Abstract
The longstanding dogma is that humans exhibit an acute reduction in haemoglobin (Hb) binding affinity for oxygen that facilitates the adaptation to moderate hypoxia. However, many animals have adapted to high-altitude through enhanced Hb binding affinity for oxygen. The objective of the study was to determine whether high-affinity haemoglobin (HAH) affects maximal and submaximal exercise capacity. To accomplish this, we recruited individuals (n=11, n=8 females) with HAH (P50=16±1 mmHg), had them perform normoxic and acute hypoxic (15% inspired oxygen) maximal exercise tests, and then compared their results to matched controls (P50=26±1, n=14, n=8 females). Cardiorespiratory and arterial blood gases were collected throughout both exercise tests. Despite no difference in end-exercise arterial oxygen tension in hypoxia (59±6 vs. 59±9 mmHg for controls and HAH, respectively), the HAH subjects’ oxyhaemoglobin saturation (SaO2) was ~7% higher. Those with HAH had an attenuated decline in maximal oxygen uptake (⩒O2max) (4±5% vs. 12±5%, p<0.001) in hypoxia and the change in ⩒O2max between trials was related to the change in SaO2 (r=−0.75, p<0.0001). Compared to normoxia, the controls’ alveolar-to-arterial oxygen gradient significantly increased during hypoxic exercise, whereas pulmonary gas exchange in HAH subjects was unchanged between the two exercise trials. However, arterial lactate was significantly higher and arterial pH significantly lower in the HAH subjects for both exercise trials. We conclude that HAH attenuates the decline in maximal aerobic capacity and preserves pulmonary gas exchange during acute hypoxic exercise. Our data supports the comparative biology literature indicating that HAH is a positive adaptation to acute hypoxia.
Keywords: maximal oxygen uptake, oxygen delivery, pulmonary gas exchange, submaximal exercise
Introduction
Exposing humans to hypoxia is a classical method to study basic and clinical physiology. The longstanding theory is that chronic exposure to moderate hypoxia in humans results in a decrease in haemoglobin (Hb) oxygen affinity or a ‘right-shift’ in the oxyhaemoglobin dissociation curve (ODC) due to the production of 2,3 bisphosphoglycerate (Aste-Salazar & Hurtado, 1944; Lenfant et al., 1968, 1971). A common metric to quantify Hb oxygen affinity is the partial pressure of oxygen at which 50% of Hb is saturated with oxygen (P50); such that a decrease in affinity results in a greater P50 and vice versa. Decreased oxygen affinity for Hb during hypoxic exposure is thought to be beneficial by facilitating oxygen unloading and consumption (⩒O2) at the tissue level in the face of suboptimal oxygen delivery (Lenfant et al., 1971). It is important to note that the metabolic stimulus from hypoxia is not necessarily the suboptimal O2 delivery, but rather the decrease PaO2 (Arthur et al., 1992; Hogan et al., 1992) Additionally, theoretical models of human alterations of P50 during exercise performance in hypoxia have led to the conclusion that maximal oxygen uptake (⩒O2max) is relatively insensitive to changes in Hb oxygen affinity (Wagner, 1997). However, published data in humans (experimental and theoretical) do not necessarily align with the comparative biology literature. For example, rats with a lower P50 (via ingestion of sodium cyanate) are better able to survive extreme hypoxia than control animals (Eaton et al., 1974). The decrease in Hb oxygen affinity in humans during hypoxia is also in stark contrast to many animals evolutionarily adapted to living and exercising at high-altitude who possess high affinity Hb (HAH) compared to their lowland counterparts (Storz, 2016). Since P50 scales with body mass in mammals, with smaller animals having a greater P50 (Schmidg-Neilsen & Larimer, 1958), comparisons between P50 should be made of similar sized animals and/or similar species.
A specific example is the bar-headed goose that has Hb with considerably higher oxygen binding affinity than its closely related lowland dwelling counterpart (Natarajan et al., 2018). The HAH adaptation, in part, facilitates exercise (e.g. flight, a vigorous form of exercise) by increasing oxygen loading at the lungs and maintaining higher arterial oxygen saturations in considerably hypoxic conditions (>4500 m) for extended periods (Scott et al., 2015).
Altered P50 in the setting of rigorous exercise can detrimentally upset the balance of pulmonary oxygen uptake and muscular extraction. With HAH, pulmonary oxygen uptake is enhanced but muscle unloading is hindered, with the opposite occurring for low affinity haemoglobin. Previous theoretical modelling indicated that enhanced pulmonary uptake was a superior alteration during hypoxia only when ⩒O2 is sufficiently high and/or in the presence of a pulmonary diffusion limitation (Bencowitz et al., 1982). Others calculated that lowering Hb affinity was beneficial up to altitudes of 5400 m (~18000 ft) (Samaja et al., 1985). The primary benefits of HAH in hypoxia was through greater arterial oxyhaemoglobin saturation (SaO2) resulting in greater arterial oxygen content (CaO2) and enhanced pulmonary gas exchange (Bencowitz et al., 1982). However, when the model was refined to include muscle diffusion capacity, the theoretical maximal ⩒O2 was determined to be insensitive to changes in P50 (Wagner, 1997).
The data from the latter model concluded that while pulmonary uptake of oxygen would be enhanced with HAH, extraction at the level of the muscle could be hindered, suggesting that the muscles would have to rely more on anaerobic metabolism, thus negating any benefits from increased CaO2 and leaving aerobic capacity unchanged. However, in contrast to the conclusions in the above models, case reports in humans align more closely with the comparative literature (Hebbel et al., 1978). Specifically, two subjects with HAH were shown to have no decrement in ⩒O2max after 10 days of hypoxic exposure (Leadville, Colorado, 3100 m), suggesting that HAH preserves exercise tolerance at altitude.
With this information as a background, the purpose of our study was to characterize the impact of HAH on the response to submaximal and maximal exercise in normoxia and acute hypoxia. We accomplished this by recruiting individuals with rare HAH. Our hypotheses were threefold. First, we hypothesized HAH in healthy humans would be associated with better maintenance of oxygen uptake during exercise in acute normobaric hypoxia. Second, during hypoxic exercise, individuals with HAH would have an alveolar to arterial oxygen gradient (AaDO2) similar to their A-aDO2 during normoxic exercise. Finally, individuals with HAH would have a greater reliance on anaerobic metabolism throughout graded exercise.
Methods
Ethical approval.
Study procedures were approved by the Mayo Clinic Institutional Review Board (IRB #16–007719) and the study is in accordance with the Declaration of Helsinki. Subjects provided written informed consent prior to beginning the study.
Participants.
Eleven subjects with rare high affinity haemoglobin (HAH) were recruited from a Mayo Clinic database. Controls with normal Hb affinity (n=14) were recruited in order to match subjects with variant Hb. None of the subjects reported any cardiovascular, respiratory, musculoskeletal or other ailments that might limit exercise capacity. Ten of the eleven subjects with HAH were related and three of the controls were also related to the ten HAH subjects. Ten HAH subjects had Hb Malmö (HBB: c.[294C>A or 294C>G] p.H97Q(FG4)) (Fairbanks et al., 1971; Berglund, 1972a) and one had Hb San Diego (HBB:c.328G>A p.V109M(G11)) (Nute et al., 1974). Both Hb Malmö and Hb San Diego are Hb beta chain variants and present with a P50 between 14–20 mmHg.
Experimental overview.
All testing occurred on a single day at ~300 m above sea level (barometric pressure 736±4 mmHg) in an air-conditioned room (ambient temperature 21±1 °C). After providing written informed consent, a baseline venous blood sample was obtained to measure P50 (see below) and resting pulmonary function assessed. Thereafter, subjects were instrumented with an arterial catheter and performed two maximal exercise tests on a cycle ergometer. One test was performed in normoxia (FiO2=0.21), and the other in acute hypoxia (FiO2=0.15). The order of the maximal exercise tests was randomized and each was separated by 60 minutes of rest. Subjects were then de-instrumented and dismissed.
Pulmonary function.
Spirometry and plethysmography were performed according to ATS/ERS standards (Coates et al., 1997; Miller et al., 2005) and expressed as absolute and normative values based on the subjects’ age, height, and weight.
Arterial blood sampling and pressure.
After administration of a local anaesthetic (2% lidocaine) a catheter was placed in either the radial or brachial artery of each subject. The catheter was kept patent using pressurized saline and was connected to a commercially available arterial blood sampling kit (model RA-04020; Arrow International, Reading, PA). To sample blood, an excess of dead space volume was withdrawn prior to the sample. The blood sample was immediately analysed using a commercial blood gas analyser (ABL90 Radiometer, Copenhagen, Denmark). Blood temperature was measured using a rapid response thin wire thermistor that was placed in a hub near the arterial catheter. Offline, arterial blood gases were corrected for blood temperature using standard equations (Severinghaus, 1958). Beat-by-beat blood pressure was obtained from a transducer (Cardiocap/5, Datex, Louisville, CO) which was connected to the arterial catheter and zeroed at heart level.
Maximal exercise testing.
To determine maximal oxygen uptake, subjects performed a step-wise maximal cycle test on an electromagnetically braked ergometer (Corival CPET, Lode, Groninge, the Netherlands). We anticipated a wide range of aerobic fitness levels due to differences in our subjects’ age, sex and training status. Therefore, we tailored the increments and initial workload for each subject. Starting workload varied from 20–120 W and the increments ranged from 10–50 W per stage. Regardless of the initial workload and increment, stages were three minutes in duration. The aim was to have each subject exercise for 15–21 min which allowed for 5–7 total stages.
During exercise, subjects wore a two-way non-breathing valve (Series 7200, Hans Rudolph, Shawnee, KS). The inspiratory port was connected to a reservoir containing humidified gas. The expiratory side of the non-rebreathing valve vented to the atmosphere. Gases were sampled at the mouth via a mass spectrometer (MGA-1100, Perkin Elmer, Waltham, MA) with a phase delay of 0.27–0.3 s. Ventilatory flow was measured from a pneumotachograph placed between the subject’s mouth and the non-rebreathing valve. Heart rate was obtained from a 12-lead ECG.
