Abstract
PURPOSE
The combination of irinotecan, temozolomide, dintuximab, and granulocyte-macrophage colony-stimulating factor (I/T/DIN/GM-CSF) demonstrated activity in patients with relapsed/refractory neuroblastoma in the randomized Children’s Oncology Group ANBL1221 trial. To more accurately assess response rate and toxicity, an expanded cohort was nonrandomly assigned to I/T/DIN/GM-CSF.
PATIENTS AND METHODS
Patients were eligible at first relapse or first designation of refractory disease. Oral T and intravenous (IV) irinotecan were administered on days 1 to 5 of 21-day cycles. DIN was administered IV (days 2-5), and GM-CSF was administered subcutaneously (days 6-12). The primary end point was objective response, analyzed on an intent-to-treat basis per the International Neuroblastoma Response Criteria.
RESULTS
Seventeen eligible patients were randomly assigned to I/T/DIN/GM-CSF (February 2013 to March 2015); 36 additional patients were nonrandomly assigned to I/T/DIN/GM-CSF (August 2016 to May 2017). Objective (complete or partial) responses were observed in nine (52.9%) of 17 randomly assigned patients (95% CI, 29.2% to 76.7%) and 13 (36.1%) of 36 expansion patients (95% CI, 20.4% to 51.8%). Objective responses were seen in 22 (41.5%) of 53 patients overall (95% CI, 28.2% to 54.8%); stable disease was also observed in 22 of 53. One-year progression-free and overall survival for all patients receiving I/T/DIN/GM-CSF were 67.9% ± 6.4% (95% CI, 55.4% to 80.5%) and 84.9% ± 4.9% (95% CI, 75.3% to 94.6%), respectively. Two patients did not receive protocol therapy and were evaluable for response but not toxicity. Common grade ≥ 3 toxicities were fever/infection (18 [35.3%] of 51), neutropenia (17 [33.3%] of 51), pain (15 [29.4%] of 51), and diarrhea (10 [19.6%] of 51). One patient met protocol-defined criteria for unacceptable toxicity (grade 4 hypoxia). Higher DIN trough levels were associated with response.
CONCLUSION
I/T/DIN/GM-CSF has significant antitumor activity in patients with relapsed/refractory neuroblastoma. Study of chemoimmunotherapy in the frontline setting is indicated, as is further evaluation of predictive biomarkers.
INTRODUCTION
Survival rates for children with high-risk neuroblastoma are poor1,2; however, targeted therapy may improve outcomes. The Children’s Oncology Group ANBL1221 trial evaluated response to targeted agents combined with irinotecan and temozolomide (I/T) in patients with relapsed/refractory neuroblastoma. Because chemoimmunotherapy has been shown to be effective in other malignancies,3-11 the combination of I/T plus dinutuximab (DIN), a chimeric antibody targeting the disialoganglioside GD2, and granulocyte-macrophage colony-stimulating factory (GM-CSF) was studied. In patients randomly assigned to receive irinotecan, temozolomide, dintuximab, and granulocyte-macrophage colony-stimulating factor (I/T/DIN/GM-CSF), the objective response (OR) rate was 53% (9 of 17 patients).12 The sample size was small (N = 17), and the 95% CI around the point estimate of response was wide (0.29 to 0.77).12 To more accurately assess the response rate, further evaluate therapy-related toxicities, evaluate potential mechanisms underlying the observed activity of this regimen, and study potential biomarkers of activity, additional patients were nonrandomly assigned to I/T/DIN/GM-CSF. Tumor response remained the primary end point.
CONTEXT
Key Objective
Survival rates for children with high-risk neuroblastoma are poor, however targeted therapy may improve outcomes. This study evaluated response to a chemoimmunotherapy regimen that included dinutuximab, an agent that targets the disialoganglioside GD2 on neuroblastoma cells, combined with irinotecan and temozolomide in patients with relapsed/refractory neuroblastoma. This combination demonstrated activity in a small cohort (n = 17) enrolled on a randomized Children’s Oncology Group trial (ANBL1221). To more accurately assess response rate and toxicity, an expanded cohort was non-randomly assigned to chemo-immunotherapy.
Knowledge Generated
Objective (complete or partial) responses were seen in 22 of 53 patients (41.5%); stable disease was also observed in 22 of 53. Common Grade ≥ 3 toxicities were fever/infection (18 of 51; 35.3%), neutropenia (17 of 51; 33.3%), pain (15 of 51; 29.4%), and diarrhea (10 of 51; 19.6%).
Relevance
I/T/DIN/GM-CSF has significant antitumor activity in patients with relapsed/refractory neuroblastoma. Study of chemoimmunotherapy in frontline treatment of patients with high-risk neuroblastoma is indicated. Evaluation of predictive biomarkers is ongoing.
PATIENTS AND METHODS
Study Design and Participants
ANBL1221 was initially designed as a prospective randomized phase II trial with a selection (ie, pick-the-winner) design.13 Because I/T/DIN/GM-CSF met the a priori benchmark for activity, this regimen was selected for further study.12 Enrollment was expanded to permit accrual of 50 eligible patients assigned to I/T/DIN/GM-CSF within approximately 2 years. This sample size would allow estimation of the response rate with a standard error of 0.07.
