Abstract
Objective
To investigate the in vitro and in vivo anticancer effects of a chalcone against KYSE-4 esophageal cancer cells.
Methods
A chalcone was synthesized via the molecular hybridization strategy based on the anticancer activity of chalcone and dithiocarbamate scaffolds. The anticancer effects of different concentrations of the chalcone derivative were compared in esophageal cancer cells.
Results
This chalcone displayed strong inhibitory effects on esophageal cancer cell growth with an IC50 of 1.06 μM in KYSE-4 cells. Analysis of the mechanism revealed that the derivative obviously inhibited KYSE-4 cell growth, migration, and invasion in a concentration-dependent manner. Furthermore, the compound regulated migration-related biomarkers (E-cadherin, N-cadherin, and Slug) and inhibited the Wnt/β-catenin pathway. According to western blotting, this chalcone suppressed the expression of proline-rich protein 11 (PRR11) in a concentration- and time-dependent manner.
Conclusions
This chalcone might be a leading candidate for suppressing the growth and metastasis of esophageal cancer by downregulating PRR11 expression and inhibiting Wnt/β-catenin signaling.
Keywords: Chalcone, esophageal cancer, cell growth, metastasis, PRR11, invasion, epithelial–mesenchymal transition, molecular hybridization
Introduction
Esophageal cancer (EC), which mainly consists of esophageal adenocarcinoma and esophageal squamous cell carcinoma, is the eighth most common cancer and the sixth leading cause of cancer death globally.1–3 Because of tumor metastasis and infiltration, the prognosis of esophageal cancer is unfavorable, and it is difficult to completely remove the tumor via surgery.4 Therefore, it is necessary to develop potent antitumor agents that inhibit the growth and metastasis of EC.
Proline-rich protein 11 (PRR11), a newly discovered gene encoding a 360-amino acid protein on human chromosome 17q22, is upregulated in various tumors.5 Silencing or downregulating PRR11 expression can suppress cellular proliferation, inhibit tumor metastasis, and reduce tumor growth in vivo.6 Thus, PRR11 might be a potential new target for antitumor agents that treat EC. Chalcone scaffolds are present in biologically active molecules including synthetic and natural products, and chalcone-containing compounds exhibit potent antitumor activity.7 In this work, we discovered a chalcone that inhibited the growth and metastasis of EC. Importantly, this chalcone suppressed PRR11 expression and regulated the Wnt/β-catenin pathway.
Materials and methods
Chemistry section
The chalcone was synthesized by our group and identified using 1H NMR and 13C NMR (Bruker 400 and 100 MHz spectrometer, Beijing China). The molecular weight was determined using high-resolution mass spectrometry (Micromass Q-Tof micro Mass Spectrometer, Waters, Beijing, China).
Cell culture and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
EC cells (KYSE-4, KYSE-180, EC-109) were maintained in Roswell Park Memorial Institute 1640 medium (Hyclone, GE Healthcare, Chicago, IL, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, GE Healthcare) and 1% penicillin-streptomycin (Hyclone, GE Healthcare) in an atmosphere of 5% CO2 at 37°C. All cell lines were purchased from the China Center for Type Culture Collection (Wuhan, China). The cells were incubated with the chalcone derivative for different intervals. Then, MTT solution (Hyclone, GE Healthcare) was added and the cells were incubated for 4 hours at 37°C. The MTT-containing medium was discarded, 150 µL of DMSO were added, and the plate was agitated until the dark violet color completely dissolved. The absorbance was measured using a microplate reader at 570 nm (PNXD-M96, Beijing Potenov Technology Co., Ltd., Beijing, China).
Migration assay
Animals were treated according to the protocols established by the ethics committee of Henan Medical College, and the experiments were conducted in accordance with approved guidelines following approval by the ethics committee of Henan Medical College (Ethics Committee reference number: HMC-2019).
KYSE-4 cells were seeded in 24-well Transwell plates (Corning, Corning, NY, USA) and treated with the chalcone for different intervals (24, 48, and 72 hours) and at different concentrations (0.5, 1, and 2 µM). Then, 1% heat-inactivated FBS and 20% FBS were added into the upper and lower chambers, respectively. After incubation, the medium was removed, and the chambers were washed with PBS. The migrating cells were fixed via incubation in methanol for 20 minutes and stained with Hoechst-33258 (Hyclone, GE Healthcare).
