Abstract
PURPOSE
Effective regimens are needed for children with relapsed acute myeloid leukemia (AML). AAML1421 is a phase I/II study of CPX-351, a liposomal preparation of daunorubicin and cytarabine. AAML1421 sought to determine the recommended phase II dose (RP2D) of CPX-351 and the response rate after up to 2 cycles of therapy.
PATIENTS AND METHODS
Children > 1 and ≤ 21 years of age with relapsed/refractory AML were eligible for dose finding; those in first relapse were eligible for the efficacy phase. Dose-limiting toxicity (DLT) assessment occurred during cycle 1. Two cycles of therapy were offered (cycle 1: CPX-351; cycle 2: FLAG [fludarabine 30 mg/m2/dose on days 1-5; cytarabine 2,000 mg/m2/dose on days 1-5; and granulocyte-colony stimulating factor 5 µg/kg/dose, days 1-5 and day 15 through absolute neutrophil count > 500/µL]). Response was assessed after each cycle.
RESULTS
Thirty-eight patients enrolled: 6 in the dose-finding phase and 32 in the efficacy phase. During dose finding, 1/6 patients experienced a DLT (grade 3 decrease in ejection fraction). The RP2D was 135 units/m2 on days 1, 3, and 5. Toxicities of grade ≥ 3 during cycle 1 included fever/neutropenia (45%), infection (47%), and rash (40%). There was no toxic mortality. Best responses included 20 complete response (CR; 54%), 5 CR with partial recovery of platelet count (CRp; 14%), and 5 CR with incomplete blood count recovery (14%). Twenty-one of 25 with CR/CRp had no detectable residual disease (RD; 84%) by flow cytometry. Hematopoietic stem cell transplantation (HSCT) was used as consolidation in 29/30 responders (96.7%); 20/25 (80%) had no RD before HSCT.
CONCLUSION
The RP2D of CPX-351 is 135 units/m2/dose on days 1, 3, and 5. Toxicity was manageable, and protocol therapy was effective. Response rates are superior to prior published North American cooperative group clinical trials for children with AML in first relapse.
INTRODUCTION
Survival in childhood acute myeloid leukemia (AML) has plateaued, despite maximally toxic standard therapy. Treatment and long-term survival are too often complicated by devastating anthracycline-induced cardiomyopathy.1-4 Until better therapeutic options are available for children with AML, methods to minimize cardiotoxicity while maintaining anthracycline dose intensity must be identified.
Context
Key Objectives
Despite intensification of de novo therapy for childhood acute myeloid leukemia (AML), relapse is prevalent, and cardiotoxicity remains a barrier to remission reinduction. Until better therapeutic options are available, methods to minimize cardiotoxicity while maintaining anthracycline dose intensity must be identified.
Knowledge Generated
The Children’s Oncology Group conducted a phase I/II study of CPX-351, a liposomal preparation of cytarabine and daunorubicin with favorable pharmacokinetic properties and demonstrable efficacy in adults. The safety phase established a recommended phase II dose of 135 units/m2 on days 1, 3, and 5. There was no toxic mortality, and toxicities were consistent with intensive AML therapy. Protocol therapy was effective for children in first relapse with complete recovery (CR) + CR with partial recovery of platelet count (CRp) rate of 68.3% (90% CI, 52.9% to 78.0%) and overall response rate (CR + CRp + CR with incomplete blood count recovery) of 81.1% (90% CI, 67.4% to 88.8%). Among responders, 96.7% were successfully bridged to hematopoietic stem cell transplantation.
Relevance
These encouraging results provide rationale for randomized study of CPX-351 in children with de novo AML.
CPX-351 is a liposomal preparation of daunorubicin and cytarabine maintained at a 1:5 molar ratio that demonstrates prolonged exposures compared with free drug. This agent has demonstrated safety and superior efficacy in adult patients with newly diagnosed secondary AML.5,6 On the basis of adult clinical trials, CPX-351 was approved by the US Food and Drug Administration in 2017 for the treatment of adults with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes.
AAML1421 was a Children’s Oncology Group (COG)–sponsored phase I/II study of CPX-351 for children with relapsed AML. The primary objectives were to determine the recommended phase II dose (RP2D) and to estimate the response rate (complete response [CR] + CR with partial recovery of platelet count [CRp]) after up to 2 cycles. CPX-351 was offered in cycle 1. To avoid additional anthracyclines, FLAG was offered in cycle 2 (fludarabine 30 mg/m2/dose on days 1-5; cytarabine 2,000 mg/m2/dose on days 1-5; and granulocyte-colony stimulating factor (G-CSF) 5 µg/kg/dose, days 1-5 and day 15 through absolute neutrophil count [ANC] > 500/µL).
PATIENTS AND METHODS
AAML1421 enrolled children > 1 and ≤ 21 years of age with relapsed/refractory AML in the dose-finding phase; the efficacy phase was restricted to first relapse with no prior reinduction attempt. A minimum of 5% leukemic burden in the marrow or a peripheral blood absolute blast count > 1,000/µL was required. Patients were required to have adequate renal and liver function. Patients who received more than the maximal cumulative anthracyclines delivered with de novo therapy (> 450 mg/m2) were excluded. The intent was to include children who received maximal cumulative anthracyclines on COG de novo AML trials, which used a conversion multiplier of 3 daunorubicin equivalents for mitoxantrone. Patients were required to have adequate cardiac function, defined as shortening fraction (SF) of ≥ 27%, ejection fraction (EF) ≥ 50%, and corrected QT interval < 500 milliseconds. Patients with > 5 blasts/µL from a nontraumatic lumbar puncture or with overt signs of CNS involvement were not eligible. Patients with acute promyelocytic leukemia, bone marrow failure syndromes, Down syndrome, or Wilson disease were ineligible. Supportive care guidelines including antifungal prophylaxis and hospitalization were provided. Site participation in the trial required approval from institutional review boards. All patients or their guardians were required to sign an informed consent document before participation. CPX-351 was supplied by Jazz Pharmaceuticals (Palo Alto, California).
