Abstract
Breast cancer is the most frequent malignancy diagnosed in premenopausal women. In this age group, breast tumors tend to be diagnosed at more advanced stages and to harbor more aggressive biological features. In addition, specific age-related issues including genetic counseling, fertility preservation, impact on social and couple relationships, working life, and management of long-term side effects should be considered highly relevant when managing early breast cancer in premenopausal women. Therefore, the care of these patients is particularly complex and a multidisciplinary approach is mandatory. The present review summarizes the current state of art in the adjuvant systemic treatment of premenopausal women with early breast cancer focusing on the optimal chemotherapy, endocrine therapy, and targeted therapy approaches in this specific patient population.
Keywords: Breast cancer, premenopausal, adjuvant, BRCA, survivorship
Introduction
Breast cancer is the most common cancer in women worldwide including among premenopausal patients.1,2 The proportion of breast cancer cases diagnosed at a young age is higher in less developed countries;3 this epidemiological difference may be related to differential genetic and environmental factors as well as reproductive behavior.4 As compared with tumors arising in older patients, breast cancer cases in premenopausal women tend to be diagnosed at more advanced stages and to harbor more aggressive biological features, with a higher incidence of the most aggressive subtypes (ie, triple-negative and HER2-positive).5,6
Young age at diagnosis has been historically considered a poor prognostic factor.7 However, more recent data have shown that the prognostic value of age may differ according to breast cancer subtype; specifically, young age at diagnosis appears to remain an independent poor prognostic factor only in luminal-like breast cancers.8-11 This divergence may reflect disparities in tumor biology, inappropriate treatment (ie, the absence of adequate endocrine therapy including the use of ovarian suppression), and lower therapeutic adherence to systemic treatments due to possible side effects.12,13
Specific age-related issues should be considered highly relevant when managing early breast cancer in premenopausal women. Among them, genetic counseling, fertility preservation, impact on social and couple relationships, working life, and management of long-term side effects are crucial factors to be taken into account at the time of treatment planning.14-20 Therefore, the care of premenopausal women with early breast cancer is particularly complex and a multidisciplinary approach is mandatory.
The present review summarizes the current state of art in the adjuvant systemic treatment of premenopausal women with early breast cancer focusing on the optimal chemotherapy, endocrine therapy, and targeted therapy approaches in this specific patient population.
Chemotherapy
Young age per se is not a criterion to use more aggressive treatments, and the indication to and choice of adjuvant chemotherapy should be made according to both individual risk of recurrence and patient’s preferences as it is done in postmenopausal women.15
Table 1 summarizes the main characteristics of the most important trials investigating modern chemotherapy regimens, focusing specifically on the results observed in premenopausal patients.21-24
Table 1.
Characteristics of the main studies investigating different adjuvant chemotherapy approaches focusing on the results available in premenopausal patients.
| Study | Patients, n | Treatment arm | Premenopausal patients, n | Results in premenopausal patients |
|---|---|---|---|---|
| EBCTCG21 | 44 251 | A vs A + T | 11 857a | Breast cancer mortality: RR: 0.91 (SE: 0.05) |
| MIG1-GIM2 pooled analysis22 | 1549 | FEC or (F)EC→P DD vs standard-interval |
1549 | OS: HR: 0.71 (95% CI: 0.54-0.95) |
| TC vs A + T Metanalysis23 | 12 741 | TC vs A + T | 1251 | DFS: HR: 0.78 (95% CI: 0.56-1.09) |
| CREATE-X24 | 910 | Adjuvant capecitabine vs no additional chemotherapy | 532b | DFS: HR: 0.72 (95% CI: 0.50-1.03) |
Abbreviations: A + T, anthracycline- and taxane-based chemotherapy; CI, confidence interval; DD, dose-dense; DFS, disease-free survival; FEC, fluoruracil, epirubicin, cyclophosphamide; HR, hazard ratio; OS, overall survival; P, paclitaxel; RR, rate ratio; SE, standard error; TC, docetaxel and cyclophosphamide; CREATE-X, Capecitabine for Residual Cancer as Adjuvant Therapy.
Patients with age < 45 years.
Patients with age < 50 years.
Chemotherapy regimens and dose scheduling
In patients with HER2-negative breast cancer, anthracycline- and taxane-based chemotherapy regimens are standard of care.25,26 The EBCTCG meta-analysis included individual patient-level data from 123 trials comparing the efficacy of different polychemotherapy regimens.21 In particular, 33 trials included in the meta-analysis assessed the benefit of adding a taxane- to anthracycline-based chemotherapy. Among 44 251 patients included in this analysis, 22 128 were allocated to taxane-based chemotherapy and 22 123 to non-taxane-based chemotherapy. Approximately 27% of women were below 45 years of age and 54% had nodal involvement. The addition of a taxane significantly reduced the risk of both recurrence (rate ratio [RR]: 0.84; 95% confidence interval [CI]: 0.78-0.91) and overall mortality (RR: 0.86; 95% CI: 0.79-0.93). No heterogeneity of treatment effect based on age at diagnosis was observed.21 Notably, the addition of a taxane to anthracycline- and cyclophosphamide-based chemotherapy in premenopausal patients may lead to an increased risk of treatment-induced amenorrhea.27-29 This is an important information to share during patients’ counseling.
In terms of optimal schedule of adjuvant chemotherapy, it is known that not only the type and the total dose of drugs but also the timing of their administration contribute to the anticancer effect of cytotoxic therapy.30,31 The possible strategies for increasing the dose intensity are either a reduction of the intervals between treatment cycles (dose-dense [DD] schedule) or the administration of individual drugs in sequence at higher doses. In patients with high-risk breast cancer, DD chemotherapy is now considered standard of care in the early setting.25,26 Recently, data from patients enrolled in the DD trials were combined in an individual patient-level meta-analysis by the EBCTCG.32 Among 37 298 patients included, 18 623 were allocated to DD and 18 750 to standard-interval chemotherapy. Approximately 28% of the patients were below 45 years of age and 77% had nodal involvement. At a median follow-up of 7.4 years, DD chemotherapy demonstrated a reduction in risk of both 10-year recurrence (RR: 0.86; 95% CI: 0.82-0.89) and overall mortality (RR: 0.87; 95% CI: 0.83-0.91). There was no heterogeneity of treatment effect based on age. Notably, a significant benefit of DD chemotherapy was observed in both patients with hormone receptor–positive disease and hormone receptor–negative disease.32 Few data are available on the efficacy of DD chemotherapy specifically in premenopausal women with breast cancer. In this group of patients, a pooled analysis of 2 DD trials (MIG1 and GIM2) was conducted.22 The MIG1 trial enrolled 1214 patients with node-positive or high-risk node-negative breast cancer, of whom 43% were premenopausal at the time of randomization.33 In this trial, patients were randomized to FEC (fluoruracil, epirubicin, cyclophosphamide) chemotherapy administered every 14 days (DD) or every 21 days (standard interval).33 The GIM2 trial enrolled 2091 patients with node-positive early breast cancer, of whom 48% were premenopausal at the time of randomization.34 In this trial, patients were randomized to chemotherapy with EC (epirubicin, cyclophosphamide) or FEC followed by paclitaxel administered every 14 days (DD) or every 21 days (standard interval).34 Data from premenopausal women enrolled in the MIG1 and GIM2 trials were combined in the pooled analysis.22 A total of 1549 premenopausal patients were included, of whom 762 received DD chemotherapy and 787 standard-interval chemotherapy. At a median follow-up of 9.1 years, a statistically significant improvement in overall survival (OS) for patients receiving DD chemotherapy was observed (hazard ratio [HR]: 0.71; 95% CI: 0.54-0.95). The risk of treatment-induced amenorrhea was not increased with the use of DD chemotherapy (OR 1.00; 95% CI: 0.80-1.25).22 Therefore, in patients with high-risk premenopausal breast cancer, DD adjuvant chemotherapy should be proposed as the preferred approach.
