Abstract
Purpose:
Although the incidence rate of epithelial ovarian cancer (EOC) is somewhat lower in African American (AA) than white women, survival is worse. The Ovarian Cancer in Women of African Ancestry (OCWAA) consortium will overcome small, study-specific sample sizes to better understand racial differences in EOC risk and outcomes.
Methods:
We harmonized risk factors and prognostic characteristics from eight U.S. studies: the North Carolina Ovarian Cancer Study (NCOCS), the Los Angeles County Ovarian Cancer Study (LACOCS), the African American Cancer Epidemiology Study (AACES), the Cook County Case-Control Study (CCCCS), the Black Women’s Health Study (BWHS), the Women’s Health Initiative (WHI), the Multiethnic Cohort Study (MEC) and the Southern Community Cohort Study (SCCS).
Results:
Determinants of disparities for risk and survival in 1,146 AA EOC cases and 2,922 AA controls will be compared to 3,368 white EOC cases and 10,270 white controls. Analyses include estimation of population attributable risk percent (PAR%) by race.
Conclusion:
OCWAA is uniquely positioned to study the epidemiology of EOC in AA women compared with white women to address disparities. Studies of EOC have been underpowered to address factors that may explain AA-white differences in the incidence and survival. OCWAA promises to provide novel insight into disparities in ovarian cancer.
Keywords: ovarian cancer, race, disparities, risk, survival
INTRODUCTION
The incidence rate of epithelial ovarian cancer (EOC) is somewhat lower in African American (AA) women (9.6/100,000) than white women (12.4/100,000), but survival is worse. [1-2] EOC is the most lethal gynecologic malignancy among women in the U.S., accounting for 5% of all cancer deaths. [3] It is the fifth-leading cause of cancer deaths among white women [3] and the sixth-leading cause of cancer deaths among AA women. [4] Statistics from the Surveillance, Epidemiology, and End Results (SEER) Program show a steady increase in relative survival over the past four decades for white women—from 35.3% to 45.6%—whereas no improvement has been seen among AA women, for whom relative survival remains at 36.4%. [2,5]
EOC comprises more than 90% of all ovarian cancer diagnoses. [6] EOC is a heterogeneous disease with five major histotypes—high-grade serous, low-grade serous, endometrioid, clear cell and mucinous—that have different epidemiologic, molecular and clinical features. [7] The most common histotype, high-grade serous carcinoma, accounts for approximately 65% of all incident EOC cases, and 79% are diagnosed at distant stages. [8]
There are several well-established EOC risk factors, including oral contraceptive (OC) use, [9] parity, [10] breastfeeding [11,12] and tubal ligation, [13,14] all of which are consistently associated with decreased risk. [15] Younger age at menarche and menopausal hormone use (particularly use of estrogen-only therapy) have been associated with increased risk of EOC in some, but not all, studies. [12,13,16-22] Family history of breast or ovarian cancer is also associated with increased risk. [23] There is a modest risk assocated with excess increased BMI among women who have not used menopausal hormones. [24,25] Other risk factors that are less well-established include aspirin use, associated with a reduced risk [26] and genital talc use, associated with increased risk. [27] Several risk factors have been associated with specific ovarian carcinoma histotypes. Smoking is associated with an increased risk of mucinous carcinoma, and endometrioisis is strongly associated with an increased risk of endometrioid and clear cell carcinomas. [28-30]
Because most published studies of EOC in AA women include fewer than 150 cases, little is known about the epidemiology in AA women. [31-35] Additionally, there has been little evaluation of differences in risk and prognostic factors between AA and white women. Most published reports have shown similar associations of established risk factors with EOC incidence in white and AA women; however, there are racial differences in the prevalence of many risk factors, particularly reproductive factors. [35,36, 37-44,45-49] Recent work by Peres, et al. [50] combined the largest case-control study of AA women with EOC, the African-American Cancer Epidemiology Study (AACES), and 11 case-control studies in the Ovarian Cancer Association Consortium (OCAC) (911 AA cases and 1,233 AA controls) to evaluate risk factor associations by race/ethnicity. Although this study included an unprecedented number of AA cases, the study was still underpowered to evaluate less prevalent risk factors and associations by histotype.
As with EOC risk, histotype is an important determinant of survival outcomes, with better survival noted for endometrioid carcinomas at any stage and the poorest survival for distant stage mucinous and clear cell carcinomas. [8,51] However, regardless of histotype or stage at diagnosis, survival from EOC is poorer among AA women than white women. [52] Analyses of Kaiser Permanente Northern California EOC patients demonstrated that disparities persist in cancer treatment, with AA women more likely than white women to experience delays in chemotherapy and undergo fewer treatments than the regimen prescribed. [53] However, another recent analysis using data from a tertiary referral center suggest that AA-white survival differences may be mitigated by equal access to highly specialized care. [54] Clearly, further investigation is needed to understand the factors that contribute to the poorer survival of AA women.
The newly formed consortium, Ovarian Cancer in Women of African Ancestry (OCWAA), brings together investigators dedicated to understanding racial differences in risk and outcomes in EOC. The goal of OCWAA is to assemble a large sample of AA and white cases and controls to better understand the determinants of differences in EOC incidence and survival by race. All eight studies in OCWAA include at least 40 AA cases, which provides the ability to assess heterogeneity across studies. Below we describe the OCWAA study population, the available risk factor and prognostic data, the process of data harmonization across studies and planned analytic approaches.
MATERIALS AND METHODS
OCWAA studies
Table 1 summarizes the characteristics of the eight participating OCWAA studies. The four case-control studies— the North Carolina Ovarian Cancer Study (NCOCS), [55] the Los Angeles County Ovarian Cancer Study (LACOCS), [56] AACES [57] and the Cook County Case-Control Study (CCCCS) [58,59]—identified cases from population-based registries using rapid case ascertainment. NCOCS used random-digit dialing to identify controls, which were then matched to cases by five-year age categories and race (white vs. non-white). LACOCS identified neighborhood controls and matched them on race and ethnicity (AA, Latina, non-Latina-white) and year of birth (± 5 years). In an early phase of LACOCS, the study identified controls from the Health Care Financing Administration (HCFA, now known as the Centers for Medicare and Medicaid Services [CMS]) and matched them by ZIP code, race and ethnicity, and year of birth to cases aged 65 years or older. AACES identified controls by random-digit dialing and matched them by five-year age categories and geographic region of residence. CCCCS matched controls by five-year age categories and race (white or black). CCCCS identified controls younger than 65 using random-digit dialing and those 65 or older using HCFA files. All studies classified race based on self-report.
Table 1.