Study Design
The study comprised 2 phases: a dose-finding phase and an efficacy phase. All patients received CPX-351 in cycle 1. It was strongly recommended that all patients receive course 2, consisting of FLAG, for consistency with other relapse trials7-9 and the potential to achieve deeper remission before hematopoietic stem cell transplantation (HSCT). Dose-limiting toxicity (DLT) assessment occurred in cycle 1 of the dose-finding phase. Dose level 1 (DL1) of CPX-351 was 135 units/m2/dose on days 1, 3, and 5. This dose was selected to test equivalent daunorubicin doses compared with standard pediatric de novo AML induction regimens and the European DaunXome (DNX) trial.8 A single dose de-escalation to 100 units/m2/dose, the adult CPX-351 MTD, would occur if DL1 was intolerable. All patients received intrathecal cytarabine within 1 week of initiating cycle 1 therapy and again within 1 week of initiating cycle 2 therapy. Additional intrathecal cytarabine was administered twice weekly after CPX-351 for 4-6 treatments in patients with any detectable leukemia in the CSF at time of screening and was allowed at the investigator’s discretion for patients at high risk for treatment failure in the CNS.
Response Criteria and Toxicity Reporting
Response was assessed after each cycle no earlier than day 28 by bone marrow aspirate and biopsy and by lumbar puncture. Evaluation of the marrow by flow cytometry for minimal residual disease (MRD) according to individual institution practices was required.
A CR was defined as the attainment of a M1 marrow (< 5% blasts) with peripheral blood recovery of ANC ≥ 1,000/µL and platelets ≥ 100,000/µL. A CRp or CR with incomplete blood count recovery (CRi) were defined as M1 marrow with either failure to recover platelets ≥ 100,000/µL or both platelets and/or ANC, respectively. Partial response (PR) was defined as an M2 bone marrow (5%-25% blasts) and at least a 50% decrease in bone marrow blast percent from baseline. Treatment failure was defined as any of the following: an absolute increase of ≥ 20% marrow blasts, > 25% marrow blasts, an M1 marrow with circulating blasts, or development of extramedullary disease. Best response was defined as CR + CRp after up to 2 cycles of therapy. The overall response rate (ORR) was defined as CR + CRp + CRi.
Toxicity was graded using version 4 of the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE). DLT was defined as any grade 3 or greater nonhematologic toxicity related to CPX-351, with the exception of commonly encountered toxicities typical of AML therapy. Hematologic DLTs were defined as failure to recover ANC > 500 /µL or platelet count > 20,000/µL by day 50 of cycle 1, unless due to persistent marrow leukemia. Sites were required to submit locally measured, echocardiogram-derived EF and SF at baseline, end of cycle 1, end of cycle 2, and during follow-up. Digital Imaging and Communications in Medicine (DICOM) echocardiogram images were collected for central measurement of cardiac function; however, safety data are based on the site assessment of left ventricular function.
Statistical Analysis
Enrollment was conducted from April 2016 to June 2018; all data are current as of June 30, 2019. The dose-finding phase used a modified rolling 6 design for DLT assessment.10 Efficacy analysis used point estimates from prior COG phase II studies to formulate the null hypothesis.7,9 A single-arm, Simon two-stage design tested the null hypothesis that the response rate (CR + CRp), was ≤ 40% with 0.1 type 1 error versus the alternative hypothesis that the response rate was ≥ 60% with 0.8 power. The response rate11 and corresponding 90% CI12 were used to define best response after up to 2 cycles, the primary outcome measure. Kaplan-Meier method was used in post hoc analysis to estimate overall survival (OS), defined as time from study entry to death. Patients alive at last contact were censored for OS analyses. For pharmacokinetic (PK) analysis, individual plasma concentrations were summarized by study cohort, day, and time point using descriptive statistics. PK concentrations were summarized including subjects with both intensive and sparse sampling schemes.
Pharmacokinetics
During the dose-finding phase, peripheral blood (2-3 mL) was obtained on day 5 before the dose of CPX-351, midinfusion (0.75 hours), immediately after the infusion (1.5 hours), and at 2, 5, 8, 12, 24, 72, and 120 hours after the infusion. An optional and abbreviated sampling schedule was used for the efficacy phase: 30 minutes after end of infusion on day 1, then on day 5: before infusion, 30 minutes after infusion, then at 6 and 72 hours after initiation of infusion. PK parameters were determined using noncompartmental methods on the basis of individual plasma concentration-time data for cytarabine and daunorubicin after day 5 CPX-351 administration in the dose-finding phase. PK parameters included area under the plasma concentration–time curve, maximum concentration (Cmax), time to Cmax, terminal phase half-life (t1/2), clearance (CL), and volume of distribution (Vss). PK parameters were calculated using Phoenix WinNonlin v.7.0 (Certara LP, Princeton, NJ). One patient was excluded from the results because the administered dosing was reported incorrectly in the PK dataset. PK data in the efficacy phase will be reported separately.