Patients of any age with documentation of a high-risk neuroblastoma diagnosis were eligible at first relapse or first designation of refractory disease status.12 Patients with bone marrow as the only site of disease were ineligible. Performance status (Lansky/Karnofsky) ≥ 50% and adequate organ function status were required. Other eligibility criteria were the same as those described for the initial cohort.12 Written informed consent was obtained. The study was approved by the National Cancer Institute Pediatric Central Institutional Review Board and local international review boards.
Randomization
The randomization process for the initial portion of ANBL1221 has been described.12 During the expansion, patients were nonrandomly assigned to I/T/DIN/GM-CSF via the National Cancer Institute OPEN system.
Procedures
Patients received oral temozolomide (100 mg/m2 per dose) and intravenous (IV) irinotecan (50 mg/m2 per dose administered over 90 minutes) on days 1 to 5 of 21-day cycles. DIN (17.5 mg/m2 per day IV on days 2-5) and GM-CSF (250 μg/m2 per dose subcutaneously on days 6-12) were administered as previously described.12 Eligible patients who received ≥ one dose of protocol therapy were evaluable for toxicity. Dose modification guidelines and toxicity assessments were the same as those previously reported.12 Disease evaluations were performed after cycles 2, 4, and 6 and every four cycles thereafter. Imaging responses were centrally reviewed.
Modified International Neuroblastoma Response Criteria (INRC) in place at the time of protocol inception were used to assess response.14 Definitions of complete (CR) and partial response (PR) and stable (SD) and progressive disease (PD) were identical to those used during the randomized portion of the trial.12 OR was defined as best overall response of CR or PR attained at or before completion of six cycles. Patients with SD were considered nonresponders but could continue protocol therapy. Patients with PD were removed from protocol therapy; additional criteria for removal from protocol therapy were previously described.12 Patients who met off-protocol therapy criteria as a result of toxicity before attaining an OR were considered nonresponders. DIN and human antichimeric antibody (HACA) levels were measured as previously described (Data Supplement).15,16
Outcomes
The primary end point was OR. All eligible patients were considered evaluable for the intent-to-treat response analysis. Progression-free (PFS) and overall survival (OS) were secondary end points. Safety and feasibility were also evaluated.
Statistical Analysis
The two-stage group sequential design (ie, activity design), the planned selection design, and the three-stage stopping rule for unacceptable toxicity relevant to the first portion of the study have been described.12 During the expanded portion, a single-stage toxicity stopping rule was applied such that if ≤ seven patients were to experience protocol-defined unacceptable toxicity, I/T/DIN/GM-CSF would be considered sufficiently tolerable. A single-stage feasibility stopping rule was also in place, such that if ≤ 20 patients required a > 25% modification of the dose of I/T/DIN because of toxicity or were to be taken off protocol therapy because of toxicity during the first two cycles of therapy, the regimen would be considered feasible.