Studies in the Ovarian Cancer in Women of African Ancestry (OCWAA) Consortium
| Study (acronym) | Location | Years of enrollment |
Age range |
Case follow-up timea (median y) |
AA cases (invasive/ borderline) |
AA controls |
White cases (invasive/ borderline) |
White controls |
|---|---|---|---|---|---|---|---|---|
| Population-Based Case Control | ||||||||
| African American Cancer Epidemiology Study (AACES) |
AL, GA, IL, LA, MI, NJ, OH, NC, SC, TN, TX | 2011-2016 | 20-79 | 3.0 | 580/12 | 752 | 0/0 | 0 |
| Los Angeles County Ovarian Cancer Study (LACOCS) | CA | 1993-2010 | 20-82 | 7.5 | 127/40 | 145 | 1180/304 | 1806 |
| Cook County Case-Control Study (CCCCS) | IL | 1994-1998 | 20-76 | 6.7 | 44/10 | 80 | 233/63 | 421 |
| North Carolina Ovarian Cancer Study (NCOCS) | NC | 1999-2008 | 20-75 | 4.9 | 117/33 | 193 | 819/189 | 867 |
| Prospective Cohort | ||||||||
| Black Women's Health Study (BWHS) | All regions of the U.S. | 1995 | 24-74 | 3.3 | 92/9 | 606 | 0/0 | 0 |
| Multiethnic Cohort Study (MEC) | CA, HI | 1993-1996 | 45-91 | 1.9 | 92/0 | 552 | 148/0 | 888 |
| Southern Community Cohort Study (SCCS) | AL, AR, FL, GA, KY, LA, MS, NC, SC, TN, VA, WV |
2002-2009 | 40-79 | 1.8 | 48/0 | 288 | 29/0 | 174 |
| Women's Health Initiative (WHI) | 40 Clinical Centers across the US | 1993-1998 | 50-79 | 2.2 | 46/5 | 306 | 959/60 | 6114 |
| Total | 4.9 | 1146/109 | 2922 | 3368/616 | 10270 | |||
OCWAA also includes 17 subjects who self-reported as multi-racial or for whom race information was not available.
64 cases have unknown tumor behavior.
Years between diagnosis and last follow-up or death.
Four studies in OCWAA are ongoing prospective cohort studies. The Multiethnic Cohort Study (MEC) [60] includes >215,000 participants aged 45-75 at baseline (1993-1996) who reside in California and Hawaii; MEC identifies incident cancer cases through linkage to the California and Hawaii cancer registries. The MEC study contributes both AA and white cases and controls, with most AA participants living in Los Angeles County, CA. The Black Women’s Health Study (BWHS) [61] enrolled 59,000 AA women aged 21-69 from across the U.S. in 1995 and follows them through biennial questionnaires. BWHS identifies EOC cases through self-report and from cancer registries in 24 states in which 95% of participants live; the study uses hospital and registry records to confirm these cases. Two additional prospective cohort studies have recently joined OCWAA: the Women’s Health Initiative (WHI) [62] and the Southern Community Cohort Study (SCCS). [63] The WHI is a multicenter longitudinal study of postmenopausal women (161,808 women; approximately 14,627 AA and 133,534 white participants) in the U.S. Women aged 50-79 enrolled in the clinical trials (CTs) or observational study (OS) between October 1993 and December 1998. The SCCS enrolled 50,342 women (32,344 non-Hispanic AA and 15,438 non-Hispanic white women) aged 40-79 years from 12 southeastern states (Alabama, Arkansas, Florida, Georgia, Kentucky, Louisiana, Mississippi, North Carolina, South Carolina, Tennessee, Virginia and West Virginia) between March 2002 and September 2009; the SCCS ascertains cases through linkage with the cancer registries in these 12 states.
Each OCWAA cohort study has constructed a nested case-control study with four to six controls per case. For each case, the studies selected eligible controls, alive at the time of case diagnosis and with at least one ovary, and matched them to the case on race, age of diagnosis and last questionnaire completed prior to EOC diagnosis (index date). We use index year to determine which of the repeated measures of a given variable we will bring into the nested case-control study.
Ovarian carcinoma histotype and clinical characteristics
NCOCS, AACES and CCCCS conducted a centralized pathology review to confirm diagnosis for EOC cases. An expert pathologist reviewed a subset of the LACOCS cases; no centralized review was performed for MEC, BWHS or SCCS. WHI identified EOC diagnoses by annual self-report and confirmed cases with medical record review adjudicated and coded by trained physician adjudicators. [64] Therefore, all eight OCWAA studies verify diagnoses through pathology reports.
To determine histotype, we applied the histotype classification scheme described in Peres, et al. [8] This schema classifies histotype using a combination of morphology and grade information to best represent the most recent diagnostic guidelines for ovarian carcinomas as detailed in the 2014 World Health Organization (WHO) Classification of Tumors of Female Reproductive Organs. [65] First, we grouped the International Classification of Diseases for Oncology, Version 3 [66] morphology codes into histology categories. Next, we categorized cases with a serous histology and grade 1 as low-grade serous, and cases with a serous histology and grade 2 or greater as high-grade serous. We classified high-grade endometrioid (grades 3 and 4) as high-grade serous since the majority of high-grade endometrioid carcinomas are biologically similar to high-grade serous carcinomas. [66,67]
Because the source of data on tumor characteristics varied by study (e.g., medical records, cancer registries), we created a summary variable to uniformly characterize tumor stage. This summary variable used all available tumor stage information—as classified by either the International Federation of Gynecologic Oncology (FIGO) staging system or the SEER staging system—to characterize stage similar to the SEER Summary Stage 2000+ variable: localized (equivalent to FIGO Stage I), regional (equivalent to FIGO Stage II), distant (equivalent to FIGO stages III and IV) or unknown.
Data collection
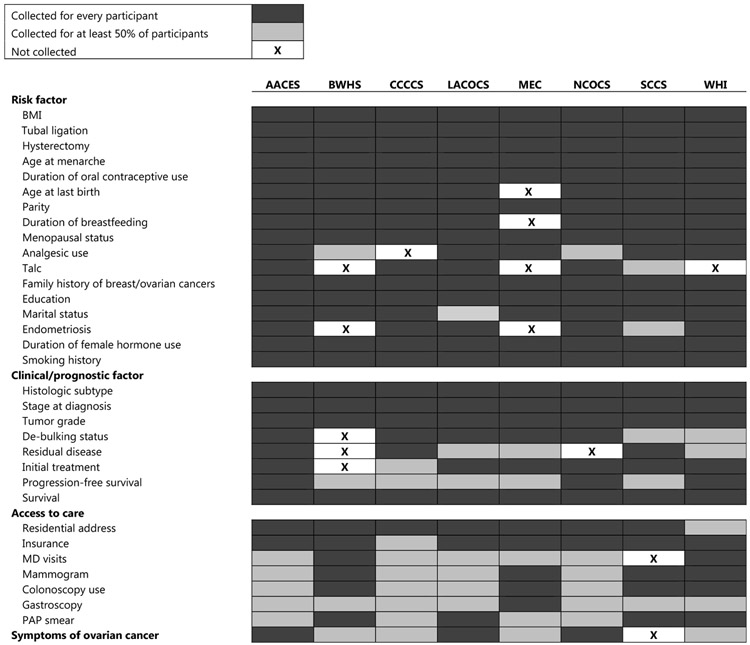
We have obtained demographic, lifestyle, reproductive history and medical history data for cases and controls for all studies in OCWAA (Figure 1). In addition, prognostic variables such as age, histotype and stage at diagnosis are available for most EOC cases in the consortium. OCWAA also has data on vital status and follow-up time for EOC patients.
