Abstract
Resistance to chemotherapy is a major obstacle in the treatment of a wide array of different types of cancer. Chemotherapeutic drug resistance is achieved by cancer cells by a variety of different mechanisms, which can be either compound specific or general. An emerging mechanism for non-specific chemotherapeutic drug resistance relies on hyperactivity of the transcription factor Nrf2. Normally Nrf2 levels are tightly regulated by the ubiquitin-proteasome system, however mutations in genes responsible for this regulation are common in many cancer types, resulting in increased expression of Nrf2, activation of its downstream target genes and resistance to a variety of chemotherapeutic agents. For this reason, there has been considerable interest in the discovery of small molecule inhibitors of Nrf2 capable of attenuating this resistance mechanism. To this end, we have screened two commercially available libraries of known biologically active small molecules to identify potential Nrf2 inhibitors. To increase the breadth of this screen we have also screened an RNAi library that targets the majority of the druggable genome to also identify Nrf2-inhibitor targets that are not currently targeted by small molecules. To complement the commercial chemical and genomic library screening, we screened a small collection of proprietary natural products isolated from marine cyanobacteria, which included actin targeting and uncharacterized but biologically active compounds. Through these efforts, we have identified three classes of compounds: cardiac glycosides; Stat3 inhibitors; and actin disrupting agents as Nrf2 inhibitors that are able to attenuate Nrf2 activity and synergize with chemotherapeutic agents in the non-small cell lung cancer cell line A549. In addition, we found that grassypeptolide A exerts Nrf2 modulatory activity via a thus far uncharacterized mechanism. In addition, we have identified a set of putative Nrf2-targets comprising the transcription factors TWIST1 and ELF4, the protein kinase NEK8, the TAK1 kinase regulator TAB1 and the dual specific phosphatase DUSP4. This study broadens the range of mechanisms through which inhibition of Nrf2 activity can be achieved, which will facilitate the characterization of novel Nrf2 inhibitors, and allow the design of target specific screening procedures with which to identify more.
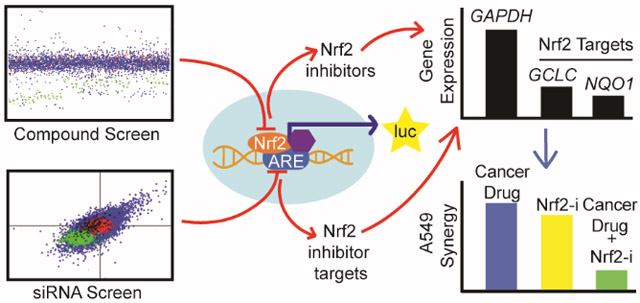
Graphical Abstract
INTRODUCTION
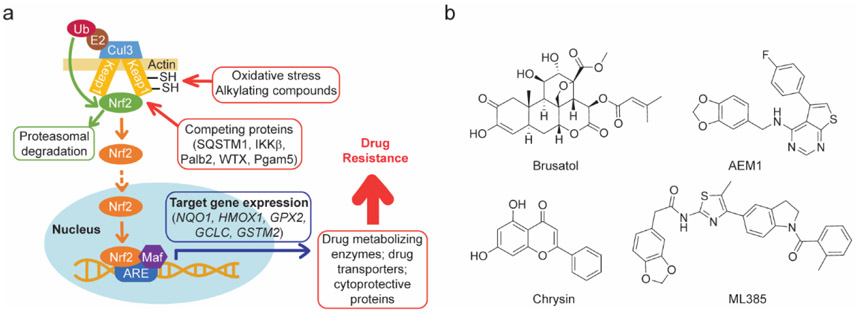
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a cap-’n’-collar type transcription factor that is responsible for expression of genes whose promoters contain the antioxidant responsive element (ARE) sequence1. Many genes have been identified as Nrf2 targets and their functions can be broadly categorized into four groups: (1) Resistance to oxidative stress; (2) Oxidant signaling; (3) Drug metabolism and transport; and (4) Intermediary metabolism2. In the absence of oxidative stress, Nrf2 is constitutively degraded by the ubiquitin-proteasome system due to its association with Kelch-like ECH-associated protein 1 (KEAP1) and an E3 ubiquitin ligase. Oxidation of key cysteine thiol side chains by reactive oxygen species (ROS) or alkylation by electrophilic compounds causes Nrf2 to dissociate from KEAP1, thereby preventing its ubiquitination and stabilizing its intracellular concentration3. The increase in cytoplasmic Nrf2 levels causes increased nuclear import and transcription of genes that are controlled by the ARE (Figure 1a). Once cellular redox balance has been restored by the action of Nrf2 target genes and the bulk of the oxidized KEAP1 has been replaced with its newly synthesized reduced equivalent, Nrf2 ubiquitination resumes and Nrf2 levels decrease to normal4.