Patient characteristics were compared between the randomly assigned and expansion cohorts using a Wilcoxon rank sum test for continuous variables and Fisher’s exact/χ2 test (depending on sample size) for categorical variables. A 95% Wald CI was placed on the response rate. Survival curves were constructed according to the Kaplan-Meier method, with standard errors according to Peto.17,18 PFS and OS were calculated as previously reported12 and are presented as 1-year point estimates ± SEs. Occurrence of toxicities was compared between cohorts using Fisher’s exact test. P values < .05 were considered statistically significant.
Analyses were performed using SAS software (SAS/STAT user’s guide, version 9.4; SAS Institute, Cary, NC). Survival curves were created using R software (https://www.r-project.org/). Statistical analyses for associations of outcome with HACA and DIN levels were performed as described in the Data Supplement and as reported previously.15,16
RESULTS
Patient Characteristics and Therapy Delivered
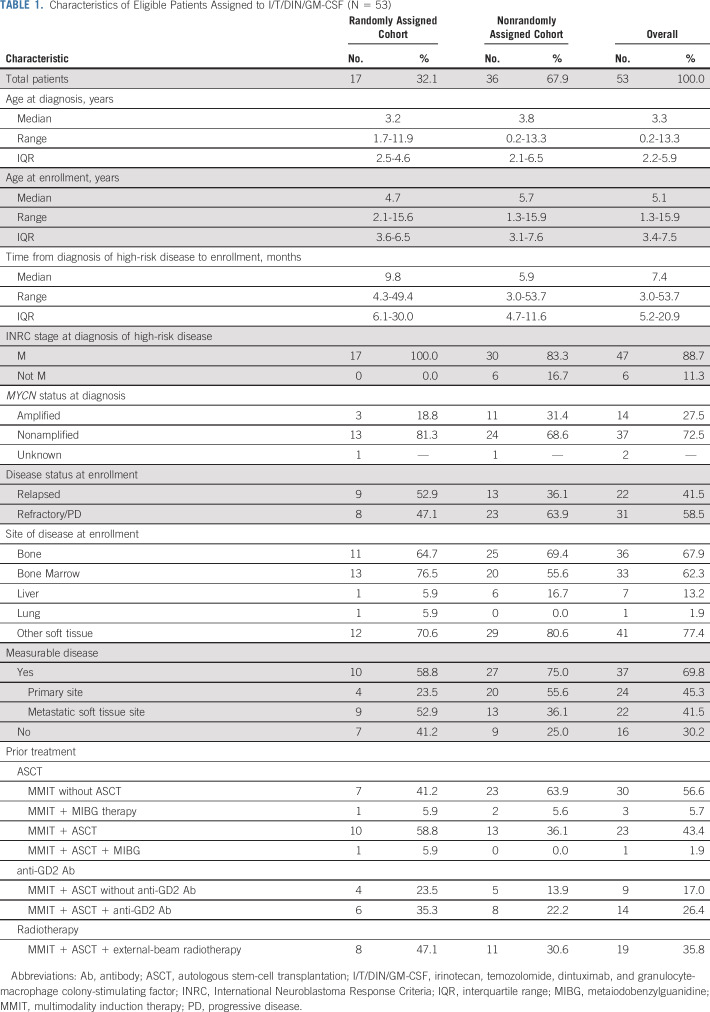
Patients were enrolled in the randomized portion of the trial from February 22, 2013, to March 23, 2015, and in the nonrandomized portion from August 26, 2016, to May 18, 2017. One patient was deemed ineligible (incorrect consent form). In total, 53 eligible patients were assigned to I/T/DIN/GM-CSF: 17 during the randomized portion and 36 during the nonrandomized portion (Table 1; Fig 1). Age at enrollment ranged from 1.3 to 15.9 years (median, 5.1 years; interquartile range [IQR], 3.4-7.5 years). Time from diagnosis of high-risk disease to enrollment ranged from 0.3 to 4.5 years (median, 0.6 years; IQR, 0.4-1.7 years). Most patients (47 [88.7%] of 53) had INRC stage M disease at diagnosis; six had localized or MS disease. MYCN status was known for 51 patients; 14 (27.5%) had MYCN-amplified tumors. At enrollment, 37 had measurable disease (69.8%); 16 had evaluable disease (30.2%). Twenty-two had relapsed neuroblastoma (41.5%); 31 had refractory disease (58.5%). Prior treatment included high-dose chemotherapy with autologous stem-cell transplantation (ASCT) in 23 patients (43.4%) and prior anti-GD2 therapy in 14 (26.4%). The cohorts were similar with respect to patient characteristics (Table 1), except that time from high-risk diagnosis to enrollment was longer (P = .0405) and prior radiotherapy was more frequent (P = .0495) in the randomized cohort.
TABLE 1.
Characteristics of Eligible Patients Assigned to I/T/DIN/GM-CSF (N = 53)
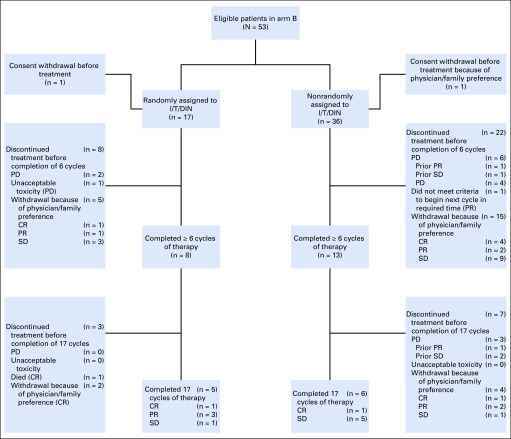
FIG 1.
Trial profile. Best response by cycle 6 was the primary end point of the trial. Patients with stable disease (SD) or better could remain in the study and receive a maximum of 17 cycles of treatment. Best response is indicated in parentheses. CR, complete response; I/T/DIN, irinotecan, temozolomide, and dintuximab; PD, progressive disease; PR, partial response.
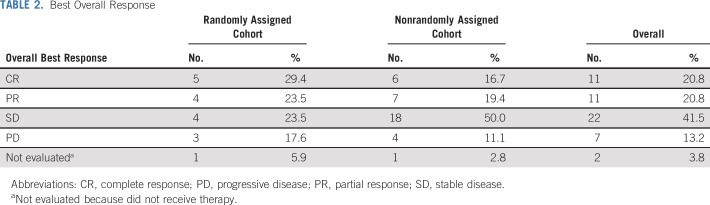
Patients randomly assigned to I/T/DIN/GM-CSF during the initial portion of the study received 148 cycles of therapy (median, six cycles; IQR, 14.0 cycles); those in the expansion cohort received 248 cycles (median, 4.5 cycles; IQR, 8.0 cycles). OR (Table 2) was observed in 9 (52.9%) of 17 patients (95% CI, 29.2% to 76.7%) randomly assigned to I/T/DIN/GM-CSF (CR, n = 5; PR, n = 4) and 13 (36.1%) of 36 patients assigned to I/T/DIN/GM-CSF in the expansion cohort (95% CI, 20.4% to 51.8%). In total, 22 (41.5%) of 53 patients had an OR (95% CI, 28.2% to 54.8%; best response: CR, n = 11; PR, n = 11; SD, n = 22; PD, n = 7; not evaluable, n = 2). Two patients (one per cohort) did not receive therapy but were included in this intent-to-treat analysis (Fig 1).