Fig. 1.
Chart describing the availability of harmonized data elements across OCWAA studies
For most cases, AACES obtained epidemiologic data during a telephone interview within a year of the diagnosis; the study also obtains important prognostic variables and updates vital status on an annual basis from cancer registries. Vital status updates are additionally supplemented using LexisNexis Accurint,[68] an electronic database that includes death records. CCCCS obtained epidemiologic questionnaire data from in-person interviews; the last vital status update was in 2008. LACOCS also obtained epidemiologic data from an in-person interview and key prognostic factors from the tumor registry. The study updated vital status in October 2018 using California Cancer Registry data. NCOCS obtained questionnaire data from an in-person interview conducted by a trained research nurse and has updated vital status using the Social Security Administration (SSA) Death Index and, more recently, the National Death Index (NDI) and, in 2018, the North Carolina Central Cancer Registry. Key prognostic variables are also available for most cases. BWHS follows its participants with self-administered questionnaires every two years for data on risk factors and incident disease. The study updates vital status every year using the NDI. All participants in MEC returned a self-administered baseline questionnaire with information on demographic, diet and lifestyle factors; MEC assesses participants’ health/disease and lifestyle prospectively through questionnaires every five years (1999-2001, 2003-2008, 2011-2013, 2015-present). MEC annually updates vital status using California and Hawaii death certificate files and, periodically, NDI. WHI utilizes clinic visits to collect an array of epidemiologic data via self-administered forms, interviews, clinical measurements and biospecimens. These visits/exams occurred through the end of the primary study period in 2005, with frequency of data collection varying according to whether participants are enrolled in the CT (yearly) or the OS (every three years) arm. WHI periodically updates vital status using the NDI. Most SCCS participants (86%) completed computer-assisted in-person interviews at community health centers for medically underserved populations; the remaining completed mailed questionnaires. In addition to baseline data, SCCS conducts active follow-up every three years by mail and telephone calls, and also conducts annual linkage to the SSA, NDI, and state cancer registries.
Most OCWAA studies also abstracted data on available clinical and prognostic factors, including treatment data (Figure 1). This abstraction has been completed for most EOC cases in AACES, MEC, LACOCS, BWHS, WHI and SCCS and a subgroup of cases in NCOCS. These studies have relied on medical records requests from hospitals, electronic medical records and cancer registry data. Treatment data are not available for the CCCCS. We are able to address missing clinical treatment data in five of the OCWAA studies and to obtain data on new ovarian cases as they are identified in the four cohort studies. Regarding treatment data, BWHS will use state tumor registry data to augment current data on initial treatment, including type of surgery and chemotherapy, and residual disease after primary debulking surgery. LACOCS and MEC obtained available first-course treatment, including surgery, chemotherapy, radiation, hormonal therapy and immunotherapy, from the California and Hawaii cancer registries, both members of SEER. WHI obtains treatment data through Medicare data, direct medical record abstraction and self-reported data from cancer survivors. [69] SCCS obtains treatment data from tumor registries. AACES and NCOCS have completed acquisition of initial treatment data; again, CCCCS did not collect treatment data.
Harmonization of risk factor data
We have constructed a centralized core database of epidemiologic risk factors, tumor characteristics and clinical follow-up information for both borderline and invasive EOC cases. To achieve harmonization of the core data, each study’s principal investigator (PI) provided copies of the data collection instrument (e.g., surveys, medical record abstraction forms). We formed working groups assigned to specific exposure variables; these groups compared the instruments, question by question, to determine the best approach to harmonize the variable(s) of interest according to the levels and categories of the data collected for each study. In some instances, data from all studies were easily mapped to a “common variable” because these data were collected in identical ways by all studies (e.g., age). In many cases, however, studies asked questions or categorized responses differently, requiring extensive discussions to develop the common variable. Based on this detailed review, we created a data dictionary identifying the variables to be used in analyses. Each study then created the common variable for each variable of interest and submitted its data to the OCWAA coordinating center at the University of Virginia.
RESULTS
Participants
Table 1 shows the distribution of cases and controls by study. In total, OCWAA includes 1,146 AA invasive EOC cases, 2,922 AA controls, 3,368 white invasive EOC cases and 10,270 white controls. Participants are of Hispanic and non-Hispanic ethnicity; 0.6% of AA participants in OCWAA are Hispanic, and 1.5% of white participants are Hispanic. Additionally, there are 109 AA cases and 616 white cases with a diagnosis of borderline EOC. For cases, the median follow-up time from diagnosis to last contact (known date of death or last date of contact) ranges from 1.8-7.5 years, with an overall median length of follow-up of 4.9 years (Table 1). We will add new cases accrued by the cohort studies to OCWAA as they are identified.
Table 2 provides the pooled distributions of histotype and stage at diagnosis for AA and white invasive EOC cases. Consistent with SEER and other national databases, [70] high-grade serous carcinoma is the most common histotype in both racial groups, with 63% and 62% in AA and white participants, respectively. Histotype distributions were also similar in AAs and whites within each study (data not shown). Similar proportions of AA women (70%) and white women (71%) were diagnosed at a distant stage.
Table 2.
Tumor characteristics among invasive OCWAA cases, by race
| Histotype | AA (n=1141) n (%) |
White (n=3340) n (%) |
|---|---|---|
| High-grade serous | 721 (63) | 2087 (62) |
| Low-grade serous | 34 (3) | 94 (3) |
| Endometrioid | 101 (9) | 225 (7) |
| Clear cell | 40 (4) | 229 (7) |
| Mucinous | 59 (5) | 160 (5) |
| Carcinosarcoma | 30 (3) | 78 (2) |
| Other epithelial | 156 (14) | 467 (14) |
| Unknown | 5 | 28 |
| Stage | AA (n=1079) n (%) |
White (n=3333) n (%) |
| Localized | 217 (20) | 576 (17) |
| Regional | 110 (10) | 382 (11) |
| Distant | 752 (70) | 2375 (71) |
| Unknown | 67 | 35 |
Note. The Unknown participants in both sections of this table are excluded from the overall denominator presented in the header, which is used to calculate the histotype- and stage-specific percentages. Including the Unknown categories, there are 1,146 AA cases and 3,364 white cases; this is reflected in the total number of AA and white invasive cases displayed in Table 1.