Figure 1.
(a) The dominant control mechanism by which Nrf2 activity is regulated occurs through its association with the E3 ubiquitin ligase KEAP1 and subsequent ubiquitination and proteasomal degradation. Reactive oxygen species and alkylating compounds react with key thiol resides on KEAP1 and promote dissociation of Nrf2 from the KEAP1 complex. Dissociation of Nrf2 from this complex can also be achieved by the expression of mutated form of KEAP1 as is common in numerous different cancer types and through the increased expression or availability of proteins that compete with Nrf2 for KEAP1 binding. Nrf2 translocates to the nucleus where it positively regulates the expression of genes required for resistance to oxidative stress, including drug metabolizing enzymes and transporters capable of detoxifying and effluxing anti-neoplastic agents, the combination of which gives rise to drug resistance. (b) Representative Nrf2 inhibitors previously reported.
The role of Nrf2 in cancer cell biology is stage dependent and apparently antithetical5. Enhancing Nrf2 activity in normal cells helps prevent neoplastic transformation by enhancing detoxification of ROS, limiting the pro-mutagenic damage that high levels of these reactive compounds can induce. In contrast, enhancing Nrf2 activity in transformed cells leads to increased cellular proliferation and tissue invasion and increased anti-neoplastic drug resistance6, 7. It is therefore not surprising that a number of different cancer types express high levels of Nrf2 and are more refractory to clinical chemotherapy. A significant proportion of lung cancers in particular have been found to harbor mutations in KEAP1, Nrf2 or other genes that affect Nrf2 function, such as CUL3 and PTEN8, 9.
While the major regulatory mechanism of Nrf2 activity by the ubiquitin-proteasome systems is mediated by the oxidation state of KEAP1 this step is also subject to regulation by the presence of proteins that compete with Nrf2 for KEAP1 binding. In this manner the p62/SQSTM1 (sequestosome1), IKKβ (IκB kinase), Palb2 (partner and localizer of Brca2), WTX (Wilms tumor gene on the X chromosome) and phosphoglycerate mutase 5 (PGAM5) have been implicated in the integration of Nrf2 activity with autophagy, DNA damage and intermediary metabolism (Figure 1a)2. Gene products that modulate Nrf2 activity can be identified through genomic screening. For example we identified DPP3 and SQSTM1 as top hits in a genomic screen for cDNA that enhance Nrf2 activation in IMR-32 cells10. DPP3 was later shown to physically interact, via its ETGE domain, with the KELCH domain of KEAP1, confirming the role of DPP3 in the regulation of Nrf2 activity11. Both Nrf2 and KEAP1 are also regulated at the post-translational level by phosphorylation by an array of different kinases which can act to increase12, decrease13 or have unknown effects14 on the expression of Nrf2 target genes2. This broad range of proteins and pathways that are able to exert a regulatory effect on Nrf2 suggests the existence of a similarly broad array of potential Nrf2 inhibitor targets.
Given the role of Nrf2 in anti-neoplastic drug resistance, there is a sustained effort to identify small molecule inhibitors of Nrf2. To date the most well characterized Nrf2 inhibitor is the natural product brusatol (Figure 1b). Treatment of A549 cells, an adenocarcinomic human alveolar basal epithelial cell line, with brusatol causes down-regulation of numerous Nrf2-target genes15.Brusatol is a translation elongation inhibitor that causes a decrease in Nrf2 protein levels by decreasing its synthesis16. This effect occurs at concentrations that are non-cytotoxic, however pretreatment of A549 cells with brusatol enhances the toxicity of various chemotherapeutic agents in both in vivo mouse xenograft tumor growth and in vitro cell culture assays 15-17. Aside from brusatol a small number of other Nrf2 inhibitors have been reported, the majority of which are analogues of the natural product flavonoid chrysin18, 19 (Figure 1b). However, the recent reports of the synthetic Nrf2 inhibitors AEM120 and ML38521, which also synergize with current chemotherapeutic agents, are a testament to how attractive the prospects of inhibiting this pathway are (Figure 1b).