TABLE 2.
Best Overall Response
Response Assessment
OR (Data Supplement) was seen among patients with measurable (eight [21.6%] of 37) and evaluable (14 [87.5%] of 16) disease and among those whose tumors were MYCN amplified (4 [28.6%] of 14) and nonamplified (17 [45.9%] 37; 45.9%). The difference in response rate based on MYCN status was not statistically significant (Fisher’s exact test P = .5232). Fourteen (60.9%) of 23 patients who had previously undergone ASCT responded to I/T/DIN/GM-CSF, as did nine (64.3%) of 14 who had received prior anti-GD2 therapy. OR was observed in 12 (54.5%) of 22 patients with relapsed disease and 10 (32.3%) of 31 with refractory/progressive disease, 21 (47.7%) of 44 with INSS stage 4 neuroblastoma diagnosed at age > 18 months, and one (11.1%) of nine with high-risk disease as a result other factors.
SD was observed in 22 (41.5%) of 53 patients: 4 (23.5%) of 17 in the initial cohort and 18 (50.0%) of 36 in the expansion cohort. Among those with SD, 21 (95.5%) of 22 had measurable disease and 15 (68.1%) of 22 had SD because of a lack of response in soft tissue, but achieved PR or CR in metastatic sites (Data Supplement). Half of the patients with SD had large primary tumors (≥ 8 cm in diameter) at enrollment; 3 (13.6%) of 22 had residual calcified lesions at enrollment that were unchanged in size after therapy. Seven patients (13.2%) experienced PD during therapy. Sites of progression included bone (n = 1), metastatic soft tissue (n = 2), primary site (n = 2), and combined sites (n = 2).
Time to Response and Duration of Therapy
Of the 22 patients with OR, 18 (81.8%) achieved OR at the post–cycle 2 evaluation. Four had an initial OR at cycle 4 or 6, but no patient with SD at cycle 6 ultimately achieved OR. In 13 (59.1%) of 22, the eventual best response was achieved at cycle 2. Among the nine patients whose best response was detected after the first evaluation time point (40.9% of responders), five had SD at cycle 2 but later had OR (PR, n = 4; CR, n = 1) and four had PR at cycle 2 but later had CR. Responders frequently went on to other neuroblastoma-directed therapies, particularly patients with refractory disease during frontline therapy. Among these (n = 31), 15 went on to surgery and/or ASCT and additional components of frontline high-risk therapy. Ten of these patients had not experienced an event, with median follow-up time of 1.6 years (range, 1.2-4.3 years).
Survival Outcomes
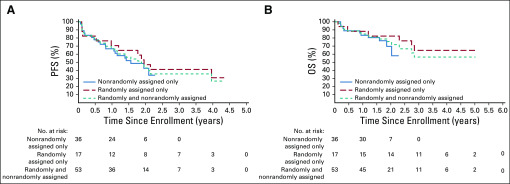
PFS and OS were evaluated on an exploratory basis. One-year PFS for the entire cohort was 67.9% ± 6.4% (95% CI, 55.4% to 80.5%; Fig 2). The 31 events occurred between 23 days and 4.0 years; median time to event was 0.8 years (IQR, 1.4 years). One-year OS was 84.9% ± 4.9% (95% CI, 75.3% to 94.6%). The 16 deaths occurred between 42 days and 2.8 years; median time to death was 1.1 years (IQR, 1.6 years). Survival by disease status (relapsed v refractory/progressive) is shown in the Data Supplement.
FIG 2.
Survival by ANBL1221 cohort. (A) Progression-free survival (PFS) and (B) overall survival (OS) for patients treated in the initial (randomly assigned) cohort, expansion (nonrandomly assigned) cohort, and combined cohort.
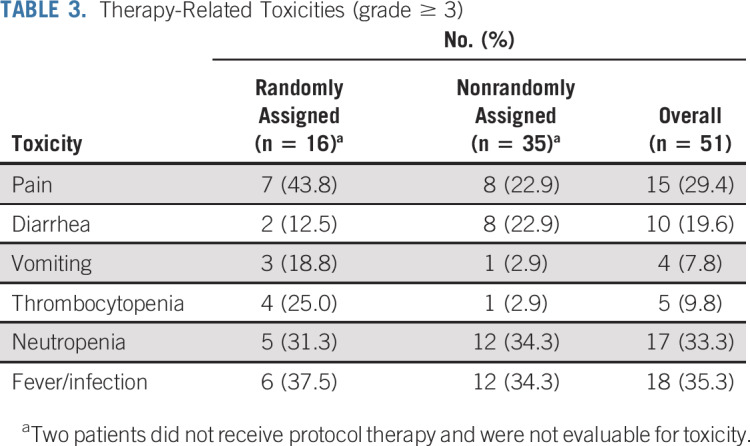
Toxicities and Dose Modifications
Grade ≥ 3 toxicities related to protocol therapy are listed in Table 3. Diarrhea was observed in 19.6% (10 of 51), vomiting in 7.8% (4 of 51), neutropenia in 33.3% (17 of 51), and thrombocytopenia in 9.8% of patients (5 of 51). Pain was more common in the initial cohort, although the difference was not statistically significant (43.8% [7 of 16] v 22.9% [8 of 35]; P = .1865). Hypoxia and peripheral motor neuropathy were previously reported in the randomized cohort.12 Two patients in the expanded cohort experienced grade 3 hypoxia; none experienced motor neuropathy.
