Abstract
Objective:
Immune dysregulation is a defining feature of sepsis, but the role for mitochondria in the development of immunoparalysis in pediatric sepsis is not known. We sought to determine if mitochondrial dysfunction measured in peripheral blood mononuclear cells (PBMCs) is associated with immunoparalysis and systemic inflammation in children with sepsis.
Design:
Prospective observational study
Setting:
Single academic pediatric intensive care unit (PICU)
Patients:
161 children with sepsis/septic shock and 18 non-infected PICU controls
Measurements and Main Results:
Mitochondrial respiration in PBMCs, markers of immune function, and plasma cytokines were measured on days 1–2 (T1), 3–5 (T2), and 8–14 (T3) after sepsis recognition, and once for controls. Immunoparalysis was defined as whole-blood ex vivo lipopolysaccharide (LPS)-induced tumor necrosis factor-alpha (TNF-α) ≤200 pg/mL or monocyte human leukocyte antigen (mHLA)-DR ≤30%. Mitochondrial respiration was lower in children with versus without immunoparalysis measured at the same timepoint. Mitochondrial respiration measured early (at T1 and T2) was also lower in those with immunoparalysis at T2 and T3, respectively. Although most patients with immunoparalysis exhibited low mitochondrial respiration, this metabolic finding was not specific to the immunoparalysis phenotype. Plasma cytokines, including IL-8, IL-10, TNF-α, and MCP-1 were highest in the subset of sepsis patients with immune paralysis or low mitochondrial respiration at T1.
Conclusions:
Children with sepsis had lower PBMC mitochondrial respiration when immunoparalysis was present compared to those without immunoparalysis. The subsets with immune paralysis and low mitochondrial respiration exhibited the highest levels of systemic inflammation.
Keywords: septic shock, child, mitochondria, immune cell
INTRODUCTION
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection (1) that is characterized by both systemic inflammation and an amplified anti-inflammatory response (2). In uncomplicated infection, a contained and coordinated process of immune activation is usually followed by down-regulation. In sepsis, the delicate homeostasis between pro- and anti-inflammatory mechanisms is disrupted, such that these functional states may co-exist and one, or the other, can manifest disproportionately (3). An exaggerated down-regulation of innate or adaptive immune function, termed “immunoparalysis”, has been associated with adverse outcomes in adults and children with sepsis (4–6).
Cellular metabolism plays a critical role in the immune response to infection. Under normal conditions, naïve leukocytes utilize mitochondrial oxidative phosphorylation for energy production, but switch to a preference for glycolysis after antigen stimulation, even when oxygen is not limiting (7). This transition to “aerobic glycolysis” enables broad metabolic reprogramming of the immune system that supports innate immune activation and lymphocyte proliferation (2, 7). Simultaneously, maintenance of mitochondrial function is necessary for cell signaling through reactive oxygen species, immunologic memory, and an eventual return to immunologic quiescence (8–10).
Alterations in cellular metabolism after infection have been increasingly implicated in the septic immune response (11–13). In particular, decreased mitochondrial respiration and redox imbalance have been reported in peripheral blood mononuclear cells (PBMCs) from patients with sepsis (14–18). Moreover, leukocytes from septic adults with diminished cytokine secretion after ex vivo stimulation by lipopolysaccharide (LPS) have been shown to exhibit broad metabolic defects in glycolysis and mitochondrial oxidative phosphorylation (11). Notably, several immunomodulatory therapies have demonstrated ability to reverse immunoparalysis and potentially improve outcomes (19–23). Therefore, clarifying the role of impaired metabolism in the immunoparalyzed phenotype may provide important biologic insights that could yield novel therapeutic targets in sepsis.
Although both mitochondrial dysfunction and immunoparalysis have been shown to occur and portend adverse outcomes in pediatric sepsis, there are currently no data linking immune cell mitochondrial dysfunction to immunoparalysis or systemic inflammation in children. We therefore sought to test the hypothesis that mitochondrial dysfunction is associated with measures of immunoparalysis and systemic inflammation in children with sepsis.
MATERIALS AND METHODS
Study Design and Population:
We performed a prospective observational study of patients <18 years treated for severe sepsis or septic shock in a single academic pediatric intensive care unit between May 2014 and June 2018. Severe sepsis and septic shock were defined using consensus pediatric criteria (24). Exclusion criteria were weight <7.5 kg (due to limited blood collection), white blood cells <0.5×103/μL, known mitochondriopathy, unrepaired cyanotic heart disease, and prior enrollment. A convenience sample of PICU patients of similar overall age and sex distribution without evidence of infection or organ dysfunction were enrolled as controls. The study was approved by The Children’s Hospital of Philadelphia Institutional Review Board and written informed consent was obtained.
Data Collection:
Data were collected about patient characteristics, clinical course, primary and secondary infections, and vital status. Severity of illness was determined by the Pediatric Risk of Mortality (PRISM)-III (25) and Pediatric Logistic Organ Dysfunction (PELOD) (26) scores. Organ dysfunction, as previously defined, was monitored daily after sepsis recognition (24). Clinical outcomes included hospital-acquired infections (HAI) within 28 days of sepsis recognition, complicated course, and all-cause 28-day mortality. HAI was defined as a new pathogen or clinically diagnosed infection that was not present within 48 hours of hospital admission or sepsis recognition. Complicated course is a composite endpoint defined as two or more organ dysfunctions present on day 7 or death by day 28 (27).
Blood Collection:
A day 1–2 sample of 7–9 mL of blood was collected as soon as possible after consent for sepsis patients and controls (T1). For sepsis patients, additional blood was collected between study days 3–5 (at least two days after first sample, T2) and again between days 8–14 (T3). Blood was collected in citrate tubes for measurement of mitochondrial respiration and content, EDTA tubes for monocyte human leukocyte antigen DR (HLA-DR) expression (mHLA-DR), and lithium heparin tubes (on ice) for measurement of whole-blood ex vivo lipopolysaccharide (LPS)-induced tumor necrosis factor-alpha (TNFα) and cytokine analyses. Blood samples were processed or utilized immediately following collection. Mitochondrial respiration, monocyte HLA-DR, and ex vivo LPS-stimulated TNF-α were measured on fresh blood samples while mitochondrial content was measured in batches using PBMCs that had been stored at −80°C. Heparinized whole blood was also centrifuged within 30 minutes of collection with aliquoted plasma stored at −80°C for batched cytokine measurements.