RESULTS
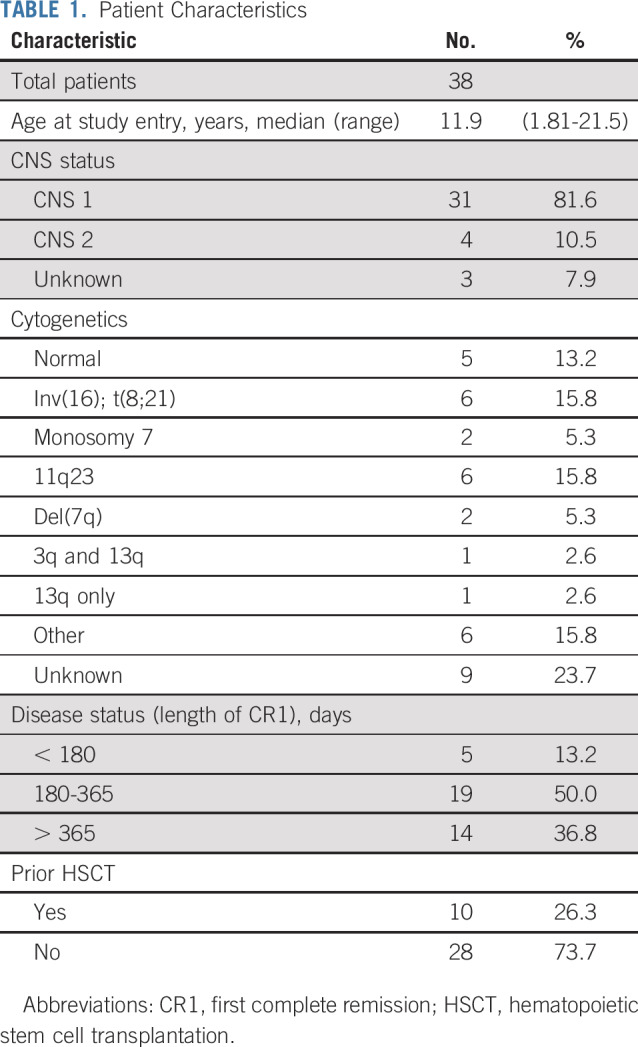
Patient Characteristics
Six patients enrolled in the dose-finding phase, and 32 participated in the efficacy phase. All those enrolled in the dose-finding phase were in first relapse and thus were eligible for efficacy determination. Patient characteristics for the entire cohort are listed in Table 1. Of note, 4 patients had CNS2 status and required additional intrathecal cytarabine, per protocol. Twenty-four patients (63.2%) had relapsed within 1 year of first CR, which is a poor prognostic indicator for OS after relapse.13 Ten patients (26.3%) had previously received allogeneic HSCT.
TABLE 1.
Patient Characteristics
Safety
Dose-Finding Phase.
In the dose-finding phase, 6 patients were evaluable for toxicity. There was one DLT of grade 3 reduction in EF at the end of cycle 1 in a patient with a prior history of cardiac dysfunction. CTCAE grade 3 or 4 bacterial infections were encountered in 4/6 participants in the dose-finding phase but did not qualify as DLTs. No other DLTs were encountered; therefore, 135 units/m2/dose was determined to be the RP2D.
Efficacy Phase
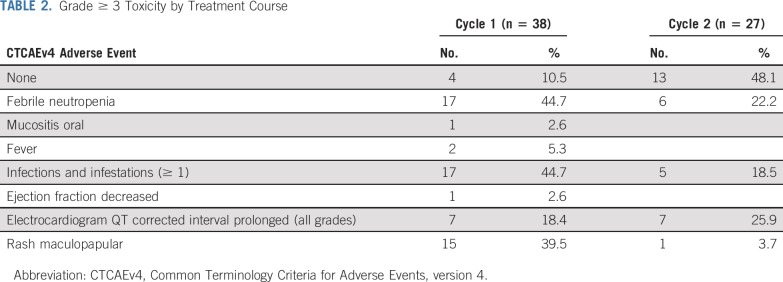
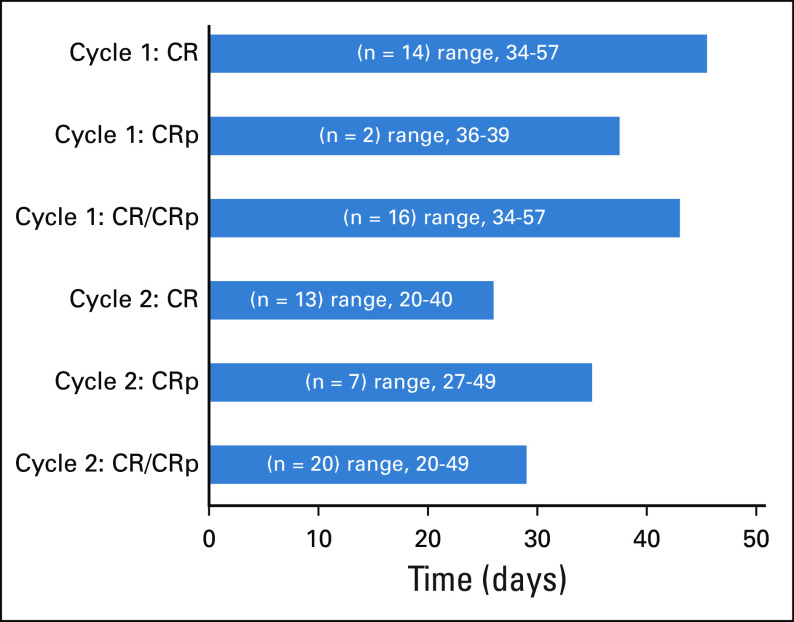
Grade 3 or greater adverse events for all 38 patients are summarized in Table 2 and were consistent with intensive AML reinduction regimens. During course 1, at least 1 infection occurred in 17 (44.7%) patients. No fungal infections were reported. Microbiologically documented pathogens are listed in Appendix Table A1 (online only). Other common toxicities included fever with neutropenia (44.7%) and maculopapular rash (39.5%). Rashes were grade 3 and expected based on adult studies with CPX-351. No additional grade 3 or greater cardiotoxicities were observed. Median ANC and platelet recovery in responders after cycle 1 was 43 days (range, 34-57 days) compared with 29 days (range, 20-49 days) after cycle 2 (Appendix Fig A1, online only). Length of hospitalization for cycle 1 (n = 38) was a median of 33.5 days (range, 5-52 days).
