Abstract
PURPOSE
Nonadherence to aromatase inhibitors (AIs) for breast cancer is common and increases the risk of recurrence. Text messaging increases adherence to medications for chronic conditions.
METHODS
We conducted a randomized clinical trial of text messaging (TM) versus no text messaging (No-TM) at 40 sites in the United States. Eligible patients were postmenopausal women with early-stage breast cancer taking an AI for > 30 days with a planned duration of ≥ 36 months. Test messages were sent twice a week over 36 months. Content themes focused on overcoming barriers to medication adherence and included cues to action, statements related to medication efficacy, and reinforcements of the recommendation to take AIs. Both groups were assessed every 3 months. The primary outcome was time to adherence failure (AF), where AF was defined as urine AI metabolite assay results satisfying one of the following: < 10 ng/mL, undetectable, or no submitted specimen. A stratified log-rank test was conducted. Multiple sensitivity analyses were performed.
RESULTS
In total, 724 patients were registered between May 2012 and September 2013, among whom,702 patients (348 in the text-messaging arm and 354 in the no–text-messaging arm) were eligible at baseline. Observed adherence at 36 months was 55.5% for TM and 55.4% for No-TM. The primary analysis showed no difference in time to AF by arm (3-year AF: 81.9% TM v 85.6% No-TM; HR, 0.89 [95% CI, 0.76 to 1.05]; P = .18). Multiple time to AF sensitivity analyses showed similar nonsignificant results. Three-year self-reported time to AF (10.4% v 10.3%; HR, 1.16 [95% CI, 0.69 to 1.98]; P = .57) and site-reported time to AF (21.9% v 18.9%; HR, 1.31 [95% CI, 0.86 to 2.01]; P = .21) also did not differ by arm.
CONCLUSION
To our knowledge, this was the first large, long-term, randomized trial of an intervention directed at improving AI adherence. We found high rates of AI AF. Twice-weekly text reminders did not improve adherence to AIs. Improving long-term adherence will likely require personalized and sustained behavioral interventions.
INTRODUCTION
Despite the proven efficacy of aromatase inhibitors (AIs) for the treatment of hormone-sensitive breast cancer,1 full adherence to therapy for the duration of 5 years is only 50%.2 Issues related to nonadherence are increasingly important, because analyses from prospective randomized trials show that adherence to endocrine therapy is associated with improved disease-free survival.3 The reasons for nonadherence to hormonal therapy are multifactorial.4 Barriers include patient-, physician-, medication-, and system-related variables, and poor adherence is usually associated with a combination of these factors.5 Although there are many factors involved, work by our group has shown that a lack of knowledge about AI efficacy and how well the benefits were explained are associated with adherence.2,6-9
CONTEXT
Key Objectives
To determine if text message reminders improve adherence to adjuvant endocrine therapy.
Knowledge Generated
We conducted a multicenter randomized trial of text messaging (TM) or no text messaging (No-TM) twice a week for 36 months among women taking adjuvant aromatase inhibitor (AI) therapy. The primary analysis showed no difference in time-to-adherence failure between patients on the TM and No-TM arms (hazard ratio, 50.89 [95% CI, 0.76 to 1.05], P = .18).
Relevance
Bi-weekly unidirectional text reminders did not improve adherence to AIs compared to usual care.
Mobile phone ownership has grown enormously over the past decade.10 As a result, the effort to use mobile phones to generate reminders for a variety of health behaviors has increased. Two early studies showed that reminder text messages could be used to enhance clinic attendance and vaccination rates.11-13 Text-messaging interventions have also been used to enhance tobacco cessation, sunscreen use, and weight loss.14-16 A recent meta-analysis was conducted on mobile telephone text messaging for medication adherence in chronic, noncancer disease. Sixteen randomized clinical trials were included. In the pooled analysis, text messaging doubled medication adherence; however, the median intervention duration was short term (12 weeks), no trials focused on cancer therapies, and self-report, which can overestimate adherence, was the most commonly used method to assess adherence.17
There are no proven interventions to improve adherence to adjuvant endocrine breast cancer therapy. We conducted a multicenter randomized trial to determine if one-way text message reminders could improve long-term adherence. If successful, text messaging could provide a low-cost, simple intervention to improve adherence outcomes.