Invasion assay
Animals were treated according to the protocols established by the ethics committee of Henan Medical College, and the experiments were conducted in accordance with approved guidelines following approval by the ethics committee of Henan Medical College (Ethics Committee reference number: HMC-2019).
KYSE-4 cells were seeded in 24-well Invasion plates (Corning) and treated with the chalcone for different intervals (24, 48, and 72 hours) and at different concentrations (0.5, 1, and 1.5 µM). The plates were wetted before use. Then, 1% heat-inactivated FBS and 20% FBS were added into the upper and lower chambers, respectively. After incubation, the medium was removed, and the chambers were washed with PBS. The cells were fixed via incubation with methanol for 20 minutes and stained with Hoechst-33258.
Western blot
Animals were treated according to the protocols established by the ethics committee of Henan Medical College, and the experiments were conducted in accordance with approved guidelines following approval by the ethics committee of Henan Medical College (Ethics Committee reference number: HMC-2019).
KYSE-4 cells were cultured with different concentrations (0.5, 1, and 1.5 µM) of the chalcone for 24, 48, or 72 hours. Equal amounts of cell lysates were denatured, and separated via sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Then, proteins were transferred to a PVDF membrane, which was blocked with milk-containing buffer for 2.5 hours at room temperature and incubated with a primary antibody overnight at 4°C. The next day, the PVDF membrane was incubated with a secondary antibody. Protein bands were visualized using an enhanced chemiluminescence kit (Thermo Fisher Scientific, Waltham, MA, USA).
Xenograft assay
BALB/c nude mice were purchased from Hunan Slack Scene of Laboratory Animal Co., Ltd. (Hunan, China). A xenograft model was established in BALB/c mice using KYSE-4 cells. The treatment group received the chalcone derivative daily at a dose of 80 mg/kg for 21 days.
Ethical approval
Animals were treated according to the protocols established by the ethics committee of Henan Medical College, and the experiments were conducted in accordance with the approved guidelines and following approval by the ethics committee of Henan Medical College (Ethics Committee reference number: HMC-2019).
Results
Chemical structure of the chalcone analog
The structure (Figure 1) was synthesized by our group. The chalcone was a yellow solid, and its melting point was 175°C. The structure was fully characterized by 1H NMR, 13C NMR, and HRMS. 1H NMR (400 MHz, CDCl3) δ 9.47 (s, 1H), 8.01 (d, J = 8.7 Hz, 2H), 7.80 (d, J = 15.7 Hz, 1H), 7.69 to 7.63 (m, 4H), 7.53 (d, J = 15.7 Hz, 1H), 7.42 (dd, J = 5.1, 2.0 Hz, 3H), 4.40 (s, 2H), 4.27 (s, 2H), 3.98 (s, 2H), 2.57 to 2.50 (m, 4H), 2.34 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 195.65, 188.91, 167.28, 144.44, 142.21, 134.97, 133.76, 130.45, 129.87, 128.94, 128.42, 121.84, 119.15, 54.29, 45.54, 40.35. HRMS (ESI) calcd. for C23H26N3O2S2 [M + H]+: 440.1466, found: 440.1469.
Figure 1.
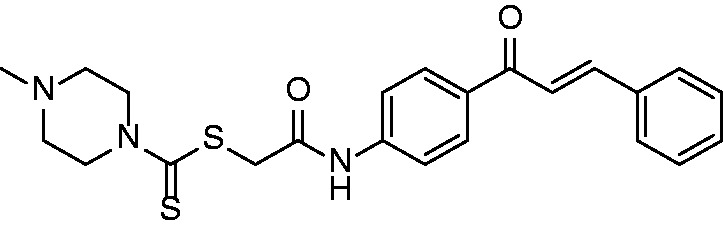
Chemical structure of chalcone.