Core database
Thus far, we have harmonized both established and suspected risk factors, sociodemographic characteristics, tumor characteristics and prognostic variables. Figure 1 lists the broad categories of available epidemiologic data by study. As indicated, not all studies in OCWAA collected data on every risk factor (e.g., MEC did not collect data on breastfeeding). We will obtain area-level characteristics by linking U.S. Census data to participants’ geocoded addresses. Geocoding is complete for six (AACES, BWHS, [71] LACOCS (cases only), MEC, [72] CCCCS [59,73] and SCCS [74]); geocoding of NCOCS addresses is underway. WHI does not have geocoded addresses.
Table 3 shows distributions of EOC risk and lifestyle factors. These factors are largely complete for all studies, with missing risk factor data generally on the order of 3% to 4%. The table shows marked differences in exposure to some of the major EOC risk factors between AA and white women. AA controls have a higher prevalence of obesity (body mass index (BMI) ≥30 kg/m2), younger age at menarche and higher prevalence of tubal ligation compared with white controls. AA controls have a lower prevalence of having a college degree, breastfeeding and using menopausal hormones. Use of OCs and family history of ovarian cancer is similar in AA and white controls. Collectively, these differences may influence the population-attributable risk of EOC by race. Additionally, because obesity may be a prognostic factor for EOC, [75-78] the increased prevalence of high BMI may affect survival to a greater degree in AA women than in white women.
Table 3.
Epidemiologic risk factors, by casea status and race
| AA cases (n=1052) n (%) |
AA controls (n=2328) n (%) |
White cases (n=2380) n (%) |
White controls (n=3982) n (%) |
pcontrolsb | |
|---|---|---|---|---|---|
| Age (y) (M (SD)) | 58.2 (11.5) | 57.9 (13.3) | 59.0 (11.2) | 58.9 (12.8) | <.01 |
| No. missing | 0 | 1 | 0 | 0 | |
| BMI ≥30 kg/m2 | 563 (54) | 1042 (45) | 504 (21) | 734 (19) | <.01 |
| No. missing | 8 | 34 | 22 | 26 | |
| College degree | 343 (33) | 821 (35) | 1224 (51) | 2216 (56) | <.01 |
| No. missing | 2 | 14 | 1 | 14 | |
| Family history of ovarian cancer | 60 (6) | 69 (3) | 120 (5) | 121 (3) | 0.94 |
| No. missing | 93 | 127 | 48 | 78 | |
| Menarche age <11 years | 107 (10) | 228 (10) | 172 (7) | 257 (6) | <.01 |
| No. missing | 9 | 20 | 6 | 12 | |
| Duration of oral contraceptive use | |||||
| Never used | 396 (38) | 861 (38) | 989 (42) | 1496 (38) | 0.52 |
| <5 yearsc | 376 (36) | 757 (33) | 833 (36) | 1258 (32) | |
| 5+ years | 261 (25) | 659 (29) | 519 (22) | 1182 (30) | |
| No. missing | 19 | 51 | 39 | 46 | |
| Full-term pregnancies | |||||
| None | 197 (19) | 354 (15) | 618 (26) | 802 (20) | <.01 |
| 1 | 186 (18) | 459 (20) | 342 (14) | 577 (15) | |
| 2 | 227 (22) | 571 (25) | 716 (30) | 1227 (31) | |
| 3 | 214 (20) | 417 (18) | 410 (17) | 752 (19) | |
| 4+ | 225 (21) | 518 (22) | 293 (12) | 614 (15) | |
| No. missing | 3 | 9 | 1 | 10 | |
| Ever breastfedd,e | 284 (39) | 677 (46) | 775 (47) | 1422 (59) | <.01 |
| No. missing | 48 | 28 | 1 | 2 | |
| Tubal ligation | 304 (29) | 699 (30) | 353 (15) | 708 (18) | <.01 |
| No. missing | 5 | 7 | 5 | 11 | |
| Ever used menopausal hormonesf | 207 (27) | 478 (31) | 991 (55) | 1509 (52) | <.01 |
| No. missing | 8 | 54 | 7 | 24 | |
Table does not include data from SCCS or WHI; these studies were added to the consortium recently, and their risk factor data have not yet been fully harmonized.
Invasive only
Testing for the difference between African American and white controls
Includes participants who used oral contraceptives for <1 year
Among parous women
Not collected by MEC
Among women older than 50
Planned analyses
Data harmonization is now complete, and we have examined the distribution of all variables by study. We will construct forest plots [79] of covariate-adjusted odds ratios (ORs), adjusted for non-missing confounders and study-specific matching variables, [80] to identify potentially biased associations due to missing confounders or coding errors. If we find heterogeneity of associations due to missing confounders, we will implement a multiple imputation method to reduce bias. To mitigate loss of power due to missing data, we will estimate regression coefficients via multiple imputation, applying the substantive model-compatible modification of the fully conditional specification (FCS). [81,82] This method modifies the FCS method [83,84] to make the imputation model compatible with the analysis model when interactions and non-linearities are present. The imputation model will incorporate outcome, analysis variables, interactions and, for Cox proportional hazard models, the cumulative hazard and censoring indicator. [85] To improve imputation of systematically missing covariates by study, we will add study-specific effects (i.e., study means) and study random effects to the imputation model following a generalized approach described by Jolani, et al. [86] We will also conduct sensitivity analyses, including the simulation of missing data to induce varying strengths of association with the dependent variable to assess the robustness of regression estimates.
We will construct a pooled risk estimate and an I2 statistic [87] for each association to assess heterogeneity across the eight studies using a conventional meta-analysis. [88,89] Because the literature advocates the use of multi-level models for meta-analysis of ORs, [89,90] we will additionally evaluate model-based pooled estimates and I2 measures for comparison purposes, using the residual pseudo-likelihood (RSPL) estimation method, which may be more unbiased for small-study meta-analysis. [91]
We will also estimate the contribution of lifestyle factors, treatment and tumor characteristics to differences in EOC survival between AA and white women. Specifically, we will assess the relationship between key prognostic factors including histotype, stage and treatment and overall survival using Cox proportional hazard models. Using geocoded data, we will also evaluate the contribution of area-level indicators such as median income, poverty and markers for access to care.
Planned analyses include estimation of population attributable risk percent (PAR%) for risk and prognostic factors, singly and in combination. We will determine adjusted PAR% using the method by Bruzzi, et al. [92] This method is especially advantageous because of its applicability to case-control data, where the distribution of exposure among the cases is used as the distribution of exposure in the population at risk. Because of its relatively large sample of AA women, OCWAA is well postioned to further investigate differences in attibutable risk incorporating temporal and dose/duration variables with refined characterization of the differences in the prevalence of risk factors, [33] thus providing more accurate estimates of attributable fractions in AA and white women than previously observed.