METHODS
Study Design
Patients were randomly assigned 1:1 to receive either text messaging (TM) or no text messaging (No-TM). The random assignment was dynamically balanced according to 2 stratification factors, length of time receiving AI therapy before random assignment (< 12 months v 12-24 months) and type of AI therapy (anastrozole v letrozole v exemestane), because these factors could be related to adherence. Patients were assessed at baseline and every 3 months for up to 36 months. A window of ± 21 days around each follow-up assessment was allowed to provide flexibility in scheduling. The study was conducted after appropriate approval by individual institutional review boards, in adherence with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The study was registered with ClinicalTrials.gov.
Patient Characteristics
Participants were women with histologically confirmed primary invasive estrogen receptor– and/or progesterone receptor–positive carcinoma of the breast (stage I-III). Patients were required to be postmenopausal (use of a gonadotropin-releasing hormone agonist was allowed), taking a third-generation AI for at least 30 days before registration, and have at least 3 years of AI therapy remaining. Patients were required to have a mobile phone that could receive text messages and be currently using or be willing to learn to use text messaging. Participants had to be willing to provide urine specimens to test for the presence of AIs and at each 3-month clinic visit for 36 months. Exclusion criteria included receipt of hormone therapy for prior diagnosis of breast cancer and Zubrod performance status > 2.
Study Intervention
Participants randomly assigned to the intervention arm received an educational text message twice a week, on 1 randomly selected weekday and on 1 randomly selected day on the weekend, over a 3-year period. The messages were sent at 8:00 am (for each time zone) by CareSpeak Communications. Text messaging education was conducted with all participants randomly assigned to the intervention arm. This involved confirmation of cell phone capability to receive text messages and instruction to the participant on how to retrieve and read text messages. A predetermined set of 40 text messages was developed from focus groups and a review of the literature; these messages had multiple formats, each with 160 characters or less. Content themes focused on overcoming potential barriers to medication adherence and included cues to action, statements related to the efficacy of the medication, reinforcements of the physician’s recommendation to take this medication, and words of support and encouragement (Data Supplement, online only). The 40 messages were randomly selected, and participants had the option to stop the text messages at any time.
Urine Assay and Outcome Measures
Assessments (patient-reported outcomes and urine collection) were conducted at baseline and every 3 months (± 21 days) for 36 months. The scheduled time window for each 3-month follow-up assessment was ± 21 days to allow for practical physician/patient considerations including (but not limited to) scheduling of visits and possible missed visits. Urine assays to detect the presence of AIs and metabolites were used for the primary end point.18 The assays used were either liquid chromatography/tandem mass spectrometry for anastrozole and exemestane and its metabolites or gas chromatography/mass spectrometry for letrozole and its metabolites (for methods, see Data Supplement). At each study visit, participants and sites were asked about hormonal medication discontinuation (discontinuation of AI without starting tamoxifen).
Statistical Considerations
Primary end point.
The primary end point for this study was time to adherence failure (AF) of AI therapy use. In this study, adherence “failure” was defined as either a negative urine test within the specified time window, indicating discontinuation over the prior 2 weeks, or failure to provide a urine specimen within the specified timeframe, which was assumed to be positively correlated with nonadherence. A positive urine test was defined as an assay result of ≥ 10 ng/mL for at least one of the AIs tested.18 A negative urine test was defined as all 3 assay results satisfying 1 of these three conditions: < 10 ng/mL, undetectable, or no submitted specimen. All 3 AIs were tested for each specimen, and the laboratory was blind to the random assignment. Assay failures (assays not performed because of technical difficulties) were treated as positive urine tests. Participants who had a newly diagnosed cancer or a cancer recurrence and those who had died were censored at the time of the last positive urine test. Patients who reached 3 years with no previous AF events, new cancer, or cancer recurrence were censored at their 3-year visit. At analysis, an additional 2 days were allowed to account for variable length of months and the different random assignment dates within the calendar year, resulting in an allowed assessment window of ± 23 days overall for analysis.
Analysis plan.
Observational studies have consistently shown a 2-year nonadherence rate of 25% and a 5-year nonadherence rate of approximately 40%-60%.2 By interpolation, the 3-year nonadherence rate was estimated to be 36%. With patients observed for 3 years, 636 eligible patients were required, to have a power of 80% to detect a hazard ratio for time to nonadherence of 1.50 on the basis of a 2-sided .05 significance level stratified log-rank test and assuming exponential decline in the rate of adherence over time. Assuming 8% of patients would be ineligible, 692 total registered patients were required to be enrolled to achieve 636 eligible patients.