The chalcone inhibited the proliferation of EC cells
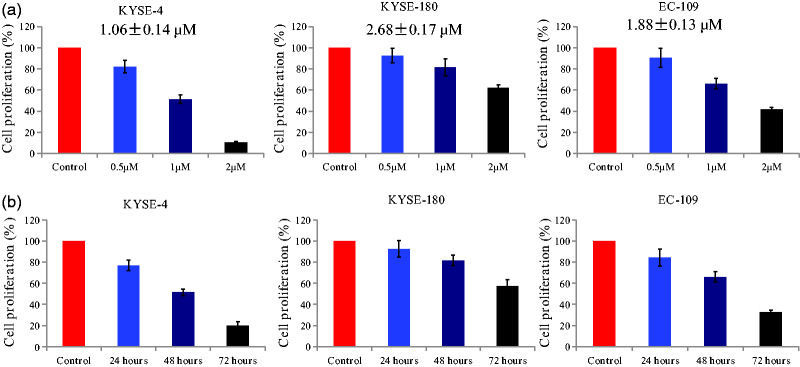
The antiproliferative activity of the compound was examined using EC cells (KYSE-4, KYSE-180, and EC-109). As presented in Figure 2a, the chalcone derivative displayed moderate-to-potent antiproliferative activity against all EC cell lines with an IC50 of 1.06 to 2.68 µM. The proliferation of EC cells was obviously decreased by 24, 48, or 72 of incubation with the chalcone at a concentration of 1 µM (Figure 2b). These results indicated that the chalcone analog inhibited the proliferation of EC cells in a concentration- and time-dependent manner.
Figure 2.
Antiproliferative activity of the chalcone derivative in esophageal cancer cells. (a) Esophageal cancer cells were treated with different concentrations of the chalcone (0.5, 1, and 2 μM) for 48 hours. (b) Esophageal cancer cells were treated with the compound at a concentration of 1 μM for different durations (24, 48, and 72 hours).
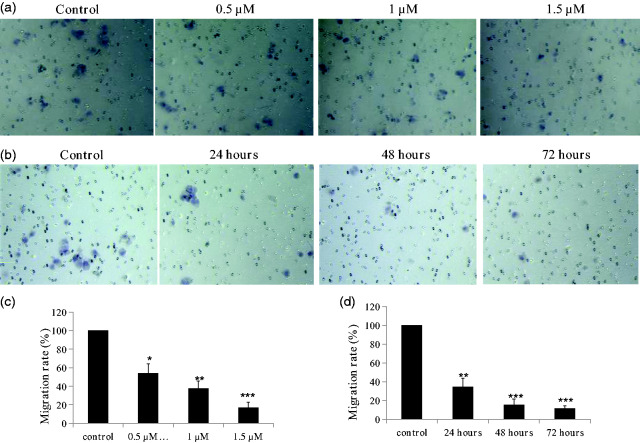
Chalcone inhibited the migration against KYSE-4 cells
Metastasis involves the detachment of cancer cells from the primary tumor and their transport as circulating tumor cells through the bloodstream or lymphatic system to other parts of the body.8 The migration of cancer is a pivotal step in the metastatic process.9 Based on the results of growth inhibition assays, KYSE-4 cells were selected to explore the inhibitory effects of the chalcone on migration (Figure 3). Treatment with the chalcone for 48 hours at concentrations of 0.5, 1 and 1.5 µM decreased the migration rate of KYSE-4 cells to 54.2%, 37.5%, and 16.7% of the control level, respectively (all P < 0.05). Meanwhile, treatment of the cells at a concentration of 1 µM for 24, 48, and 72 hours decreased the migration rate to 34.6%, 15.4%, and 11.5% of the control level, respectively (all P < 0.05).
Figure 3.
Inhibitory effects of the chalcone on the migration of KYSE-4 cells. (a, c) KYSE-4 cells were treated with the different concentrations of the chalcone (0.5, 1, and 1.5 μM) for 48 hours. (b, d) KYSE-4 cells were treated with the compound at a concentration of 1 μM for different durations (24, 48, and 72 hours). The data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001, significantly different versus the control.