Finally, we will pursue a novel approach to understand the differences in risk and survival that uses a simulation model estimating the relative contribution of variable exposure to potentially modifiable risk factors that have differential effects by race on EOC incidence and survival. This approach builds on a semi-Markov state-transition model of the natural history of ovarian cancer that has been used in previous studies of ovarian cancer screening. [93-95] The model was subsequently adapted to assess the impact of interventions to improve adherence to ovarian cancer treatment guidelines on survival in white and AA women [93] and the impact of OC use on ovarian cancer incidence and mortality when combined with estimates of the association of OCs with either risk-decreasing (e.g., endometrial and colorectal cancers) or risk-increasing (e.g., breast and cervical cancer, thromboembolic events) health outcomes. [96-97] Incorporating the effect of OCs on competing risks for both mortality and EOC risk is important for model calibration.
DISCUSSION
As EOC is a leading cause of cancer death in women, there is a need for increased understanding of factors related to its incidence and survival to improve prevention and treatment. This research is particularly relevant for AA women, who have a lower incidence than white women but much worse five-year relative survival. To date, no study has enrolled enough AA women to conduct adequately powered analyses either of risk factors for incidence or of factors influencing survival of this difficult-to-detect and -treat cancer. The small sample sizes are particularly evident within EOC histotypes, reflecting the logistical challenges of studying less common cancers in minority populations.
Racial disparities in ovarian cancer involve a combination of inter-related lifestyle and sociodemographic factors. OCWAA is uniquely positioned to compare the epidemiology of ovarian cancer in AA and white women, and our planned analyses will address multiple contributors to disparities, focusing on the evaluation of associations with risk factors; treatment and other prognostic factors; and the exploration of these relationships among the most common histotype, high-grade serous carcinoma. Because of the marked differences in risk factor prevalence, attributable fractions for specific risk factors related to ovarian cancer incidence likely differ by race.
Along with harmonized data and large samples size, strengths of OCWAA includes well-designed case-control and cohort studies that, in total, represent the largest number of AA women with EOC among existing epidemiologic studies in the U.S. OCWAA provides ~200 more AA cases and more than a doubling of the AA controls compared to OCAC. Since six of the eight studies also collected data for white women, OCWAA is well situated to compare relationships of risk and prognostic factors between racial groups. In addition, the OCWAA studies are a more representative sample of the US population. Because each of the eight studies contributes at least 40 cases compared to 3 of the 11 studies included in the OCAC paper, there is also greater power to assess whether results differ across studies. The EOC cases in OCWAA are a diverse sample of women across the U.S., representing distinct geographic regions and a range of socioeconomic conditions, providing the opportunity to examine how individual- and area-level measures of socioeconomic status influence risk and survival and expanding opportunities to assess the impact of societal-level factors on risk and prognosis of ovarian cancer. Although currently not a focus, several OCWAA studies have obtained biospecimens and we may incorporate these resources into future research. Seven of the eight studies collected biospecimens; all seven collected germline DNA (blood or saliva) and five collected or plan to collect tumor tissue.
Although OCWAA includes the largest number of EOC cases diagnosed in AA women, it is still underpowered for histotype-specific analyses of the less common subtypes. It will be important to continue to identify AA EOC cases, as these new cases will better represent changes in environmental exposures and current treatment practices. All four cohort studies have active follow-up for participants. Another limitation in OCWAA is incomplete clinical data, including treatment, debulking status and residual disease. The OCWAA studies did not uniformly obtain these data, thus reducing the available sample size for the survival analyses. Namely, CCCCS did not obtain data on treatment, and other studies have treatment data on only a subset of cases. We hope to use simulation models to ameliorate this deficiency.
In summary, EOC is a rare, heterogeneous disease with worse survival among AAs. Previous studies of ovarian cancer have been underpowered to address factors that may explain AA-white differences in incidence and survival. OCWAA provides a unique resource for the evaluation of the causes of disparities in EOC risk and survival between AA and white women.
Supplementary Material
Acknowledgements
The authors thank the WHI investigators and staff for their dedication and the study participants for making the program possible. A full listing of WHI investigators can be found at: http://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf. The authors would also like to thank Alpana Kaushiva for harmonizing the data from the Cook County Case-Control Study.
Additionally, the authors sincerely thank the state cancer registries that contributed to the OCWAA studies: Arizona, Arkansas, California, Colorado, Connecticut, Delaware, District of Columbia, Florida, Georgia, Hawaii, Illinois, Indiana, Kentucky, Louisiana, Maryland, Massachusetts, Michigan, Mississippi, New Jersey, New York, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia and West Virginia.
Funding: This project is supported by grant R01-CA207260 (L.A. Rosenberg, J.M. Schildkraut) from the National Cancer Institute (NCI), National Institutes of Health (NIH), U.S. Department of Health and Human Services. In addition, AACES was funded by NCI (R01-CA142081; J.M. Schildkraut); BWHS is funded by NIH (R01-CA058420 and UM1CA164974); CCCCS was funded by NIH/NCI (R01-CA61093; K.A. Rosenblatt); LACOCS was funded by NCI (R01- CA17054 [Pike], R01-CA58598 [Goodman, A. Wu] and Cancer Center Core Grant P30-CA014089 [Henderson, A. Wu]) and the California Cancer Research Program (2II0200 [A. Wu]); and NCOCS was funded by NCI (R01-CA076016; J.M. Schildkraut). SCCS is supported by the NCI (grants R01-CA092447 and U01-CA202979; W.J. Blot and W. Zheng), and data collection is performed by the Survey and Biospecimen Shared Resource, which is supported by the Vanderbilt-Ingram Cancer Center (P30-CA68485). In addition, support to bring SCCS to OCWAA was provided by a Pilot Award (PI: Beeghly-Fadiel) from the NIH Precision Medicine and Health Disparities Collaborative (NIMHD/NHGRI U54-MD010722). The WHI program is funded by the National Heart, Lung, and Blood Institute, NIH through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C and HHSN268201600004C. Additionally, WHI, “The women’s health Initiative Cancer Survivor Cohort, is funded by NCI (5UM1CA173642-05; G.L. Anderson).