The primary analysis was conducted under a modified intention-to-treat principle using all eligible randomly assigned patients. A stratified log-rank test was conducted. We adjusted for the design-specified stratification factors used in intervention assignments as covariates. To provide additional interpretation, we also examined the time to AF outcomes using Cox regression, adjusted for the stratification factors. In addition, we examined whether the relationship between adherence outcomes and random assignment arm differed by the following baseline factors (categorized as binary indicator variables to aid in interpretation across the panel of factors) using interaction tests: age (< 65 v ≥ 65 years), stage (I v II-III), years of prior therapy (≤ 1.0 v > 1.0), education (< college education v college or graduate school), race (Black v other), ethnicity (Hispanic v non-Hispanic), teaching hospital versus community hospital, insurance status, and copayment amount.
We conducted additional analyses modifying the definition of adherence to evaluate the sensitivity of results to modeling assumptions. To limit potential misclassification caused by counting a single missing specimen as a failure, we required 2 consecutive missing, negative, or out-of-window specimens to indicate AF, with failure time specified at the target follow-up time for the first urine assessment. We also used the urine test data only to determine AF (ie, failure to provide a urine test within the specified timeframe was not considered a failure). In addition, we allowed the requirement for urine AF of 10 ng/mL to vary from 1 to 50 ng/mL in 1 ng/mL increments. Finally, we examined time to AF on the basis of both patient self-report and site-reported AI discontinuation and evaluated cross-sectional assessments of adherence at 12, 24, and 36 months.
RESULTS
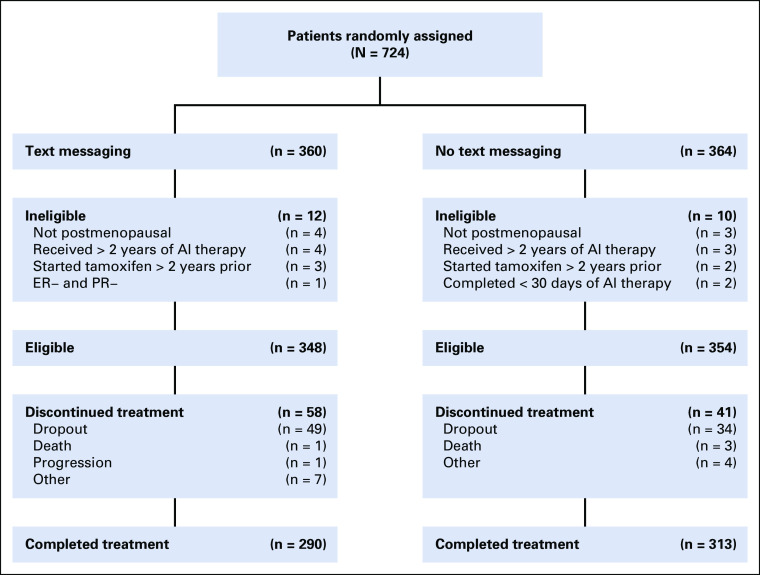
A total of 724 patients from 40 institutions were registered between May 2012 and September 2013. Of the 724 patients randomly assigned, 22 were ineligible for the reasons listed, 12 in the text-messaging arm and 10 in the no–text-messaging arm. Therefore, 348 patients in the text-messaging arm and 354 patients in the no–text-messaging arm were eligible (Fig 1). In the text-messaging arm, 12 patients were coded as major deviations: did not receive text messages (n = 8), began the text messaging intervention late (n = 3), and removed from the protocol because the site could not reach the patient (n = 1). One patient in the no–text-messaging arm who received text messages in error was coded as a major deviation.
FIG 1.
Consort diagram of patient flow. AI, aromatase inhibitor; ER, estrogen receptor; PR, progesterone receptor.