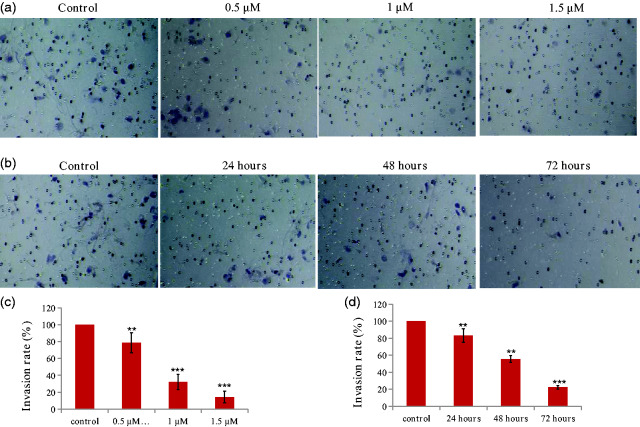
The chalcone inhibited the invasion of KYSE-4 cells
Local tumor invasion is a key event during tumor progression that can lead to metastasis and poor patient outcomes.10 We evaluated the inhibitory effects of this chalcone analog on the migration of KYSE-4 cells. As shown in Figure 4, the chalcone analog inhibited the invasiveness of KYSE-4 cells in a concentration- and time-dependent manner. Treatment with the compound at concentrations of 0.5, 1, and 1.5 µM for 48 hours reduced invasion rates to 78.6%, 32.1%, and 14.3% of the control level, respectively (all P < 0.05). When KYSE-4 cells were exposed to 1 µM for 24, 48, and 72 hours, the invasion rates versus the control were 83.3%, 55.6%, and 22.2%, respectively.
Figure 4.
Inhibitory effects of the chalcone on the invasion of KYSE-4 cells. (a, c) KYSE-4 cells were treated with different concentrations of the chalcone (0.5, 1, and 1.5 μM) for 48 hours. (b, d) KYSE-4 cells were treated with the compound at a concentration of 1 μM for different durations (24, 48, and 72 hours). **P < 0.01 and ***P < 0.001, significantly different compared with the control by test.
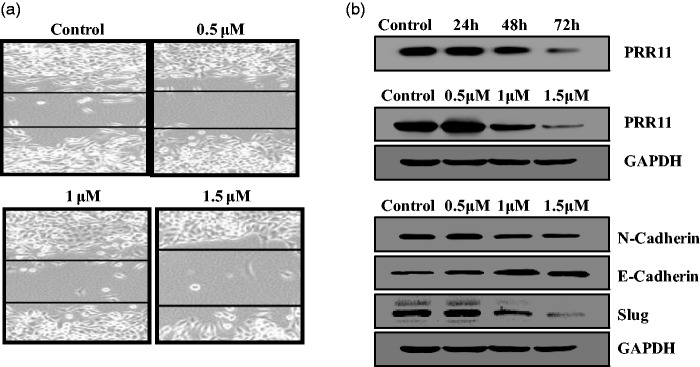
Chalcone decreased the expression of PRR11 and inhibited epithelial-mesenchymal transition (EMT) in KYSE-4 cells
EMT is a significantly pathophysiological process in the development of esophageal squamous cell carcinoma.11 Recent studies illustrated that PRR11 regulates cancer cell invasion and proliferation.12 In this work, we found that the chalcone analog decreased the expression of PRR11 (Figure 5b). Morphological changes induced by the compound were evaluated using a wound-healing assay. As shown in Figure 5a, the chalcone obviously inhibited wound healing in KYSE-4 cells. In addition, the treatment increased the expression of E-cadherin and decreased that of EMT-related markers (N-cadherin and Slug).
Figure 5.
(a) Chalcone blocked epithelial-mesenchymal transition (EMT) in KYSE-4 cells in a wound healing assay. (b) Western blot analysis of proline-rich protein (PRR11) and EMT-related markers (E-cadherin, N-cadherin, and Slug) in cells treated with the chalcone. GAPDH served as a loading control.
