Figure 2:
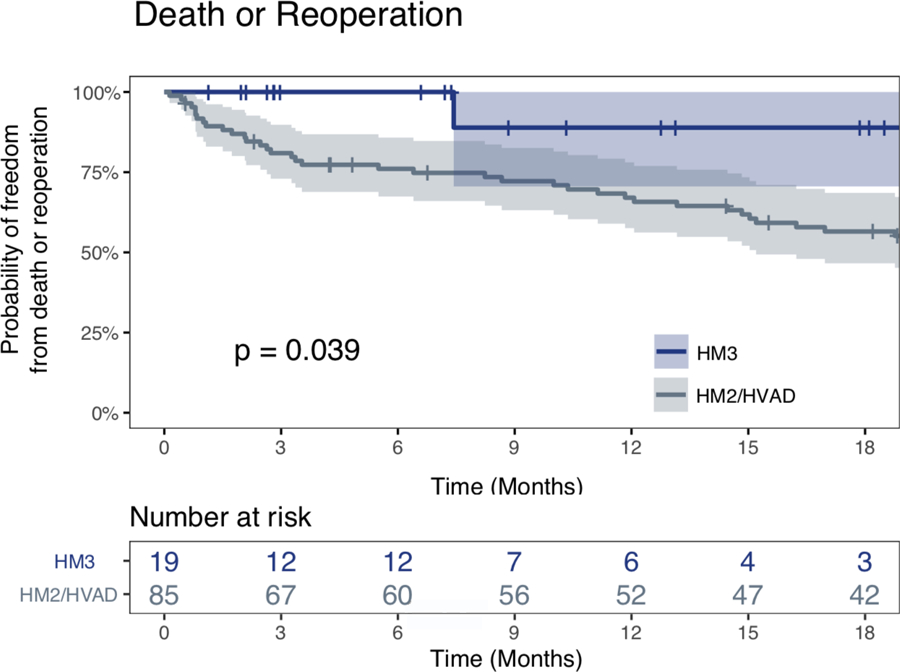
Kaplan Meier curves demonstrating freedom from death or need for reoperation segregated by the type of the pump replaced (Group 1 versus Group 2). The patients with HM3 replacements (group 1) versus group 2, experienced significantly greater freedom from either death or need for another replacement during the follow up period (P=0.039).
Pump thrombosis patients
14 (74%) patients in group 1 presented with pump thrombosis with a median serum LDH level before the pump replacement of 1000 U/L, which came down to a mean of 258 U/L two weeks after the HM3 replacement, and to a mean of 170 U/L at six weeks following replacement.
Figure 3 shows only data for the 14 LVAD thrombosis cases which were eventually replaced with HM3. Over a mean follow up of 221 days after HM3 replacement, there were no thrombosis events after HM3 replacement. For comparison, time to need for replacement due to thrombosis is shown for those 14 patients, after the replacements which were conducted with non-HM3 devices, p = 0.048.
Figure 3:
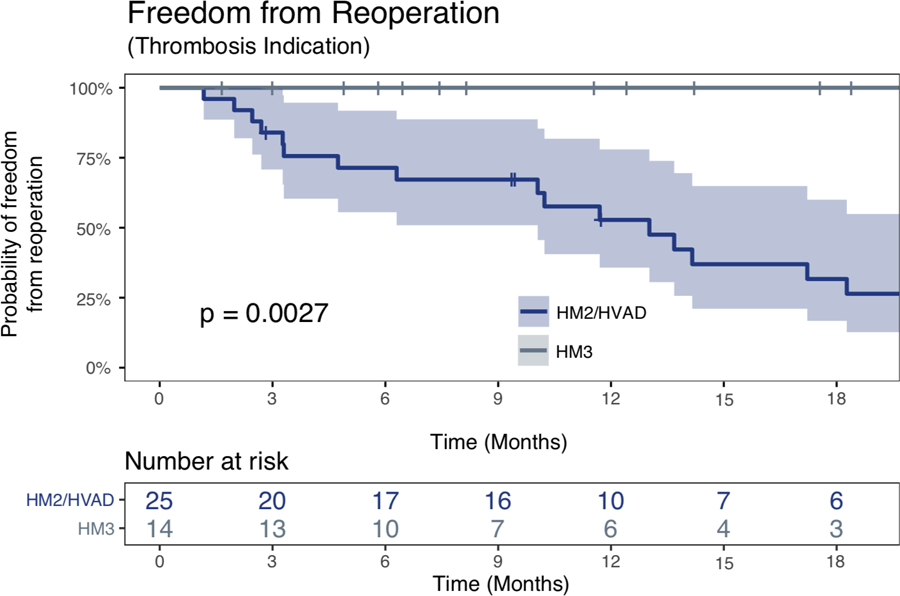
Patients that were implanted with HM3 post Pump thrombosis; Kaplan Meier curves for freedom from reoperation following LVAD implantation segregated by the type of the LVAD; patients are used as their own control. Once implanted with HM3 the risk for pump replacement was substantially lower when compared to the period of time these patients were on either HMII/HVAD; p = 0.048
Discussion
In this single-center study, we report intermediate outcomes for patients who required LVAD replacement with the newer generation HM3 device versus the HMII or HVAD. Pump thrombosis involving the HMII device, was the leading cause for replacement. Kirklin et al. reviewed the INTERMACS registry and showed that after LVAD replacement, overall survival and freedom from adverse events were diminished relative to a primary implant cohort. Furthermore, they identified risk factors for LVAD thrombosis and these included later year of implant, higher creatinine, larger body mass index (1). Others have reported that the rate of second pump thrombosis and need for replacement was greater in a cohort of patients, who had already experienced one thrombosis event (3,4). Although the MOMENTUM trial demonstrated that the rate of thrombosis and overall need for replacement was significantly reduced with the HM3 versus HMII device, high risk patients who had experienced one device failure requiring replacement, were notably excluded from the trial (2). Therefore, in this report, we examine outcomes among patients who require durable LVAD replacement and compare HM3 replacements to replacement with either HMII or HVAD. Asking the question do the benefits of HM3 extend to a high-risk cohort that required replacement?