To reduce the long-term adverse events of anthracyclines, which can be of particular importance to premenopausal patients, many trials have tried to evaluate the possibility to spare their use. An alternative regimen is the combination of docetaxel and cyclophosphamide (TC).35,36 Several trials have compared the TC regimen to anthracycline- and taxane-based chemotherapy regimens.37-40 Results from seven randomized controlled trials comparing TC with sequential anthracycline- and taxane-based chemotherapy were combined in a meta-analysis.23 No difference in terms of disease-free survival (DFS) (HR: 1.08; 95% CI: 0.96-1.20) and OS (HR: 1.05; 95% CI: 0.90-1.22) were observed among all enrolled patients. In the subgroup analysis, use of anthracycline- and taxane-based chemotherapy was associated with a larger benefit in high-risk patients such as those with hormone receptor–negative disease (HR: 1.12; 95% CI: 0.93-1.34) and with more than 4 positive axillary nodes (HR: 1.25; 95% CI: 0.82-1.90).23 Only 2 studies included in the meta-analysis reported results of DFS according to menopausal status.38,39 In both trials, no significant difference was observed between pre- and postmenopausal patients, with a trend favoring TC in premenopausal patients (HR: 0.78; 95% CI: 0.56-1.09).23 This could be partially explained by the fact that patients treated with the TC regimen received a higher cumulative dose of cyclophosphamide (6 cycles in the TC regimen vs 3-4 cycles with anthracycline- and taxane-based chemotherapy) which is known to be the chemotherapy agent associated with the highest risk of gonadoxicity.41,42 A higher dose of cyclophosphamide and the subsequent potential increased incidence of chemotherapy-related amenorrhea with TC may have led to a possible chemoendocrine effect of cytotoxic therapy in premenopausal women with hormone receptor–positive disease that were probably treated with tamoxifen alone as adjuvant endocrine therapy within these trials.
Use of genomic tests
The decision on the need to administer adjuvant chemotherapy may be complex in some circumstances. Although tools based on traditional clinicopathological factors can be helpful to predict an individual’s prognosis and to aid clinical decision-making, there are some concerns about their performance in the younger population.6,43 The decision to add adjuvant chemotherapy is particularly challenging in the setting of patients with hormone receptor–positive HER2-negative disease. For these women, in addition to standard clinicopathological features, genomic tests have shown to allow a further refinement in the selection of patients that may benefit from the addition of adjuvant chemotherapy to endocrine therapy, including among young patients.44 Recent prospective data from the TAILORx trial with the use of the 21-gene recurrence score (RS) assay Oncotype DX are of particular relevance for premenopausal women.45 In node-negative patients with hormone receptor–positive HER2-negative breast cancer and intermediate RS (11-25), the addition of chemotherapy to endocrine therapy did not show to provide any advantage in the overall breast cancer population.46 However, the study showed that patients below 50 years of age and RS between 16 and 25 had apparent additional benefit with the use of adjuvant chemotherapy.46 The benefit was more pronounced in premenopausal women older than 45 years of age, suggesting a potential chemoendocrine effect of chemotherapy that is likely to induce more frequently premature ovarian insufficiency in older premenopausal women closer to their natural age at menopause.47 The addition of tumor size and histologic grade to RS may help to further stratify this group of patients in terms of expected benefit from the addition of chemotherapy.45 Cytotoxic therapy seems to be associated with a significant benefit only in patients with RS of 21 to 25, irrespective of their clinical risk, and in those with RS of 16 to 20 but in the presence of less favorable tumor size and/or histologic grade.45
These findings should be interpreted with cautious considering that most of the premenopausal patients enrolled in the TAILORx trial received tamoxifen alone. Hence, in this specific intermediate RS population, the benefit of adding chemotherapy remains unclear, also considering the widespread use of ovarian function suppression (OFS) as part of adjuvant endocrine therapy in current clinical practice.
Post-neoadjuvant chemotherapy
Over the past years, the use of chemotherapy before surgery instead of in the adjuvant setting has gained more attention.48 Achieving a pathological complete response (pCR), defined as the absence of invasive disease in the breast and axillary lymph nodes following neoadjuvant systemic therapy, has a strong prognostic value particularly for HER2-positive and triple-negative disease.49 It should be highlighted that, although no survival advantage has been shown for the neoadjuvant versus adjuvant approach in old trials,50 nowadays, chemotherapy regimens may differ in these 2 settings for some patients. For example, the use of platinum salts in addition to standard chemotherapy has become more established only in the neoadjuvant setting mostly for patients with triple-negative disease.51 This can be relevant for premenopausal patients considering that they are more often diagnosed with this disease subtype and can be usually considered fit for this regimen.