Footnotes
Conflict of Interest: Patricia Moorman has received compensation for work related to litigation in regard to talc and ovarian cancer.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- [1].DeSantis C, Naishadham D, and Jemal A, “Cancer statistics for African Americans, 2013.,” CA. Cancer J. Clin, vol. 63, no. 3, pp. 151–66, 2013. [DOI] [PubMed] [Google Scholar]
- [2].Noone A et al. , “SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. Based on November 2017 SEER data submission, posted to the SEER web site, April 2018.,” Bethesda, MD, 2018. [Google Scholar]
- [3].Siegel RL, Miller KD, and Jemal A, “Cancer statistics, 2019,” CA. Cancer J. Clin, vol. 69, no. 1, pp. 7–34, January 2019. [DOI] [PubMed] [Google Scholar]
- [4].DeSantis CE et al. , “Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities,” CA. Cancer J. Clin, vol. 66, no. 4, pp. 290–308, July 2016. [DOI] [PubMed] [Google Scholar]
- [5].Siegel RL, Miller KD, and Jemal A, “Cancer statistics, 2018.,” CA. Cancer J. Clin, vol. 68, no. 1, pp. 7–30, January 2018. [DOI] [PubMed] [Google Scholar]
- [6].“Surveillance Epidemiology and End Results (SEER) Program. Seer*Stat Database: Incidence-SEER 18 Regs Research Data+Hurricane Katrina impacted Louisiana Cases, Nov 2015 sub (2000–2013). National Cancer Institute, DCCPS, Surveillance Research Program, Surve.”. [Google Scholar]
- [7].Doherty JA, Peres LC, Wang C, Way GP, Greene CS, and Schildkraut JM, “Challenges and Opportunities in Studying the Epidemiology of Ovarian Cancer Subtypes.,” Curr. Epidemiol, reports, vol. 4, no. 3, pp. 211–220, September 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Peres LC et al. , “Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage,” JNCI J. Natl. Cancer Inst, April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beral V, Doll R, Hermon C, Peto R, and Reeves G, “Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls.,” Lancet (London, England), vol. 371, no. 9609, pp. 303–14, January 2008. [DOI] [PubMed] [Google Scholar]
- [10].Adami H-O et al. , “Parity, age at first childbirth, and risk of ovarian cancer,” Lancet, vol. 344, no. 8932, pp. 1250–1254, November 1994. [DOI] [PubMed] [Google Scholar]
- [11].Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, and Hankinson SE, “Breastfeeding and risk of ovarian cancer in two prospective cohorts,” Cancer Causes Control, vol. 18, no. 5, pp. 517–523, June 2007. [DOI] [PubMed] [Google Scholar]
- [12].Luan N-N, Wu Q-J, Gong T-T, Vogtmann E, Wang Y-L, and Lin B, “Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies.,” Am. J. Clin. Nutr, vol. 98, no. 4, pp. 1020–31, October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rice MS, a Murphy M, and Tworoger SS, “Tubal ligation, hysterectomy and ovarian cancer: A meta-analysis,” J. Ovarian Res, vol. 5, no. 1, p. 13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sieh W et al. , “Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies.,” Int. J. Epidemiol, vol. 42, no. 2, pp. 579–89, April 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cooper GS, Schildkraut JM, Whittemore AS, and Marchbanks PA, “Pregnancy recency and risk of ovarian cancer,” Cancer Causes Control, vol. 10, no. 5, pp. 397–402. [DOI] [PubMed] [Google Scholar]
- [16].Beral V, Bull D, Green J, and Reeves G, “Ovarian cancer and hormone replacement therapy in the Million Women Study.,” Lancet (London, England), vol. 369, no. 9574, pp. 1703–10, May 2007. [DOI] [PubMed] [Google Scholar]
- [17].Coughlin SS, Giustozzi A, Smith SJ, and Lee NC, “A meta-analysis of estrogen replacement therapy and risk of epithelial ovarian cancer,” J. Clin. Epidemiol, vol. 53, no. 4, pp. 367–375, April 2000. [DOI] [PubMed] [Google Scholar]
- [18].Gong T-T, Wu Q-J, Vogtmann E, Lin B, and Wang Y-L, “Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies.,” Int. J. Cancer, vol. 132, no. 12, pp. 2894–900, June 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pearce CL, Chung K, Pike MC, and Wu AH, “Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin.,” Cancer, vol. 115, no. 3, pp. 531–9, February 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, and Weiss NS, “Menopausal hormone therapy and risk of epithelial ovarian cancer.,” Cancer Epidemiol. Biomarkers Prev, vol. 16, no. 12, pp. 2548–56, December 2007. [DOI] [PubMed] [Google Scholar]
- [21].Trabert B et al. , “Ovarian cancer and menopausal hormone therapy in the NIH-AARP diet and health study.,” Br. J. Cancer, vol. 107, no. 7, pp. 1181–7, September 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou B et al. , “Hormone replacement therapy and ovarian cancer risk: a meta-analysis.,” Gynecol. Oncol, vol. 108, no. 3, pp. 641–51, March 2008. [DOI] [PubMed] [Google Scholar]
- [23].Schildkraut JM, Risch N, and Thompson WD, “Evaluating genetic association among ovarian, breast, and endometrial cancer: evidence for a breast/ovarian cancer relationship.,” Am. J. Hum. Genet, vol. 45, no. 4, pp. 521–9, October 1989. [PMC free article] [PubMed] [Google Scholar]
- [24].“Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies.,” PLoS Med., vol. 9, no. 4, p. e1001200, January 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Collaborative Group On Epidemiological Studies Of Ovarian Cancer et al. , “Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies,” Lancet, vol. 385, no. 9980, pp. 1835–1842, May 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Trabert B et al. , “Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium.,” J. Natl. Cancer Inst, vol. 106, no. 2, p. djt431, February 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Terry KL et al. , “Genital powder use and risk of ovarian cancer: A pooled analysis of 8,525 cases and 9,859 controls,” Cancer Prev. Res, vol. 6, no. 8, pp. 811–821, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wentzensen N et al. , “Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium,” J. Clin. Oncol, vol. 34, no. 24, pp. 2888–2898, August 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Faber MT et al. , “Cigarette smoking and risk of ovarian cancer: a pooled analysis of 21 case-control studies.,” Cancer Causes Control, vol. 24, no. 5, pp. 989–1004, May 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pearce CL et al. , “Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies.,” Lancet. Oncol, vol. 13, no. 4, pp. 385–94, April 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].John EM, Whittemore AS, Harris R, and Itnyre J, “Characteristics Relating to Ovarian Cancer Risk: Collaborative Analysis of Seven U.S. Case-Control Studies. Epithelial Ovarian Cancer in Black Women,” JNCI J. Natl. Cancer Inst, vol. 85, no. 2, pp. 142–147, January 1993. [DOI] [PubMed] [Google Scholar]
- [32].Hoyo C et al. , “Anthropometric measurements and epithelial ovarian cancer risk in African-American and White women.,” Cancer Causes Control, vol. 16, no. 8, pp. 955–63, October 2005. [DOI] [PubMed] [Google Scholar]
- [33].Moorman PG, Palmieri RT, Akushevich L, Berchuck A, and Schildkraut JM, “Ovarian cancer risk factors in African-American and white women.,” Am. J. Epidemiol, vol. 170, no. 5, pp. 598–606, September 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ness RB, Grisso JA, Klapper J, and Vergona R, “Racial differences in ovarian cancer risk.,” J. Natl. Med. Assoc, vol. 92, no. 4, pp. 176–82, April 2000. [PMC free article] [PubMed] [Google Scholar]