Mitochondrial Respiration:
PBMCs were isolated by density centrifugation gradient and mitochondrial respiration was measured in 2–4 × 106 intact PBMCs using a high-resolution oxygraph (Oxygraph-2k, Oroboros Instruments, Innsbruck, Austria) as previously described (18). PBMC cell counts using trypan blue exclusion yielded median viability of 88% (interquartile range 76–95%). The median purity of PBMCs was 85% (IQR 63–87%) among random samples tested at the start and conclusion of the study. Intact cells were utilized to maintain the cellular microenvironment such that respiration relied on endogenous substrates. We directly measured baseline (routine) respiration, proton leak after inhibition of ATP synthase (LEAK), and maximal uncoupled respiration through the electron transport system (ETSmax). Non-mitochondrial respiration that persisted after addition of antimycin-A and sodium azide was subtracted from all parameters. ATP-linked respiration was calculated as routine respiration minus LEAK, and spare respiratory capacity (SRC) was calculated as ETSmax minus routine respiration. SRC is the mitochondrial bioenergetic reserve available for cells to produce ATP in response to a stress-induced increase in metabolic demand, and low SRC indicates a potential cellular bioenergetic crisis (28). Low levels of respiration are often used as a proxy to indicate mitochondrial dysfunction and have been linked to adverse clinical outcomes (15, 16, 28, 29). For example, we have demonstrated that persistently low SRC measured in PBMCs over the first week of septic illness was associated with prolonged organ dysfunction in children (18). Mitochondrial respiration was normalized to PBMC number, rather than to mitochondrial content, to assess overall level of mitochondrial function at the cellular level.
Immune Function:
Several pediatric studies have demonstrated an association between reduced capacity of whole blood to produce the pro-inflammatory cytokine TNFα after ex vivo stimulation with LPS with increased risk for hospital-acquired infections and death (4, 30, 31). As a result, this immunologic assay is considered a reliable marker of immunoparalysis in critically ill children. To a lesser extent, reduced expression of the monocyte cell surface molecule HLA-DR (involved with antigen presentation) has also been linked immunoparalysis in children (4), but studies have preferentially used this measure in adults (23, 32). Thus, we measured both ex vivo LPS-stimulated TNF-α and monocyte HLA-DR as indicators of sepsis-induced immunoparalysis.
Ex vivo LPS-stimulated whole blood TNFα was measured by mixing 50 μL heparinized whole blood with 500 μL (250 pg) of phenol-extracted LPS from Salmonella enterica abortus equi (Sigma-Aldrich, L5886) within 60–90 minutes of blood collection as previously described (4). The sample was then incubated for four hours at 37°C, followed by centrifugation at 1,000g for five minutes. The supernatant was stored at −80°C for batched analysis. TNFα was measured, in duplicate, using an enzyme-linked immunosorbent assay kit (Invitrogen KHC3011C). Monocyte HLA-DR was measured using a whole-blood lysis technique. The sample was then stained with labeled anti-HLA-DR and anti-CD14 antibodies and the percentage of HLA-DR positive cells among the CD-14 positive population was determined using flow cytometry. Immunoparalysis was a priori defined as LPS-stimulated TNF-α ≤200 pg/mL or mHLA-DR ≤30% based on prior studies (4, 33).
Cytokines:
Four cytokines (interleukin [IL]-8, IL-10, TNF-α, and monocyte chemoattractant protein [MCP]-1) involved in the systemic inflammatory response were measured using commercially-available enzyme-linked immunosorbent assay assays (Life Technologies, Carlsbad, CA; EMD Millipore, Darmstadt, Germany. Samples were analyzed in duplicate, and the mean value was used as the measured biomarker concentration. FLUOstar software® (Ortenberg, Germany) was used to calculate standard curves for each analyte. The standard curve for each analyte was required to have R2 >95%, and the results from control samples were required to be within the 95% confidence interval of the expected range provided by the manufacturer. For samples that remained below or above range after serial dilutions, the value was set to the lowest or highest value produced for that analyte in each run, respectively.
Statistical Analysis:
Analyses were performed using STATA (Version 12.1, College Station, TX). Data are presented as median with interquartile range (IQR) for continuous variables and percentages for categorical variables. Comparisons were performed using Wilcoxon rank sum and Fisher’s exact tests. Correlations were analyzed using Spearman’s rank. The criterion validity for mitochondrial respiration to discriminate between sepsis patients with versus without immunoparalysis was tested using the area under the receiver operating characteristic curve (AUROC). We then calculated the sensitivity and specificity using varying percentiles derived from non-septic control values. Finally, to account for potential down-regulatory effects of corticosteroids on both mitochondrial and immune function, we performed a sensitivity analysis excluding sepsis patients treated with corticosteroids at each timepoint. Statistical significance was set at p-value <0.05, with Bonferroni corrections for analyses that included multiple comparisons.
RESULTS
Study Patients:
A total of 6,535 patients were screened, 471 met eligibility criteria, and 204 were enrolled. Forty-three patients were excluded due to a final diagnosis other than sepsis or lack of sufficient study measures, leaving 161 patients available for analysis (Figure, Supplemental Digital Content 1, http://links.lww.com/SHK/A994). One hundred twenty and 80 patients had data to analyze on study days 3–5 (T2) and 8–14 (T3), respectively. Twelve patients died; thus, missing longitudinal data was largely attributable to clinical improvement. Eighteen controls with mitochondrial and immune function data were also included.
Patient characteristics are shown in Table 1, with clinical and laboratory characteristics at the time of blood sampling shown in Table, Supplemental Digital Content 2, http://links.lww.com/SHK/A995. Sepsis patients had slightly lower LPS-stimulated TNF-α levels and significantly lower mHLA-DR levels than controls at T1 (Figure 1A). The proportion of controls and sepsis patients with immunoparalysis by either LPS-stimulated TNF-α ≤200 pg/mL, mHLA-DR ≤30%, or both at each timepoint is shown in Figure 1B. Fourteen (9%) sepsis patients had secondary HAIs, 30 (19%) had complicated course, and 10 (6%) died within 28 days. No control patients experienced these clinical outcomes. Immunoparalysis defined using LPS-stimulated TNF-α at T1 or T2 was associated with a higher rate of complicated course (T1: 27% versus 13%, p=0.04; T2: 30% versus 13%, p=0.04), but not with HAI or 28-day mortality (Figure, Supplemental Digital Content 3, http://links.lww.com/SHK/A996). Immunoparalysis defined using mHLA-DR was not associated with clinical outcomes.