Inspired gas concentrations.
For the normoxic trial, subjects inspired compressed room air that was humidified prior to inhalation. Compressed room air was utilized to blind the subjects to the condition. Hypoxia was induced using commercially available pre-mixed tanks containing 15% O2, balance N2. The breathing apparatus, including all tubing and humidification method, was identical between trials. We chose 15% O2 (average inspired partial pressure of oxygen 110±1 mmHg) for our hypoxic condition for two reasons: 1) it approximates a realistic altitude (~3000 m/ 10000 ft) that an individual would commonly be acutely exposed to for recreation or vocation; 2) it is a similar level of hypoxia to previous studies investigating HAH variants and hypoxic exercise (Hebbel et al., 1978).
Data processing and analysis.
To determine the P50, heparinized venous blood samples were analyzed for haemoglobin oxygen saturation and partial pressure of oxygen by dual wavelength spectrophotometry and a Clark electrode, respectively (Hemox™ Analyzer, TCS Medical Products, Huntington Valley, PA, USA). After deoxygenation with nitrogen, the compressed air reoxygenation curve was measured at pH 7.6 and 37°C using a laboratory developed protocol (Winslow et al., 1977).
All variables, except arterial blood gases, were collected using a 16-channel analog-to-digital data acquisition system (PowerLab 16/35, AD Instruments, Colorado Springs) that sampled at 200 Hz and was recorded using LabChart 8.1.13 software. The alveolar gas equation was used to calculate ideal alveolar oxygen tension and the A-aDO2, with water vapor pressure calculated using core temperature (Stickland et al., 2013). Base excess was calculated accounting for differences in Hb concentration (Davenport, 1974). To compare variables at different fractions of ⩒O2peak, we pooled the subjects into bins corresponding to 50, 65, 80, 90 and 100% of ⩒O2max in normoxia. For hypoxic exercise, subjects were pooled at 50, 65, 80 and 100% of ⩒O2max in normoxia.
Statistics.
Descriptive variables were compared using unpaired Student’s t-tests. We were a priori interested in both the submaximal and maximal response to exercise and therefore elected to compare the responses independently. This was done, in part, because we expected the decline in exercise capacity to be different between controls and HAH subjects. To compare variables at maximal exercise, we utilized a two-way (Inspirate [hypoxia, normoxia], Hb type [normal, HAH]) repeated measures analysis of variance. To compare variables throughout the two different exercise groups, we employed a three-way (Inspirate [hypoxia, normoxia], Hb type [normal, HAH], exercise intensity [50, 65, 80, 100% normoxic max] mixed model analysis of variance. For both, when significant F-ratios were detected, a Tukey HSD was performed to determine where the differences lay. The relationship between change in ⩒O2 and the change in SaO2 was determined using a Pearson product-moment correlation. The relationship between A-aDO2 and ⩒O2 was calculated by determining the slope and intercept for each subject, and then comparing those means using an unpaired Student’s t-test. Significance was set at P<0.05 and all data are presented as means±SD.
Results
Subjects.
Resting demographics are presented in Table 1. Controls were significantly taller than HAH variants, but otherwise were not different in terms of sex, age, weight or body mass index. As expected, the P50 was significantly lower in HAH subjects. While PaO2 was not different and within the normal range for both groups (Crapo et al., 1999), arterial oxyhaemoglobin saturation (SaO2) and arterial oxygen content (CaO2) were significantly higher in HAH subjects. The higher CaO2 was predominantly due to the polycythaemia commonly observed in individuals with HAH (Shepherd et al., 2019). Females in both HAH and control groups had Hb concentrations ~1–2 g dL−1 lower than male subjects. Pulmonary function was unremarkable in both groups (Table 1). Controls had several pulmonary function values that approached being statistical significantly different from HAH group (p=0.06–0.10). However, any differences represent normal biological variation, and are unlikely to be of physiological significant due to the close proximity of HAH variant normal values (>95%).
Table 1.
Resting subject demographics, pulmonary function and haematological variables while breathing room air
| Controls (n=14) | High-affinity variants (n=11) | |
|---|---|---|
| Sex | 7M:8F | 3M:8F |
| Age (years) | 40±9 | 35±11 |
| Height (cm) | 174±12 | 165±8* |
| Weight (kg) | 82±14 | 89±27 |
| BMI (kg·m−2) | 26.9±3.5 | 32.7±9.8 |
| FVC (L) | 4.8±1.3 | 3.9±0.8 |
| FVC (% pred) | 103±11 | 96±14 |
| FEV1 (L) | 3.8±0.9 | 3.2±0.6 |
| FEV1 (% pred) | 104±10 | 96±12 |
| FEV1/FVC (%) | 82±8 | 83±4 |
| PEF (L sec−1) | 8.7±2.0 | 8.3±1.4 |
| PEF (% pred) | 103±15 | 107±13 |
| TLC (L) | 6.6±1.7 | 5.5±1.1 |
| TLC (% pred) | 107±10 | 100±16 |
| VC (L) | 4.9±1.4 | 3.9±0.8 |
| VC (% pred) | 105±12 | 98±14 |
| RV (L) | 1.8±0.4 | 1.6±0.5 |
| P50 (mmHg) | 26.0±1.4 | 15.6±1.1* |
| Hb (g·dL−1) | 14.3±1.4 | 19.1±1.7* |
| PaO2 (mmHg) | 96±6 | 92±7 |
| SaO2 (%) | 96.0±0.8 | 97.2±0.6* |
| CaO2 (ml dl) | 18.9±1.8 | 25.5±2.1* |
| pH | 7.42±0.01 | 7.41±0.02 |
| MAP (mmHg) | 109±13 | 107±12 |
Values are mean±SD. BMI, body mass index; FVC, forced vital capacity; FEV1, forced expired volume in 1 second; PEF, peak expiratory flow; TLC, total lung capacity; VC, vital capacity; RV, residual volume; P50, pressure at which oxyhaemoglobin is 50% saturated; Hb, haemoglobin; PaO2, arterial oxygen tension; SaO2, arterial oxyhaemoglobin saturation; CaO2, arterial oxygen content; MAP, mean arterial pressure
, significantly different from controls (p<0.05)
Maximal exercise.
Data from maximal exercise in normoxia and hypoxia are presented in Tables 2–3 and Figures 1–3. Arterial and alveolar PO2 were not different between groups for both exercise trials, but the HAH subjects maintained significantly higher SaO2 and CaO2 (Table 2). Despite no difference in pH at rest, those with HAH had a lower pH during all exercise trials (Table 2, Figure 1), with both groups having a significantly greater maximal pH during hypoxic exercise. Arterial lactate was significantly higher in the HAH at maximal intensity for both exercise trials (Figure 1). Notably, the HAH had no change in lactate between normoxic and hypoxic trials, whereas the controls had a significant decline. Despite differences in pH and lactate, arterial bicarbonate and base excess were not different between the groups (Table 2).
Table 2.
Arterial blood gases, oximetry and electrolytes at maximal exercise
| Controls | High-affinity variants | ||||||
|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Hb type | Inspirate | Interaction | |
| PaO2 (mmHg) | 102±7 | 59±6 | 98±11 | 59±9 | NS | <0.001 | NS |
| PaCO2 (mmHg) | 33±6 | 29±7 | 32±5 | 30±4 | NS | 0.002 | NS |
| PAO2 (mmHg) | 114±6 | 75±6 | 115±5 | 74±5 | NS | <0.001 | NS |
| AaDO2 (mmHg) | 12±5 | 16±6 | 17±6 | 16±6 | NS | NS | 0.007 |
| Hb (g dL−1) | 15.4±1.3 | 14.9±1.7 | 20.1±2.5 | 20.4±1.9 | <0.001 | NS | NS |
| Hct (%) | 47±4 | 46±5 | 62±8 | 63±6 | <0.001 | NS | NS |
| SaO2 (%) | 95.4±0.9 | 86.1±3.0 | 96.8±0.7 | 93±2.0 | <0.001 | <0.001 | <0.001 |
| FCOHb (%) | 1.0±0.6 | 1.1±0.5 | 0.7±0.2 | 0.9±0.1 | NS | 0.004 | NS |
| FMetHb (%) | 0.8±0.2 | 0.9±0.2 | 0.8±0.2 | 0.6±1 | 0.006 | NS | NS |
| CaO2 (ml·dL−1) | 20.0±1.6 | 17.4±2.0 | 26.4±3.2 | 25.6±2.2 | <0.001 | <0.001 | <0.001 |
| K+ (meq·dL−1) | 5.6±0.5 | 4.9±0.8 | 5.6±1.1 | 5.5±0.5 | 0.023 | 0.034 | NS |
| Glua (mg·dL−1) | 114±50 | 101±28 | 97±22 | 110±29 | NS | NS | NS |
Values are mean±SD. PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension; PAO2, alveolar oxygen tension; AaDO2, alveolar to arterial oxygen gradient; Hb, hemoglobin concentration; Hct, hematocrit; SaO2, arterial oxyhaemoglobin saturation; FCOHb, carboxyhaemoglobin fraction; FMetHb, methemoglobin fraction; CaO2, arterial oxygen content Glua, arterial glucose concentration; HCO3, arterial bicarbonate concentration;
Table 3.