The discussion around neoadjuvant therapy goes beyond the scope of this review. However, notably, the preference for the neoadjuvant approach is also highlighted by the opportunity to module the adjuvant treatment in patients without optimal response to the treatment given before surgery.48 Specifically, in patients not achieving a pCR, additional adjuvant chemotherapy has been investigated for trying to improve their poorer prognosis.48 Among these efforts, the most important results so far have been obtained with the use of capecitabine. In the CREATE-X trial, patients with HER2-negative breast cancer and residual invasive disease after neoadjuvant systemic treatment were randomized to receive or not capecitabine for 6 to 8 cycles in addition to standard of care (radiotherapy and/or endocrine therapy).24 A total of 910 patients were enrolled; median age was 48 years, with 57.5% of premenopausal women. At a median follow-up of 3.6 years, a statistically significant improvement in DFS (HR: 0.70; 95% CI: 0.53-0.92) and OS (HR: 0.59; 95% CI: 0.39-0.90) was observed for patients treated with capecitabine. In the subgroup analysis, the benefit of capecitabine was larger for patients with triple-negative disease, irrespective of their age.24 Different trials in the post-neoadjuvant setting are currently ongoing to investigate the role of other chemotherapy drugs (including platinum salts), immunotherapy, or targeted agents as additional adjuvant treatments in patients without a pCR after standard neoadjuvant therapy.48 The impact of these additional treatments should be assessed also on a safety perspective (in terms of irreversible and/or long-term side effects), particularly in the setting of premenopausal women. Based on all these observations including the important prognostic information and the possibility to adapt the subsequent adjuvant treatment, in the presence of clear indication for chemotherapy, the neoadjuvant approach should be preferred in patients with breast cancer overall including among premenopausal women.
Endocrine Therapy
Despite the higher risk of developing triple-negative and HER2-positive subtypes, hormone receptor–positive disease remains the most common form of breast cancer diagnosed in premenopausal patients.6,52,53 Considering the apparent negative prognostic value of young age in luminal-like breast cancer, a correct choice among the multiple available endocrine therapy options is pivotal to properly manage these patients.54
Tamoxifen has been the standard of care for many years as adjuvant endocrine therapy of premenopausal women with hormone receptor–positive breast cancer.55,56 More recently, several studies have reported on the role of OFS in addition to tamoxifen or to an aromatase inhibitor (AI) and have helped to elucidate the most appropriate endocrine treatment option in this subgroup of patients.
OFS plus tamoxifen
Three studies analyzed the combination of OFS plus tamoxifen (Table 2).57,59,60 While negative results emerged from the E-3193 INT-0142 study, probably due to the small sample size and the low-risk population included,59 an improvement in terms of DFS and OS by adding OFS to tamoxifen was observed in both the ASTRRA and SOFT studies.58,61 In the ASTRRA trial, a total of 1289 premenopausal patients with hormone receptor–positive breast cancer were randomly allocated to receive tamoxifen alone for 5 years or tamoxifen for 5 years plus OFS for 2 years.58 Median age was 39 years and 53% of the patients had nodal involvement. All patients in this study were previously exposed to adjuvant or neoadjuvant chemotherapy and had to have a recovery of ovarian function within 2 years after the end of cytotoxic treatment for being eligible to the trial.60 An improvement in DFS (HR: 0.69; 95% CI: 0.48-0.97) and OS (HR: 0.31; 95% CI: 0.10-0.94) was observed for patients that received tamoxifen plus OFS.58 The SOFT study was a 3-arm trial that randomized 3066 premenopausal women with hormone receptor–positive early breast cancer to tamoxifen alone, OFS plus tamoxifen, or OFS plus exemestane.57 Median age was 43 years; approximately 35% of patients had nodal involvement and about 53% were previously exposed to chemotherapy. After a median follow-up of 8 years, when considering the comparison between tamoxifen plus OFS versus tamoxifen alone, a significant improvement in DFS (HR: 0.76; 95% CI: 0.62-0.93) and OS (HR: 0.67; 95% CI: 0.48-0.92) was observed favoring the addition of OFS in the overall population. The subgroup of patients who appeared to benefit the most were those exposed to prior chemotherapy, the youngest population (age below 35 years), and women with HER2-positive breast cancer.61
Table 2.
Characteristics of the main studies investigating different adjuvant endocrine therapy approaches in premenopausal patients with breast cancer.
| Study | Patients, n | Treatment arm | Premenopausal patients, n | Results in premenopausal patients |
|---|---|---|---|---|
| E-3193 INT-014259 |
345 | Tamoxifen vs Tamoxifen + OFS | 345 | DFS: HR: 1.17 (95% CI: 0.64-2.12) OS: HR: 1.19 (95% CI: 0.53-2.65) |
| ASTRRA60 | 1289 | Tamoxifen + OFS vs Tamoxifen | 1289 | DFS: HR: 0.69 (95% CI: 0.48-0.97) OS: HR: 0.31 (95% CI: 0.10-0.94) |
| SOFT61 | 2033a | Tamoxifen + OFS vs Tamoxifen | 2033 | DFS: HR: 0.76 (95% CI: 0.62-0.93) OS: HR: 0.67 (95% CI: 0.48-0.92) |
| ABCSG-1262 | 1803 | Tamoxifen + OFS (±zoledronic acid) vs Anastrozole + OFS (±zoledronic acid) | 1803 | DFS: HR: 1.13 (95% CI: 0.88-1.45) OS: HR: 1.63 (95% CI: 1.05-2.52) |
| Joint analysis SOFT & TEXT61 | 4690 | Exemestane + OFS vs Tamoxifen + OFS | 4690 | DFS: HR: 0.77 (95% CI: 0.67-0.90) OS: HR: 0.98 (95% CI: 0.79-1.22) |
| HOBOE63 | 710b | Letrozole + OFS vs Tamoxifen + OFS | 710 | DFS: HR: 0.72 (95% CI: 0.48-1.07) |
| NCIC CTG MA17 trial64 | 5166 | Extended therapy with letrozole vs placebo | 877 | DFS: HR: 0.26 (95% CI: 0.13-0.55) OS: HR: 0.43 (95% CI: 0.08-2.22) |
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; OFS, ovarian function suppression; OS, overall survival.
Patients in the exemestane plus OFS arm (n = 1014) were considered in the joint analysis with the TEXT trial.
Patients in the letrozole plus OFS plus zoledronic acid arm (n = 355) were not included in the table.