Table 1:
Patient Characteristics
| Variablea | Controls | Sepsis | P-value |
|---|---|---|---|
| N | 18 | 161 | |
| Age, years | 9.8 (2.1–13.4) | 8.3 (3.6–13.7) | 0.96 |
| Sex, male | 9 (50) | 83 (52) | 0.99 |
| Race | 0.28 | ||
| White | 13 (72) | 82 (51) | |
| Black | 3 (17) | 44 (27) | |
| Other or not reported | 2 (11) | 35 (22) | |
| Previously healthy | 2 (11) | 20 (12) | 0.99 |
| Cancer or HSCT | 2 (11) | 26 (16) | 0.74 |
| PRISM-III score | 1 (0–3) | 11 (6–17) | <0.001 |
| PIM-2 risk of mortality | 0.2 (0.1–0.8) | 3.3 (1.1–4.7) | <0.001 |
| PELOD score, admission | 0 (0–10) | 11 (10–12) | <0.001 |
| Lactate (mmol/L), max day 1–2 | 1.3 (1.0 −2.6) | 2.9 (1.9–4.8) | 0.02 |
| No. of organ dysfunctions at study enrollment | 0 (0–0) | 2 (2–3) | <0.001 |
| Reason for PICU admission | <0.001 | ||
| Sepsis | 161 (100) | ||
| Neurosurgery | 15 (83) | ||
| Intracranial hemorrhage | 2 (11) | ||
| Deep vein thrombosis | 1 (6) | ||
| Site of infection | <0.001 | ||
| None | 19 (100) | ||
| Bacteremia, primary | 32 (20) | ||
| Respiratory | 61 (38) | ||
| Abdominal | 14 (9) | ||
| Genitourinary | 14 (9) | ||
| Central nervous system | 3 (2) | ||
| Skin or soft tissue | 4 (2) | ||
| Other | 7 (4) | ||
| Unknown | 26 (16) | ||
| Type of infection | <0.001 | ||
| None | 19 (100) | ||
| Bacterial | 69 (43) | ||
| Viral | 44 (29)d | ||
| Unknown | 47 (28) | ||
| Hospital-acquired infectionb | 0 | 14 (9) | 0.37 |
| Organ dysfunction, day 14 | 0 | 59 (37) | 0.001 |
| Complicated coursec | 0 | 30 (19) | 0.046 |
| Mortality, day 28 | 0 | 10 (6) | 0.60 |
HSCT, hematopoietic stem cell transplant; PRISM, pediatric risk of mortality; PIM, pediatric index of mortality; PELOD, pediatric logistic organ dysfunction; PICU, pediatric intensive care unit
Data presented as median (interquartile range) or n (%)
Hospital-acquired infection within 28 days after index sepsis episode
Complicated course defined as ≥2 organ system dysfunctions by day 7 or death by day 28 from study enrollment
Viral infection was defined as a viral pathogen identified as part of microbiologic diagnostic testing in the absence of a primary bacterial pathogen
Figure 1: Immunoparalysis By Group.
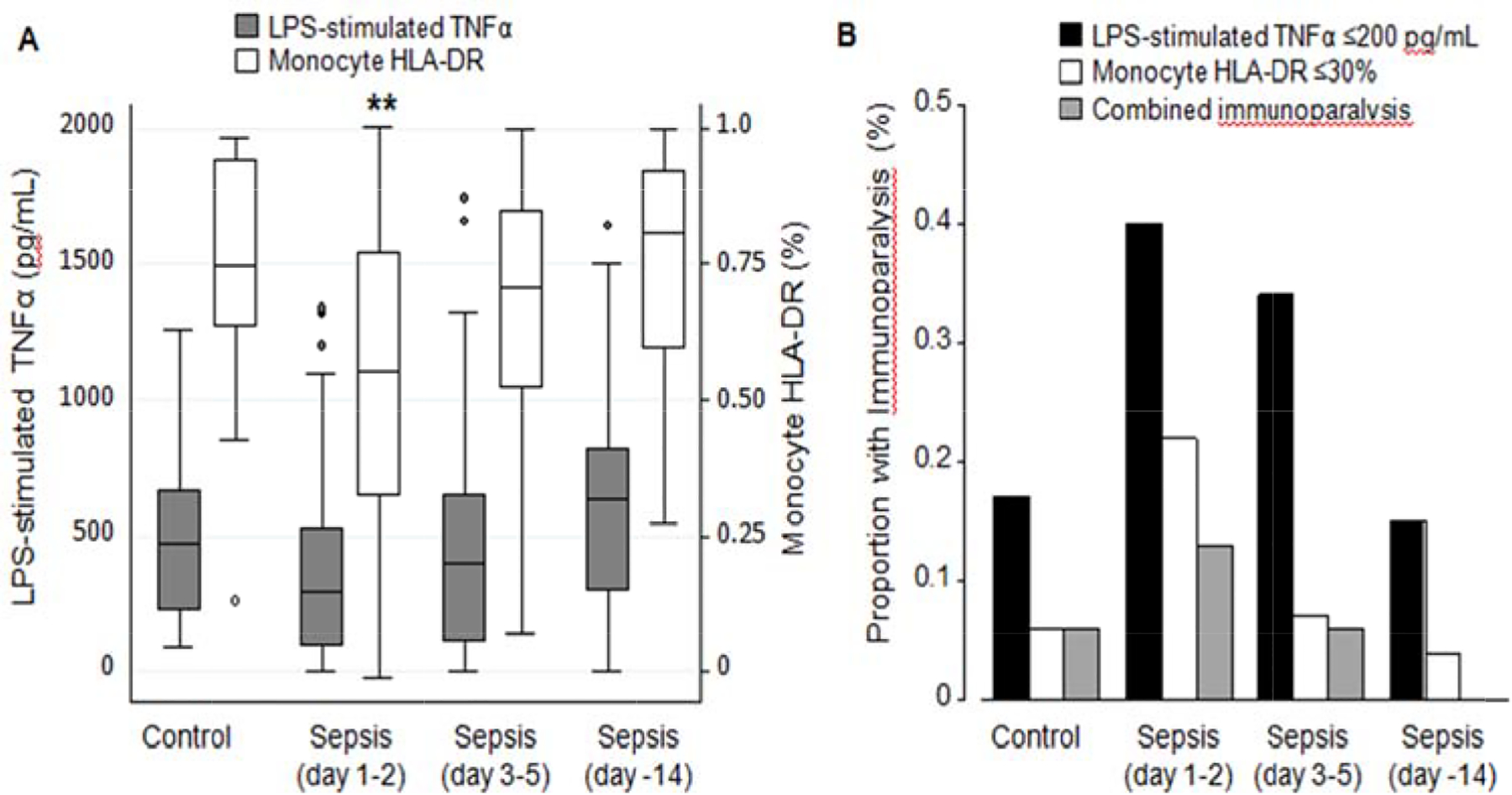
(A) Ex vivo lipopolysaccharide (LPS)-stimulated whole blood tumor necrosis factor (TNF)-α levels and monocyte HLA-DR percentage in non-septic controls and sepsis patients by study day. Data are presented in box plot analysis with the central line indicating the median and boxes indicating the interquartile range. (B) The proportion of non-septic controls and sepsis patients at each timepoint with immunoparalysis as defined by ex vivo lipopolysaccharide-stimulated whole blood TNF-α ≤200 pg/mL, monocyte HLA-DR ≤30%, or both. Data are presented as proportion of the total group. *p<0.05 **p<0.01 compared to controls after Bonferroni correction for multiple comparisons
Mitochondrial Respiration and Immune Function:
The correlation of PBMC mitochondrial respiration with concurrently measured immune function at each timepoint is shown in Table 2. In control patients, mitochondrial respiration was not associated with either LPS-stimulated TNF-α or mHLA-DR. In sepsis patients, ETSmax and SRC were positively correlated with LPS-stimulated TNF-α at T1, such that patients with low ETSmax and SRC exhibited low LPS-stimulated TNF-α. At T2, all measures of mitochondrial respiration trended towards association with LPS-stimulated TNF-α, though none remained significant after correction for multiple comparisons. Mitochondrial respiration was not associated with mHLA-DR in controls or sepsis patients at any timepoint.