Ventilatory, metabolic and cardiovascular variables at maximal exercise
| Controls | High-affinity variants | ||||||
|---|---|---|---|---|---|---|---|
| Normoxia | Hypoxia | Normoxia | Hypoxia | Hb type | Inspirate | Interaction | |
| ⩒A (L·min−1) | 80±15 | 80±24 | 74±17 | 73±17 | NS | NS | NS |
| ⩒E (L·min−1) | 96±20 | 89±19 | 91±15 | 93±17 | NS | 0.01 | <0.001 |
| ⩒O2 (L min−1) | 2.65±0.6 | 2.32±0.5 | 2.35±0.6 | 2.25±0.5 | NS | <0.001 | 0.002 |
| ⩒O2 (mL·kg−1·min−1) | 33±7 | 29±6 | 28±7 | 27±6 | NS | <0.001 | 0.002 |
| ⩒CO2 (L·min−1) | 2.97±0.6 | 2.60±0.6 | 2.67±0.6 | 2.53±0.5 | NS | <0.001 | <0.001 |
| RER | 1.12±0.05 | 1.11±0.06 | 1.14±0.05 | 1.13±0.06 | NS | NS | NS |
| ⩒E/⩒O2 | 37±6 | 39±6 | 40±7 | 42±7 | NS | <0.001 | NS |
| ⩒E/⩒CO2 | 33±5 | 35±5 | 35±5 | 37±5 | NS | <0.001 | NS |
| Peak power (W) | 173±49 | 152±42 | 132±34 | 127±31 | NS | <0.001 | 0.001 |
| VO2/W (mL·W−1) | 12.3±1.0 | 11.8±1.2 | 13.5±2.3 | 13.7±2.6 | 0.037 | NS | NS |
| HR (bpm) | 176±13 | 173±13 | 183±12 | 181±10 | NS | 0.002 | NS |
| MAP (mmHg) | 131±20 | 131±17 | 142±23 | 140±25 | NS | NS | NS |
Values are mean±SD.; ⩒A, alveolar ventilation; ⩒E, expired minute ventilation; ⩒O2, oxygen uptake; ⩒CO2, carbon dioxide production; RER, respiratory exchange ratio; W, Watts; HR, heart rate; MAP, mean arterial pressure.
Figure 1.
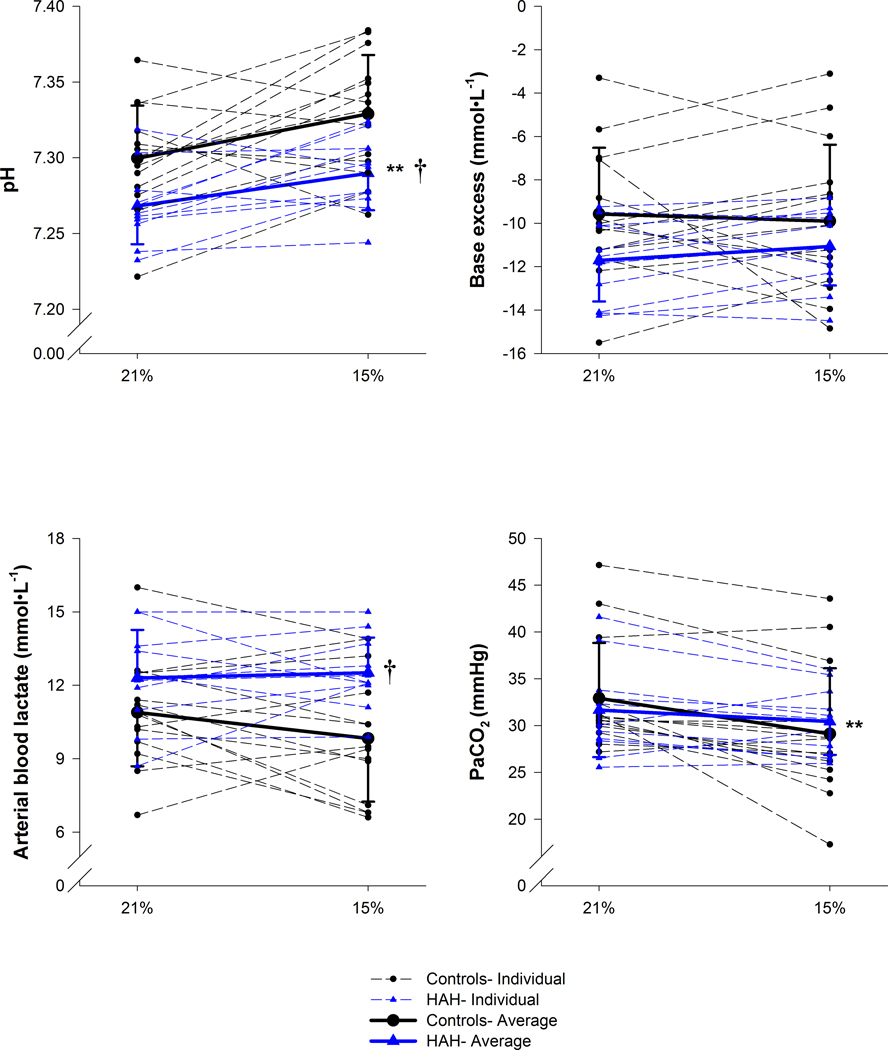
Variables relating to acid base status at the end of exercise. Thin dashed lines are individual points. The 21% and 15% represent the inspired oxygen concentrations for the normoxia and hypoxia trial respectively. PaCO2, arterial carbon dioxide tension. **, significantly effect of haemoglobin; †, significantly effect of inspirate. p<0.05.
Figure 3.
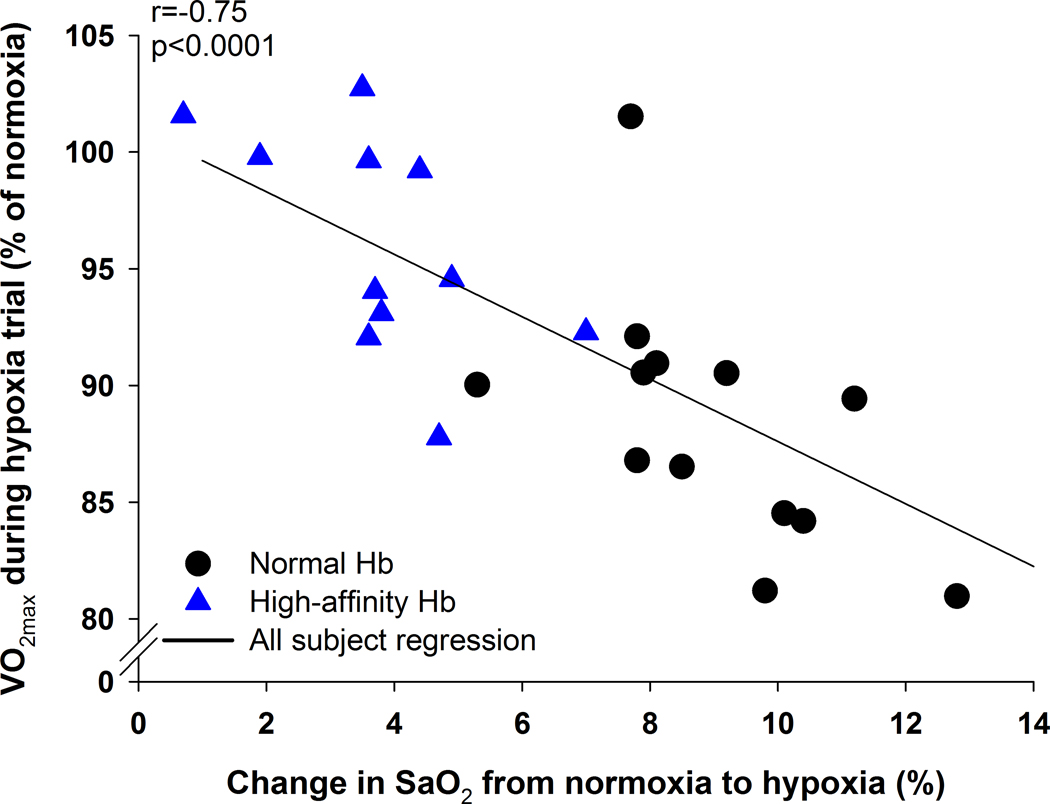
The relationship between the change in maximal oxygen uptake between the exercise trials and the change in oxyhaemoglobin saturation between trials at maximal exercise. ⩒O2max, maximal oxygen uptake; SaO2, arterial oxygen saturation.
For the normoxic exercise trial, the HAH group was statistically not different in terms of maximal oxygen uptake and peak power output to controls (Table 3). There was no relationship between Hb and ⩒O2max for either group (R=0.10 and 0.28, P>0.05 for controls and HAH respectively). The HAH subjects also had a poorer exercise efficiency as indicated by a higher ⩒O2/W (Table 3). As expected, hypoxia resulted in decreased peak ⩒O2, carbon dioxide production and peak power in both groups (Table 3). However, there was a significantly greater decrease in ⩒O2max and peak power in the controls compared to the HAH (Table 3 & Figure 2). The attenuated decline in ⩒O2max in the HAH was not caused by differences in ventilation, heart rate, or arterial blood gas tensions (Table 2 and 3). Rather, those with HAH had a significantly higher SaO2 which resulted in greater CaO2. In support of this, there was a significant relationship (r=−0.75, p<0.001) between the change in SaO2 and change in ⩒O2 (Figure 3), signifying that subjects with greatest decrements in SaO2 had higher decrements in maximal ⩒O2.
Figure 2.