Patient Characteristics
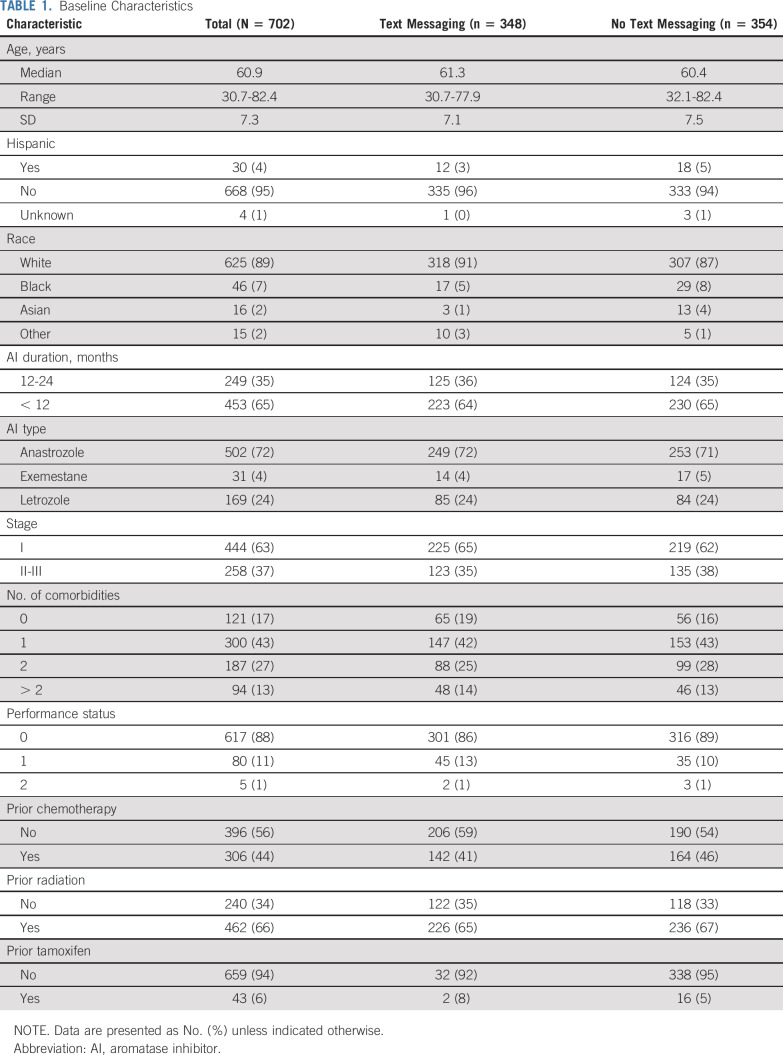
The median age was 60.9 years. Thirty patients (4.3%) were of Hispanic origin, and 46 (6.6%) were Black. The majority of patients (64.5%) had received AI therapy less than 12 months before random assignment. The predominant AI type was anastrozole (71.5%). Patient characteristics were well balanced by arm (Table 1). Overall, 338 patients (97.1% of eligible patients) in the text-messaging arm and 338 patients (95.5% of eligible patients) in the no–text-messaging arm were adherent at baseline (ie, evaluable) on the basis of the urine assay.
TABLE 1.
Baseline Characteristics
Primary End Point Results
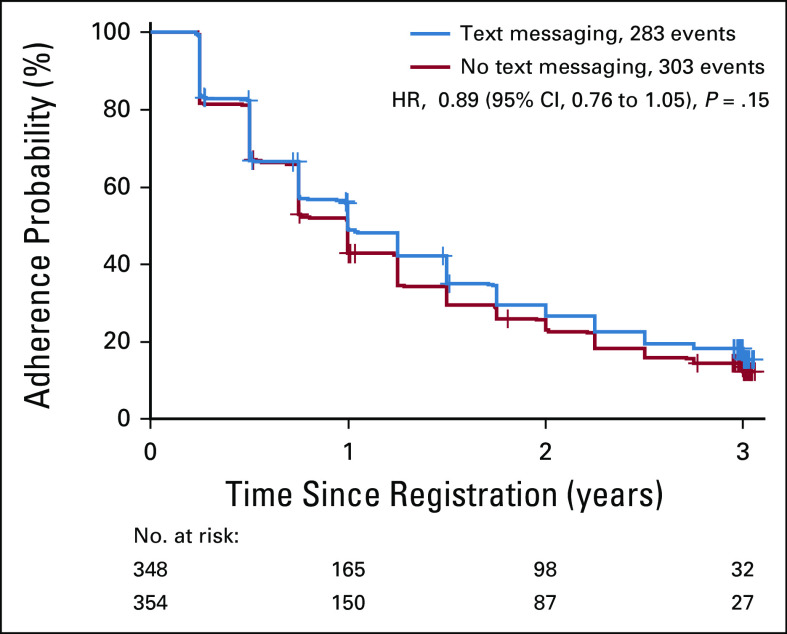
The estimates of AF by arm (TM v No-TM) accounting for censoring were 50.9% versus 57.2% for year 1, 70.4% versus 74.4% for year 2, and 81.9% versus 85.6% for year 3 (Fig 2). The estimates of AF between those in the TM arm and those in the No-TM arm were not different from each other after adjusting for stratification factors (P = .15 by stratified log-rank test).