Table 2:
Correlation of Mitochondrial Respiration with Concurrent Immune Function Measured at the Same Timepoint
| Mitochondrial Respiration | LPS-stimulated TNFαa | Monocyte HLA-DRa | ||||
|---|---|---|---|---|---|---|
| n | rho | P-value | n | rho | P-value | |
| Control | ||||||
| Routine | 18 | 0.09 | 0.74 | 18 | −0.08 | 0.75 |
| ATP-linked | 18 | −0.01 | 0.98 | 18 | −0.09 | 0.72 |
| LEAK | 18 | 0.12 | 0.64 | 18 | 0.03 | 0.91 |
| ETSmax | 18 | −0.15 | 0.56 | 18 | 0.10 | 0.70 |
| SRC | 18 | −0.15 | 0.56 | 18 | 0.19 | 0.45 |
| Sepsis Day 1–2 (T1) | ||||||
| Routine | 161 | 0.12 | 0.13 | 125 | −0.08 | 0.36 |
| ATP-linked | 161 | 0.13 | 0.12 | 125 | −0.06 | 0.47 |
| LEAK | 161 | 0.08 | 0.33 | 125 | −0.08 | 0.36 |
| ETSmax | 161 | 0.26 | 0.001b | 125 | 0.01 | 0.92 |
| SRC | 161 | 0.27 | <0.001b | 125 | 0.01 | 0.89 |
| Sepsis Day 3–5 (T2) | ||||||
| Routine | 120 | 0.19 | 0.03 | 88 | 0.12 | 0.27 |
| ATP-linked | 120 | 0.19 | 0.04 | 88 | 0.08 | 0.46 |
| LEAK | 120 | 0.19 | 0.04 | 88 | 0.16 | 0.13 |
| ETSmax | 120 | 0.17 | 0.06 | 88 | 0.18 | 0.10 |
| SRC | 120 | 0.13 | 0.15 | 88 | 0.21 | 0.06 |
| Sepsis Day 8–14 (T3) | ||||||
| Routine | 80 | 0.21 | 0.06 | 55 | −0.21 | 0.12 |
| ATP-linked | 80 | 0.21 | 0.06 | 55 | −0.20 | 0.15 |
| LEAK | 80 | 0.17 | 0.13 | 55 | 0.17 | 0.49 |
| ETSmax | 80 | 0.13 | 0.24 | 55 | −0.21 | 0.13 |
| SRC | 80 | 0.07 | 0.56 | 55 | −0.16 | 0.25 |
ATP, adenine triphosphate; LEAK, proton leak across the inner mitochondrial membrane after inhibition of ATP synthase; ETSmax, maximal uncoupled respiration through the electron transport system after addition of chemical uncoupler; SRC, spare respiratory capacity
Immune function measured at the same timepoint as mitochondrial respiration
P-value remained significant at ≤0.01 level after correction for multiple comparisons (0.05/5 tests per study day)
Mitochondrial respiration was generally lower in patients with concurrent evidence of immunoparalysis measured at the same timepoint, using either LPS-stimulated TNF-α or mHLA-DR (Figure 2). Mitochondrial respiration measured at T1 and T2 was also lower in patients who exhibited immunoparalysis at subsequent timepoints (i.e., T2 and T3, respectively), defined using LPS-stimulated TNF-α (Figure 3). The small number of patients with immunoparalysis defined using mHLA-DR at T2 and T3 precluded meaningful analyses at these later timepoints.
Figure 2: PBMC Mitochondrial Respiration in Sepsis Patients According to Presence of Concurrently Measured Immunoparalysis.
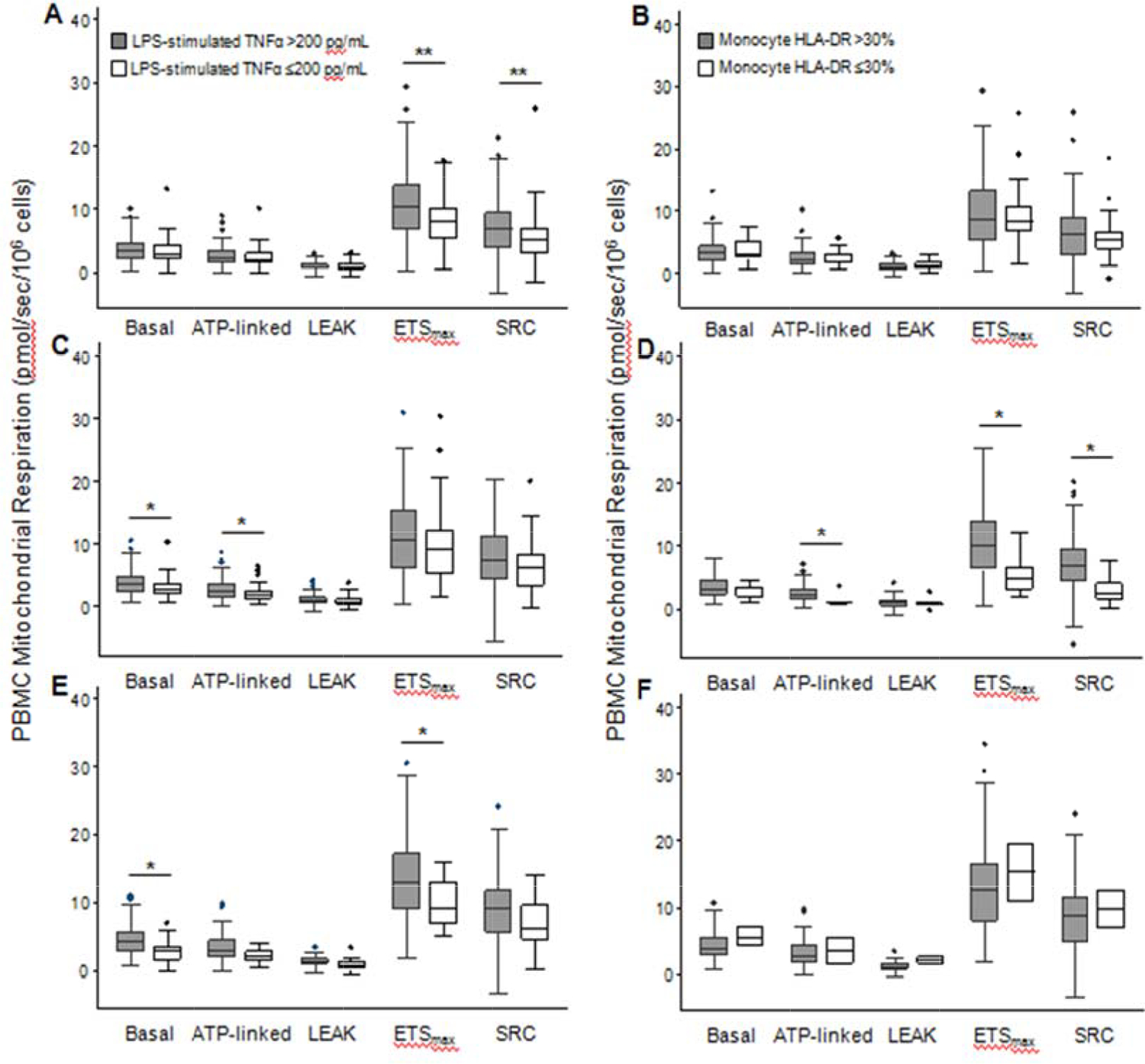
Basal, ATP-linked, LEAK, ETSmax, and SRC mitochondrial respiration in peripheral blood mononuclear cells (PBMCs) from sepsis patients with immunoparalysis, as defined by ex vivo lipopolysaccharide-stimulated whole blood TNF-α ≤200 pg/mL (A, C, and E) or monocyte HLA-DR ≤30% (B, D, and F) measured at the same timepoint as respiration. Respiration by immunoparalytic phenotype is shown for study day 1–2 (A, B), 3–5 (C, D), and 8–14 (E, F). Data are presented in box plot analysis with the central line indicating the median and boxes indicating the interquartile range. *p<0.05 ** p<0.01 after Bonferroni correction for multiple comparisons
Figure 3: PBMC Mitochondrial Respiration in Sepsis Patients According to Presence of Immunoparalysis Measured at Subsequent Timepoints.