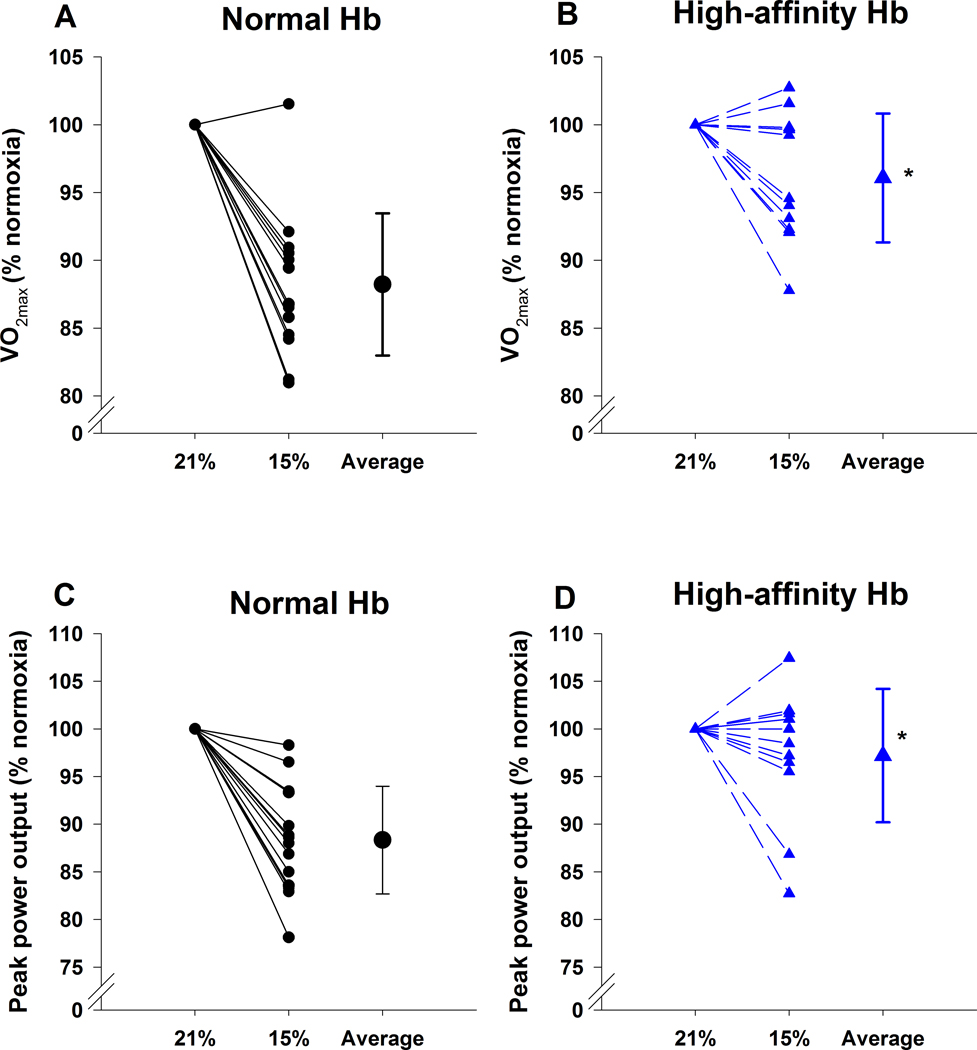
Relative change in maximal oxygen uptake (Panels A and B) and peak power output (Panels C and D) during hypoxic exercise for those with normal haemoglobin (Panels A and C) and high affinity haemoglobin (Panels B and D). Individual values are presented for each trial along with the group means±SD (Average). The 21% and 15% represent the inspired oxygen concentrations for the normoxic and hypoxic trial, respectively. Hb, haemoglobin, ⩒O2max, maximal oxygen uptake. *, significantly different from the normal haemoglobin.
Pulmonary gas exchange.
As exercise intensity increased, there was a progressive widening of the AaDO2 with considerable between-subject variability in the absolute value (Figure 4). At maximal exercise, there was a significant interaction between Hb and inspirate, whereby the A-aDO2 for the controls normoxia trial was significantly lower than both HAH trials and the control’s hypoxic trial. In hypoxia however, the absolute A-aDO2 was not different between groups at maximal exercise. While the controls had a wider A-aDO2 during hypoxia, the HAH variants had no change in their A-aDO2 between normoxic and hypoxic exercise (Table 2 & Figure 4). A similar result was also found during submaximal intensities (Figure 4 and 5C). Specifically, at a given ⩒O2 the A-aDO2 was significantly lower for the controls in normoxia compared to the control’s hypoxic and both HAH trials (Figure 4), whereas there was no change in the A-aDO2 for the HAH subjects between normoxic and hypoxic trials.
Figure 4.
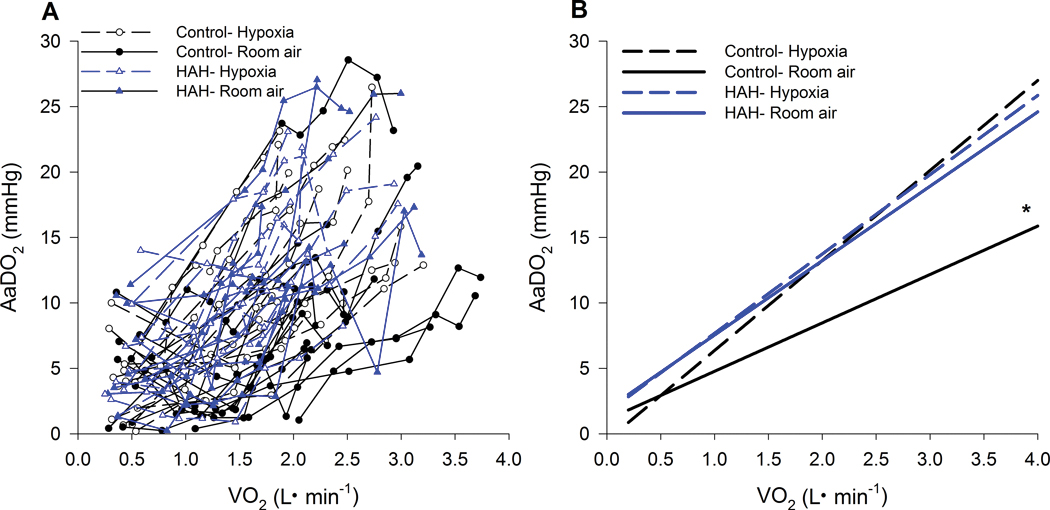
Pulmonary gas exchange throughout both exercise trials. Panel A depicts individual values for each subject whereas Panel B is the average regression for the groups. AaDO2, alveolar to arterial oxygen difference; ⩒O2, oxygen uptake. *, significantly different from all other groups. P<0.05.
Figure 5.
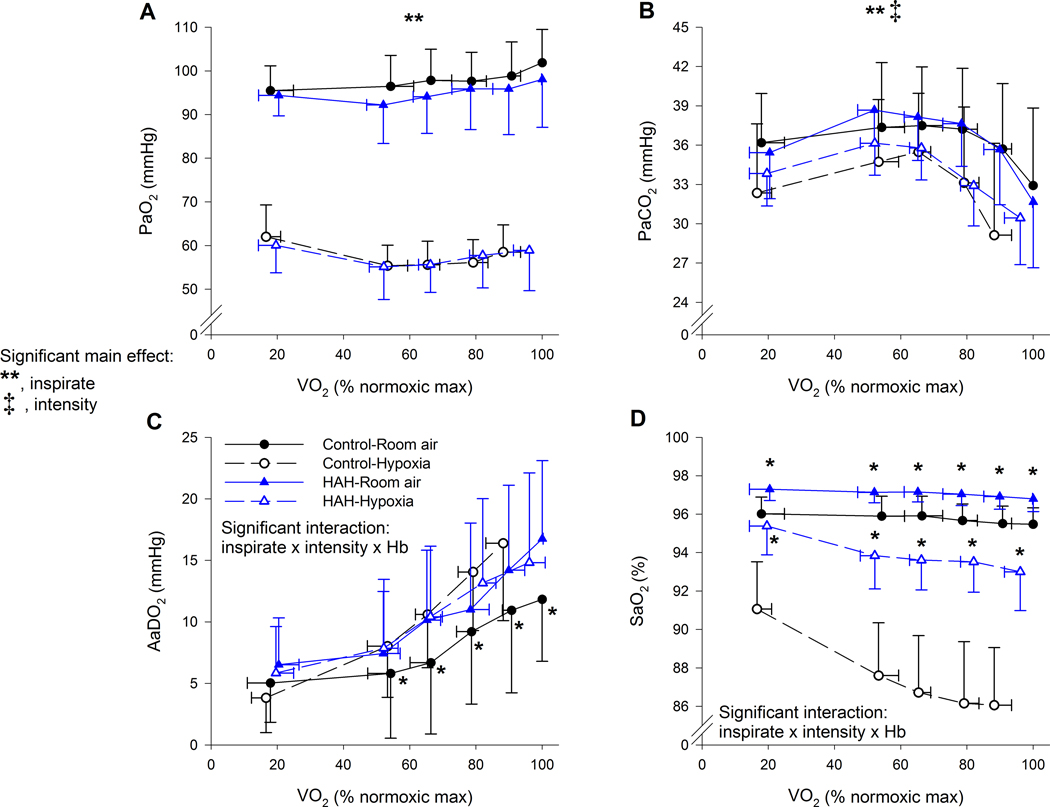
Arterial blood gases, alveolar to arterial oxygen gradients and oxyhaemoglobin saturation for both exercise trials in controls and high affinity haemoglobin variants. There was a significant three-way interaction for Panels C and D. For Panel C, the asterix (*) represents the controls on room air were significantly lower than all other groups. For Panel D, the Asterix (*) represents that the high affinity subjects had significantly greater oxyhaemoglobin saturation for each inspirate. PaO2, arterial oxygen tension; PaCO2, arterial carbon dioxide tension; AaDO2, alveolar to arterial oxygen difference; FO2Hb, fraction of haemoglobin saturated with oxygen. ⩒O2, oxygen uptake. **, significant main effect of inspirate; ‡, significant main effect of intensity type. P<0.05.
Submaximal exercise.