TABLE 3.
Therapy-Related Toxicities (grade ≥ 3)
Twelve patients across cohorts required I/T dose modifications. Doses were reduced because of hematologic toxicity (neutropenia or thrombocytopenia, n = 5), drug administration issue (n = 2), diarrhea (n = 2), nausea/emesis (n = 1), or other/unknown (n = 2). Fourteen patients required DIN dose modifications. Doses were modified because of pulmonary toxicity (n = 4), pain (n = 1), hypotension (n = 1), visual loss (n = 1), elevated transaminases (n = 1), drug administration issue (n = 1), or other/unknown (n = 5). Among patients requiring dose modifications, only those in the initial cohort who had hypoxia and bronchospasm required treatment discontinuation because of toxicity. There were no deaths during therapy or events that met protocol-defined criteria for unacceptable toxicity other than those reported previously.12 The stopping rule for unacceptable toxicity was not met.
DIN Levels and HACA Response
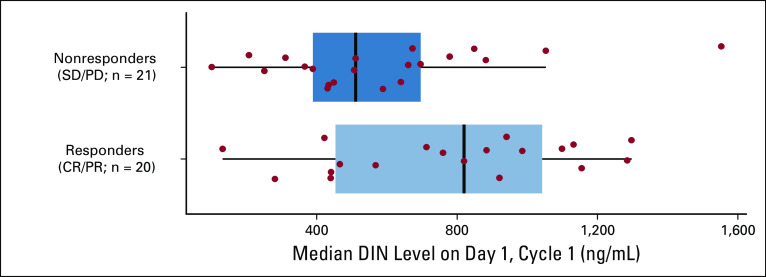
DIN levels were evaluated using plasma samples (n = 41) from day 1, cycle 2. These values reflect the level of drug detectable 17 days after the last cycle-1 DIN dose. Median DIN trough level in patients with OR was higher than that of nonresponders (P < .001; Fig 3; Data Supplement).
FIG 3.
Association of dintuximab (DIN) trough level and response in patients with an available plasma sample on day 1 of cycle 2. Relationship between response (complete response [CR]/partial response [PR]; n = 20 v stable disease [SD]/progressive disease [PD]; n = 21) and median DIN level (ng/mL) measured on day 1 of cycle 2. P value calculated using the Mann-Whitney-Wilcoxon test (P < .001). Data for 40 patients are shown. One responder had a DIN level of 12,595 ng/mL (off the scale for this figure). Values for all 41 patients were included in statistical analyses.
Serial plasma samples were available for HACA testing from 31 patients in the nonrandomized cohort. An HACA response was detected in only two patients; both were among the eight patients who had received DIN before enrollment. In comparison, none of the 23 patients without prior DIN treatment developed HACA (P = .060; Data Supplement). Among eight patients in whom extended HACA surveillance through six cycles of therapy was performed, none developed HACA. In the two HACA-positive patients, median DIN level at the start of cycles administered after HACA development was lower than median level in HACA-negative patients (P = .017; Data Supplement). In contrast, DIN levels in HACA-negative patients treated with DIN before study enrollment did not differ from those in HACA-negative DIN-naïve patients (Data Supplement). One HACA-positive patient had an OR, and one did not; association of HACA status with response was not possible to determine because of small numbers.
DISCUSSION
In the overall cohort of patients with relapsed/refractory neuroblastoma, OR rate to I/T/DIN/GM-CSF exceeded 40% in an intent-to-treat analysis. This response rate compares favorably with rates of response to commonly used treatments, including chemotherapy19-22 and iodine-131 metaiodobenzylguanidine.23 Administration of DIN with cytokines in the frontline setting improves event-free survive (EFS),24-26 and important work has been done to mitigate toxicity and potentially improve efficacy of GD2-directed therapy.26-29 End-induction response rates and early EFS data in patients treated with a humanized anti-GD2 antibody–containing induction on a single-center phase II trial are also encouraging.30 However, our study is the first multicenter trial to our knowledge to evaluate anti-GD2 therapy combined with chemotherapy in a relatively large relapsed/refractory cohort.