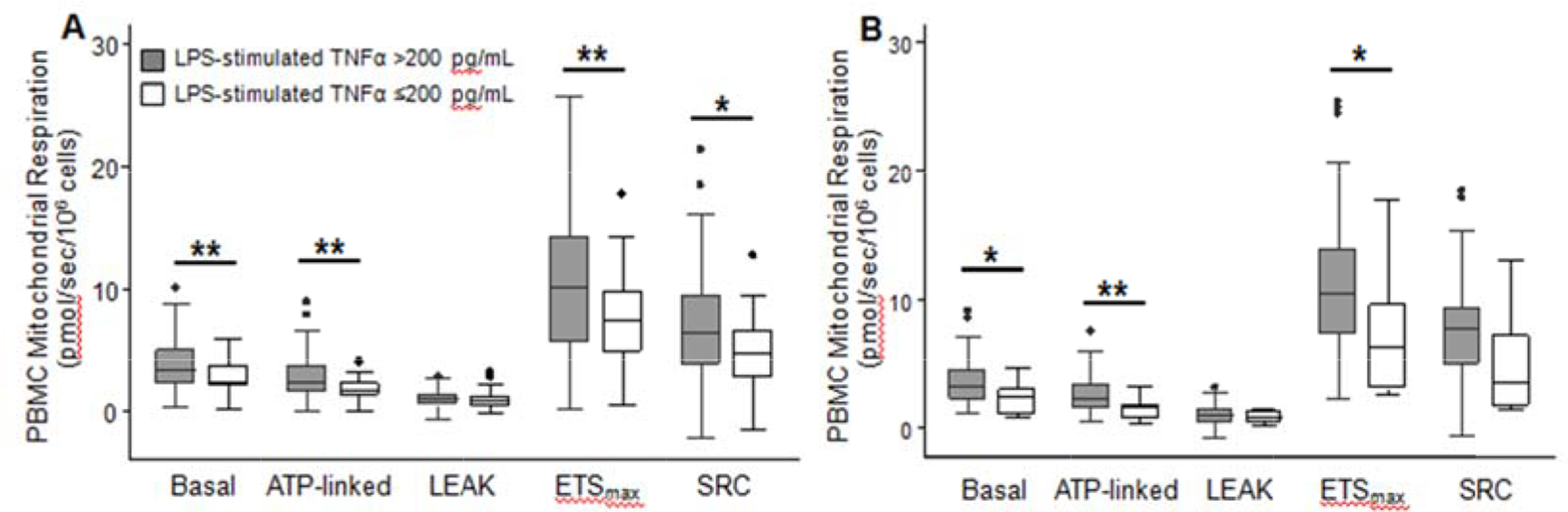
Basal, ATP-linked, LEAK, ETSmax, and SRC mitochondrial respiration in peripheral blood mononuclear cells (PBMCs) from sepsis patients with immunoparalysis, as defined by ex vivo lipopolysaccharide-stimulated whole blood TNF-α ≤200 pg/mL, measured at subsequent timepoints. Respiration measured on study day 1–2 was lower in sepsis patients with immunoparalysis on day 3–5 (B), and respiration measured on day 3–5 was lower in sepsis patients with immunoparalysis on day 8–14 (B). *p<0.05 **p<0.01 after Bonferroni correction for multiple comparisons
The AUROC for mitochondrial SRC measured at T1 to discriminate between those with versus without immunoparalysis, defined using LPS-stimulated TNF-α, was 0.63 (95% CI 0.54, 0.72) at T1, 0.63 (95% CI 0.53, 0.73) at T2, and 0.68 (95% CI 0.52, 0.84) at T3. The sensitivity and specificity of mitochondrial SRC at T1, dichotomized at different percentiles of control values, to identify immunoparalysis are shown in Table 3. SRC cut-points below the 25th and 50th percentile of control values exhibited high sensitivity to identify immunoparalysis, but all cut-points exhibited low specificity (i.e., high rate of false-positives). These findings suggest that, while most patients with immunoparalysis exhibited low mitochondrial SRC, this metabolic finding was not specific to the immunoparalysis phenotype.
Table 3:
Cut-points for Mitochondrial Spare Respiratory Capacity to Differentiate Sepsis Patients with Immunoparalysis
| Mitochondrial Respiration Measured on Study Day 1–2 (T1) | Immunoparalysis on Study Day 1–2 (T1) | Immunoparalysis on Study Day 3–5 (T2) | Immunoparalysis on Study Day 8–14 (T3) | |||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | Sensitivity | Specificity | |
| SRC ≤10th percentile of control valuesa | 0.61 | 0.60 | 0.63 | 0.58 | 0.64 | 0.52 |
| SRC ≤25th percentile of control valuesb | 0.83 | 0.46 | 0.85 | 0.42 | 0.91 | 0.38 |
| SRC ≤50th percentile of control valuesc | 0.95 | 0.28 | 0.95 | 0.28 | 1.0 | 0.22 |
SRC, spare respiratory capacity
SRC ≤5.8 pmol/sec/106 cells
SRC ≤7.5 pmol/sec/106 cells
SRC ≤9.5 pmol/sec/106 cells
Cytokines:
Plasma levels of IL-8, IL-10, and MCP-1, but not TNF-α, were higher in sepsis compared to controls (Figure, Supplemental Digital Content 4, http://links.lww.com/SHK/A997). In non-septic controls, mitochondrial respiration and immune function were not correlated with plasma cytokines (Table 4). In sepsis patients, mitochondrial SRC and LPS-stimulated TNF-α demonstrated a weak inverse correlation with plasma cytokines at T1 and T2, and mHLA-DR demonstrated a similar pattern at T2 (Table 4). Plasma cytokines were also higher in the subset of sepsis patients with LPS-stimulated TNF-α ≤200 pg/mL or low mitochondrial SRC at T1 (Figure 4).