Indices of arterial oxygen content and delivery are shown in Figures 5 and 6. There was a significant main effect of inspirate on PaO2, but no other significant main or interactive effects (Figure 5A). For PaCO2, there was the expected main effect of inspirate and intensity with both hypoxia and increasing exercise intensity resulting in lower PaCO2 (Figure 5B). There was a significant three-way interaction for SaO2, with the HAH having a higher SaO2 at every intensity and inspirate (Figure 5D). Furthermore, while those with HAH had a decline in SaO2 during the hypoxic trial, it was significantly less compared to the decline observed in the control group. CaO2 was significantly higher in the HAH subjects for both exercise trials (Figure 6A), due to the significantly higher Hb/Hct (Figure 6B) and SaO2 (Figure 5D). Both groups also showed the expected haemoconcentration (Figure 6B) and subsequent rise in CaO2 during exercise (Figure 6A). Mean arterial pressure rose progressively with exercise intensity in both groups with no other significant effects noted (Figure 6C). Heart rate rose throughout exercise in both groups, but to a lesser degree in hypoxia, with no significant difference between groups (Figure 6D).
Figure 6.
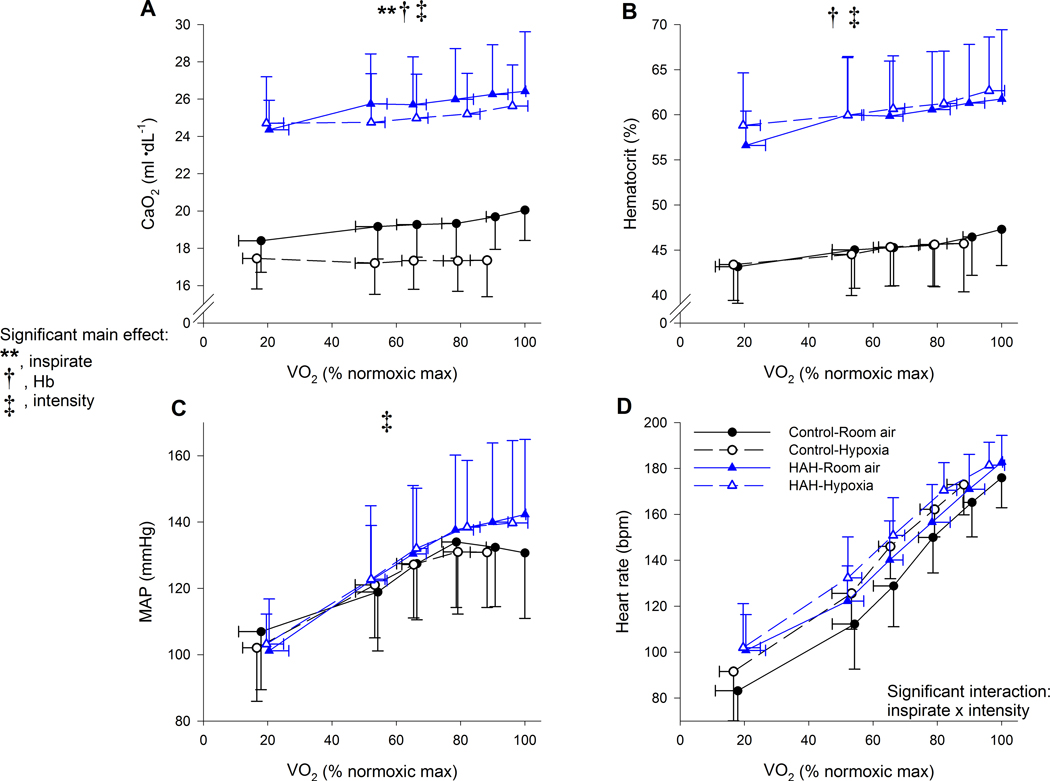
Arterial oxygen content, haematocrit, and mean arterial pressure along with heart rate for the two exercise trials. CaO2, arterial oxygen content; MAP, mean arterial pressure. ⩒O2, oxygen uptake. There was a significant main effect for exercise intensity for all panels. **, significant main effect of inspirate; †, significant main effect of haemoglobin type, ‡ significant main effect of intensity P<0.05.
Arterial metabolites are presented in Figure 7. There was a significant interaction between Hb and intensity for arterial lactate (Figure 7A). Above 80% of normoxic ⩒O2 the control subjects had significantly lower arterial lactate values than HAH. A similar result was found for pH (Figure 7B). Significant main effects of inspirate and intensity were found for arterial bicarbonate and base excess (Figure 7D and E) with no main or interactive effects of Hb, whereby hypoxia and increasing exercise intensity resulted in lower levels for both. There were no significant differences for arterial glucose or potassium (Figure 7C and F).
Figure 7.
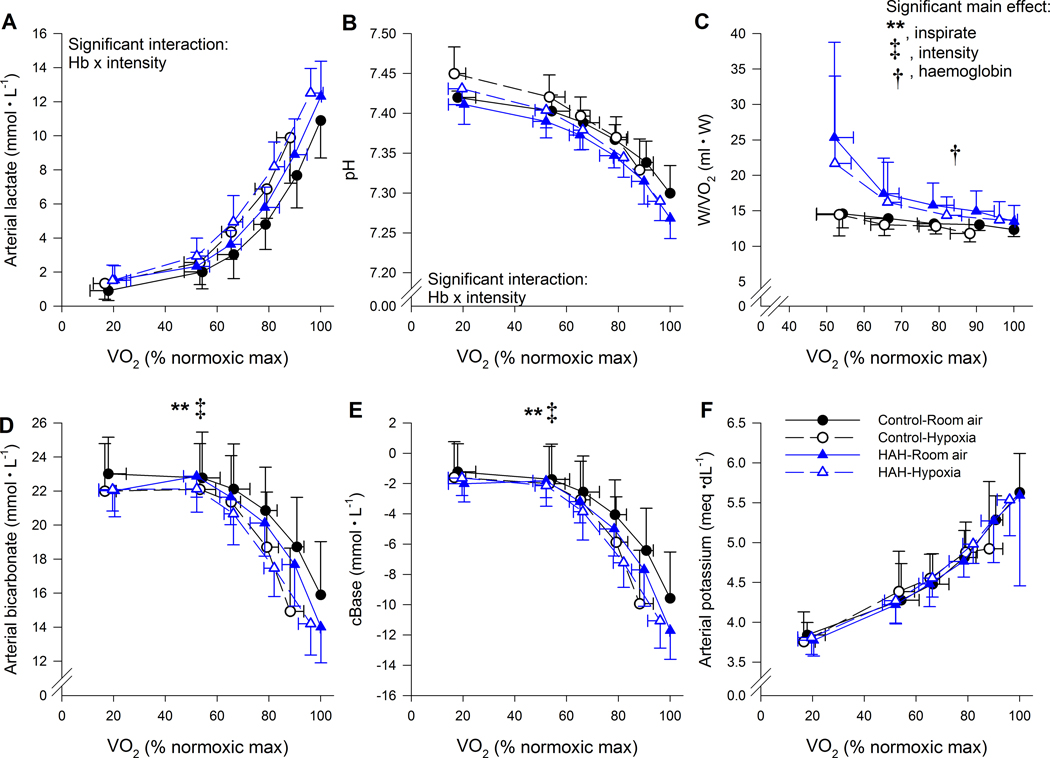
Arterial metabolite variables and economy during both exercise trials. ⩒O2, oxygen uptake. **, significant main effect of inspirate, †, significant main effect of haemoglobin type ‡ significant main effect of intensity. P<0.05.
Variables of pulmonary ventilation are shown in Figure 8. For all of the displayed variables (⩒E, respiratory exchange ratio, ⩒E /⩒O2 and ⩒E /⩒CO2), there were main effects of inspirate and intensity, each of which increased in hypoxia and as exercise intensity progressed. There were no main or interactive effects of Hb.
Figure 8.
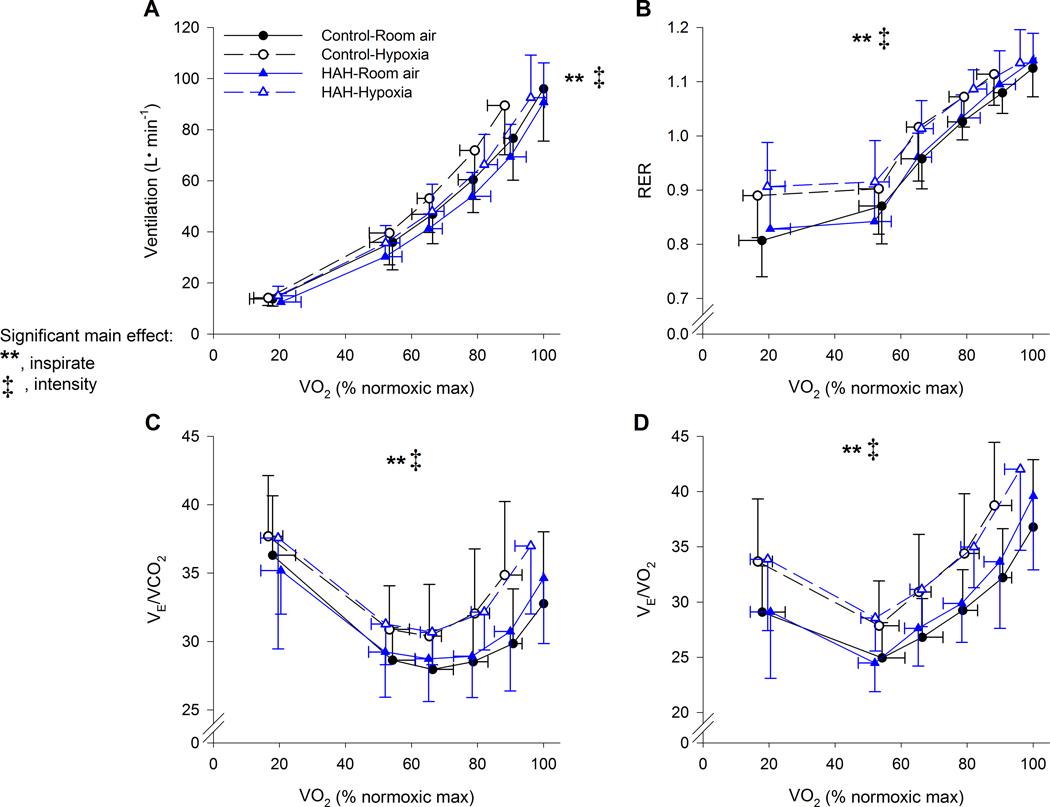
Ventilatory variables during both exercise trials in controls and high affinity haemoglobin variants. ⩒E, minute ventilation; RER, respiratory exchange ratio; ⩒O2, oxygen uptake; ⩒CO2, carbon dioxide production. **, significant main effect of inspirate, ‡ significant main effect of intensity. P<0.05.