OFS plus an AI
Three trials compared the efficacy and safety of OFS combined with tamoxifen or an AI (Table 2).62,63,65 In the ABCSG-12 trial, a total of 1803 premenopausal patients with hormone receptor–positive breast cancer were randomized to 3 years of OFS plus anastrozole or tamoxifen with or without zoledronic acid.62 The patient population included in this trial had low risk of disease recurrence: median age was 45 years, 30% had nodal involvement, and 5% were previously exposed to chemotherapy. At a median follow-up of 8 years, when considering the comparison between anastrozole and tamoxifen, no significant differences in terms of DFS were observed (HR: 1.13; 95% CI: 0.88-1.45), with a negative effect for anastrozole in terms of OS (HR: 1.63; 95% CI: 1.05-2.52).62 The TEXT study was a phase 3 trial that randomly allocated 2672 premenopausal patients with hormone receptor–positive breast cancer to OFS plus exemestane or OFS plus tamoxifen for 5 years.65 Median age of patients was 44 years; about 48% had nodal involvement and approximately 60% were previously exposed to chemotherapy.65 Differently from SOFT, TEXT did not have a third arm with tamoxifen alone and, when chemotherapy was given, OFS had to be started concurrently with cytotoxic therapy (and not only in patients with recovery of ovarian function up to 8 months following chemotherapy completion as in SOFT). In the joint analysis of SOFT and TEXT, for the comparison between OFS plus exemestane or OFS plus tamoxifen, after a median follow-up of 9 years, an improvement in DFS (HR: 0.77; 95% CI: 0.67-0.90) was observed for the AI arm with no difference in OS (HR: 0.98; 95% CI: 0.79-1.22).61 In the subgroup analysis, a larger benefit for OFS plus exemestane was observed in patients with HER2-negative disease who received prior chemotherapy, whereas an opposite trend favoring OFS plus tamoxifen was observed for the small population of patients with HER2-positive disease.61 Notably, when OFS plus an AI is chosen as the preferred treatment approach in premenopausal patients, a complete suppression of ovarian function must be obtained. Within the SOFT study, the SOFT-Estrogen (SOFT-EST) substudy demonstrated that approximately 20% of patients treated with OFS plus exemestane did not obtain a complete OFS (estradiol levels greater than 2.72 pg/mL).66 Potential risk factors for not obtaining a complete OFS were younger age, no prior exposure to chemotherapy, and a higher body mass index. These data raise the issue of the need to monitor estradiol levels during at least the first months of treatment; in the case of incomplete ovarian suppression, switching to OFS plus tamoxifen should be considered.
In the HOBOE study, a total of 1065 premenopausal patients with hormone receptor–positive breast cancer were randomized to receive 5 years of treatment with OFS plus letrozole or OFS plus tamoxifen with or without zoledronic acid.63 Median age at randomization was 45 years; about 45% of patients had nodal involvement and 62% were previously exposed to chemotherapy. At a median follow-up of 5 years, a favorable but nonstatistically significant advantage for OFS plus letrozole as compared with OFS plus tamoxifen was observed in terms of DFS (HR: 0.72; 95% CI: 0.48-1.07) with no significant differences in OS (P = .14).63
Taken together, the available evidence suggests that OFS (with tamoxifen or an AI) should be considered in patients with higher risk of disease recurrence, whereas tamoxifen alone is still a valid option for those with favorable clinicopathological features.15,26,67 The higher the risk of recurrence, the larger is the expected benefit and thus the preference for the combination of OFS plus an AI.68,69 In patients exposed to prior chemotherapy, OFS can be started during cytotoxic therapy instead of waiting for its completion. Concurrent administration of OFS with chemotherapy does not impair survival outcomes 70-72 and has the potential benefit of increasing the chances of menstrual function recovery and future pregnancies after treatment completion.72,73
Endocrine therapy and bone health
In patients receiving OFS for 5 years, and particularly in those exposed also to an AI, bone health should be carefully monitored and managed.26
Two trials evaluated the potential anticancer effect of bisphosphonates added to endocrine therapy in the specific cohort of premenopausal patients undergoing OFS.62,63 In the ABCSG-12 trial, at a median follow-up of 8 years, the addition of zoledronic acid improved DFS compared with endocrine therapy alone (HR: 0.77; 95% CI: 0.60-0.99) with a trend in OS (HR: 0.66; 95% CI: 0.43-1.02).62 In the HOBOE trial, a statistically significant benefit in DFS was observed for OFS plus letrozole plus zoledronic acid compared with OFS plus tamoxifen (HR: 0.52 95% CI: 0.34-0.80) without significant difference in OS.63
Extended adjuvant endocrine therapy
Considering the increased risk of late recurrences among patients with hormone receptor–positive breast cancer,74,75 extended adjuvant endocrine therapy should be considered in high-risk patients.76 In the NCIC CTG MA17 trial, a total of 5166 patients (of whom 17% were premenopausal) were randomized to receive extended adjuvant endocrine therapy for 5 years with letrozole or placebo after the completion of 5 years of tamoxifen (Table 2).64 Approximately 56% of premenopausal women had nodal involvement and 80% were previously exposed to chemotherapy. Data from this study showed that the benefit of extending adjuvant endocrine therapy was observed in both premenopausal and postmenopausal patients but it was particularly important for those who were premenopausal at diagnosis. In this subgroup of patients, a significant improvement in DFS was observed (HR: 0.26; 95% CI: 0.13-0.55), particularly in those with nodal involvement (HR: 0.40; 95% CI: 0.18-0.85), with a trend toward better OS (HR: 0.43; 95% CI: 0.08-2.22).64
Therefore, in premenopausal patients at high risk of disease recurrence, extended endocrine therapy should be considered although no proper data are available to counsel women exposed to OFS in the first 5 years.
Quality of life
Importantly, during patients’ counseling on the available endocrine treatment options, it is important to discuss also their different safety profile considering the potential negative effect on patients’ quality of life (QoL). As recently shown in the CANTO study, endocrine therapy has a major detrimental impact on QoL.77 Endocrine therapy showed to negatively impact role and social function, insomnia, pain, side effects of systemic treatment, and breast symptoms; in addition, a further limitation in emotional function and future perspective recovery was observed. Although this study showed that in premenopausal patients chemotherapy was more frequently associated with a deterioration in QoL domains than endocrine therapy, most of the women included in CANTO received tamoxifen alone as part of endocrine therapy.77 In premenopausal patients, adding OFS to tamoxifen is associated with a relevant worsening of endocrine symptoms (hot flashes, sweat, and sleep disturbance) and health-related QoL, whereas OFS plus exemestane leads to significantly more frequent bone and joint pain, vaginal dryness, and loss of sexual interest but with apparent similar global health-related QoL as compared with OFS plus tamoxifen.78,79 Therefore, in the current era, escalation of endocrine therapy with OFS may add a substantial burden on patients’ QoL and treating physicians should pay a great attention on this regard. Other important issues to be considered in premenopausal patients are their potential concerns about fertility and the possibility to have a subsequent pregnancy that have been shown to be significantly associated with both noninitiation of endocrine therapy and its early discontinuation.80,81 To increase patients’ compliance with endocrine therapy and, consequently, their long-term outcomes, it is essential to adequately inform patients about the toxicity profile of different options, monitor them carefully during treatment, and switch to a different option when the ongoing therapy is not tolerated.53
Targeted Therapy
In patients with HER2-positive breast cancer, the advent of adjuvant trastuzumab has revolutionized the natural history and outcomes of this disease and remains standard of care in the adjuvant setting.82 In a retrospective analysis of the HERA trial, the potential prognostic and predictive value of age at diagnosis was investigated.83 Among the 3401 patients randomized to trastuzumab for 1 year or observation, 21% of them were below 40 years of age at the time of randomization. At a median follow-up of 2 years, results showed that age had no prognostic value and was not associated with prediction of benefit from trastuzumab treatment.83 Therefore, chemotherapy plus trastuzumab-based treatment for 1 year is the current standard of care for most of patients with HER2-positive disease.15,25,26,84 Importantly, in premenopausal patients, the use of anti-HER2 therapy does not appear to increase the rate of chemotherapy-induced premature ovarian insufficiency.28,85 On this regard, the possibility to administer weekly paclitaxel plus trastuzumab (without cyclophosphamide and anthracyclines) in selected patients should be considered of great importance for premenopausal patients considering the low risk of treatment-induced premature ovarian failure with this regimen.86 However, the efficacy of this de-escalated regimen has been shown only for patients with small tumors (<2 cm) and node-negative disease.87 On the contrary, several efforts in escalating treatment effect have been conducted in the past years to further improve patients’ outcomes.82
Anti-HER2 agents beyond trastuzumab
Among the different escalating efforts, 3 trials have led to the approval of additional anti-HER2 agents beyond trastuzumab (ie, pertuzumab, neratinib, and trastuzumab-emtansine [T-DM1]) in the adjuvant setting (Table 3).