Table 4:
Correlation of Immune Function and Mitochondrial Respiration with Plasma Cytokines
| Immune Function or Mitochondrial Respiration | IL-8 | IL-10 | TNF-α | INF-γ | MCP-1 |
|---|---|---|---|---|---|
| Control (n = 18) | |||||
| LPS-stim TNF-α | −0.26 | −0.13 | 0.10 | 0.34 | 0.15 |
| mHLA-DR | −0.48 | −0.32 | 0.02 | −0.08 | −0.07 |
| SRCc | −0.01 | −0.16 | 0.14 | −0.03 | 0.08 |
| Sepsis Day 1–2 (T1, n = 137) | |||||
| LPS-stim TNF-α | −0.31b | −0.27a | −0.26a | −0.10 | −0.34b |
| mHLA-DR | −0.07 | 0.04 | 0.01 | 0.06 | −0.12 |
| SRCc | −0.28a | −0.18 | −0.12 | −0.20 | −0.23a |
| Sepsis Day 3–5 (T2, n = 104) | |||||
| LPS-stim TNF-α | −0.28a | −0.23 | −0.17 | −0.04 | −0.28a |
| mHLA-DR | −0.39b | −0.25 | 0.32a | 0.26 | 0.08 |
| SRCc | −0.31a | −0.05 | −0.09 | −0.01 | −0.06 |
| Sepsis Day 8–14 (T3, n = 70) | |||||
| LPS-stim TNF-α | −0.08 | −0.03 | −0.05 | −0.08 | −0.19 |
| mHLA-DR | −0.31 | −0.10 | 0.25 | 0.21 | −0.04 |
| SRCc | −0.16 | −0.18 | −0.01 | −012 | 0.04 |
LPS-stim TNF-α, ex vivo LPS-stimulated whole blood TNF-α; mHLA-DR, monocyte HLA-DR; SRC, spare respiratory capacity
Statistical significance remained at p<0.01 after correction for multiple comparisons
Statistical significance remained at p<0.001 after correction for multiple comparisons
SRC demonstrated the strongest correlation with plasma cytokines among all mitochondrial respiration parameters and therefore only these data are shown
Figure 4: Plasma Cytokines in Sepsis Patients With versus Without Immunoparalysis or Low Mitochondrial Respiration.
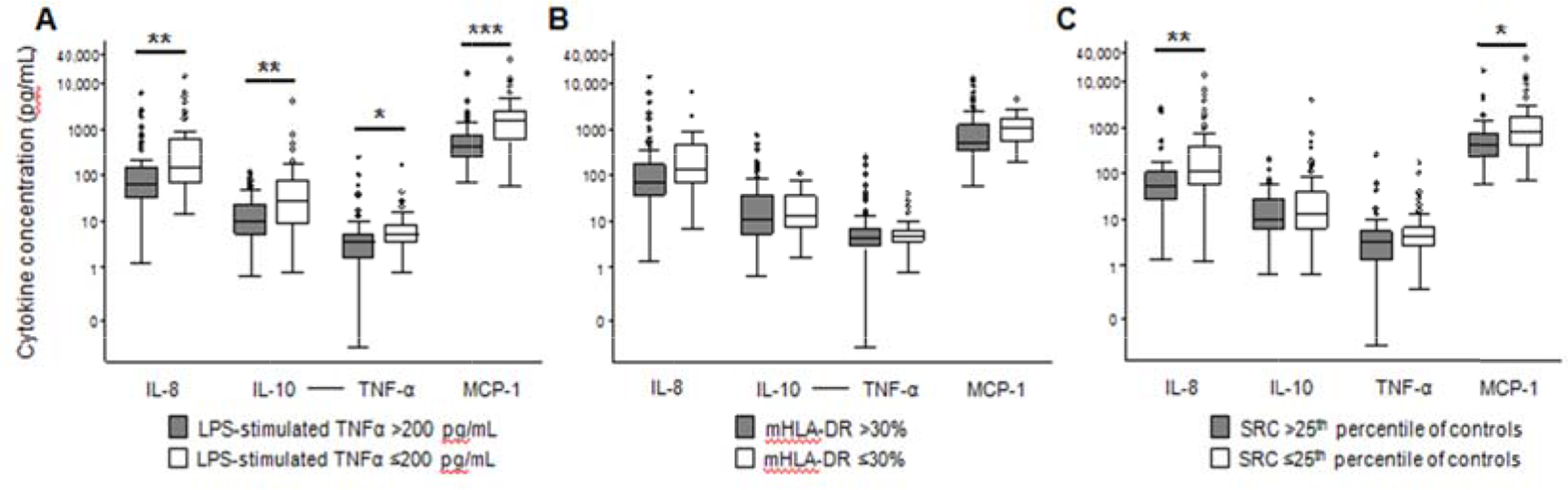
(A) Interleukin (IL)-8, IL-10, tumor necrosis factor (TNF)-α, and monocyte chemoattractant protein (MCP)-1 were higher in sepsis patients with ex vivo lipopolysaccharide (LPS)-stimulated whole blood TNF-α ≤200 pg/mL (white bar) versus >200 pg/mL (grey bar) at T1.
(B) None of the cytokines were different between sepsis patients with monocyte HLA-DR ≤30% versus >30% at T1. (C) IL-8, IL-10, and MCP-1 were higher in sepsis patients with mitochondrial spare respiratory capacity (SRC) <25th percentile (white bar) versus ≥25th percentile (grey bar) of control values at T1. Data are presented using a logarithmic scale in box plot analysis with the central line indicating the median and boxes indicating the interquartile range. *p<0.05 **p<0.01 ***p<0.001 after Bonferroni correction for multiple comparisons
Sensitivity Analysis Excluding Corticosteroid Exposure:
There was no difference in the proportion of sepsis patients with versus without corticosteroid treatment who had immunoparalysis at T1 defined by LPS-stimulated TNF-α (42% with corticosteroids versus 38% without corticosteroids, p=0.63) or mHLA-DR (21% with corticosteroids versus 22% without corticosteroids, p=0.56). Similar to the overall analysis, ETSmax and SRC were positively correlated with LPS-stimulated TNF-α at T1 in the subgroup not treated with corticosteroids (Table, Supplemental Digital Content 5, http://links.lww.com/SHK/A998). Mitochondrial respiration also followed a similar pattern in non-corticosteroid patients as for the primary analysis with generally lower values in these with concurrent (Figure, Supplemental Digital Content 6, http://links.lww.com/SHK/A999) and subsequent (data not shown) evidence of immunoparalysis measured at the same timepoint, though power to detect statistical significance was reduced for this sensitivity analysis. Finally, plasma cytokines remained higher in the subset of sepsis patients not treated with corticosteroids with LPS-stimulated TNF-α ≤200 pg/mL or low mitochondrial SRC at T1 (Figure, Supplemental Digital Content 7, http://links.lww.com/SHK/A1000).
DISCUSSION
Among children with septic shock and other sepsis-associated organ dysfunction, immunoparalysis was common and, as has been previously shown, was associated with a composite endpoint of organ dysfunction and death. Our novel finding was that mitochondrial dysfunction, denoted by low PBMC respiration, was associated with both concurrent and later immunoparalysis in children with sepsis. However, decreased mitochondrial respiration was not specific for the immunoparalysis phenotype; indeed, many children with sepsis without immunoparalysis also exhibited low PBMC mitochondrial respiration. Although systemic inflammation was evident in sepsis patients irrespective of immune paralysis or degree of PBMC mitochondrial respiration on day 1–2, the subset with immunoparalysis or low mitochondrial respiration exhibited the highest levels of plasma inflammatory cytokines.