FIG 2.
Kaplan-Meier curves of time to adherence failure by treatment arm. Adherence failure is defined as it was for the primary analysis. +, censoring; HR, hazard ratio.
Additional Analyses
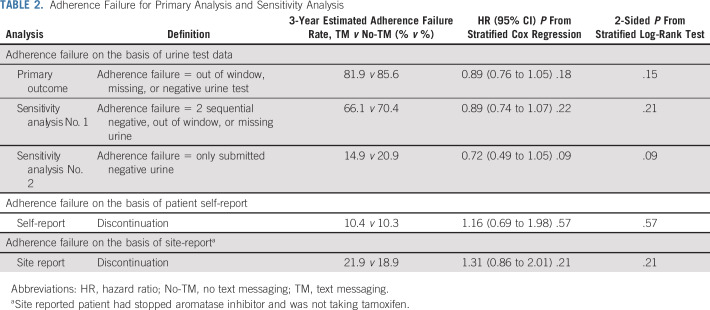
Using a Cox regression model rather than log-rank statistic, the CIs for the hazard ratio for 3-year AF included 1.0 (HR, 0.89 [95% CI, 0.76 to 1.05], P = .18; Table 2). In a sensitivity analysis requiring 2 consecutive missing, negative, or out-of-window specimens to indicate AF, no difference in time to AF was observed between groups, but the rate of 3-year AF changed (66.1% TM v 70.4% No-TM; HR, 0.89 [95% CI, 0.74 to 1.07], P = .22). Similarly, in an analysis relying only on submitted urine samples to indicate AF, no difference in time to AF was observed between groups (3-year AF estimates, 14.9% TM v 20.9% No-TM; HR, 0.72 [95% CI, 0.49 to 1.05], P = .09).
TABLE 2.
Adherence Failure for Primary Analysis and Sensitivity Analysis
At each study visit, participants and sites were asked about hormonal medication discontinuation. Using time-to-event analysis, self-reported discontinuation at 3 years was 10.4% (TM) and 10.3% (No-TM), and site-reported discontinuation at 3 years was 21.9% versus 18.9%. No statistical difference was observed between the intervention group and usual care (Table 2). Furthermore, there was no evidence that varying the cut-point indicating urine assay failure changed the results; there was no evidence of a statistically significant benefit of TM at any level of urine assay failure cut-point (Data Supplement).
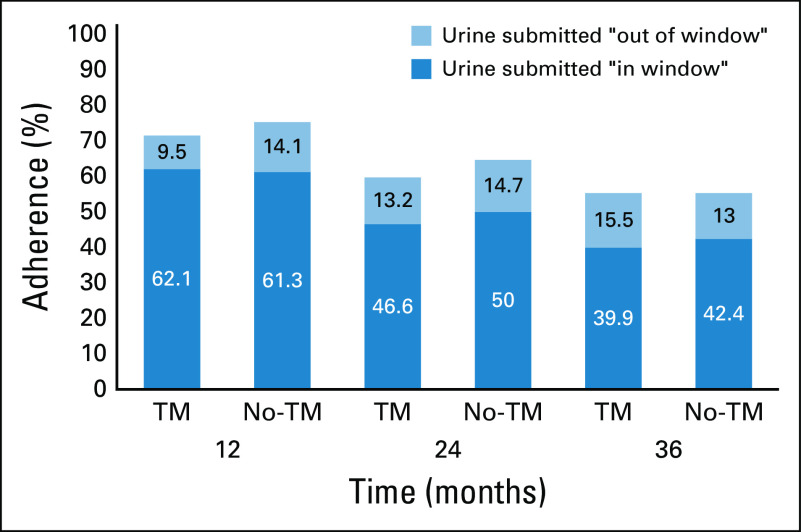
Among eligible patients, the observed proportions (irrespective of prior adherence status or whether the specimen was submitted within the specified time window) who were adherent at 12, 24, and 36 months were 73.5%, 62.3%, and 55.4%, respectively. Although annual cross-sectional adherence decreased over time, no differences were observed by arm at any of these points (Fig 3).
FIG 3.