TABLE 2.
Grade ≥ 3 Toxicity by Treatment Course
Cardiotoxicity Data
As above, 1 patient with history of cardiac dysfunction during de novo therapy developed a grade 3 reduction in EF (defined as EF 20%-39%) after cycle 1, which subsequently resolved after initiation of enalapril. Grade 2 reduction in EF (defined as EF 40%-50%) occurred in 6 additional patients, 2 after cycle 1 and 4 after cycle 2. Subsequent EF measurements were available for 6/7 patients with grade ≥ 2 EF reduction; all demonstrated recovery of EF to > 50% during follow-up. There were no reports of symptomatic left ventricular systolic dysfunction (LVSD).
Pharmacokinetics
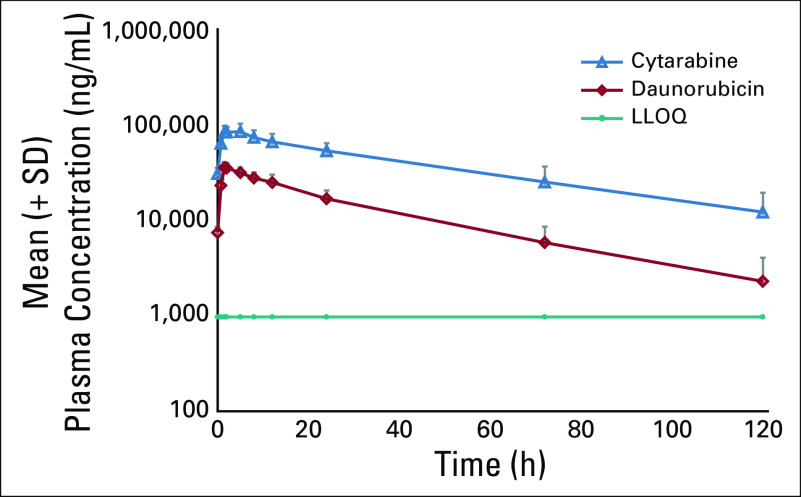
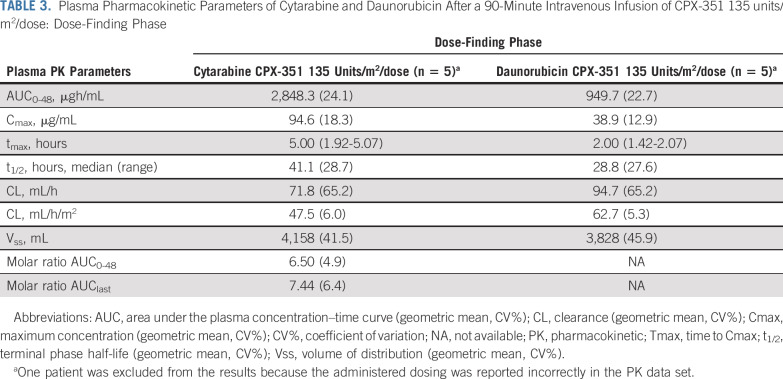
During the dose-finding phase, mean molar plasma concentration ratio was maintained close to the ratio of 1:5 (daunorubicin:cytarabine) over the first 72 hours after the start of the day 5 infusion (Fig 1). After administration of 135 units/m2 CPX-351, plasma exposures in pediatric patients were similar to those observed in adults.5 PK parameters for total cytarabine and daunorubicin in pediatric patients, such as t1/2, CL, and Vss, were also similar to those observed in adults (Table 3).
FIG 1.
Mean plasma concentrations of cytarabine and daunorubicin versus time after a 90-minute intravenous infusion of CPX-351 135 units/m2/dose (semi-log scale) dose-finding phase, day 5. LLOQ, lower limit of quantification.
TABLE 3.