We present over 100 patients requiring durable LVAD replacement, with 19 patients undergoing replacement with HM3. Notably, 14 of the 19 cases had experienced thrombosis with over half of these patients needing multiple replacements. Previously, we described the technique for these procedures which in most instances involved complete replacement of the system via redo sternotomy (5). Group 1 patients (HM3 cohort) had more previous devices and greater number of prior sternotomies compared to group 2 patients. There was only one death in follow up for group 1, and the primary composite outcome of freedom from death or need for replacement significantly favored group 1 (HM3). Additionally, Group 1 patients, who had higher surgical risk characteristics of larger BMI and multiple prior surgeries, experienced similar post-operative outcomes as Group 2 patients. Among the 14 HM3 cases in which the indication was thrombosis, there were no episodes of thrombosis following replacement and serum LDH levels appeared to decline to a new lower baseline relative to pre-HM3. Mean follow up for group 1 was of 221 days, compared to the mean time to HMII thrombosis of approximately 90 days reported by Starling et al (6). Therefore, while further follow up is needed, one would have anticipated some thrombosis events had these replacements been conducted with the HMII device.
While there have been many publications on outcomes and surgical techniques for durable pump replacements, there is no consensus on the best strategy (7–11). Furthermore, the optimal surgical technique may depend on the reason for replacement (12,13). One limitation of the current analysis is that surgical approach (redo-sternotomy versus lateral thoracotomy or sub costal) is not controlled for. Previously, we have published a detailed report of our redo sternotomy technique for replacement to HM3, in which all components of the old device are removed (5), others have published minimal approaches to perform such an exchange (14). In this series the vast majority of replacements to HM3 were conducted via a redo sternotomy. This has the advantage of removing all old elements which might contribute to subsequent issues with the new device. However, redo sternotomies have the disadvantages of being invasive and having greater risk of RV injury and bleeding (12). Most of the HMII to HMII and HVAD to HVAD replacements were conducted via a lateral thoracotomy or subcostal approach. This has the advantage of being less invasive (perhaps more beneficial for RV function) but retained elements from the old device could contribute to re infection or thrombosis of the new device. Going forward, it is anticipated that some replacements to HM3, may be conducted via a lateral approach (14,15). Furthermore, the best surgical approach may depend on the individual circumstances of the patient and case. The benefits of replacements with HM3, shown in this study, are probably related to the device type, however it is possible that differences in surgical techniques between the two groups contributed to the outcome.
Limitations of this study are that it is retrospective, and the overall number of patients who underwent replacement with HM3 is small. There may have been bias leading to use of the HM3 for replacement relative to replacement with HMII or HVAD, although, the review of baseline characteristics suggests that the HM3 group may have been a higher risk group. Furthermore, greater follow up may reveal differences which are not apparent with current follow up. Finally, the sample sizes of the two groups and number of outcomes limited our ability to perform any meaningful risk adjustment in our analyses.
In conclusion, it can be expected that a small percentage of patients supported with durable LVAD, will need a pump replacement (16). High risk patients undergoing HM3 replacement did not experience any thrombosis events and demonstrated significantly lower risk for death and reoperation relative to patients replaced with either HMII or HVAD. While greater follow up is warranted, we conclude that LVAD replacement with HM3 can be performed safely and may be considered as the pump of choice in patients requiring LVAD replacement (Graphical abstract).
Supplementary Material
Supplemental Figure 1: Kaplan Meier curves demonstrating freedom from death or need for reoperation segregated by the type of the pump replaced (Group 1 versus Group 2). The patients with HM3 replacements (group 1) versus all the pump replacements of group 2 (not merely the last one), experienced significantly greater freedom from either death or need for another replacement during the follow up period (P=0.018).
Central Message:
LVAD replacement with HM3 can be performed safely and may be considered as the pump of choice in patients requiring LVAD replacement.