The exciting potential of Nrf2 inhibitors to constitute part of an anti-neoplastic combination therapy certainly warrants efforts to discover these compounds. It is reasonable to suggest, given the ever evolving and already multi-faceted nature of the regulatory network that surrounds Nrf2, that a wide array of potential Nrf2 inhibitor targets remain to be discovered. Knowledge of these targets and the mechanism by which they regulate Nrf2 activity would greatly enhance efforts to identify the targets of novel Nrf2 inhibitors in the future. These assertions motivated us to screen two libraries of commercially available, biologically active small molecules to identify potential inhibitors of Nrf2. In order to broaden the scope of this screen, and due to our previous success in using a genomic screening approach to identify novel Nrf2 regulators10, we also screened a library of siRNA that targets the majority of the known or predicted druggable genome to identify potential Nrf2-inhibitor drug targets.
RESULTS AND DISCUSSION
Small molecule library screen
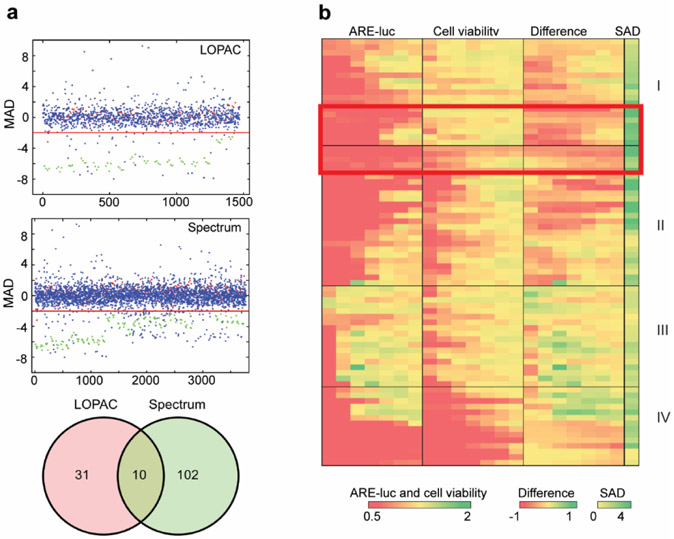
In order to identify putative inhibitors of Nrf2, the MDA-MB-231 reporter cell line expressing luciferase under the control of the ARE-containing NQO1 promoter22 (referred to hereafter as MDA-MB-231 ARE-luc) was used to screen the Sigma LOPAC and Spectrum Collection libraries of biologically active small molecules as we previously did to identify indirect inhibitors of the HIF pathway in colon cancer cells23. We used a two-step screening process since any Nrf2 inhibitor could also be cytotoxic at concentrations greater than those that modulate Nrf2 activity, and that the relative concentrations at which these two distinct phenotypes occur will likely differ in a compound dependent manner, as previously seen with brusatol15. The primary screen for Nrf2 inhibitors was performed in quadruplicate at a single concentration of 10 μM in 384-well plates for 24 h and luciferase expression was quantified using the BriteLite reagent. The raw luciferase data was processed and hit compounds identified as outlined in the methods (MAD score ≤ −2). Hit compounds from the primary screen were retested in a secondary screen that consisted of 24-h parallel dose-response luciferase and viability assays (Figure 2b and Figure 3a). Dose-response curves were plotted and compounds that showed a range of concentrations at which the luciferase activity was inhibited to a greater extent and at lower concentrations than the viability assay were further investigated. The primary screen yielded 31 and 102 structurally distinct potential Nrf2 inhibitors from the Sigma LOPAC and Spectrum Collection libraries, respectively, plus 10 hit compounds present in both libraries (Figure 2a and Table S1). This overlap is greater than what would be expected by random chance given the overlap between the two libraries screened (Figure S1c). In total 77 compounds were retested in the secondary screen (Figure 2b). The selection of these compounds aimed to maximize diversity of biological activities, targets, and chemical structures within the secondary screen. Of all the compounds retested, 15 showed a clear window of specific Nrf2 inhibitory activity (Figure 2b). Due to target and mechanism redundancy, 12 of these compounds were selected for further characterization based on their drug-like properties (Figures 3b and S1).
Figure 2.