Table 3.
Characteristics of the main studies investigating adjuvant-targeted therapy approaches focusing on the results available in premenopausal patients.
| Study | Patients, n | Treatment arm | Premenopausal patients, n | Results in premenopausal patients |
|---|---|---|---|---|
| APHINITY88 | 4804 | CT + trastuzumab + pertuzumab vs CT + trastuzumab + placebo | 2325 | IDFS: HR: 0.99 (95% CI: 0.75-1.32) |
| EXTENET89 | 2840 | Neratinib vs placebo | 1327 | IDFS: HR: 0.74 (95% CI: 0.53-1.04) |
| KATHERINE90 | 1486 | T-DM1 vs trastuzumab | 296a | IDFS: HR: 0.50 (95% CI: 0.29-0.86) |
Abbreviations: CI, confidence interval; CT, chemotherapy; HR, hazard ratio; IDFS, invasive disease-free survival.
Patients < 40 years.
In the APHINITY trial, a total of 4805 patients were randomized to receive adjuvant chemotherapy and dual anti-HER2 blockade with trastuzumab plus pertuzumab or single anti-HER2 agent trastuzumab.88 About 13% of patients were younger than 40 years, 48% were premenopausal, and approximately 62% had nodal involvement. At a median follow-up of 45.4 months, the addition of pertuzumab increased invasive DFS (IDFS) (HR: 0.81; 95% CI: 0.66-1.00) with a greater benefit observed in patients with nodal involvement (HR: 0.77; 95% CI: 0.62-0.96). The IDFS benefit was shown irrespective of age and menopausal status.88
In the ExteNET trial, a total of 2840 patients who had completed chemotherapy and 1 year of adjuvant trastuzumab were randomized to receive neratinib or placebo for an additional year.89,91 At a median follow-up of 5.2 years, a statistically significantly improvement in IDFS was observed in the neratinib group comparing with placebo (HR: 0.73; 95% CI: 0.57-0.92). In the subgroup analyses, a larger benefit of neratinib was observed in patients with hormone receptor–positive disease (HR: 0.60; 95% CI: 0.43-0.83). No heterogeneity of treatment effect was shown in relation to menopausal status.89,91 The benefit of extending adjuvant therapy with neratinib should be balanced with increased risk of gastrointestinal toxicity especially diarrhea. Without appropriate prophylaxis, 40% of patients developed grade 3 diarrhea and 1% grade 4. Other common adverse effects were nausea and vomiting.89
The most important results among the escalation efforts have been recently presented with the use of adjuvant T-DM1 in patients with HER2-positive breast cancer and residual disease after standard neoadjuvant chemotherapy plus anti-HER2 treatment. In the KATHERINE trial, patients previously treated with neoadjuvant taxane-based chemotherapy plus anti-HER2 trastuzumab-based therapy and with residual disease at the time of surgery were randomized to receive adjuvant trastuzumab alone or T-DM1 to complete 1 year of treatment.90 A total of 1486 patients were enrolled; median age was 49 years (approximately 20% of the patients were younger than 45 years). Most of the patients (76.9%) received anthracycline- and taxane-based neoadjuvant chemotherapy; neoadjuvant pertuzumab was added to trastuzumab in 18.3% of the patients. At a median follow-up of 41.4 months, a statistically significant improvement in IDFS was demonstrated for T-DM1 compared with trastuzumab (HR: 0.50; 95% CI: 0.39-0.64). In the subgroup analysis, the significant improvement with the use of TDM1 was observed irrespective of patients’ age at the time of treatment.90 Based on the evidence from this study, T-DM1 should be considered the preferred adjuvant anti-HER2 treatment in patients with residual disease after neoadjuvant chemotherapy. With the expected increased use of T-DM1 in the early setting, the potential gonadotoxicity of this agent needs to be investigated for better counseling premenopausal patients who are candidates to this treatment.
Conclusions
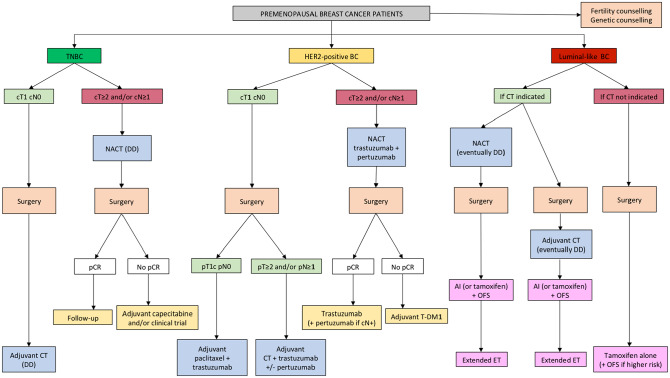
In the current era of rapidly emerging treatment options, the adjuvant management of premenopausal women with early breast cancer is becoming more complex also considering the specific age-related issues of this patient population (Figure 1).
Figure 1.
Algorithm for the management of premenopausal patients with early breast cancer.
Abbreviations: BC, breast cancer; CT, chemotherapy; DD, dose-dense; ET, endocrine therapy; HER2, Human Epidermal growth factor Receptor 2; NACT, neoadjuvant chemotherapy; OFS, ovarian function suppression; pCR, pathological complete response; Tam, tamoxifen; TDM1, trastuzumab-emtansine; TNBC, triple-negative breast cancer.