- [35].Wu AH, Pearce CL, Tseng C-C, and Pike MC, “African Americans and Hispanics Remain at Lower Risk of Ovarian Cancer Than Non-Hispanic Whites after Considering Nongenetic Risk Factors and Oophorectomy Rates.,” Cancer Epidemiol. Biomarkers Prev, vol. 24, no. 7, pp. 1094–100, July 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chumlea WC et al. , “Age at Menarche and Racial Comparisons in US Girls,” Pediatrics, vol. 111, no. 1, pp. 110–113, January 2003. [DOI] [PubMed] [Google Scholar]
- [37].Flegal KM, Carroll MD, Kit BK, and Ogden CL, “Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010.,” JAMA, vol. 307, no. 5, pp. 491–7, February 2012. [DOI] [PubMed] [Google Scholar]
- [38].Ogden CL, Carroll MD, Kit BK, and Flegal KM, “Prevalence of childhood and adult obesity in the United States, 2011-2012.,” JAMA, vol. 311, no. 8, pp. 806–14, February 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bandera E et al. , “Obesity, weight gain, and ovarian cancer risk in African American women,” Int. J. Cancer, vol. 139, no. 3, pp. 593–600, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cramer DW, Vitonis AF, Terry KL, Welch WR, and Titus LJ, “The association between talc use and ovarian cancer: a retrospective case-control study in two US states.,” Epidemiology, December 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schildkraut JM et al. , “Association between body powder use and ovarian cancer: The African American Cancer Epidemiology Study (AACES),” Cancer Epidemiol. Biomarkers Prev, vol. 25, no. 10, pp. 1411–1417, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Martin T and Osterman M, “Preterm Births - United States, 2006 and 2010. Morbidity and Mortality Weekly Report.,” vol. 62, no. Suppl 3, pp. 136–138, 2013. [PubMed] [Google Scholar]
- [43].“Progress in Increasing Breastfeeding and Reducing Racial/Ethnic Differences — United States, 2000–2008 Births.” [PMC free article] [PubMed]
- [44].Daniels K, Mosher W, and Jones J, “Contraceptive methods women have ever used: United States, 1982-2010. National Health Statistics Reports.,” vol. 62, 2013. [PubMed] [Google Scholar]
- [45].Setiawan VW et al. , “Use of nonsteroidal anti-inflammatory drugs and risk of ovarian and endometrial cancer: the Multiethnic Cohort.,” Cancer Epidemiol. Biomarkers Prev, vol. 21, no. 9, pp. 1441–9, September 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Peres LC et al. , “Analgesic medication use and risk of epithelial ovarian cancer in African American women,” Br. J. Cancer, vol. 114, no. 7, pp. 819–825, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Powell LH et al. , “Ethnic differences in past hysterectomy for benign conditions.,” Womens. Health Issues, vol. 15, no. 4, pp. 179–86, January 2005. [DOI] [PubMed] [Google Scholar]
- [48].Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, and Hunter DJ, “Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors.,” Am. J. Epidemiol, vol. 160, no. 8, pp. 784–96, October 2004. [DOI] [PubMed] [Google Scholar]
- [49].Olsen CM et al. , “Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium.,” Endocr. Relat. Cancer, vol. 20, no. 2, pp. 251–62, April 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peres LC et al. , “Racial/ethnic differences in the epidemiology of ovarian cancer: a pooled analysis of 12 case-control studies,” Int. J. Epidemiol, vol. 47, no. 2, pp. 460–472, April 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jemal A et al. “Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival,” J. Natl. Cancer Inst, vol. 109, no. 9, September 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Torre LA et al. , “Ovarian cancer statistics, 2018,” CA. Cancer J. Clin, vol. 68, no. 4, pp. 284–296, July 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].V Bandera E, Lee VS, Rodriguez-Rodriguez L, Powell CB, and Kushi LH, “Racial/Ethnic Disparities in Ovarian Cancer Treatment and Survival,” Clin. Cancer Res, vol. 22, no. 23, pp. 5909–5914, December 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bristow RE, Ueda S, Gerardi MA, Ajiboye OB, and Ibeanu OA, “Analysis of racial disparities in stage IIIC epithelial ovarian cancer care and outcomes in a tertiary gynecologic oncology referral center.,” Gynecol. Oncol, vol. 122, no. 2, pp. 319–23, August 2011. [DOI] [PubMed] [Google Scholar]
- [55].Schildkraut JM, Moorman PG, Halabi S, Calingaert B, Marks JR, and Berchuck A, “Analgesic drug use and risk of ovarian cancer.,” Epidemiology, vol. 17, no. 1, pp. 104–7, January 2006. [DOI] [PubMed] [Google Scholar]
- [56].Wu AH, Pearce CL, Tseng C-C, Templeman C, and Pike MC, “Markers of inflammation and risk of ovarian cancer in Los Angeles County.,” Int. J. Cancer, vol. 124, no. 6, pp. 1409–15, March 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Schildkraut JM et al. , “A multi-center population-based case-control study of ovarian cancer in African-American women: The African American Cancer Epidemiology Study (AACES),” BMC Cancer, vol. 14, no. 1, p. 688, September 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kim S, Dolecek TA, and Davis FG, “Racial differences in stage at diagnosis and survival from epithelial ovarian cancer: a fundamental cause of disease approach.,” Soc. Sci. Med, vol. 71, no. 2, pp. 274–281, July 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Peterson CE et al. , “The effect of neighborhood disadvantage on the racial disparity in ovarian cancer-specific survival in a large hospital-based study in cook county, Illinois.,” Front. public Heal, vol. 3, p. 8, January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kolonel LN et al. , “A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics.,” Am. J. Epidemiol, vol. 151, no. 4, pp. 346–357, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bethea TN, Palmer JR, Adams-Campbell LL, and Rosenberg L, “A prospective study of reproductive factors and exogenous hormone use in relation to ovarian cancer risk among Black women,” Cancer Causes Control, vol. 28, no. 5, pp. 385–391, May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hays J et al. , “The women’s health initiative recruitment methods and results,” Ann. Epidemiol, vol. 13, no. 9, pp. S18–S77, October 2003. [DOI] [PubMed] [Google Scholar]
- [63].Signorello LB et al. , “Southern community cohort study: establishing a cohort to investigate health disparities.,” J. Natl. Med. Assoc, vol. 97, no. 7, pp. 972–9, July 2005. [PMC free article] [PubMed] [Google Scholar]
- [64].Curb JD et al. , “Outcomes Ascertainment and Adjudication Methods in the Women’s Health Initiative,” Ann Epidemiol, vol. 13, pp. 122–128, 2003. [DOI] [PubMed] [Google Scholar]
- [65].Kurman RJ, Carcangiu ML, Herrington CS, and Young RH, WHO Classification of Tumours of Female Reproductive Organs., 4th ed. Lyon: IARC, 2014. [Google Scholar]
- [66].Fritz A et al. , Eds., International Classification of Diseases for Oncology, Third Edition. Geneva: World Health Organization, 2000. [Google Scholar]
- [67].Clarke AA and Gilks B, “Ovarian Carcinoma: Recent Developments in Classification of Tumour Histological Subtype,” Can. J. P athology, pp. 33–42, 2011. [Google Scholar]
- [68].“LexisNexis® Accurint®.” [Online]. Available: http://www.accurint.com/. [Accessed: 17-May-2019].