Screen for small molecule modulators of Nrf2 activity. (a) Primary screen for Nrf2 modulators from the Sigma LOPAC and Spectrum Collection libraries using the MDA-MB-231 ARE-luc reporter cells line. Compound index number plotted against the median absolute deviation (MAD) from quadruplicate samples, the red line indicates the Nrf2 inhibitor MAD cutoff value (MAD ≤ −2). Blue: library compounds tested at 10 μM. Red: Negative control (0.5% DMSO). Green: Nrf2 inhibitor positive control (125 nM brusatol). The Venn diagram summarizes the common and unique Nrf2 inhibitor hits from each library. (b) Secondary screen for Nrf2 inhibitors. Primary screen hits were retested in parallel ARE-luc and cell viability seven-point dose-response assays. The ARE-luc and cell viability assay raw values are expressed relative to the negative control (0.5% DMSO) and the sum of the absolute difference across all seven concentrations was used to identify: non-toxic Nrf2 inhibitors (group I); Nrf2 inhibitors that are also toxic (group II); compounds with negligible activity (group III); toxic compounds (groups IV). The red box indicates the compounds that were further characterized.
Figure 3.
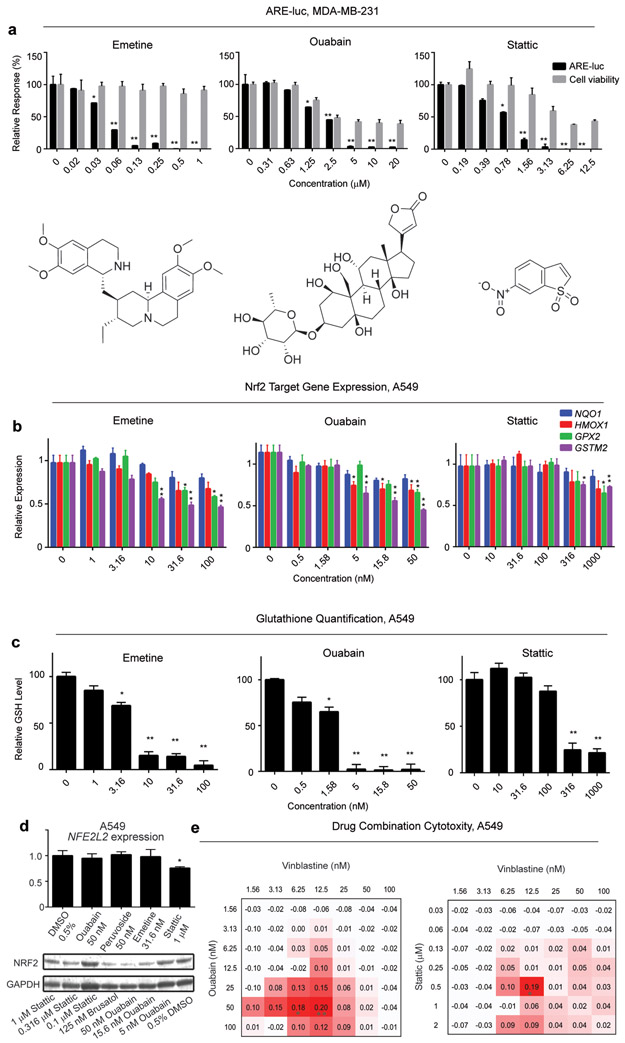
(a) Representative secondary screen dose-response plots for the putative ARE inhibitors emetine, stattic and ouabain in the MDA-MB-231 ARE-luc reporter cell line expressed relative to the negative control (0.5% DMSO). (b) Nrf2 target gene expression in A549 cells after exposure to emetine, stattic and ouabain. A549 cells were exposed to the putative Nrf2 inhibitors for 24 h and the expression of the indicated Nrf2 target genes was quantified by RT-qPCR using GAPDH as the reference gene. (c) Total glutathione content of A549 cells after exposure to putative Nrf2 inhibitors. A549 cells were exposed to emetine, stattic and ouabain for 24 h and total glutathione was quantified with the colorimetric DTNB assay. Error bars represent ± SEM, * P ≤ 0.05, ** P ≤ 0.01 relative to negative control (One way ANOVA followed by Dunnett’s test). (d) Upper: NFE2L2 gene expression quantification by RT-PCR in response to ARE inhibitor treatment in A549 cells using GAPDH as the endogenous control. Error bars represent ± SD, *P ≤ 0.05, relative to 0.5% DMSO treatment (one way ANOVA followed by Dunnett’s test). Lower Nrf2 protein expression in response to 24 h treatment of the indicated Nrf2 (e) Δ Bliss independence calculations for A549 cells co-treated with oubain (left) and stattic (right) and vinblastine. A549 cells were treated with ouabain or stattic at the indicated concentrations for 6 h, followed by treatment with vinblastine at the indicated concentrations and viability was quantified using the MTT assay after a total of 72 h drug exposure. “Δ Bliss independence” is the difference between observed growth inhibition and Bliss expectation. Values greater than zero represent a synergistic response. Bliss expectation equals to C = (A+B) - (A×B), where A and B are the growth inhibition fractions of two compounds at a given dose and expressed relative to the negative control (0.5% DMSO). * P ≤ 0.05, ** P ≤ 0.01 relative the expected inhibitory effect of the two compounds (Student’s t-test).