Discussion
Major findings.
The major findings from our study are three-fold. First, HAH and the associated polycythaemia attenuates the decrements in aerobic exercise capacity during acute hypoxia. We interpret this to indicate that the benefits of HAH in enhancing oxygen uptake in the lungs outweighs deficits in oxygen unloading in the muscle. Second, HAH protects against worsening pulmonary gas exchange during acute hypoxic exercise. The lack of change in pulmonary gas exchange efficiency for HAH subjects is due to the shape of their ODC, which preserves the equilibrium index in hypoxia despite the lack of difference in PaO2. Third, subjects with HAH had a greater reliance on anaerobic metabolism throughout exercise in both normoxia and hypoxia. The greater reliance on anaerobic metabolism is most likely the consequence of impaired oxygen offloading at the level of the working muscle. Overall, our findings support the comparative biology literature and the notion that HAH may be a strategy for preserving exercise tolerance in acute hypoxia.
Maximal oxygen uptake.
Acute hypoxic exposure results in a consistent decline in ⩒O2max. In line with this, our controls subjects had a ~12% decline in their ⩒O2max when breathing 15% O2 (equivalent to ~3000 m). The decline is within a similar range of studies that report changes in ⩒O2max with increasing altitude (Buskirk et al., 1967), but it important to note that our groups had a lower ⩒O2max in normoxia, which will impact the decline in hypoxia (Lawler et al., 1988; Martin & O’Kroy, 1993). However, comparing ⩒O2max decline in response to absolute levels of hypoxia (e.g. 15% O2 or 3000m exposure) does not account for other aspects of hypoxic exposure that can impact exercise capacity. For example, hypoxic ventilatory response is variable between individuals (Teppema & Dahan, 2010), and those who have greater ventilatory responses have smaller declines in SaO2 and ⩒O2max (Gavin et al., 1998). As such, a better comparison would be the decrease in ⩒O2max compared to the change in SaO2. Previously, it was demonstrated that for every 1% decline in SaO2 below ~95%, there is a 1–2% decline in ⩒O2max (Harms et al., 2000).
Next, we compared the decline in SaO2 between normoxic and hypoxic exercise with the % change in ⩒O2max (Figure 3). Consistent with others, our controls on average had a 12±5 % decline in ⩒O2max and a corresponding 9±2% difference in SaO2 or 1.2±0.5 % decline in ⩒O2max per 1% decline in SaO2. Conversely, the HAH group only had a 4±5% decline in ⩒O2max resulting from a 4±2% difference in SaO2 or 0.7±1.5 % decline in ⩒O2max per 1% decline in SaO2, with no statistical difference in these slopes for controls and HAH, respectively. The relationship between SaO2 and decline in aerobic capacity is further illustrated by the clear relationship shown in Figure 3, whereby those with the greatest decrease in SaO2 have the greatest decrement in ⩒O2max, independent of Hb type.
Although not statistically significant, the aerobic fitness of our HAH was lower than controls (Table 3). There is a known significant relationship between initial ⩒O2max and its decrement in hypoxia (Buskirk et al., 1967; Lawler et al., 1988; Martin & O’Kroy, 1993). As such, part of the attenuation in ⩒O2max in our HAH subjects could be due to their lower initial aerobic fitness levels. To explore this idea, we utilized a published model that accounts for both sea-level ⩒O2max and level of hypoxia to estimate maximal aerobic capacity at altitude (MacInnis et al., 2015). Using our subjects who fell within the range of the model, the control subjects real vs. predicted decline in ⩒O2max were in close agreement to the model, being within 1.1 ml kg−1 min−1 or 3%, with subjects equally distributed above and below predicted levels. Conversely, the HAH subjects’ actual hypoxic ⩒O2max was 5.2 ml kg−1 min−1 (~18%) higher than predicted and in every subject was greater than predicted. Therefore, the attenuated decline in ⩒O2max in the HAH subjects appears to be independent of their initial aerobic fitness. Another potential confounder was the compensatory polycythaemia in the HAH subjects. However, we found no relationship between Hb concentration and decline in ⩒O2max during hypoxic exercise. The lack of effect of Hb concentrations is similar to others who found that Hb did not impact ⩒O2max in acclimatized low-landers (Calbet et al., 2002). However, it should be appreciated that the haematocrit of the microvasculature is considerable lower than that of the systemic circulation (Poole et al., 2011). It is possible, that the higher haematocrit in the HAH subjects permeated into the microcirculation and also improved diffusive oxygen transport. Overall, most likely the increased oxyhaemoglobin affinity of the HAH group, and not the increased Hb, likely played the major role in preserved acute hypoxic exercise performance.
Pulmonary gas exchange.
As exercise progressed in normoxia, there was a progressive widening of the A-aDO2 in both groups (Figure 4). In normoxia, the controls had a significantly lower A-aDO2 at submaximal and maximal exercise intensities, compared to the HAH subjects. Based on insights from our previous model, most likely, the lower mixed venous PO2 required to sufficiently extract oxygen in the HAH resulted in poorer pulmonary end-capillary equilibrium resulting in the greater A-aDO2 (Stickland et al., 2013; Shepherd et al., 2019). It is also possible that the higher Hb levels could hinder gas exchange, as haemodilution can lower A-aDO2 (Deem et al., 1999). Nonetheless, there was considerable between-subject variability in the absolute A-aDO2 for a given workload as previously reported (Dempsey & Wagner, 1999; Stickland et al., 2013). For a given workload, hypoxia results in a greater A-aDO2 (Torre-Bueno et al., 1985; Wagner et al., 1986; Olfert et al., 2004; Lovering et al., 2008). The greater A-aDO2 is due to impaired lung diffusion (Wagner et al., 1987), resulting in a poorer equilibrium index [see (Piiper & Scheid, 1981; Stickland et al., 2013)].
Indeed, we found that compared to normoxic exercise, the A-aDO2 during in hypoxia was significantly greater in the control subjects (Figure 4 & 5C). However, there was no change in pulmonary gas exchange efficiency between normoxic and hypoxic exercise in the HAH subjects. The lack of a difference in gas exchange efficiency between normoxia and hypoxia in those with HAH is likely due to differences in the average slope of the ODC or the diffusive to convective conductance ratio (Kobayashi et al., 1991; Stickland et al., 2013). Specifically, in control subjects, hypoxia results in the oxyhaemoglobin saturation being on the ‘steep’ part of the ODC, which hinders diffusive gas exchange. Conversely, the HAH allows those subjects to stay on the relatively flat upper portion of their ODC curve during exercise in normoxia, especially during hypoxia. Although the HAH subjects’ gas exchange efficiency is statistically worse in normoxia, it is likely of little physiological consequence. This is because PaO2 is maintained near resting levels at submaximal and maximal exercise intensities as compensatory hyperpnea offsets worsening A-aDO2. In hypoxia, the significantly greater A-aDO2 in controls contributes to the lower SaO2, which is not the case in the HAH subjects.
Anaerobic metabolism.
As expected, for both groups in hypoxia and normoxia, there was a progressive rise in arterial lactate and lowering of arterial pH, together resulting in lower arterial bicarbonate and base excess (Figure 7). At both submaximal and maximal exercise, those with HAH had a greater arterial lactate concentration and lower pH (Figure 1 & 7). Yet, despite group differences regarding changes in lactate and pH, bicarbonate and base excess were not different between groups (Figure 1 & 7). The lack of difference in base excess is likely due to higher Hb concentration in the HAH group which increases the buffering capacity of blood (Davenport, 1974). Thus, although the HAH subjects are potentially producing more anaerobic metabolites, they are similarly able to buffer the acidic changes. The control subjects also had lower arterial blood lactate concentration during hypoxic exercise compared to normoxic exercise (Figure 1D). This counterintuitive decrease in lactate concentration has been observed during hypoxic exercise in acclimatized subjects; this phenomenon is known as the “lactate paradox” (Hochachka et al., 2002), and its interpretation is less clear during acute exposure (Calbet et al., 2003). Conversely, the HAH group had nearly identical arterial lactates for both exercise trials (Figure 1). The lack of change in lactate could be due to the considerable reliance on anaerobic metabolism in the HAH group, and/or that their absolute exercise intensity (in Watts) during hypoxia was similar to the intensity in their normoxic exercise trial.
We sought to further explore how a lower pH in HAH subjects impacts the integrative exercise response. We verified that the Bohr effect of pH on SaO2 was similar and performed the P50 analysis at a pH of 7.2 in a subset of subjects (including both control and HAH). Both the control and the HAH group showed the expected rightward shift of the ODC with a pH of 7.2, and the increases in P50 for both was equivalent at ~ 7 mmHg. Thus, with the lower pH developed during exercise (especially in active muscles), we expect the HAH variants to still maintain a left-shifted (increased affinity) Hb compared with the control subjects. This confirmation of a similar Bohr effect is significant because it indicates that HAH subjects have a comparable ability to decrease Hb oxygen affinity in the muscle, thus facilitating oxygen unloading and utilization. Since the arterial pH (and presumably muscle pH) was significantly lower in those with HAH, HAH subjects will experience a relatively greater increase in P50 in the muscle compared to the controls. The relatively greater increase of in vivo P50 would potentially facilitate oxygen diffusion into the muscle, because the HAH subjects would have an ability to ‘unload’ oxygen that is similar to controls. Conversely, in subjects with normal Hb, in vivo shifts in Hb affinity have little impact on oxygen transport and utilization during hypoxic exercise due to the close proximity of the respective ODC at muscle PO2 (Dempsey et al., 1975). Specifically, although the P50 increases during hypoxic exercise, venous PO2 is at the lower part of the ODC (~10–20 mmHg) where it ‘converges’ with a resting curve (Dempsey et al., 1975). A caveat to the above is it assumes homogenous distribution of oxygen delivery matching demand throughout the muscle. As such, any heterogeneity would potentially amplify the effects of the ODC on venous PO2.