In the randomized cohort, the point estimate of the OR rate was 52.9% (95% CI, 29.2% to 76.7%). The point estimates of OR in the expansion cohort alone and in the combined cohort fell within this interval, at 36.1% and 41.5%, respectively. In the randomized cohort, 8 (47.1%) of 17 patients had refractory disease, and only 2 (11.8%) of 17 had residual primary site disease. In the expansion cohort, 23 (63.9%) of 36 had refractory disease; 18 of these (50%) had residual primary site disease. Variability in the approach to primary tumor resection is expected in the context of a cooperative group trial. The nature of primary site involvement may have contributed to the increased proportion of patients with SD in the expansion cohort. Further study of survival based on overall versus metastatic site response in patients treated with I/T/DIN may help determine whether SD status resulting from residual primary tumor has implications for those with incomplete surgical resections. Our data suggest that for patients with substantial primary site disease and response in metastatic sites, clinical benefit may be difficult to estimate using the INRC alone.
A majority of responders achieved what would eventually be their best OR at the first disease evaluation time point, but approximately 40% of responders experienced best response at cycle ≥ 4. Although brisk responses can be seen, evolving responses may also be observed. However, the earliest OR was seen at the first evaluation in > 80% of those who experienced OR, and no patient first achieved OR beyond the post–cycle 6 time point. The risk/benefit ratio of I/T/DIN in patients with SD after the first four cycles of therapy should be carefully considered.
A limitation of this study is that duration of response could not be accurately assessed, particularly in patients with refractory disease. Additional studies are needed to determine whether response to I/T/DIN/GM-CSF in this setting ultimately improves OS. This study was not designed to address the question of whether chemoimmunotherapy can obviate the need for ASCT among responders. Four patients with refractory disease received the maximum duration of therapy and never went on to ASCT; one subsequently experienced PD. Further study is needed to determine the role of chemoimmunotherapy in the frontline setting.
The mechanisms of response or resistance to I/T/DIN/GM-CSF are currently unknown. Median DIN level before the start of cycle 2 was higher in responders than in nonresponders, which may reflect greater antibody exposure during therapy. Only two of 31 HACA-evaluable patients had an HACA response; levels of DIN were low after HACA development. I/T administered concurrently with DIN may have attenuated the development of HACA, particularly in DIN-naïve patients, because HACA was more frequently observed in adults treated with DIN without prior or concurrent chemotherapy.15
Chemotherapy-induced alterations in the tumor microenvironment and in circulating immune cells could also explain the I/T/DIN/GM-GCSF response rate. In lymphoma and breast cancer, chemoimmunotherapy has improved survival; however, the mechanisms underlying improved outcomes are poorly understood.31,32 Downregulation of CD20 through receptor internalization and decreased mRNA expression may be a mechanism of resistance in lymphoma.33,34 Expression of GD2 on neuroblastoma cells is not uniform at diagnosis or during treatment,35 and studies of alterations in expression of GD2 after antibody therapy are ongoing.
Although chemotherapy is considered immunosuppressive, specific chemotherapeutic agents may augment tumor immunity. Induction of immunogenic cell death with promotion of tumor-specific immunity and modulation of the tumor microenvironment may play a role. Irinotecan modulates the immune microenvironment by reducing Foxp3-positive regulatory T (Treg) cells and myeloid-derived suppressor cells, leading to increased proliferation and interferon gamma production by tumor-specific CD8 T cells.36 In addition, irinotecan upregulates major histocompatibility complex class I and programmed death 1 ligand (PD-L1) expression in tumor cells. This results in a synergistic antitumor effect when irinotecan is combined with anti–PD-L1 antibodies in preclinical models.36 Temozolomide induces downregulation of PD-L1 in glioblastoma cells, consistent with reduced PD-L1 levels in tumors from patients with recurrent glioblastoma.37 Low-dose temozolomide also depletes Treg cells.37 Further study is required to determine whether these effects underlie the activity of I/T/DIN/GM-CSF in neuroblastoma and whether such effects can be observed when DIN is combined with other chemotherapeutics.38
The role of GM-CSF also bears further evaluation, particularly given results from a trial of humanized anti-GD2 antibody with GM-CSF.28 In the postchemotherapy setting, GM-CSF leads to an early increase in number and activation of circulating neutrophils that could augment antibody-dependent cellular cytotoxicity. GM-CSF is an M1 macrophage polarizing factor, and when administered after chemotherapy, it also increases expression and release of tumor necrosis factor.39 Increases in number and functionality of circulating myeloid cells postchemotherapy could also reshape the tumor microenvironment and augment antitumor cytotoxic T-cell responses.
Mechanistic studies may lead to the design of newer combination therapies, including anti-GD2 antibodies with optimized chemotherapy regimens, other monoclonal antibodies, or immunomodulatory agents. Whether the effect of chemoimmunotherapy can be augmented via the use of other modalities, such as external-beam radiotherapy or targeted radionuclide therapy, is not yet known but merits investigation.40,41 In addition to elucidating mechanisms that may underlie the responses observed during this trial, ongoing work may also identify biomarkers that can be used to select patients likely to respond to chemoimmunotherapy as well as those in whom responses are likely to be durable.
In conclusion, I/T/DIN/GM-CSF has significant antitumor activity in patients with relapsed/refractory neuroblastoma. Chemoimmunotherapy is being evaluated as a component of frontline therapy in children with newly diagnosed high-risk disease.
PRIOR PRESENTATION
Presented in abstract form at the 54th ASCO Annual Meeting, Chicago, IL, June 1-5, 2018, and the Advances in Neuroblastoma Research Meeting, San Francisco, CA, May 9-12, 2018.