Plasma Pharmacokinetic Parameters of Cytarabine and Daunorubicin After a 90-Minute Intravenous Infusion of CPX-351 135 units/m2/dose: Dose-Finding Phase
Efficacy
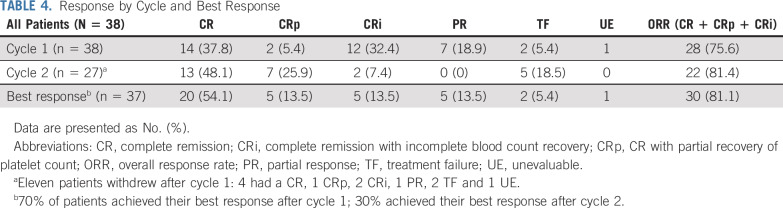
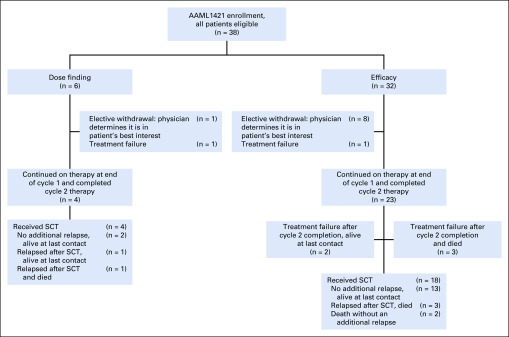
Responses by course and best response after up to 2 cycles are listed in Table 4. Among 38 patients eligible for efficacy determination, one was unevaluable. In that case, the patient received nonprotocol therapy with a tyrosine kinase inhibitor on day 15 of cycle 1, before a disease evaluation at an adequate time point could be performed. Of 37 evaluable patients, 28 patients (75.7%) achieved CR, CRp, or CRi with course 1 (CPX-351). Eleven patients withdrew from protocol therapy after course 1, 7 of whom demonstrated a response (4 CR, 1 CRp, 2 CRi) and withdrew to receive HSCT. In addition, 1 patient withdrew after a PR, 2 patients had treatment failure (TF), and 1 patient received off-protocol therapy and was unevaluable, as previously described. All children with 5%-25% blasts at study entry responded (4CR, 1CRp, 1CRi). Twenty-seven patients received both cycles of therapy; 7 with a PR after cycle 1 continued in the study to receive FLAG in cycle 2. Two of these 7 patients achieved a CR after cycle 2, and the rest either progressed to TF or withdrew from the study.
TABLE 4.
Response by Cycle and Best Response
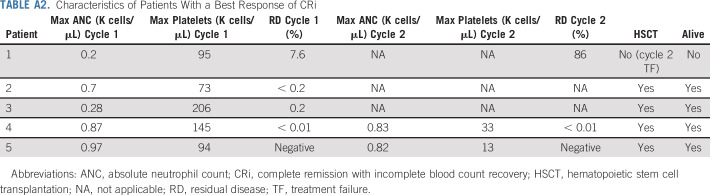
Seventy percent of responders achieved their best response with course 1. Best responses after up to 2 cycles were 20 CR (54%), 5 CRp (13.5%), and 5 CRi (13.5%). Of 5 patients with best response of CRi, one had disease progression (TF) after course 2. Two of the remaining 4 were MRD negative, and all were successfully bridged to HSCT. Each of these 4 patients had evidence of neutrophil and platelet recovery (Appendix Table A2).
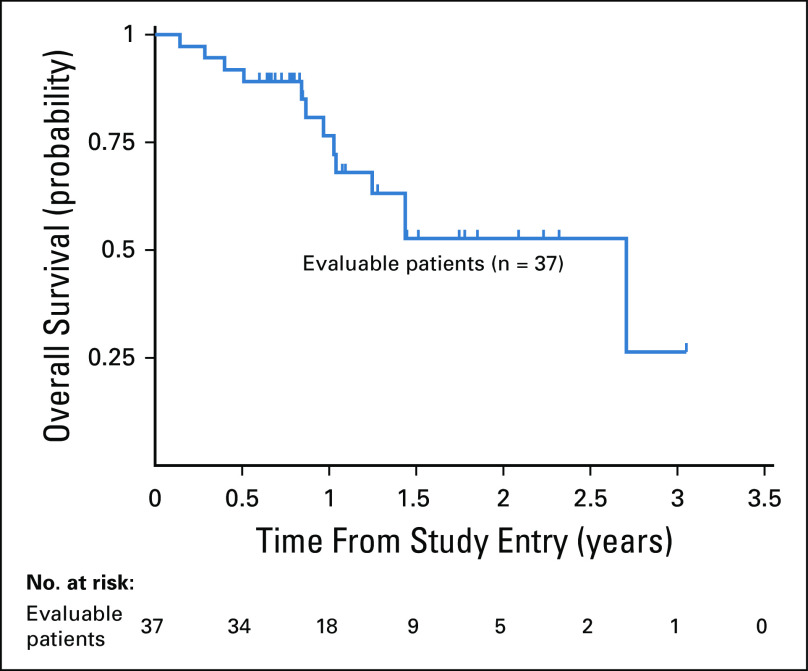
Characteristics of responders are listed in Table 5. Responses were observed in all cytogenetic risk groups. All 14 patients with AML in late relapse (initial CR > 12 months) achieved a CR, CRp, or CRi, and 16/24 (67%) with early relapse (initial CR < 12 months) achieved a CR, CRp, or CRi. Allogeneic HSCT was used as consolidation in 96.7% of patients with a CR, CRp, or CRi. Overall survival at 2 years for evaluable patients was 52.7% ± 21.1% (Fig 2).
TABLE 5.