The higher ⩒O2 for a given relative power output in the HAH subjects indicates a worse exercise economy (Table 3 & Figure 7 Panel C). The lower economy in HAH is potentially a function of poor oxygen unloading at the level of the working muscles which results in a greater reliance on less economical energy pathways. Alternatively, those with HAH could have a lower mitochondrial density, which could be due to the lower aerobic fitness or lack of appropriate stimulus at the level of the muscle. Regardless of the cellular cause, it is clear that those HAH subjects have a greater reliance on anaerobic metabolism and poor exercise economy. However, identifying definitive mechanisms responsible for the cellular consequences of HAH awaits testing with muscle biopsies.
Ventilation.
There were no differences in pulmonary ventilation or ventilatory equivalents between the groups during normoxic exercise (Figure 8). As expected, exercise in acute hypoxia resulted in a greater ventilation (Panel A) and higher metabolic equivalents (Panels C and D) for a given workload, but this too was not different between groups. Recently, we found that resting chemosensitivity to acute hypoxia and hypercapnia was not different between controls and subjects with HAH (Dominelli et al., 2019). Although resting chemosensitivity does not necessarily translate into the control of exercise hyperpnoea, we assume that it would be similar between groups. During exercise there are a multitude of interactive ventilatory stimuli (Forster et al., 2012), as such, it is difficult to determine the exact role of each on exercise hyperpnoea in exercising humans. With the above in mind, we did find significant differences in some known ventilatory stimuli (i.e. pH) while others were not different (i.e. potassium ion concentration), yet ventilation was not different (Figure 8).
Although submaximal ventilation was significantly greater in both groups during hypoxic exercise, maximal ⩒E was unchanged. Most likely this was due to the lower metabolic production as ventilatory equivalents for both O2 and CO2 were greater during hypoxic exercise (Table 3). While no significant effects of Hb affinity were observed, it is noteworthy that the controls’ end-exercise ⩒E in hypoxia was lower than their ⩒E in normoxia, whereas the HAH subjects were similar had minimal change in ⩒E between the two inspirates (Figure 8 & Table 3). As the controls had a greater decrease in their workload (thus metabolic rate) in the hypoxia trial, it is expected that their ⩒E would also decrease. In line with this, there was a significant relationship between the change in ⩒E and the change in ⩒O2 (r=0.69, p<0.0001) or ⩒CO2 (r=0.77, p<0.0001), whereby those subjects that had the largest decrease in either ⩒O2 or ⩒CO2 also had the greatest decline in ⩒E. As the HAH subjects had minimal change in their maximal workload in hypoxia, it is unsurprising that their end-exercise ⩒E was also relative unchanged. The lack of increased ⩒E could also be due to mechanical constraints to hyperpnea which were not assessed in the current study. Overall, we interpret our results to indicate that that the overall integrative ventilatory output does not appear to be influenced by HAH per se.
Perspectives.
Our HAH group consisted of two known beta chain mutated Hb variants: Haemoglobin Malmö (Fairbanks et al., 1971; Berglund, 1972b) (n=11, P50 ~15–16 mmHg) and Haemoglobin San Diego (Nute et al., 1974) (n=1, P50 ~17 mmHg). While these two HAH variants have different P50 values, we cautiously note that the subjects with a higher P50 showed no or very minimal decline in either ⩒O2 or peak power output during exercise in hypoxia. We emphasize though, that the change in ⩒O2 in acute hypoxia is quite variable and our observation is speculative. Nonetheless, it is possible that there is an ideal P50 for a given level of hypoxic exposure, whereby any decrement in oxygen unloading in the muscle from HAH is balanced by enhanced pulmonary oxygen uptake. In other words, for our level of hypoxia (15% O2), it is possible that the greater P50 provided the ideal balance between preserved arterial oxygenation and tissue oxygen extraction. Evidence for this comes from a case study by Hebbel et al. (Hebbel et al., 1978), which demonstrated that two siblings (under 18 years old) with the beta chain variant Hb-Andrew-Minneapolis (P50= 17 mmHg) had no decline in maximal ⩒O2 during exercise after two weeks exposure to 3100 m (~14.5% O2 equivalent at sea-level). An important distinction though is the subjects in the previous study were exposed to hypoxia much longer (2 weeks) than our study (<1 hr) and it is unknown the extent to which HAH subject acclimatize to long-term hypoxia. We again reinforce that this notion is speculative and requires experimental testing with individuals possessing a greater range of P50.
Limitations.
Our study has limitations that merit comment. First, while we clearly demonstrate that those with HAH have an attenuated decrease in ⩒O2 at altitude, we are currently unable to dissociate the individual influence of HAH versus the associated polycythaemia. Others have tested this hypothesis in normoxia by having individuals with the beta chain variant Hb Linköping (P50=16.5 mmHg) perform a maximal exercise test before and after haemodilution (3 g dL−1 reduction in Hb ) (Wranne et al., 1991). The maximal ⩒O2 in subjects with Hb Linköping was similar to our subjects, and they reported no significant decline in maximal ⩒O2 or peak power output after haemodilution (Wranne et al., 1991). While the polycythaemia associated with HAH appears to have minimal impact on normoxic exercise tolerance, we acknowledge that this hypothesis requires confirmation during hypoxic exercise. However, we note that there was no within group relationship between Hb concentration and ⩒O2max. Second, due to logistical constraints, we performed both maximal exercise tests on the same day, which could have resulted in lower values for the second exercise test. To ensure this did not impact our principal hypothesis, we randomized the order of testing and ensured it was balanced between controls and HAH subjects. Third, most of our subjects had little prior experience performing maximal exercise tests and we did not perform a ⩒O2 validation test (Poole & Jones, 2017). As such, exercise may have been terminated due to a psychological or non-energetic factor. While not all subjects may have attained their ⩒O2max, both groups had several indicators of (near) maximal effort) (respiratory exchange ratio>1.1, a maximal heart rate >95% predicted, arterial lactate > 9 mmol L−1) with no differences between them. A such, we do not believe this limitation predominantly impacted one group. Finally, our HAH subjects as a group would be classified as obese, which itself impacts exercise performance. However, if we examine a subset of HAH subjects (n=6) with a BMI under 30 kg m2, we find that they were even better able to maintain their ⩒O2max in acute hypoxia (99.5±3.0 % of normoxia). Thus, the obese HAH subject may have attenuated our main finding of HAH preserving ⩒O2max in hypoxia with obesity specific limitation to exercise tolerance.
Conclusions.
We conclude that in otherwise healthy humans, HAH preserves exercise performance under acute hypoxia conditions. The attenuated decline in ⩒O2max during hypoxic exercise in our HAH subjects was achieved with higher SaO2 and maintained pulmonary gas exchange. Overall, our data supports the assertion consistent with the comparative biology literature, that increased oxyhaemoglobin affinity is a superior strategy to preserve exercise tolerance in acute hypoxia.
Supplementary Material
Key Points Summary:
Theoretical models suggest there is no benefit of high affinity haemoglobin to preserve maximal oxygen uptake in acute hypoxia. Conversely, the comparative biology literature has many examples of species that are evolutionarily adapted to hypoxia and have high affinity haemoglobin.
We studied humans with high affinity haemoglobin and compensatory polycythaemia. These subjects performed maximal exercise tests in normoxia and hypoxia to determine how their altered affinity impacts hypoxic exercise tolerance.
The high affinity haemoglobin participants demonstrated an attenuated decline in maximal aerobic capacity in acute hypoxia.
Those with high affinity haemoglobin had no worsening of pulmonary gas exchange during hypoxic exercise, but had a greater lactate and lower pH for all exercise bouts.
High affinity haemoglobin and compensatory polycythaemia mitigated the decline in exercise performance in acute hypoxia through a higher arterial oxygen content and an unchanged pulmonary gas exchange.
Acknowledgements.
We are thankful for the enthusiastic participation of our research subjects. We would like to acknowledge the contribution of the Human Integrative Physiology Laboratory and the Clinical Research and Trials Unit at the Mayo Clinic. We would like to thank Andrew Miller, Zachariah Scruggs, Pamela Engrav, Nancy Meyer, and Christopher Johnson for their continued assistance throughout the project.
Funding: PBD was supported by a post-doctoral fellowship from the Natural Science and Engineering Council of Canada. CCW was supported by NIH-T32-DK-007352-39. SEB was supported by NIH-F32-HL131151. The work was funded by National Instituted of Health R-35-HL139854 and the Mayo Foundation.