A chalcone regulated the Wnt/β-catenin pathway in KYSE-4 cells
Wnt/β-catenin signaling pathways comprise a group of signal transduction pathways that play significant roles in cell proliferation and metastasis.13 Wnt activation has been observed in lung cancer, breast cancer, and EC, and it contributes to tumor recurrence.14 In this work, we next examined the protein expression of Wnt pathway-related makers such as β-catenin, GSK-3β, and TCF4. As shown in Figure 6, the chalcone analog upregulated the expression of GSK-3β and downregulated that of β-catenin and TCF4. These results indicated that this chalcone could inhibit the Wnt/β-catenin pathway in KYSE-4 cells.
Figure 6.
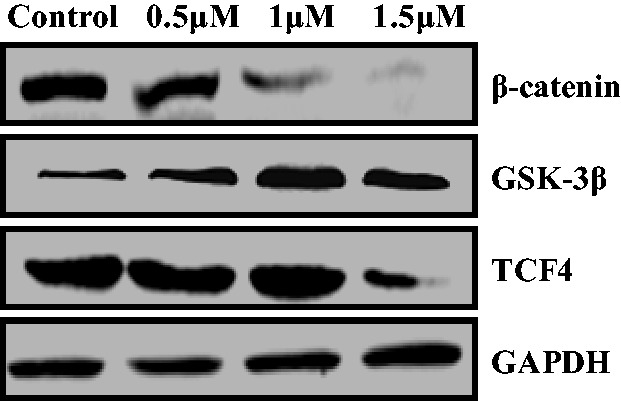
Western blot analysis of Wnt/β-catenin signaling components. GAPDH served as a loading control.
In vivo antitumor effects of the chalcone
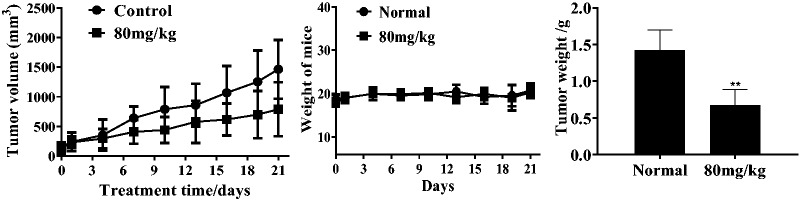
Given the obvious inhibitory effects of the chalcone on KYSE-4 cells, we also evaluated its in vivo antitumor effects in mice bearing KYSE-4 tumors following subcutaneous implantation. After treatment with the chalcone, body weight, tumor weight, and tumor volume were measured and recorded. As shown in Figure 7, treatment with the chalcone remarkably inhibited tumor growth, whereas the body weight of mice was almost unchanged, supporting the antitumor efficacy and low global toxicity of the therapy.
Figure 7.
In vivo antitumor effects. **P < 0.01 denoted statistical significance compared with the control.
Discussion
EC is a common cancer type globally that is frequently fatal.15–17 Thus, the development of potent anticancer drugs is urgently needed for EC treatment. Chalcones, as the core of many biologically interesting compounds from natural sources and synthetic agents, have attracted substantial attention because of their anticancer effects.7 In addition, the dithiocarbamate moiety has been used to design anticancer hybrids.18 Based on the anticancer activity of chalcone and dithiocarbamate scaffolds, we designed a chalcone–dithiocarbamate hybrid via the molecular hybridization strategy.
In this work, we discovered a chalcone derivative with potent antiproliferative activity against EC. The IC50 values of the compound in KYSE-4, KYSE-180, and EC-109 cells were 1.06, 2.68, and 1.88 µM, respectively. The derivative also inhibited EC cell proliferation in a concentration- and time-dependent manner.
Based on the study results, the chalcone inhibited the invasion and migration of KYSE-4 cells in a concentration- and time-dependent manner. In addition, the treatment obviously decreased the protein expression of PRR11 and inhibited EMT in KYSE-4 cells. Furthermore, the chalcone downregulated the expression of β-catenin and TCF4 and upregulated that of GSK-3β. In summary, this novel chalcone inhibited the growth and metastasis of EC cells by inhibiting PRR11 expression and Wnt/β-catenin signaling. This compound might be a promising anticancer agent with potential clinical applications for several malignancies.