Annual cross-sectional assessment of adherence as determined by submitted urine for biomarkers. Annual observed adherence by arm, including all eligible patients, regardless of prior adherence status and not accounting for censoring. The difference in adherence at each time point between the text messaging (TM) and the no text messaging (No-TM) groups was not statistically significant.
Using interaction tests, we examined whether the effect of the intervention differed by age, stage of disease, years receiving AI, education, race, ethnicity, institution type, copayment amount, and insurance type (Data Supplement). There was evidence that the effect of the intervention differed by race (P = .04) and, marginally, by the amount of AI copay (P = .09). Furthermore, examinations within subgroups suggested a potential beneficial impact (P < .05) of the intervention on adherence among patients who were older than 65 years, those treated at a teaching hospital, those with a copayment above $10, and those without private insurance. These exploratory analyses were hypothesis generating, requiring confirmation in independent studies, and no adjustments for multiple comparisons were made
DISCUSSION
In this multicenter randomized clinical trial of patients with early-stage breast cancer receiving AIs, an intervention consisting of biweekly unidirectional text messages for 36 months focused on overcoming potential barriers to medication adherence did not reduce AI AF. Using multiple definitions of AF, including self-report, no differences in adherence between patients assigned to the text message intervention or usual care were observed.
There are a limited number of studies evaluating interventions to improve adherence to hormonal therapy for breast cancer. A systematic review found 5 articles of 356 articles screened, and none reported that the interventions significantly enhanced adherence compared with usual care.19 All evaluated educational interventions with or without reminders or navigation. For example, one of the studies of more than 4,000 women tested a standardized information program and found no difference in 12-month adherence (88.5% v 88.8%) or persistence (40.5% v 43%).20 Another trial of 2,757 women randomly assigned patients to a similar educational intervention and found that 1- and 2-year rates of adherence were similar for patients in the education and usual care arms according to self-report.21 These studies evaluated a single point in time, did not account for censoring, and relied on a single self-report outcome. A third trial randomly assigned 181 patients to reminder letters and educational books versus telephone calls versus usual care. At 12 months, self-reported adherence was similar.22 The text messages in this trial were developed with similar educational themes.
In this context, it may not be surprising that our intervention did not result in long-term adherence. Our meta-analysis of 16 randomized clinical trials assessed the effect of mobile telephone text messaging on medication adherence in chronic disease.17 The median intervention duration was 12 weeks, and self-report was the most commonly used method to assess medication adherence. In the pooled analysis, there was a twofold significant improvement in medication adherence. The authors concluded that these results should be interpreted with caution given the short duration of the trials and the reliance on self-reported medication adherence measures. Short-term adherence does not likely translate to improved outcomes in breast cancer, and no prior study has evaluated the long-term effects of an intervention. Furthermore, the simple receipt of text messages represents a passive experience for patients, and the messages themselves may become repetitive. Thus, the unidirectional text-messaging intervention used in this study that did not actively engage the patient may have been insufficient to produce behavioral change. A review of interventional trials to improve medication adherence stressed that reminders are most effective when personalized and interactive.23 In addition, it has been reported that the primary reason for AI discontinuation is musculoskeletal symptoms.24 Although the text messages directed patients to inform their provider if they developed adverse effects, the current treatments for AI arthralgias are limited. Finally, the intervention did not target patients with a known history of nonadherence, and prior studies have shown that behavioral interventions are more effective when they target the specific reason for nonadherence.25
A strength of our study was the use of a biomarker over time to assess endocrine therapy adherence, in addition to other measures of discontinuation. We found that approximately 4% of patients had negative urine tests at baseline despite stating they were taking their medications. Determining adherence to adjuvant hormonal therapy and assessing treatment discontinuation can be methodologically challenging because many measures result in bias, which can make it difficult to compare results across studies.5,18 Patient self-reports and medical record reviews are susceptible to misrepresentation and tend to overestimate adherence.8 Physician assessment of discontinuation is reliable, and although adherence may be may overestimated, it may be the most reliable measure for future studies.8 Electronic pharmacy refill data abstraction is commonly used for pharmacoepidemiology, but the use of automated refills can overestimate adherence because it does not measure that which is actually being taken. A disadvantage of the reliance on urine assessments in our study was the missing data caused by a lack of submitted urine samples. Because these missing data are likely correlated with AF, and are therefore informative, we chose to specify these occurrences as AFs. Although this approach improved the internal validity of the randomized comparison by arm, it likely underestimated the overall adherence rates. But regardless of how we measured adherence in this study, we found no evidence of a benefit of the text messaging intervention. Given the complexity of adherence measurement, future interventions should examine multiple outcome measures when possible.