Abbreviation list:
- A-aDO2
alveolar to arterial oxygen difference
- Hb
haemoglobin
- ODC
oxyhaemoglobin dissociation curve
- PaO2
arterial oxygen tension
- PaCO2
arterial carbon dioxide tension
- P50
partial pressure which results in 50% of haemoglobin to be saturated with oxygen
- SaO2
oxyhaemoglobin saturation
- ⩒E
ventilation
- ⩒O2
oxygen uptake
References
- Arthur PG, Hogan MC, Bebout DE, Wagner PD & Hochachka PW (1992). Modeling the effects of hypoxia on ATP turnover in exercising muscle. J Appl Physiol 73, 737–742. [DOI] [PubMed] [Google Scholar]
- Aste-Salazar H & Hurtado A (1944). The affinity of hemoglobin for oxygen at sea level and at high altitudes. Am J Physiol 142, 733–743. [Google Scholar]
- Bencowitz HZ, Wagner PD & West JB (1982). Effect of change in P50 on exercise tolerance at high altitude: a theoretical study. J Appl Physiol 53, 1487–1495. [DOI] [PubMed] [Google Scholar]
- Berglund S (1972a). The oxygen dissociation curve of whole blood contraining haemoglobin Malmo. Scand J Haemat 9, 377–386. [DOI] [PubMed] [Google Scholar]
- Berglund S (1972b). Erythrocytosis associated with haemoglobin Malmo, accompanied by pulmonary changes, occuring in the same family. Scand J Haemat 9, 355–369. [DOI] [PubMed] [Google Scholar]
- Buskirk ER, Kollias J, Akers RF, Prokop EK & Reategui EP (1967). Maximal performance at altitude and on return from altitude in conditioned runners. J Appl Physiol 23, 259–266. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Boushel R, Radegran G, Sondergaard H, Wagner PD & Saltin B (2003). Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol-Reg Integ Comp Physiol 284, R291–R303. [DOI] [PubMed] [Google Scholar]
- Calbet JAL, Rådegran G, Boushel R, Søndergaard H, Saltin B & Wagner PD (2002). Effect of blood haemoglobin concentration on VO2max and cardiovascular function in lowlanders acclimatised to 5260 m. J Physiol 545, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates AL, Peslin R, Rodenstein D & Stocks J (1997). Measurement of lung volumes by plethysmography. Eur Respir J 10, 1415–1427. [DOI] [PubMed] [Google Scholar]
- Crapo R, Jensen R, Hegewald M & Tashkin D (1999). Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Resp Crit Care Med 160, 1525–1531. [DOI] [PubMed] [Google Scholar]
- Davenport HW (1974). The ABC of Acid-Base Chemistry, 6th edn The University of Chicago Press. [Google Scholar]
- Deem S, Hedges RG, McKinney S, Polissar NL, Alberts MK & Swenson ER (1999). Mechanisms of improvement in pulmonary gas exchange during isovolemic hemodilution. J Appl Physiol 87, 132–141. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Thomson JM, Forster H V, Cerny FC & Chosy LW (1975). HbO2 dissociation in man during prolonged work in chronic hypoxia. J Appl Physiol 38, 1022–1029. [DOI] [PubMed] [Google Scholar]
- Dempsey JA & Wagner PD (1999). Exercise-induced arterial hypoxemia. J Appl Physiol 87, 1997–2006. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Baker SE, Wiggins CC, Stewart GM, Sajgalik P, Shepherd JRA, Roberts SK, Roy TK, Curry TB, Hoyer JD, Oliveira JL, Foster GE & Joyner MJ (2019). Dissociating the effects of oxygen pressure and content on the control of breathing and acute hypoxic response. J Appl Physiol; In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton JW, Skelton TD & Berger E (1974). Survival at Extreme Altitude: Protective Effect of Increased Hemoglobin-Oxygen Affinity. Science (80- ) 183, 743–744. [DOI] [PubMed] [Google Scholar]
- Fairbanks VF, Maldonado JE, Charache S & Boyer S (1971). Familial erthrocytosis due to electrophoretically undectable hemoglobin with impaired oxygen dissociations (hemoglobin Malmo, alpha 2 beta 2 97 gln). Mayo Clin Proc 46, 721–727. [PubMed] [Google Scholar]
- Forster HV, Haouzi P & Dempsey JA (2012). Control of Breathing During Exercise. Comp Physiol 2, 743–777. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Derchak PA & Stager JM (1998). Ventilation’s role in the decline in VO2max and SaO2 in acute hypoxic exercise. Med Sci Sport Exerc 30, 195–199. [DOI] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB & Dempsey JA (2000). Effect of exercise-induced arterial O2 desaturation on O2max in women. Med Sci Sport Exer 32, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG & Berger EM (1978). Human llamas: adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest 62, 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Beatty CL, Burelle Y, Trump ME, McKenzie DC & Matheson GO (2002). The lactate paradox in human high-altitude physiological performance. New Physiol Sci 17, 122–126. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Arthur PG, Bebout DE, Hochachka PW & Wagner PD (1992). Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol 73, 728–736. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Pelster B, Piiper J & Scheid P (1991). Diffusion and perfusion limitation in alveolar O2 exchange: shape of the blood O2 equilibrium curve. Resp Physiol 83, 23–34. [DOI] [PubMed] [Google Scholar]
- Lawler J, Powers SK & Thompson D (1988). Linear relationship between VO2max and VO2max decrement during exposure to acute hypoxia. J Appl Physiol 64, 1486–1492. [DOI] [PubMed] [Google Scholar]
- Lenfant C, Torrance J, English E, Finch CA, Reynafarje C, Ramos J & Faura J (1968). Effect of altitude on oxygen binding by hemoglobin and on organic phosphate levels. J Clin Invest 47, 2652–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C, Torrance JD & Reynafarje C (1971). Shift of the O2-Hb dissociation curve at altitude: mechanism and effect. J Appl Physiol 30, 625–631. [DOI] [PubMed] [Google Scholar]
- Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS & Eldridge MW (2008). Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol 104, 1418–1425. [DOI] [PubMed] [Google Scholar]
- MacInnis MJ, Nugent SF, MacLeod KE & Lohse KR (2015). Methods to Estimate VO2max upon Acute Hypoxia Exposure. Med Sci Sport Exerc 47, 1869–1876. [DOI] [PubMed] [Google Scholar]
- Martin D & O’Kroy J (1993). Effects of acute hypoxia on the VO2 max of trained and untrained subjects. J Sport Sci 11, 37–42. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G & Wanger J (2005). Standardisation of spirometry. Eur Resp J 26, 319 LP – 338. [DOI] [PubMed] [Google Scholar]
- Natarajan C, Jendroszek A, Kumar A, Weber RE, Tame JRH, Fago A & Storz JF (2018). Molecular basis of hemoglobin adaptation in the high-flying bar-headed goose. PLOS Genet 14, e1007331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nute PE, Stamatoyannopoulos G, Hermodson MA & Roth D (1974). Hemoglobinopathic erythrocytosis due to a new electrophoretically silent variant, hemoglobin San Diego (beta109 (G11)val--met). J Clin Invest 53, 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, Balouch J, Kleinsasser A, Knapp A, Wagner H, Wagner PD & Hopkins SR (2004). Does gender affect human pulmonary gas exchange during exercise? J Physiol 557, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piiper J & Scheid P (1981). Model for capillary-alveolar equilibration with special reference to O2 uptake in hypoxia. Resp Physiol 46, 193–208. [DOI] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Hirai DM & Musch TI (2011). Dynamics of muscle microcirculatory and blood-myocyte O(2) flux during contractions. Acta Physiol 202, 293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC & Jones AM (2017). Measurement of the maximum oxygen uptake ⩒o2max: ⩒o2peak is no longer acceptable. J Appl Physiol 122, 997–1002. [DOI] [PubMed] [Google Scholar]
- Samaja M, DiPrampero PE & Cerretelli P (1985). Simulation of oxygen delivery to tissues: The role of the hemoglobin oxygen equilibrium curve at altitude. Int J Clin Monit Comp 2, 95–99. [DOI] [PubMed] [Google Scholar]
- Schmidg-Neilsen K & Larimer JL (1958). Oxygen dissociation curves of mammalian blood in relation to body size. Am J Physiol 195, 424–428. [DOI] [PubMed] [Google Scholar]
- Scott GR, Hawkes LA, Frappell PB, Butler PJ, Bishop CM & Milsom WK (2015). How Bar-Headed Geese Fly Over the Himalayas. Physiol 30, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinghaus JW (1958). Oxyhemoglobin dissociation curve correction for temperature and pH variation in human blood. J Appl Physiol 12, 485–486. [DOI] [PubMed] [Google Scholar]
- Shepherd JRA, Dominelli PB, Roy TK, Secomb TW, Hoyer JD, Oliveira JL & Joyner MJ (2019). Modelling the relationships between haemoglobin oxygen affinity and the oxygen cascade in humans. J Physiol 59716, 4193–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Lindinger MI, Olfert IM, Heigenhauser GJF & Hopkins SR (2013). Pulmonary Gas Exchange and Acid-Base Balance During Exercise In Comp Physiol. John Wiley & Sons, Inc; Available at: 10.1002/cphy.c110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF (2016). Hemoglobin–oxygen affinity in high-altitude vertebrates: is there evidence for an adaptive trend? J Exp Biol 219, 3190–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ & Dahan A (2010). The Ventilatory Response to Hypoxia in Mammals: Mechanisms, Measurement, and Analysis. Physiol Rev 90, 675–754. [DOI] [PubMed] [Google Scholar]
- Torre-Bueno JR, Wagner PD, Saltzman HA, Gale GE & Moon RE (1985). Diffusion limitation in normal humans during exercise at sea level and simulated altitude. J Appl Physiol 58, 989–995. [DOI] [PubMed] [Google Scholar]
- Wagner PD (1997). Insensitivity of ⩒O2max to hemoglobin-P50 at sea level and altitude. Resp Physiol 107, 205–212. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW & Saltzman HA (1986). Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol 61, 260–270. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Sutton JR, Reeves JT, Cymerman A, Groves BM & Malconian MK (1987). Operation Everest II: pulmonary gas exchange during a simulated ascent of Mt. Everest. J Appl Physiol 63, 2348–2359. [DOI] [PubMed] [Google Scholar]
- Winslow RM, Swenberg ML, Berger RL, Shrager RI, Luzzana M, Samaja M & Rossi-Bernardi L (1977). Oxygen equilibrium curve of normal human blood and its evaluation by Adair’s equation. J Biol Chem 252, 2331–2337. [PubMed] [Google Scholar]
- Wranne B, Jorfeldt L, Berlin G, Ryding E, Lansimies E, Kuikka J, Rautio P, Vainio P & Lahtinen R (1991). Effect of hemodilution on maximal oxygen consumption, blood lactate response to exercise and cerebral blood flow in subjects with a high-affinity hemoglobin. Eur J Haematol 47, 268–276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