SUPPORT
Supported by National Cancer Institute, National Institutes of Health (NIH), Grants No. U10CA180899 (Children’s Oncology Group Statistics and Data Center), U10CA98543 (Children’s Oncology Group Chair’s Grant), and U10CA180886 (National Clinical Trial Network Operations Center Grant) and NIH Grants No. R35 CA197078 and R35 CA220500 (St Baldrick’s Foundation) and by funds from the Midwest Athletes Against Childhood Cancer to the University of Wisconsin.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Rajen Mody, Alice L. Yu, Fan F. Zhang, Wendy B. London, Marguerite T. Parisi, Paul M. Sondel, Julia Glade-Bender, John M. Maris, Julie R. Park, Rochelle Bagatell
Collection and assembly of data: Rajen Mody, Arlene Naranjo, Fan F. Zhang, Wendy B. London, Barry L. Shulkin, Mitchell B. Diccianni, Jacquelyn A. Hank, Mildred Felder, Paul M. Sondel, Shahab Asgharzadeh, Rochelle Bagatell
Data analysis and interpretation: Rajen Mody, Alice L. Yu, Arlene Naranjo, Fan F. Zhang, Wendy B. London, Barry L. Shulkin, Marguerite T. Parisi, Sabah-E-Noor Servaes, Mitchell B. Diccianni, Jacquelyn A. Hank, Jennifer Birstler, Paul M. Sondel, Shahab Asgharzadeh, Howard Katzenstein, John M. Maris, Julie R. Park, Rochelle Bagatell
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children’s Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rajen Mody
Consulting or Advisory Role: Amgen
Alice L. Yu
Leadership: OPKO Health
Stock and Other Ownership Interests: OPKO Health/GeneDx, OBI Pharma
Honoraria: EUSA Pharma
Consulting or Advisory Role: OBI Pharma
Speakers’ Bureau: EUSA Pharma
Research Funding: United Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Globo H-diphtheria toxoid vaccine for cancer therapy; NKT stimulatory phenyl-glycolipids for cancer therapy and vaccine adjuvant; cancer-targeting peptides
Travel, Accommodations, Expenses: EUSA Pharma
Arlene Naranjo
Consulting or Advisory Role: Novartis
Wendy B. London
Consulting or Advisory Role: ArQule, Jubilant Draximage
Research Funding: Agios, Bristol-Myers Squibb, Novartis, Aileron Therapeutics, Bluebird Bio
Travel, Accommodations, Expenses: ArQule
Barry L. Shulkin
Consulting or Advisory Role: Navidea
Paul M. Sondel
Patents, Royalties, Other Intellectual Property: Partial interest in patents related to work at the University of Wisconsin-Madison (Madison, WI) that are held by and managed by the University of Wisconsin Foundation; unpaid medical advisor to Invenra, a monoclonal antibody biotech firm (Madison, WI) with which University of Wisconsin laboratory is collaborating and from which it is receiving research reagents for mutual research (Inst)
Uncompensated Relationships: Invenra
Julia Glade-Bender
Consulting or Advisory Role: AbbVie (Inst)
Research Funding: Celgene (Inst), Merck (Inst), Pfizer (Inst), Amgen (Inst), Ignyta (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Eli Lilly (Inst), Loxo (Inst), Roche/Genentech (Inst)
Travel, Accommodations, Expenses: Novartis, Amgen, Merck, Bayer, Roche/Genentech
Uncompensated Relationships: Springworks, Bristol-Myers Squibb
John M. Maris
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs (Inst); neuroblastoma antigens (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladenstein R, Pötschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500–514. doi: 10.1016/S1470-2045(17)30070-0. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 4.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 5.Haynes NM, van der Most RG, Lake RA, et al. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:545–557. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 8.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreri AJ, Cwynarski K, Pulczynski E, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3:e217–e227. doi: 10.1016/S2352-3026(16)00036-3. [DOI] [PubMed] [Google Scholar]
- 12.Mody R, Naranjo A, Van Ryn C, et al. Irinotecan-temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017;18:946–957. doi: 10.1016/S1470-2045(17)30355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinberg SM, Venzon DJ. Early selection in a randomized phase II clinical trial. Stat Med. 2002;21:1711–1726. doi: 10.1002/sim.1150. [DOI] [PubMed] [Google Scholar]
- 14.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 15.Albertini MR, Hank JA, Schiller JH, et al. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3:1277–1288. [PubMed] [Google Scholar]
- 16.Hank JA, Gan J, Ryu H, et al. Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin Cancer Res. 2009;15:5923–5930. doi: 10.1158/1078-0432.CCR-08-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan ER, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.London WB, Frantz CN, Campbell LA, et al. Phase II randomized comparison of topotecan plus cyclophosphamide versus topotecan alone in children with recurrent or refractory neuroblastoma: A Children’s Oncology Group study. J Clin Oncol. 2010;28:3808–3815. doi: 10.1200/JCO.2009.27.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: A Children’s Oncology Group study. J Clin Oncol. 2011;29:208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubie H, Chisholm J, Defachelles AS, et al. Phase II study of temozolomide in relapsed or refractory high-risk neuroblastoma: A joint Société Française des Cancers de l’Enfant and United Kingdom Children Cancer Study Group-New Agents Group Study. J Clin Oncol. 2006;24:5259–5264. doi: 10.1200/JCO.2006.06.1572. [DOI] [PubMed] [Google Scholar]