The trial has many unique strengths, including a large sample size, representation from community and academic sites that were geographically diverse, long-term follow-up, and a prospectively specified end point that accounted for both disease recurrence and missed patient appointments. However, the study (like almost any study in this research setting) was also limited by an inability to differentiate missed appointments from actual medication nonadherence. In addition, as text messaging and electronic alerts have increased in frequency, the impact of interventions that use these technologies may be limited because of “alert fatigue” and information overload, which was the rationale for twice-weekly as opposed to daily messages.
In conclusion, a biweekly unidirectional text-messaging intervention did not reduce AI AF in women with breast cancer. This finding was consistent regardless of the definition of AF. Medication nonadherence is a health care challenge and is associated with significant societal costs.26 This trial has design implications for other medication adherence interventions that are often of short duration. Future trials may focus on enhancing two-way communication to increase early interventions to prevent discontinuation. Improving long-term adherence will likely require sustained and personalized behavioral interventions, symptom management, and support.
SUPPORT
Supported by National Institutes of Health/National Cancer Institute/Division of Cancer Prevention grant UG1CA189974 and legacy grant U10CA37429; and by ASCO’s Conquer Cancer Foundation and the Breast Cancer Research Foundation.
CLINICAL TRIAL INFORMATION
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
See accompanying editorial on page 2117
AUTHOR CONTRIBUTIONS
Conception and design: Dawn L. Hershman, Joseph M. Unger, Grace Clarke Hillyer, Julie R. Gralow, Alfred I. Neugut
Financial support: Dawn L. Hershman, Joseph M. Unger, Alfred I. Neugut
Administrative support: Dawn L. Hershman
Provision of study material or patients: Dawn L. Hershman, Shaker R. Dakhil, Benjamin T. Esparaz, Ming C. Kuan
Collection and assembly of data: Dawn L. Hershman, Joseph M. Unger, Grace Clarke Hillyer, Anna Moseley, Kathryn B. Arnold, Shaker R. Dakhil, Benjamin T. Esparaz, Mark L. Graham II, Douglas M. Lackowski, Zoneddy R. Dayao, Alfred I. Neugut
Data analysis and interpretation: Dawn L. Hershman, Anna Moseley, Kathryn B. Arnold, Shaker R. Dakhil, Ming C. Kuan, William J. Edenfield, N. Lynn Henry, Julie R. Gralow, Scott D. Ramsey
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Trial of Text Messaging to Reduce Early Discontinuation of Adjuvant Aromatase Inhibitor Therapy in Women With Early-Stage Breast Cancer: SWOG S1105
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Dawn L. Hershman
Consulting or Advisory Role: AIM Specialty Health
William J. Edenfield
Consulting or Advisory Role: Chimerix
Zoneddy R. Dayao
Consulting or Advisory Role: Amgen, bioTheranostics
N. Lynn Henry
Research Funding: Innocrin Pharma (Inst), Pfizer (Inst), AbbVie (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/27894/summary
Julie R. Gralow
Consulting or Advisory Role: Novartis, Genentech, Pfizer, Merck, Puma Biotechnology, Sandoz, AstraZeneca, Immunomedics, Genomic Health
Scott D. Ramsey
Employment: Flatiron Health
Consulting or Advisory Role: Kite Pharma, Bayer, Genentech, Bristol-Myers Squibb, AstraZeneca, Merck & Company, Epigenomics, GRAIL
Research Funding: Bayer (Inst), Bristol-Myers Squibb (Inst), Microsoft (Inst)
Travel, Accommodations, Expenses: Bayer Schering Pharma, Bristol-Myers Squibb, Flatiron Health, Bayer, GRAIL
Alfred I. Neugut
Stock and Other Ownership Interests: Stemline Therapeutics
Consulting or Advisory Role: Otsuka, United Biosource, EHE International
Expert Testimony: Hospira
No other potential conflicts of interest were reported.