In terms of best chemotherapy approach, in patients with high-risk breast cancer, the use of an anthracycline- and taxane-based regimen with a DD schedule should be considered the preferred choice. In patients considered in the gray zone for chemotherapy indication, anthracyclines can be spared and the TC regimen is a valid option. For women with hormone receptor-positive HER2-negative breast cancer, including among premenopausal patients, the use of genomic tests adds valuable prognostic and predictive information to help identifying patients requiring adjuvant chemotherapy in addition to endocrine therapy. Importantly, whenever there is clear indication for chemotherapy, considering the prognostic information and the possibility to tailor the subsequent adjuvant treatment, the neoadjuvant approach should be preferred.
For the choice of endocrine therapy, not only the individual risk of recurrence but also the toxicity profile and treatment adherence should be considered as crucial factors when counseling premenopausal patients about the optimal approach. The addition of OFS to AI or tamoxifen should be considered standard of care in women at higher risk of recurrence, with a preference for an AI in the population of patients with the worst prognostic features. Tamoxifen alone remains the preferred approach in women at low risk of disease recurrence.
No major difference exists for the management of premenopausal patients with HER2-positive disease as compared with older patients, with the same indication and expected benefit from the approved adjuvant anti-HER2-targeted therapies. With the only exception of patients with small tumor without nodal involvement for whom weekly paclitaxel and trastuzumab alone can be given, a neoadjuvant approach should be preferred in all the other cases considering the possibility to customize the adjuvant treatment according to the pathologic results at surgery and the substantial benefit of T-DM1 in those with residual disease.
In view of the complexity and the sensitive issues pertaining this age group, a multidisciplinary approach is mandatory to establish the appropriate adjuvant treatment of premenopausal women with early breast cancer. More prospective research efforts dedicated to premenopausal patients with breast cancer (including powered subgroup analyses in randomized trials according to age and menopausal status at diagnosis) are required to further improve care, outcomes, and QoL of these women.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:M.T. received travel grants from Roche, Bristol-Myers Squibb, AstraZeneca, Takeda, and Honoraria as medical writer from Novartis outside the submitted work. M.L. served as consultant for Roche and Novartis, and received speaker honoraria from Roche, Takeda, Lilly, Novartis and Theramex outside the submitted work. The other authors declare no conflicts of interests.
Author Contributions: All the authors contributed in reviewing the literature, interpreting the results of the included studies and writing the manuscript.
ORCID iDs: Luca Arecco  https://orcid.org/0000-0002-3818-0364
https://orcid.org/0000-0002-3818-0364
Marco Tagliamento  https://orcid.org/0000-0001-7461-023X
https://orcid.org/0000-0001-7461-023X
Matteo Lambertini  https://orcid.org/0000-0003-1797-5296
https://orcid.org/0000-0003-1797-5296
References
- 1. Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100:1643-1648. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18:1579-1589. [DOI] [PubMed] [Google Scholar]
- 3. Ghiasvand R, Adami H-O, Harirchi I, Akrami R, Zendehdel K. Higher incidence of premenopausal breast cancer in less developed countries; myth or truth? BMC Cancer. 2014;14:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poggio F, Lambertini M, Bighin C, et al. Management of young women with early breast cancer. ESMO Open. 2018;3:e000458. doi: 10.1136/esmoopen-2018-000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collins LC, Marotti JD, Gelber S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061-1066. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 6. Lambertini M, Pinto AC, Ameye L, et al. The prognostic performance of Adjuvant! Online and Nottingham Prognostic Index in young breast cancer patients. Br J Cancer. 2016;115:1471-1478. doi: 10.1038/bjc.2016.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azim HA, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014;16:427. doi: 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308-3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 9. Yoon TI, Hwang U-K, Kim ET, et al. Survival improvement in hormone-responsive young breast cancer patients with endocrine therapy. Breast Cancer Res Treat. 2017;165:311-320. doi: 10.1007/s10549-017-4331-4. [DOI] [PubMed] [Google Scholar]
- 10. Sopik V, Sun P, Narod SA. The prognostic effect of estrogen receptor status differs for younger versus older breast cancer patients. Breast Cancer Res Treat. 2017;165:391-402. doi: 10.1007/s10549-017-4333-2. [DOI] [PubMed] [Google Scholar]
- 11. Kan Z, Ding Y, Kim J, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018;9:1725. doi: 10.1038/s41467-018-04129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lambertini M, Viglietti G, de Azambuja E. Impact of ovarian function suppression in premenopausal women with estrogen receptor-positive early breast cancer. Curr Opin Oncol. 2019;31:43-51. doi: 10.1097/CCO.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 13. Huiart L, Bouhnik A-D, Rey D, et al. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer. 2012;48:1939-1946. doi: 10.1016/j.ejca.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 14. Rossi L, Mazzara C, Pagani O. Diagnosis and treatment of breast cancer in young women. Curr Treat Options Oncol. 2019;20:86. doi: 10.1007/s11864-019-0685-7. [DOI] [PubMed] [Google Scholar]
- 15. Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. 2017;35:203-217. doi: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 16. Rosenberg SM, Vaz-Luis I, Gong J, et al. Employment trends in young women following a breast cancer diagnosis. Breast Cancer Res Treat. 2019;177:207-214. doi: 10.1007/s10549-019-05293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lambertini M, Goldrat O, Clatot F, Demeestere I, Awada A. Controversies about fertility and pregnancy issues in young breast cancer patients: current state of the art. Curr Opin Oncol. 2017;29:243-252. doi: 10.1097/CCO.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 18. Tung N, Lin NU, Kidd J, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34:1460-1468. doi: 10.1200/JCO.2015.65.0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosenberg SM, Ruddy KJ, Tamimi RM, et al. BRCA1 and BRCA2 mutation testing in young women with breast cancer. JAMA Oncol. 2016;2:730-736. doi: 10.1001/jamaoncol.2015.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Copson ER, Maishman TC, Tapper WJ, et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): a prospective cohort study. Lancet Oncol. 2018;19:169-180. doi: 10.1016/S1470-2045(17)30891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Peto R, Davies C, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432-444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lambertini M, Ceppi M, Cognetti F, et al. Dose-dense adjuvant chemotherapy in premenopausal breast cancer patients: a pooled analysis of the MIG1 and GIM2 phase III studies. Eur J Cancer. 2017;71:34-42. doi: 10.1016/j.ejca.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 23. Caparica R, Bruzzone M, Poggio F, Ceppi M, de Azambuja E, Lambertini M. Anthracycline and taxane-based chemotherapy versus docetaxel and cyclophosphamide in the adjuvant treatment of HER2-negative breast cancer patients: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res Treat. 2019;174:27-37. doi: 10.1007/s10549-018-5055-9. [DOI] [PubMed] [Google Scholar]