Characteristics of Responders
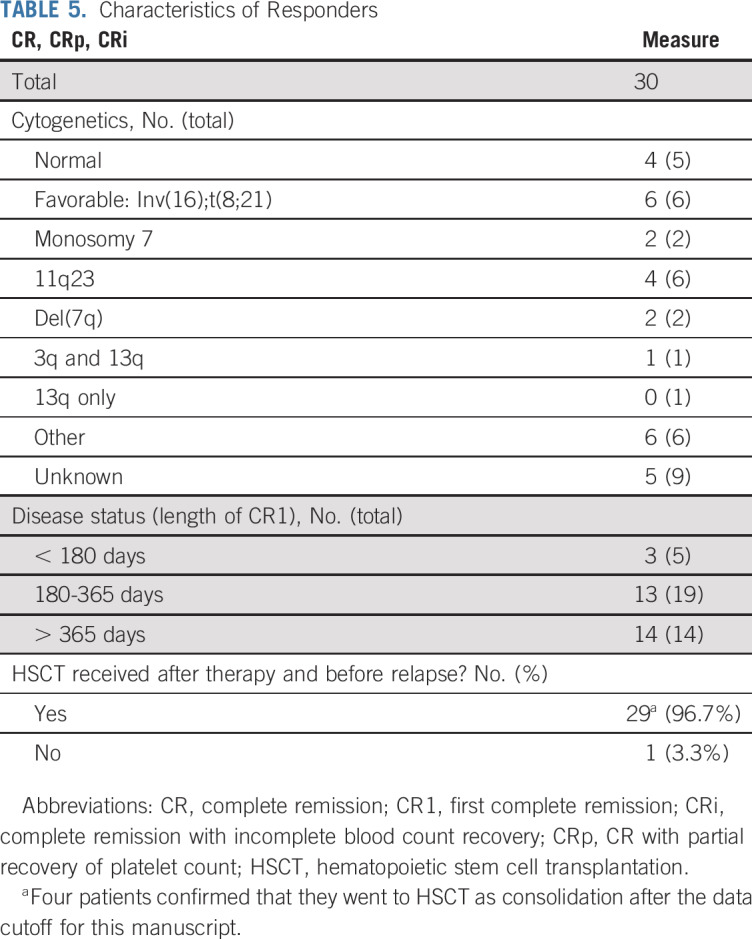
FIG 2.
Overall survival, including evaluable patients (n = 37).
Blast Quantification by Flow Cytometry
Flow cytometry for residual disease (RD) was required after each cycle for more precise blast quantification. The protocol determined response using morphology by Cheson’s criteria,14 but guidance was provided that flow cytometry could be used to differentiate between marrow recovery and leukemic blasts. Flow cytometry was either performed locally (n = 15) or sent to Clinical Laboratory Improvement Amendments–approved reference centers at Hematologics (Seattle, Washington) or the University of Washington (n = 23). Lower level of detection of local flow cytometry ranged from 0.01%-0.5%. Of 16 patients with CR or CRp after cycle 1, 12 (75%) had no RD. Overall, 21 of 25 patients (84%) with CR or CRp reported as best response had no RD. Twenty-four of 30 patients (80%) with CR, CRp, or CRi had no detectable RD by flow cytometry.
DISCUSSION
Treatment of children with relapsed AML is complicated by the intensity of de novo therapies, including anthracyclines, which have deleterious cardiac effects.2 It is essential that strategies for mitigating anthracycline-induced cardiotoxicity in children are pursued. The COG conducted AAML1421, a phase I/II study of CPX-351, to determine an RP2D and estimate its efficacy in children with AML in first relapse. CPX-351 is a liposomal preparation of daunorubicin and cytarabine designed to maintain an optimally synergistic 1:5 molar ratio with a half-life considerably longer compared with free drug, resulting in higher uptake in leukemia cells.15-17 Supporting AAML1421 was NCT01943862, a first-in-child phase I study conducted at a single institution, which included children with acute lymphoblastic leukemia/AML/mixed phenotype leukemia with refractory disease or first or greater relapse.18 Rationale for pediatric development of CPX-351 is further strengthened by the adult phase III study results, which reported tolerability and superior efficacy in adult patients with newly diagnosed secondary AML.19
The AAML1421 CR + CRp rate of 68.3% (90% CI, 52.9% to 78.0%) is superior to prior COG studies for AML in first relapse.7,9 Although these response rates are encouraging, direct statistical comparisons to non-COG cooperative group trials are not possible because of differences in study design and response criteria. Although our primary objective of determining CR + CRp response was selected for statistical comparison with prior COG trials, here we also report ORR (CR/CRp/CRi), because CRi responses in our study were clinically significant. Four of 5 patients with CRi were successfully bridged to transplantation, 2 of whom had no residual disease. All 4 had evidence of count recovery at the time of response assessment but did not meet Cheson’s criteria for CR (Appendix Table A2, online only).14 Therefore, the ORR of 81.1% (90% CI, 67.4% to 88.8%) is a valid assessment of CPX-351 activity in children with AML in first relapse. As clinicians become less likely to wait until complete peripheral blood count recovery before HSCT, consideration of alternatives to Cheson’s criteria for response needs to be incorporated. In addition, alternative methods to morphologic assessment of response, such as flow cytometry, should be incorporated into clinical trials as technologic advances continue. Although not performed centrally, CPX-351 did induce negative RD responses in 80% of children with CR + CRp + CRi.
CPX-351 demonstrated a toxicity profile similar to other published intensive AML reinduction regimens. Infection and fever/neutropenia were common; however, no treatment-related mortality (TRM) or 100-day TRM after HSCT was observed. Given the long half-life of CPX-351, as well as the higher RP2D compared with adults, peripheral blood count recovery was carefully monitored. Although ANC and platelet recovery were longer after cycle 1 (Appendix Fig A1), these results may be skewed because of G-CSF administration during cycle 2 with FLAG chemotherapy.
A devastating consequence of therapy for patients receiving AML therapy and for childhood survivors of AML is anthracycline-induced cardiotoxicity. Liposomal delivery systems for anthracyclines are a promising strategy to mitigate cardiotoxicity while maintaining efficacy.20-22 The potential advantages of liposomal preparations include prolonged time in circulation due to protection of the drug from enzymatic inactivation, circumvention of drug efflux transporters responsible for drug resistance,23,24 and altered bio-distribution of the liposome formulation, with potential sparing of normal tissue to mitigate toxicity.25 Adult studies have demonstrated decreased cardiotoxicity with liposomal anthracyclines, yet this benefit is not yet well studied for CPX-351.26,27
Our study required close monitoring of cardiac function by echocardiogram at baseline, after each cycle of therapy, and during follow-up. All patients with grade 2 or greater LVSD with echocardiogram data available during follow-up (5/6 patients) demonstrated recovery of EF to > 50%. There were no patients with symptomatic LVSD. Analysis of other centrally measured parameters of left ventricular systolic function and cardiac biomarkers (natriuretic peptides and high-sensitivity cardiac troponin) will be reported in a separate article. Although these results offer important insight into the cardiac effects of liposomal anthracyclines in the relapse setting, it is important to recognize that this population is heavily anthracycline pretreated and at high risk for cardiotoxicity.