Evidence supports that the pathophysiology of sepsis involves a “fundamental reorganization of immune and metabolic cell processes, and that measures of inflammation and suppression are a reflection of this acute cellular reprogramming” (2). We, and others, have previously shown that mitochondrial respiration is often decreased in immune cells from patients with sepsis early in the disease course (15–18, 29). This finding is not necessarily indicative of dysfunction. A shift from oxidative phosphorylation in quiescent and naïve mononuclear cells to glycolysis is believed to be an important part of the initial activation of innate immune function (7, 8, 34). However, activation of Toll-like receptor (TLR)-4 by exposure to LPS can also render leukocytes immune tolerant, which has been characterized by a generalized down-regulation of both glycolysis and oxidative phosphorylation, along with changes in fatty acid metabolism that can impair phagocytic activity and reduce cytokine production (11, 35, 36).
Our study is the first to investigate the association of mitochondrial respiration, immune function, and systemic inflammation in children with sepsis. We observed the cooccurrence of depressed mitochondrial respiration in PBMCs with ex vivo tolerance to TNF-α production and elevated inflammatory cytokines early in sepsis, a pattern that has been reported previously in shock and sepsis models (13, 37). Although our findings are not able to delineate a temporal relationship between metabolic and immunologic changes, we speculate that the decrease in mitochondrial respiration evident on day 1–2 in children with sepsis (as previously described (18)) is generally indicative of an acute immune response early in sepsis given the inverse correlation with SRC and the common pro-inflammatory cytokines, IL-8 and MCP-1. Low PBMC respiration is consistent with the transition of naïve monocytes and lymphocytes to an effector phenotype that is accompanied by a metabolic switch from mitochondrial oxidative phosphorylation to glycolysis (13, 36). However, because low respiration was sensitive for either having or progressing to immunoparalysis, the down-regulation of mitochondrial metabolism likely also included a subset of patients with an early, potentially unfavorable, level of induced immune tolerance. Conversely, few patients with PBMC mitochondrial respiration preserved at >25–50th percentile of control values on day 1–2 developed an immune paralysis phenotype, suggesting that while mitochondrial dysfunction may contribute to or herald immunoparalysis in pediatric sepsis, it is not sufficient to do so on its own.
Understanding of the role of metabolism in the immunologic response to infection holds promise for enhancing precision medicine in sepsis (3, 38). To date, efforts to identify distinct biologic phenotypes in sepsis have focused largely on immuno-inflammatory, endothelial, and coagulation profiles (33, 39, 40). However, we previously demonstrated that children with septic shock characterized by repression of genes related to adaptive immunity and glucocorticoid receptor signaling and a high rate of mortality also had suppression of mitochondrial genes (41). Another study found that changes in the metabolome, particularly in mitochondrial fatty acid oxidation, did not mirror the clinical progression from uncomplicated infection to septic shock, but did distinguish sepsis non-survivors from survivors (12). This suggests that metabolic changes in sepsis can provide additional insights into the pathobiology of sepsis to further refine phenotypes and identify novel mechanisms for targeted therapies (11).
Our data highlight the importance of integrating multiple pathways in the complex syndrome of sepsis, as the decrease in mitochondrial respiration observed on study day 1–2 was associated with both immune paralysis and increased systemic inflammation. The presence of immune paralysis, as measured by LPS-stimulated TNF-α, with increased circulating levels of plasma inflammatory cytokines is somewhat paradoxical, but has been reported previously (30). Interestingly, research into neurodegenerative disorders suggests that mitochondrial dysfunction itself can stimulate inflammatory pathways (42). This suggests an interesting, but as yet untested, hypothesis as to whether mitochondrial dysfunction in immune cells can simultaneously account for diminished immune responsiveness to external ligands while still propagating a systemic inflammatory response (43).
There are several limitations to this study. First, heterogeneity of patients in comorbid conditions, type and site of infection, and other factors may have blurred together several different immunometabolic patterns. We do note, however, that treatment with corticosteroids did not substantially confound our results, as a sensitivity analysis excluding patients who receive corticosteroids closely paralleled the primary analysis. Second, only two measures of immune function were included. Although both LPS-stimulated TNF-α and mHLA-DR have been used to characterize the immune paralysis phenotype in sepsis, these measures largely focus on innate, rather than adaptive, immunity. Third, there is no acceptable definition of mitochondrial “dysfunction”. Because mitochondrial respiration is linked to ATP production and energy availability, a decrease in this measure is often presumed to indicate dysfunction. However, mitochondria are multifaceted organelles with numerous functions, which are not completely represented by respiration alone. Indeed, several studies have demonstrated that altered redox state and fatty acid oxidation are more indicative of mitochondrial dysfunction than respiration (12, 14). Moreover, because we studied intact cells and normalized mitochondrial respiration to PBMC number, we are not able to differentiate between abnormalities within the mitochondrial electron transport system itself versus changes in mitochondrial content as a cause of the observed decrease in respiration. Fourth, the timepoints at which study measurements were performed were established a priori and may not have captured the full evolution of immunometabolic changes in sepsis. In addition, longitudinal data was missing from 25% and 50% of sepsis patients at T2 and T3, respectively. Although missing data over time do not affect the internal validity of comparisons of study measures performed at the same timepoint (since patients either had all or none of the study measures at any one timepoint), loss of study measures at later timepoints due largely to drop-out of patients who clinically improved led to a “sicker” patient sample at T2 and T3. Thus, missing data not only reduce statistical power, but could introduce a selection bias at later timepoints. However, bias in comparing study measures at T1 to later timepoints is expected to be toward the null because more “less sick” patients at T1 were compared to “more sick” patients at T2 and T3. Last, limitations in blood volume collection precluded accounting for variation in PBMC composition between patients or over time despite known variability in the metabolism of immune cell subsets.
CONCLUSIONS
Mitochondrial respiration within circulating PBMCs was lower in children who had sepsis with versus without immunoparalysis, though the decrease in mitochondrial respiration was not specific for immunoparalysis. While systemic inflammation was evident in sepsis patients irrespective of immune paralysis or degree of PBMC mitochondrial respiration on day 1–2, the subset with immunoparalysis or low mitochondrial respiration exhibited the highest levels of plasma inflammatory cytokines. The temporal association and mechanisms linking metabolic changes to immune function in pediatric sepsis warrant further investigation to assist with improved phenotyping and identify novel therapies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Fang Chen and Natalka Kengle from the Center for Immunotherapies at the University of Pennsylvania for their contributions to this study.
Financial Support: Financial support was provided by NICHD K12HD047349 (SLW), NIGMS K23GM110496 (SLW), and the Center for Mitochondrial and Epigenomic Medicine (though grants awarded to DCW including NIH NS021328, MH108592, OD010944 and US Department of Defense grant W81XWH-16-1-0401) and Department of Anesthesiology and Critical Care at the Children’s Hospital of Philadelphia.