Perspective Statement:
It can be expected that a small percentage of patients supported with durable LVAD, will need a pump replacement. High risk patients undergoing pump replacement with HM3 did not experience any thrombosis events and demonstrated significantly lower risk for death and reoperation relative to patients replaced with either HMII or HVAD.
Acknowledges:
A Disclosure/Conflict of Interest:
C.M has received funds from Abbott Inc and Medtronic Inc for education and consulting.
References:
- 1.Kirklin JK, Naftel DC, Kormos RL et al. Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of pump thrombosis in the Heartmate II left ventricular assist device. JHLT 33(2014):12–22. [DOI] [PubMed] [Google Scholar]
- 2.Mehra MR, Naka Y, et al. , A Fully Magnetically Levitated Left Ventricular Assist Device - Final Report. N Engl J Med. 2019. March. [DOI] [PubMed]
- 3.Shi M, Moreno J, Raymer D et al. Risk of Recurrent Pump Thrombosis after Heartmate II LVAD Implantation: a Large High Acuity Cohort. JACC 71 (11S) [Google Scholar]
- 4.Andruska AM, Nassif M, Novak E et al. LVAD exchange for Thrombosis is associated with Higher Recurrent Rates of Hemolysis, Thrombosis and Death. JHLT 2014;33(4): S131–S132. [Google Scholar]
- 5.Barac YD, Schroder JN, Daneshmand MA, Patel CB, Milano CA. Heartmate III Replacement for Recurring Left Ventricular Assist Device Pump Thrombosis. ASAIO J. 2018. May-Jun;64(3):424–426. [DOI] [PubMed] [Google Scholar]
- 6.Starling R, Moazami N, Silvestry SC et al. Unexpected abrupt increase in LVAD pump thrombosis NEJM 2014;370:33–40. [DOI] [PubMed] [Google Scholar]
- 7.Barac YD, Nevo A, Schroder JN, Milano CA, Daneshmand MA. LVAD Outflow Graft Role in Pump Thrombosis. ASAIO J. 2018. December 18. [DOI] [PubMed]
- 8.Dang G, Epperla N, Muppidi V, Sahr N, Pan A, Simpson P, Baumann Kreuziger L. Medical Management of Pump-Related Thrombosis in Patients with Continuous-Flow Left Ventricular Assist Devices: A Systematic Review and Meta-Analysis. ASAIO J. 2017. Jul-Aug;63(4):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toda K, Sawa Y. Clinical management for complications related to implantable LVAD use. Gen Thorac Cardiovasc Surg. 2015. January;63(1):1–7. [DOI] [PubMed] [Google Scholar]
- 10.Hanke JS, Dogan G, Wert L, Ricklefs M, Heimeshoff J, Chatterjee A, Feldmann C, Haverich A, Schmitto JD. Left ventricular assist device exchange for the treatment of HeartMate II pump thrombosis. J Thorac Dis. 2018. June;10(Suppl 15):S1728–S1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsubota H, Ribeiro RVP, Billia F, Cusimano RJ, Yau TM, Badiwala MV, Stansfield WE, Rao V. Left ventricular assist device exchange: the Toronto General Hospitalexperience. Can J Surg. 2017. August;60(4):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schechter MA, Patel CB, Blue LJ, Welsby I, Rogers JG, Schroder JN, Milano CA. Improved early survival with a nonsternotomy approach for continuous-flow left ventricular assist device replacement. Ann Thorac Surg. 2015. February;99(2):561–6. [DOI] [PubMed] [Google Scholar]
- 13.Pawale AA, Farkash A, Pandis D, Anyanwu AC. Axial to Centrifugal Continuous Flow LVAD Pump Exchange Using Minimally Invasive Technique. Innovations (Phila). 2017. Nov-Dec;12(6):496–498. [DOI] [PubMed] [Google Scholar]
- 14.Hanke JS, Rojas SV, Dogan G, Feldmann C, Beckmann E, Deniz E, Wiegmann B, Michaelis JE, Napp LC, Berliner D, Shrestha M, Bauersachs J, Haverich A, Schmitto JD. First series of left ventricular assist device exchanges to HeartMate 3. Eur J Cardiothorac Surg. 2017. May 1;51(5):887–8 [DOI] [PubMed] [Google Scholar]
- 15.Khayata M, ElAmm CA, Sareyyupoglu B, Zacharias M, Oliveira GH, Medalion B. HeartMate II pump exchange with HeartMate III implantation to the descending aorta. J Card Surg. 2019. January;34(1):47–49. [DOI] [PubMed] [Google Scholar]
- 16.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017. October;36(10):1080–1086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Kaplan Meier curves demonstrating freedom from death or need for reoperation segregated by the type of the pump replaced (Group 1 versus Group 2). The patients with HM3 replacements (group 1) versus all the pump replacements of group 2 (not merely the last one), experienced significantly greater freedom from either death or need for another replacement during the follow up period (P=0.018).