Importantly, the prevalence of grade ≥ 2 LVSD (defined as EF < 50%) was 10.6% at the end of the first year after up-front treatment on AAML1031.28 Given that the majority of patients treated in this trial were within 1 year of initial remission, the cardiac toxicities observed may be in part related to the natural evolution of cardiotoxicity due to up-front anthracycline exposure and the impact of concurrent infections. The optimal setting for a prospective evaluation of the cardioprotection of liposomal anthracyclines is a randomized phase III study in anthracycline-naïve patients. COG is planning a randomized study of CPX-351 versus standard anthracycline-containing induction given with dexrazoxane, which has emerged as a potentially effective cardioprotective strategy in pediatric AML.28 Prospective evaluation and comparison of cardioprotective strategies will be an integral aim of the trial.
Treatment with CPX-351 followed by FLAG in AAML1421 demonstrated encouraging response rates and an acceptable toxicity profile. These results support the efficacy of CPX-351 for children with AML in first relapse and provide excellent rationale for phase III study in childhood de novo AML.
Appendix
FIG A1.
Median time to absolute neutrophil count (ANC) recovery ≥ 1,000/µL in days for all patients achieving a complete response (CR) or CR with partial recovery of platelet count (CRp) in cycles 1 and 2.
FIG A2.
AAML1421 CONSORT diagram.
TABLE A1.
Microbiologically Documented SS and NSS Infections Reported on AAML1421
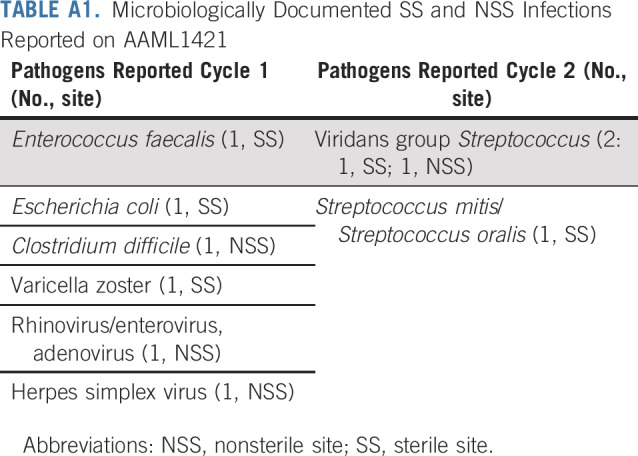
TABLE A2.
Characteristics of Patients With a Best Response of CRi
PRIOR PRESENTATION
Presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
Supported by National Clinical Trials Network (NCTN) Operations Center Grant No. U10CA180886, NCTN Statistics & Data Center Grant U10CA180899, and the St Baldrick’s Foundation.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Todd M. Cooper, Michael J. Absalon, Todd A. Alonzo, Kasey J. Leger, Jessica Pollard, Bassem I. Razzouk, E. Anders Kolb
Provision of study material or patients: Michael J. Absalon, Kasey J. Leger
Collection and assembly of data: Todd M. Cooper, Michael J. Absalon, Todd A. Alonzo, Betsy A. Hirsch, E. Anders Kolb
Data analysis and interpretation: Todd M. Cooper, Michael J. Absalon, Todd A. Alonzo, Robert B. Gerbing, Kasey J. Leger, Jessica Pollard, Bassem I. Razzouk, Richard Aplenc, E. Anders Kolb
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I/II Study of CPX-351 Followed by Fludarabine, Cytarabine, and Granulocyte-Colony Stimulating Factor for Children with Relapsed Acute Myeloid Leukemia: A Report from the Children’s Oncology Group
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Todd M. Cooper
Employment: Juno Therapeutics/Celgene (I)
Stock and Other Ownership Interests: Juno Therapeutics/Celgene (I)
Michael J. Absalon
Research Funding: Jazz Pharmaceuticals (Inst)
Kasey J. Leger
Honoraria: Boston Scientific
Consulting or Advisory Role: Jazz Pharmaceuticals, BTG
Research Funding: Abbott Laboratories
Travel, Accommodations, Expenses: Jazz Pharmaceuticals, BTG
Richard Aplenc
Honoraria: Sigma-Tau
Expert Testimony: Wiggin and Dana
Travel, Accommodations, Expenses: Sigma-Tau
E. Anders Kolb
Travel, Accommodations, Expenses: Roche/Genentech
No other potential conflicts of interest were reported.