- [69].Paskett ED et al. , “The Women’s Health Initiative (WHI) Life and Longevity After Cancer (LILAC) Study: Description and Baseline Characteristics of Participants,” Cancer Epidemiol. Biomarkers Prev, vol. 27, no. 2, pp. 125–137, February 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Park HK, Ruterbusch JJ, and Cote ML, “Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes,” Cancer Epidemiol. Biomarkers Prev, vol. 26, no. 10, pp. 1511–1518, October 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Krishnan S, Cozier YC, Rosenberg L, and Palmer JR, “Socioeconomic status and incidence of type 2 diabetes: results from the Black Women’s Health Study.,” Am. J. Epidemiol, vol. 171, no. 5, pp. 564–70, March 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].“Project Information: 3R01CA154644-05S1,” NIH Research Portfolio Online Reporting Tools (RePort), 2015. .
- [73].Peterson CE et al. , “The association between neighborhood socioeconomic status and ovarian cancer tumor characteristics,” Cancer Causes Control, vol. 25, no. 5, pp. 633–637, May 2014. [DOI] [PubMed] [Google Scholar]
- [74].Sonderman JS, Mumma MT, Cohen SS, Cope EL, Blot WJ, and Signorello LB, “A multi-stage approach to maximizing geocoding success in a large population-based cohort study through automated and interactive processes.,” Geospat. Health, vol. 6, no. 2, pp. 273–84, May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Protani MM, Nagle CM, and Webb PM, “Obesity and ovarian cancer survival: a systematic review and meta-analysis.,” Cancer Prev. Res. (Phila)., vol. 5, no. 7, pp. 901–10, July 2012. [DOI] [PubMed] [Google Scholar]
- [76].Nagle CM et al. , “Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium.,” Br. J. Cancer, vol. 113, no. 5, pp. 817–26, September 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Minlikeeva AN et al. , “History of hypertension, heart disease, and diabetes and ovarian cancer patient survival: evidence from the ovarian cancer association consortium,” Cancer Causes Control, vol. 28, no. 5, pp. 469–486, May 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Minlikeeva AN et al. , “Joint exposure to smoking, excessive weight, and physical inactivity and survival of ovarian cancer patients, evidence from the Ovarian Cancer Association Consortium,” Cancer Causes Control, vol. 30, no. 5, pp. 537–547, May 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hedges LV and Olkin I, Statistical methods for meta-analysis. Academic Press, 1985. [Google Scholar]
- [80].Mansournia MA, Hernán MA, and Greenland S, “Matched designs and causal diagrams,” Int. J. Epidemiol, vol. 42, no. 3, pp. 860–869, June 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Bartlett JW, Seaman SR, White IR, Carpenter JR, and for the A. D. N. Alzheimer’s Disease Neuroimaging Initiative*, “Multiple imputation of covariates by fully conditional specification: Accommodating the substantive model.,” Stat. Methods Med. Res, vol. 24, no. 4, pp. 462–87, August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bartlett J and Morris T, “Multiple imputation of covariates by substantive-model compatible fully conditional specification,” STATA J., 15 pp 437–456. ,2015. [Google Scholar]
- [83].Liu Y and De A, “Multiple Imputation by Fully Conditional Specification for Dealing with Missing Data in a Large Epidemiologic Study.,” Int. J. Stat. Med. Res, vol. 4, no. 3, pp. 287–295, August 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].He Y, Zaslavsky AM, Landrum MB, Harrington DP, and Catalano P, “Multiple imputation in a large-scale complex survey: a practical guide.,” Stat. Methods Med. Res, vol. 19, no. 6, pp. 653–70, December 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].White IR and Royston P, “Imputing missing covariate values for the Cox model,” Stat. Med, vol. 28, no. 15, pp. 1982–1998, July 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jolani S, Debray TPA, Koffijberg H, van Buuren S, and Moons KGM, “Imputation of systematically missing predictors in an individual participant data meta-analysis: a generalized approach using MICE,” Stat. Med, vol. 34, no. 11, pp. 1841–1863, May 2015. [DOI] [PubMed] [Google Scholar]
- [87].Higgins JPT and Thompson SG, “Quantifying heterogeneity in a meta-analysis,” Stat. Med, vol. 21, no. 11, pp. 1539–1558, June 2002. [DOI] [PubMed] [Google Scholar]
- [88].DerSimonian R and Laird N, “Meta-analysis in clinical trials.,” Control. Clin. Trials, vol. 7, no. 3, pp. 177–88, September 1986. [DOI] [PubMed] [Google Scholar]
- [89].Chang B-H and Hoaglin DC, “Meta-Analysis of Odds Ratios: Current Good Practices.,” Med. Care, vol. 55, no. 4, pp. 328–335, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Thompson SG, Turner RM, and Warn DE, “Multilevel models for meta-analysis, and their application to absolute risk differences,” Stat. Methods Med. Res, vol. 10, no. 6, pp. 375–392, December 2001. [DOI] [PubMed] [Google Scholar]
- [91].McNeish D, “Estimation Methods for Mixed Logistic Models with Few Clusters,” Multivariate Behav. Res, pp. 1–15, November 2016. [DOI] [PubMed] [Google Scholar]
- [92].Bruzzi P, Green SB, Byar DP, Brinton LA, and Schairer C, “Estimating the population attributable risk for multiple risk factors using case-control data,” Am. J. Epidemiol, vol. 122, no. 5, pp. 904–914, 1985. [DOI] [PubMed] [Google Scholar]
- [93].Dottino JA, Cliby WA, Myers ER, Bristow RE, and Havrilesky LJ, “Improving NCCN guideline-adherent care for ovarian cancer: Value of an intervention.,” Gynecol. Oncol, vol. 138, no. 3, pp. 694–9, September 2015. [DOI] [PubMed] [Google Scholar]
- [94].Havrilesky LJ, Sanders GD, Kulasingam S, and Myers ER, “Reducing ovarian cancer mortality through screening: Is it possible, and can we afford it?,” Gynecol. Oncol, vol. 111, no. 2, pp. 179–187, 2008. [DOI] [PubMed] [Google Scholar]
- [95].Havrilesky LJ et al. , “Development of an ovarian cancer screening decision model that incorporates disease heterogeneity,” Cancer, vol. 117, no. 3, pp. 545–553, 2011. [DOI] [PubMed] [Google Scholar]
- [96].Havrilesky LJ et al. , “Oral contraceptive use for the primary prevention of ovarian cancer.,” Evid. Rep. Technol. Assess. (Full. Rep), no. 212, pp. 1–514, June 2013. [PMC free article] [PubMed] [Google Scholar]
- [97].Gierisch JM et al. , “Oral Contraceptive Use and Risk of Breast, Cervical, Colorectal, and Endometrial Cancers: A Systematic Review,” Cancer Epidemiol. Biomarkers Prev, vol. 22, no. 11, pp. 1931–1943, November 2013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.