- 24. Masuda N, Lee S-J, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147-2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 25. Denduluri N, Chavez-MacGregor M, Telli ML, et al. Selection of optimal adjuvant chemotherapy and targeted therapy for early breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2018;36:2433-2443. doi: 10.1200/JCO.2018.78.8604. [DOI] [PubMed] [Google Scholar]
- 26. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1194-1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 27. Zavos A, Valachis A. Risk of chemotherapy-induced amenorrhea in patients with breast cancer: a systematic review and meta-analysis. Acta Oncol. 2016;55:664-670. doi: 10.3109/0284186X.2016.1155738. [DOI] [PubMed] [Google Scholar]
- 28. Lambertini M, Campbell C, Bines J, et al. Adjuvant anti-HER2 Therapy, treatment-related amenorrhea, and survival in premenopausal HER2-Positive early breast cancer patients. J Natl Cancer Inst. 2019;111:86-94. doi: 10.1093/jnci/djy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambertini M, Olympios N, Lequesne J, et al. Impact of taxanes, endocrine therapy, and deleterious germline BRCA mutations on anti-müllerian hormone levels in early breast cancer patients treated with anthracycline- and cyclophosphamide-based chemotherapy. Front Oncol. 2019;9:575. doi: 10.3389/fonc.2019.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Citron ML. Dose-dense chemotherapy: principles, clinical results and future perspectives. Breast Care (Basel). 2008;3:251-255. doi: 10.1159/000148914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Almeida FK, Rosa DD. Adjuvant dose-dense chemotherapy for breast cancer: available evidence and recent updates. Breast Care (Basel). 2018;13:447-452. doi: 10.1159/000488026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gray R, Bradley R, Braybrooke J, et al. Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet. 2019;393:1440-1452. doi: 10.1016/S0140-6736(18)33137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blondeaux E, Lambertini M, Michelotti M, et al. Dose-dense adjuvant chemotherapy in early breast cancer patients: 15-year results of the phase 3 Mammella InterGruppo (MIG)-1 study. Br J Cancer. 2020;122:1611-1617. doi:10.1038/s41416-020-0816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Del Mastro L, De Placido S, Bruzzi P, et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised phase 3 trial. Lancet. 2015;385:1863-1872. doi: 10.1016/S0140-6736(14)62048-1. [DOI] [PubMed] [Google Scholar]
- 35. Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of us oncology research trial 9735. J Clin Oncol. 2009;27:1177-1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 36. Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381-5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 37. Blum JL, Flynn PJ, Yothers G, et al. Anthracyclines in early breast cancer: the ABC trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol. 2017;35:2647-2655. doi: 10.1200/JCO.2016.71.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mavroudis D, Matikas A, Malamos N, et al. Dose-dense FEC followed by docetaxel versus docetaxel plus cyclophosphamide as adjuvant chemotherapy in women with HER2-negative, axillary lymph node-positive early breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2016;27:1873-1878. doi: 10.1093/annonc/mdw274. [DOI] [PubMed] [Google Scholar]
- 39. Ejlertsen B, Tuxen MK, Jakobsen EH, et al. Adjuvant cyclophosphamide and docetaxel with or without epirubicin for early TOP2A-normal breast cancer: DBCG 07-READ, an open-label, phase III, randomized trial. J Clin Oncol. 2017;35:2639-2646. doi: 10.1200/JCO.2017.72.3494. [DOI] [PubMed] [Google Scholar]
- 40. Janni W, Nitz U, Rack BK, et al. Pooled analysis of two randomized phase III trials (PlanB/SuccessC) comparing six cycles of docetaxel and cyclophosphamide to sequential anthracycline taxane chemotherapy in patients with intermediate and high risk HER2-negative early breast cancer (n=5,923). J Clin Oncol. 2018;36:522-522. doi: 10.1200/JCO.2018.36.15_suppl.522. [DOI] [Google Scholar]
- 41. Zhao J, Liu J, Chen K, et al. What lies behind chemotherapy-induced amenorrhea for breast cancer patients: a meta-analysis. Breast Cancer Res Treat. 2014;145:113-128. doi: 10.1007/s10549-014-2914-x. [DOI] [PubMed] [Google Scholar]
- 42. Lambertini M, Del Mastro L, Pescio MC, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14:1. doi: 10.1186/s12916-015-0545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Engelhardt EG, Garvelink MM, de Haes JHCJM, et al. Predicting and communicating the risk of recurrence and death in women with early-stage breast cancer: a systematic review of risk prediction models. J Clin Oncol. 2014;32:238-250. doi: 10.1200/JCO.2013.50.3417. [DOI] [PubMed] [Google Scholar]
- 44. Poorvu PD, Gelber SI, Rosenberg SM, et al. Prognostic impact of the 21-gene recurrence score assay among young women with node-negative and node-positive ER-positive/HER2-negative breast cancer. J Clin Oncol. 2020;38:725-733. doi: 10.1200/JCO.19.01959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380:2395-2405. doi: 10.1056/NEJMoa1904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111-121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lambertini M, Blondeaux E, Perrone F, Del Mastro L. Improving adjuvant endocrine treatment tailoring in premenopausal women with hormone receptor-positive breast cancer. J Clin Oncol. 2020;38:1258-1267. doi: 10.1200/JCO.19.02242. [DOI] [PubMed] [Google Scholar]
- 48. Caparica R, Lambertini M, Pondé N, Fumagalli D, de Azambuja E, Piccart M. Post-neoadjuvant treatment and the management of residual disease in breast cancer: state of the art and perspectives. Ther Adv Med Oncol. 2019;11:1758835919827714. doi: 10.1177/1758835919827714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 50. Asselain B, Barlow W, Bartlett J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27-39. doi: 10.1016/S1470-2045(17)30777-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29:1497-1508. doi: 10.1093/annonc/mdy127. [DOI] [PubMed] [Google Scholar]
- 52. Thomas A, Rhoads A, Pinkerton E, et al. Incidence and survival among young women with stage I-III breast cancer: SEER 2000-2015. JNCI Cancer Spectr. 2019;3:pkz040. doi: 10.1093/jncics/pkz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shoemaker ML, White MC, Wu M, Weir HK, Romieu I. Differences in breast cancer incidence among young women aged 20-49 years by stage and tumor characteristics, age, race, and ethnicity, 2004-2013. Breast Cancer Res Treat. 2018;169:595-606. doi: 10.1007/s10549-018-4699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lambertini M, Viglietti G, de Azambuja E. Controversies in oncology: which adjuvant endocrine therapy is to be given to premenopausal patients with hormone receptor-positive breast cancer? ESMO Open. 2018;3:e000350. doi: 10.1136/esmoopen-2018-000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255-2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771-784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436-446. doi: 10.1056/NEJMoa1412379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim H-A, Lee JW, Nam SJ, et al. Adding ovarian suppression to tamoxifen for premenopausal breast cancer: a randomized phase III trial. J Clin Oncol. 2020;38:434-443. doi: 10.1200/JCO.19.00126. [DOI] [PubMed] [Google Scholar]