Authors’ contributions
Jie Chen, Chun-Yan Kang, Hong-Mei Yang, Zhao-Xia Niu, and Hui-Cong Zhou performed all experiments. Hong-Mei Yang designed the study and analyzed the experimental data. All authors read and approved the final manuscript.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This study was funded by the Foundation Project of Young Backbone Teachers Training Program in Henan Higher Education Institutions (2016GGJS-261) and the Key Scientific and Technological Research Projects in Henan Province (192102310103).
ORCID iD
Hong-Mei Yang https://orcid.org/0000-0003-2071-9605
References
- 1.Li H, Jiang C, Wu D, et al. The prognostic and clinicopathologic characteristics of CD147 and esophagus cancer: a meta-analysis. PLoS One 2017; 12: e0180271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng KC, Chen YL, Lai SW, et al. Risk of esophagus cancer in diabetes mellitus: a population-based case-control study in Taiwan. BMC Gastroenterol 2012; 12: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin EW, Karakasheva TA, Hicks PD, et al. The tumor microenvironment in esophageal cancer. Oncogene 2016; 35: 5337–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang L, Zhao X, Meng X, et al. Involved field irradiation for the treatment of esophageal cancer: is it better than elective nodal irradiation? Cancer Lett 2015; 357: 69–74. [DOI] [PubMed] [Google Scholar]
- 5.Tan S, Jiang Z, Hou A, et al. Expression of PRR11 protein and its correlation with pancreatic cancer and effect on survival. Oncol Lett 2017; 13: 4117–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Zhang Y, Zhang C, et al. The gene pair PRR11 and SKA2 shares a NF-Y-regulated bidirectional promoter and contributes to lung cancer developent. Biochim Biophys Acta 2015; 1849: 1133–1144. [DOI] [PubMed] [Google Scholar]
- 7.Zhuang C, Zhang W, Sheng C, et al. Chalcone: a Privileged Structure in Medicinal Chemistry. Chem Rev 2017; 117: 7762–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munkley J, McClurg UL, Livermore KE, et al. The cancer-associated cell migration protein TSPAN1 is under control of androgens and its upregulation increases prostate cancer cell migration. Sci Rep 2017; 7: 5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul CD, Mistriotis P, Konstantopoulos K. Cancer cell motility: lessons from migration in confined spaces. Nat Rev Cancer 2017; 17: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow CR, Ebine K, Knab LM, et al. Cancer Cell Invasion in Three-dimensional Collagen Is Regulated Differentially by Gα13 Protein and Discoidin Domain Receptor 1-Par3 Protein Signaling. J Biol Chem 2016; 291: 1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Chen J, Tang H, et al. EGCG Suppresses ERK5 Activation to Reverse Tobacco Smoke-Triggered Gastric Epithelial-Mesenchymal Transition in BALB/c Mice. Nutrients 2016; 8: E380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Z, Liu W, Xiao Y, et al. PRR11 Is a Prognostic Marker and Potential Oncogene in Patients with Gastric Cancer. PLoS One 2015; 10: e0128943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed MK, Shao C, Wang J, et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis 2016; 3: 11–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnamurthy N, Kurzrock R. Targeting the Wnt/beta-catenin pathway in cancer: update on effectors and inhibitors. Cancer Treat Rev 2018; 62: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang ZH, Ma XW, Zhang J, et al. Cetuximab for esophageal cancer: an updated meta-analysis of randomized controlled trials. BMC Cancer 2018; 18: 1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeser H, Waldschmidt D, Kuetting F, et al. Somatic BRCA1-associated protein 1 (BAP1) loss is an early and rare event in esophageal adenocarcinoma. Mol Clin Oncol 2017; 7: 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang T, Guo P, Zhang Y, et al. The Antidiabetic Drug Metformin Inhibits the Proliferation of Bladder Cancer Cells in Vitro and in Vivo. Int J Mol Sci 2013; 14: 24603–24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li RD, Wang HL, Li YB, et al. Discovery and optimization of novel dual dithiocarbamates as potent anticancer agents. Eur J Med Chem 2015; 93: 381–391. [DOI] [PubMed] [Google Scholar]