- 22.Di Giannatale A, Dias-Gastellier N, Devos A, et al. Phase II study of temozolomide in combination with topotecan (TOTEM) in relapsed or refractory neuroblastoma: A European Innovative Therapies for Children with Cancer-SIOP-European Neuroblastoma study. Eur J Cancer. 2014;50:170–177. doi: 10.1016/j.ejca.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 24.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. doi: 10.3389/fimmu.2018.01355. Ozkaynak MF, Gilman AL, London WB, et al: A comprehensive safety trial of chimeric antibody 14.18 with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: Children’s Oncology Group study ANBL0931. Front Immunol 9:1355, 2018 [Erratum: Front Immunol 9:1641, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ladenstein R, Pötschger U, Valteau-Couanet D, et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617–1629. doi: 10.1016/S1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 27.Navid F, Sondel PM, Barfield R, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, Hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol. 2014;32:1445–1452. doi: 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushner BH, Cheung IY, Modak S, et al. Humanized 3F8 anti-GD2 monoclonal antibody dosing with granulocyte-macrophage colony-stimulating factor in patients with resistant neuroblastoma: A phase 1 clinical trial. JAMA Oncol. 2018;4:1729–1735. doi: 10.1001/jamaoncol.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceylan K, Jahns LJ, Lode BN, et al. Inflammatory response and treatment tolerance of long-term infusion of the anti-GD2 antibody ch14.18/CHO in combination with interleukin-2 in patients with high-risk neuroblastoma. Pediatr Blood Cancer. 2018;65:e26967. doi: 10.1002/pbc.26967. [DOI] [PubMed] [Google Scholar]
- 30.Furman WL, Federico SM, McCarville MB, et al. A phase II trial of Hu14.18K322A in combination with induction chemotherapy in children with newly diagnosed high-risk neuroblastoma. Clin Cancer Res. 2019;25:6320–6328. doi: 10.1158/1078-0432.CCR-19-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahl B. Chemotherapy combinations with monoclonal antibodies in non-Hodgkin’s lymphoma. Semin Hematol. 2008;45:90–94. doi: 10.1053/j.seminhematol.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nami B, Maadi H, Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers (Basel) 2018;10:E342. doi: 10.3390/cancers10100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jilani I, O’Brien S, Manshuri T, et al. Transient down-modulation of CD20 by rituximab in patients with chronic lymphocytic leukemia. Blood. 2003;102:3514–3520. doi: 10.1182/blood-2003-01-0055. [DOI] [PubMed] [Google Scholar]
- 34.Hiraga J, Tomita A, Sugimoto T, et al. Down-regulation of CD20 expression in B-cell lymphoma cells after treatment with rituximab-containing combination chemotherapies: Its prevalence and clinical significance. Blood. 2009;113:4885–4893. doi: 10.1182/blood-2008-08-175208. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher-Kuckelkorn R, Volland R, Gradehandt A, et al. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr Blood Cancer. 2017;64:46–56. doi: 10.1002/pbc.26184. [DOI] [PubMed] [Google Scholar]
- 36.Iwai T, Sugimoto M, Wakita D, et al. Topoisomerase I inhibitor, irinotecan, depletes regulatory T cells and up-regulates MHC class I and PD-L1 expression, resulting in a supra-additive antitumor effect when combined with anti-PD-L1 antibodies. Oncotarget. 2018;9:31411–31421. doi: 10.18632/oncotarget.25830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heynckes S, Daka K, Franco P, et al. Crosslink between temozolomide and PD-L1 immune-checkpoint inhibition in glioblastoma multiforme. BMC Cancer. 2019;19:117. doi: 10.1186/s12885-019-5308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Federico SM, McCarville MB, Shulkin BL, et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–6449. doi: 10.1158/1078-0432.CCR-17-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams MA, Kouroumoussis I, Syndercombe-Court D, et al. Administration of recombinant human granulocyte-macrophage colony-stimulating factor after chemotherapy regulates the expression and secretion of monocyte tumor necrosis factor (TNF) and TNF receptors p55 and p75. Blood. 1995;86:4234–4242. [PubMed] [Google Scholar]
- 40.Morris ZS, Guy EI, Francis DM, et al. In situ tumor vaccination by combining local radiation and tumor-specific antibody or immunocytokine treatments. Cancer Res. 2016;76:3929–3941. doi: 10.1158/0008-5472.CAN-15-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hernandez R, Walker KL, Grudzinski JJ, et al. 90Y-NM600 targeted radionuclide therapy induces immunologic memory in syngeneic models of T-cell Non-Hodgkin’s Lymphoma. Commun Biol. doi: 10.1038/s42003-019-0327-4. 10.1038/s42003-019-0327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]