- 59. Tevaarwerk AJ, Wang M, Zhao F, et al. Phase III comparison of tamoxifen versus tamoxifen plus ovarian function suppression in premenopausal women with node-negative, hormone receptor-positive breast cancer (E-3193, INT-0142): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2014;32:3948-3958. doi: 10.1200/JCO.2014.55.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim H-A, Ahn SH, Nam SJ, et al. The role of the addition of ovarian suppression to tamoxifen in young women with hormone-sensitive breast cancer who remain premenopausal or regain menstruation after chemotherapy (ASTRRA): study protocol for a randomized controlled trial and progress. BMC Cancer. 2016;16:319. doi: 10.1186/s12885-016-2354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Francis PA, Pagani O, Fleming GF, et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med. 2018;379:122-137. doi: 10.1056/NEJMoa1803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gnant M, Mlineritsch B, Stoeger H, et al. Zoledronic acid combined with adjuvant endocrine therapy of tamoxifen versus anastrozol plus ovarian function suppression in premenopausal early breast cancer: final analysis of the Austrian Breast and Colorectal Cancer Study Group Trial 12. Ann Oncol. 2015;26:313-320. doi: 10.1093/annonc/mdu544. [DOI] [PubMed] [Google Scholar]
- 63. Perrone F, De Laurentiis M, De Placido S, et al. Adjuvant zoledronic acid and letrozole plus ovarian function suppression in premenopausal breast cancer: HOBOE phase 3 randomised trial. Eur J Cancer. 2019;118:178-186. doi: 10.1016/j.ejca.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 64. Goss PE, Ingle JN, Martino S, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol. 2013;24:355-361. doi: 10.1093/annonc/mds330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107-118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bellet M, Gray KP, Francis PA, et al. Twelve-month estrogen levels in premenopausal women with hormone receptor-positive breast cancer receiving adjuvant triptorelin plus exemestane or tamoxifen in the suppression of ovarian function trial (SOFT): the SOFT-EST substudy. J Clin Oncol. 2016;34:1584-1593. doi: 10.1200/JCO.2015.61.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American society of clinical oncology clinical practice guideline update on ovarian suppression. J Clin Oncol. 2016;34:1689-1701. doi: 10.1200/JCO.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 68. Regan MM, Francis PA, Pagani O, et al. Absolute benefit of adjuvant endocrine therapies for premenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative early breast cancer: TEXT and SOFT trials. J Clin Oncol. 2016;34:2221-2231. doi: 10.1200/JCO.2015.64.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pagani O, Francis PA, Fleming GF, et al. Absolute improvements in freedom from distant recurrence to tailor adjuvant endocrine therapies for premenopausal women: results from TEXT and SOFT. J Clin Oncol. 2020;38:1293-1303. doi: 10.1200/JCO.18.01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lambertini M, Boni L, Michelotti A, et al. Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: a randomized clinical trial. JAMA. 2015;314:2632-2640. doi: 10.1001/jama.2015.17291. [DOI] [PubMed] [Google Scholar]
- 71. Regan MM, Walley BA, Francis PA, et al. Concurrent and sequential initiation of ovarian function suppression with chemotherapy in premenopausal women with endocrine-responsive early breast cancer: an exploratory analysis of TEXT and SOFT. Ann Oncol. 2017;28:2225-2232. doi: 10.1093/annonc/mdx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol. 2018;36:1981-1990. doi: 10.1200/JCO.2018.78.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lambertini M, Ceppi M, Poggio F, et al. Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: a meta-analysis of randomized studies. Ann Oncol. 2015;26:2408-2419. doi: 10.1093/annonc/mdv374. [DOI] [PubMed] [Google Scholar]
- 74. Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836-1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lambertini M, Campbell C, Gelber RD, et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res Treat. 2019;177:103-114. doi: 10.1007/s10549-019-05284-y. [DOI] [PubMed] [Google Scholar]
- 76. Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol. 2019;37:423-438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 77. Ferreira AR, Di Meglio A, Pistilli B, et al. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: a prospective patient-reported outcomes analysis. Ann Oncol. 2019;30:1784-1795. doi: 10.1093/annonc/mdz298. [DOI] [PubMed] [Google Scholar]
- 78. Ribi K, Luo W, Bernhard J, et al. Adjuvant tamoxifen plus ovarian function suppression versus tamoxifen alone in premenopausal women with early breast cancer: patient-reported outcomes in the suppression of ovarian function trial. J Clin Oncol. 2016;34:1601-1610. doi: 10.1200/JCO.2015.64.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bernhard J, Luo W, Ribi K, et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16:848-858. doi: 10.1016/S1470-2045(15)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ruddy KJ, Gelber SI, Tamimi RM, et al. Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol. 2014;32:1151-1156. doi: 10.1200/JCO.2013.52.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ruggeri M, Pagan E, Bagnardi V, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European Centers. Breast. 2019;47:85-92. doi: 10.1016/j.breast.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 82. Lambertini M, Pondé NF, Solinas C, de Azambuja E. Adjuvant trastuzumab: a 10-year overview of its benefit. Expert Rev Anticancer Ther. 2017;17:61-74. doi: 10.1080/14737140.2017.1264876. [DOI] [PubMed] [Google Scholar]
- 83. Partridge AH, Gelber S, Piccart-Gebhart MJ, et al. Effect of age on breast cancer outcomes in women with human epidermal growth factor receptor 2-positive breast cancer: results from a herceptin adjuvant trial. J Clin Oncol. 2013;31:2692-2698. doi: 10.1200/JCO.2012.44.1956. [DOI] [PubMed] [Google Scholar]
- 84. Burstein HJ, Curigliano G, Loibl S, et al. Estimating the benefits of therapy for early-stage breast cancer: the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30:1541-1557. doi: 10.1093/annonc/mdz235. [DOI] [PubMed] [Google Scholar]
- 85. Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH. The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer. 2010;116:791-798. doi: 10.1002/cncr.24835. [DOI] [PubMed] [Google Scholar]
- 86. Ruddy KJ, O’Neill A, Miller KD, et al. Biomarker prediction of chemotherapy-related amenorrhea in premenopausal women with breast cancer participating in E5103. Breast Cancer Res Treat. 2014;144:591-597. doi: 10.1007/s10549-014-2891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tolaney SM, Guo H, Pernas S, et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2019;37:1868-1875. doi: 10.1200/JCO.19.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377:122-131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688-1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 90. von Minckwitz G, Huang C-S, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617-628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 91. Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:367-377. doi: 10.1016/S1470-2045(15)00551-3. [DOI] [PubMed] [Google Scholar]