REFERENCES
- 1.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orgel E, Zung L, Ji L, et al. Early cardiac outcomes following contemporary treatment for childhood acute myeloid leukemia: A North American perspective. Pediatr Blood Cancer. 2013;60:1528–1533. doi: 10.1002/pbc.24498. [DOI] [PubMed] [Google Scholar]
- 3.Getz KD, Sung L, Ky B, et al. Occurrence of treatment-related cardiotoxicity and its impact on outcomes among children treated in the AAML0531 clinical trial: A report from the Children’s Oncology Group. J Clin Oncol. 2019;37:12–21. doi: 10.1200/JCO.18.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jarfelt M, Andersen NH, Hasle H. Is it possible to cure childhood acute myeloid leukaemia without significant cardiotoxicity? Br J Haematol. 2016;175:577–587. doi: 10.1111/bjh.14374. [DOI] [PubMed] [Google Scholar]
- 5.Feldman EJ, Lancet JE, Kolitz JE, et al. First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29:979–985. doi: 10.1200/JCO.2010.30.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper TM, Alonzo TA, Gerbing RB, et al. AAML0523: A report from the Children’s Oncology Group on the efficacy of clofarabine in combination with cytarabine in pediatric patients with recurrent acute myeloid leukemia. Cancer. 2014;120:2482–2489. doi: 10.1002/cncr.28674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: Results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31:599–607. doi: 10.1200/JCO.2012.43.7384. [DOI] [PubMed] [Google Scholar]
- 9.Horton TM, Perentesis JP, Gamis AS, et al. A Phase 2 study of bortezomib combined with either idarubicin/cytarabine or cytarabine/etoposide in children with relapsed, refractory or secondary acute myeloid leukemia: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2014;61:1754–1760. doi: 10.1002/pbc.25117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skolnik JM, Barrett JS, Jayaraman B, et al. Shortening the timeline of pediatric phase I trials: The rolling six design. J Clin Oncol. 2008;26:190–195. doi: 10.1200/JCO.2007.12.7712. [DOI] [PubMed] [Google Scholar]
- 11.Jung SH, Kim KM. On the estimation of the binomial probability in multistage clinical trials. Stat Med. 2004;23:881–896. doi: 10.1002/sim.1653. [DOI] [PubMed] [Google Scholar]
- 12.Koyama T, Chen H. Proper inference from Simon’s two-stage designs. Stat Med. 2008;27:3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells RJ, Adams MT, Alonzo TA, et al. Mitoxantrone and cytarabine induction, high-dose cytarabine, and etoposide intensification for pediatric patients with relapsed or refractory acute myeloid leukemia: Children’s Cancer Group Study 2951. J Clin Oncol. 2003;21:2940–2947. doi: 10.1200/JCO.2003.06.128. [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 15.Lim WS, Tardi PG, Dos Santos N, et al. Leukemia-selective uptake and cytotoxicity of CPX-351, a synergistic fixed-ratio cytarabine:daunorubicin formulation, in bone marrow xenografts. Leuk Res. 2010;34:1214–1223. doi: 10.1016/j.leukres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Tardi P, Johnstone S, Harasym N, et al. In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33:129–139. doi: 10.1016/j.leukres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Kim HP, Gerhard B, Harasym TO, et al. Liposomal encapsulation of a synergistic molar ratio of cytarabine and daunorubicin enhances selective toxicity for acute myeloid leukemia progenitors as compared to analogous normal hematopoietic cells. Exp Hematol. 2011;39:741–750. doi: 10.1016/j.exphem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Absalon M, O’Brien MM, Phillips CL, et al. A phase I/pilot study of CPX-351 for children, adolescents and young adults with recurrent or refractory hematologic malignancies. J Clin Oncol. 2016;34(15_suppl; abstr 10541) [Google Scholar]
- 19.Cortes JE, Goldberg SL, Feldman EJ, et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine:daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121:234–242. doi: 10.1002/cncr.28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forssen EA, Coulter DM, Proffitt RT. Selective in vivo localization of daunorubicin small unilamellar vesicles in solid tumors. Cancer Res. 1992;52:3255–3261. [PubMed] [Google Scholar]
- 21.Lowis S, Lewis I, Elsworth A, et al. A phase I study of intravenous liposomal daunorubicin (DaunoXome) in paediatric patients with relapsed or resistant solid tumours. Br J Cancer. 2006;95:571–580. doi: 10.1038/sj.bjc.6603288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellott R, Auvrignon A, Leblanc T, et al. Pharmacokinetics of liposomal daunorubicin (DaunoXome) during a phase I-II study in children with relapsed acute lymphoblastic leukaemia. Cancer Chemother Pharmacol. 2001;47:15–21. doi: 10.1007/s002800000206. [DOI] [PubMed] [Google Scholar]
- 23.Thierry AR, Vigé D, Coughlin SS, et al. Modulation of doxorubicin resistance in multidrug-resistant cells by liposomes. FASEB J. 1993;7:572–579. doi: 10.1096/fasebj.7.6.8097173. [DOI] [PubMed] [Google Scholar]
- 24.Rahman A, Husain SR, Siddiqui J, et al. Liposome-mediated modulation of multidrug resistance in human HL-60 leukemia cells. J Natl Cancer Inst. 1992;84:1909–1915. doi: 10.1093/jnci/84.24.1909. [DOI] [PubMed] [Google Scholar]
- 25.Ewer MS, Martin FJ, Henderson C, et al. Cardiac safety of liposomal anthracyclines. Semin Oncol. 2004;31(suppl 13):161–181. doi: 10.1053/j.seminoncol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Rafiyath SM, Rasul M, Lee B, et al. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp Hematol Oncol. 2012;1:10. doi: 10.1186/2162-3619-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 28. doi: 10.1200/JCO.19.02856. Getz KD, Sung L, Leger K, et al: Effect of dexrazoxane on left ventricular function and treatment outcomes in patients with acute myeloid leukemia: A Children’s Oncology Group report. J Clin Oncol 36, 2018 (15_suppl; abstr 10501) [DOI] [PMC free article] [PubMed] [Google Scholar]