Footnotes
This study was performed at The Children’s Hospital of Philadelphia.
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. : The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801–10, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Poll T, van de Veerdonk FL, Scicluna BP and Netea MG: The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol 17(7):407–420, 2017. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G and Payen D: Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 13(12):862–74, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD and Carcillo JA: Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 37(3):525–32, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzoli TF, Troster EJ, Ferranti JF and Sales MM: Prolonged suppression of monocytic human leukocyte antigen-DR expression correlates with mortality in pediatric septic patients in a pediatric tertiary Intensive Care Unit. J Crit Care 33:84–9, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohe J, Vanhems P and Monneret G: Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med 36(11):1859–66, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Pearce EL and Pearce EJ: Metabolic pathways in immune cell activation and quiescence. Immunity 38(4):633–43, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Windt GJ and Pearce EL: Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev 249(1):27–42, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ and Pearce EL: Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36(1):68–78, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. : Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38(2):225–36, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR, Wagenaars JA, Cremer OL, et al. : Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 17(4):406–13, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, Chen B, Carin L, Suarez A, Mohney RP, et al. : An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 5(195):195ra95, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao J, Zhang J, Ling Y, McCall CE and Liu TF: Mitochondrial Sirtuin 4 Resolves Immune Tolerance in Monocytes by Rebalancing Glycolysis and Glucose Oxidation Homeostasis. Front Immunol 9:419, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T, Lin X, Xu J, Tan R, Ji J and Shen P: Redox imbalance provokes deactivation of macrophages in sepsis. Proteomics Clin Appl 3(8):1000–9, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Belikova I, Lukaszewicz AC, Faivre V, Damoisel C, Singer M and Payen D: Oxygen consumption of human peripheral blood mononuclear cells in severe human sepsis. Crit Care Med 35(12):2702–8, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Japiassu AM, Santiago AP, d’Avila JC, Garcia-Souza LF, Galina A, Castro Faria-Neto HC, Bozza FA and Oliveira MF: Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5’-triphosphate synthase activity. Crit Care Med 39(5):1056–63, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Weiss SL, Selak MA, Tuluc F, Perales Villarroel J, Nadkarni VM, Deutschman CS and Becker LB: Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med 16(1):e4–e12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss SL, Zhang D, Bush J, Graham K, Starr J, Henrickson S, Kilbaugh T, Deutschman CS, Murdock D, McGowan FX, et al. : Persistent mitochondrial dysfunction linked to prolonged organ dysfunction in pediatric sepsis. Crit Care Med 47(10):1433–1441, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bo L, Wang F, Zhu J, Li J and Deng X: Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: a meta-analysis. Crit Care 15(1):R58, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Kong BB, Yang WP, Zhao X and Zhang R: Immunomodulatory intervention with Gamma interferon in mice with sepsis. Life Sci 185:85–94, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, Monneret G, Rimmele T, Blood T, Morre M, et al. : Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight 3(5), 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, Martin GS, Coopersmith CM, Brakenridge S, Mayr FB, et al. : Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Antiprogrammed Cell Death-Ligand 1 Antibody (BMS-936559). Crit Care Med 47(5):632–642, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, et al. : Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 180(7):640–8, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein B, Giroir B and Randolph A: International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 6(1):2–8, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Pollack MM, Patel KM and Ruttimann UE: PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 24(5):743–52, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, et al. : Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet 362(9379):192–7, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Wong HR, Weiss SL, Giuliano JS Jr., Wainwright MS, Cvijanovich NZ, Thomas NJ, Allen GL, Anas N, Bigham MT, Hall M, et al. : The temporal version of the pediatric sepsis biomarker risk model. PLoS One 9(3):e92121, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand MD and Nicholls DG: Assessing mitochondrial dysfunction in cells. Biochem J 435(2):297–312, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garrabou G, Moren C, Lopez S, Tobias E, Cardellach F, Miro O and Casademont J: The effects of sepsis on mitochondria. J Infect Dis 205(3):392–400, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Hall MW, Geyer SM, Guo CY, Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, Shay DK, Nateri J, Greathouse K, Sullivan R, et al. : Innate immune function and mortality in critically ill children with influenza: a multicenter study. Crit Care Med 41(1):224–36, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davila S, Halstead ES, Hall MW, Doctor A, Telford R, Holubkov R, Carcillo JA, Storch GA and Eunice Kennedy Shriver Collaborative Pediatric Critical Care Research Network I: Viral DNAemia and Immune Suppression in Pediatric Sepsis. Pediatr Crit Care Med 19(1):e14–e22, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drewry AM, Ablordeppey EA, Murray ET, Dalton CM, Fuller BM, Kollef MH and Hotchkiss RS: Monocyte Function and Clinical Outcomes in Febrile and Afebrile Patients With Severe Sepsis. Shock 50(4):381–387, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carcillo JA, Halstead ES, Hall MW, Nguyen TC, Reeder R, Aneja R, Shakoory B, Simon D, Eunice Kennedy Shriver National Institute of Child H and Human Development Collaborative Pediatric Critical Care Research Network I: Three Hypothetical Inflammation Pathobiology Phenotypes and Pediatric Sepsis-Induced Multiple Organ Failure Outcome. Pediatr Crit Care Med 18(6):513–523, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krauss S, Brand MD and Buttgereit F: Signaling takes a breath--new quantitative perspectives on bioenergetics and signal transduction. Immunity 15(4):497–502, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Lachmandas E, Boutens L, Ratter JM, Hijmans A, Hooiveld GJ, Joosten LA, Rodenburg RJ, Fransen JA, Houtkooper RH, van Crevel R, et al. : Microbial stimulation of different Toll-like receptor signalling pathways induces diverse metabolic programmes in human monocytes. Nat Microbiol 2:16246, 2016. [DOI] [PubMed] [Google Scholar]
- 36.Liu TF, Vachharajani VT, Yoza BK and McCall CE: NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem 287(31):25758–69, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villarroel JP, Guan Y, Werlin E, Selak MA, Becker LB and Sims CA: Hemorrhagic shock and resuscitation are associated with peripheral blood mononuclear cell mitochondrial dysfunction and immunosuppression. J Trauma Acute Care Surg 75(1):24–31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters van Ton AM, Kox M, Abdo WF and Pickkers P: Precision Immunotherapy for Sepsis. Front Immunol 9:1926, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, et al. : Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong HR, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Willson DF, Freishtat RJ, Anas N, Meyer K, Checchia PA, et al. : Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med 7:34, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss SL, Cvijanovich NZ, Allen GL, Thomas NJ, Freishtat RJ, Anas N, Meyer K, Checchia PA, Shanley TP, Bigham MT, et al. : Differential expression of the nuclear-encoded mitochondrial transcriptome in pediatric septic shock. Crit Care 18(6):623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, et al. : Parkin and PINK1 mitigate STING-induced inflammation. Nature 561(7722):258–262, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arulkumaran N, Deutschman CS, Pinsky MR, Zuckerbraun B, Schumacker PT, Gomez H, Gomez A, Murray P, Kellum JA and Workgroup AX: Mitochondrial Function in Sepsis. Shock 45(3):271–81, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