REFERENCES
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the Breast International Group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34:2452–2459. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hershman DL. Sticking to it: Improving outcomes by increasing adherence. J Clin Oncol. 2016;34:2440–2442. doi: 10.1200/JCO.2016.67.7336. [DOI] [PubMed] [Google Scholar]
- 5.Accordino MK, Hershman DL. Disparities and challenges in adherence to oral antineoplastic agents. Am Soc Clin Oncol Educ Book. 2013:271–276. doi: 10.14694/EdBook_AM.2013.33.271. [DOI] [PubMed] [Google Scholar]
- 6.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. J Natl Cancer Inst. 2014;106:dju319. doi: 10.1093/jnci/dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 9.Neugut AI, Zhong X, Wright JD, et al. Nonadherence to medications for chronic conditions and nonadherence to adjuvant hormonal therapy in women with breast cancer. JAMA Oncol. 2016;2:1326–1332. doi: 10.1001/jamaoncol.2016.1291. [DOI] [PubMed] [Google Scholar]
- 10. https://www.pewinternet.org/fact-sheet/mobile/ Pew Research Center: Mobile fact sheet.
- 11.Martin C, Perfect T, Mantle G. Non-attendance in primary care: The views of patients and practices on its causes, impact and solutions. Fam Pract. 2005;22:638–643. doi: 10.1093/fampra/cmi076. [DOI] [PubMed] [Google Scholar]
- 12.Downer SR, Meara JG, Da Costa AC. Use of SMS text messaging to improve outpatient attendance. Med J Aust. 2005;183:366–368. doi: 10.5694/j.1326-5377.2005.tb07085.x. [DOI] [PubMed] [Google Scholar]
- 13.Stockwell MS, Kharbanda EO, Martinez RA, et al. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: A randomized controlled trial. JAMA. 2012;307:1702–1708. doi: 10.1001/jama.2012.502. [DOI] [PubMed] [Google Scholar]
- 14.Gerber BS, Stolley MR, Thompson AL, et al. Mobile phone text messaging to promote healthy behaviors and weight loss maintenance: A feasibility study. Health Informatics J. 2009;15:17–25. doi: 10.1177/1460458208099865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36:165–173. doi: 10.1016/j.amepre.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AW, Watson AJ, Makredes M, et al. Text-message reminders to improve sunscreen use: A randomized, controlled trial using electronic monitoring. Arch Dermatol. 2009;145:1230–1236. doi: 10.1001/archdermatol.2009.269. [DOI] [PubMed] [Google Scholar]
- 17.Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: A meta-analysis. JAMA Intern Med. 2016;176:340–349. doi: 10.1001/jamainternmed.2015.7667. [DOI] [PubMed] [Google Scholar]
- 18.Clarke Hillyer G, Neugut AI, Crew KD, et al. Use of a urine anastrozole assay to determine treatment discontinuation among women with hormone-sensitive breast cancer: A pilot study. J Oncol Pract. 2012;8:e100–e104. doi: 10.1200/JOP.2011.000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hurtado-de-Mendoza A, Cabling ML, Lobo T, et al: Behavioral interventions to enhance adherence to hormone therapy in breast cancer survivors: A systematic literature review. Clin Breast Cancer 16:247-255.e3, 2016. [DOI] [PMC free article] [PubMed]
- 20.Hadji P, Blettner M, Harbeck N, et al. The Patient’s Anastrozole Compliance to Therapy (PACT) Program: A randomized, in-practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early breast cancer. Ann Oncol. 2013;24:1505–1512. doi: 10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 21.Markopoulos C, Neven P, Tanner M, et al. Does patient education work in breast cancer? Final results from the global CARIATIDE study. Future Oncol. 2015;11:205–217. doi: 10.2217/fon.14.179. [DOI] [PubMed] [Google Scholar]
- 22.Ziller V, Kyvernitakis I, Knöll D, et al. Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment--The COMPAS study. BMC Cancer. 2013;13:407. doi: 10.1186/1471-2407-13-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kini V, Ho PM. Interventions to improve medication adherence: A review. JAMA. 2018;320:2461–2473. doi: 10.1001/jama.2018.19271. [DOI] [PubMed] [Google Scholar]
- 24.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derose SF, Green K, Marrett E, et al. Automated outreach to increase primary adherence to cholesterol-lowering medications. JAMA Intern Med. 2013;173:38–43. doi: 10.1001/2013.jamainternmed.717. [DOI] [PubMed] [Google Scholar]
- 26.Zullig LL, Mendys P, Bosworth HB. Medication adherence: A practical measurement selection guide using case studies. Patient Educ Couns. 2017;100:1410–1414. doi: 10.1016/j.pec.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]