Nrf2 protein levels and therefore expression of ARE controlled genes is higher in MDA-MB-231 cells than in non-transformed cell lines despite the presence of wild type copies of NFE2L2, KEAP1 and CUL3. Overexpression of Nrf2 still acts to enhance drug resistance in MDA-MB-231 cells, indicating that this cell line has not reached the maximal capacity for Nrf2 expression24. To assess whether the putative Nrf2 inhibitors identified using the MDA-MB-231 ARE-luc reporter cell line would act in the same way in a cell line with considerably higher basal Nrf2 protein levels and ARE gene expression, they were tested for the ability to inhibit Nrf2-target gene expression in the non-small cell lung cancer A549 cell line. The A549 cell line, which was derived from an adenocarcinoma of the human alveolar basal epithelium, expresses high levels of Nrf2 due to a somatic mutation of the KEAP1 gene, in combination with hypermethylation in the KEAP1 promoter, and has been used extensively to study the role of Nrf2 in cancer biology25-28. In this cell line Nrf2 has been found to be required for long term acquired resistance to cisplatin29, and attenuating Nrf2 activity by shRNA knockdown or pharmacologically enhances chemotherapeutic sensitivity both in vitro and in vivo15, 24, 30 by enhancing cellular ROS levels17. Compounds were first tested for their ability to inhibit the expression of the Nrf2-target genes GSTM2, NQO1, HMOX1 and GPX2 (Figure 3b). The translation elongation inhibitor emetine inhibited the expression all of Nrf2 target genes tested to varying extents (Figure 3b). This result was expected since the inhibitory effect of brusatol on cellular Nrf2 activity is due to preferential inhibition of Nrf2 translation and lends validity to the screen design used16, 31. However, compounds that generally perturb transcription such as the topoisomerase I and II inhibitors topotecan, and mitoxantrone failed to specifically inhibit the expression of Nrf2 target genes in A549 cells (Figure S2). This discrepancy could indicate cell type specificity with regards to Nrf2 inhibitor mechanism or more likely it is due to the transcription centered mechanism of all of these compounds.
The hit compound with the largest Nrf2 inhibitory window aside from the protein synthesis inhibitors emetine32 and anisomycin was the Stat3 inhibitor stattic33. Stattic was the third most potent inhibitory hit in the Sigma LOPAC library screen and showed minimal toxicity at concentrations that almost completely inhibited expression of the ARE-luc reporter (Figure 3a). Stattic modestly decreased the expression of Nrf2 target genes in A549 cells (Figure 3a) as well as a slight effect on the expression of NFE2L2 itself that did not translate to a decrease in Nrf2 protein levels (Figure 3d). Stattic displayed statistically significant cooperativity with vinblastine in A549 cell cytotoxicity and a trend towards cooperativity with cisplatin (Figure 3e and Figure S3).
One interesting class of compound identified as putative Nrf2 inhibitors was the cardiac glycosides. While this class is particularly over-represented in the Spectrum Collection library, all of the five cardiac glycosides (lantoside C, strophanthidin, peruvoside, proscillaridin and ouabain) tested showed a window of specific ARE-luc inhibition in the MDA-MB-231 reporter cell line, and also inhibited the expression of Nrf2 target genes to varying extents in A549 cells (Figures 3a, 3b and Figure S1) including genes involved in glutathione biosynthesis and recycling. Furthermore, exposure to ouabain also causes a dose dependent decrease in A549 cellular glutathione levels (Figure 3c), consistent with inhibition of Nrf2 target gene expression20, 34, 35. Furthermore, ouabain treatment causes a significant decrease in cellular Nrf2 protein levels in A549 cells without effecting NFE2L2 gene expression (Figure 3d). The cardiac glycosides are well characterized inhibitors of the plasma membrane sodium-potassium ATPase pump (Na+,K+-ATPase)36, however the structurally unrelated Na+,K+-ATPase inhibitor 3,4,5,6-tetrahydroxyxanthone37 failed to inhibit ARE-luc expression in MDA-MB-231 cells, nor does it inhibit the expression of the Nrf2 target genes tested in A549 cells (Figure S4). Finally, to investigate the potential for cardiac glycoside drugs to be used as a part of an anti-cancer drug combination treatment regime, the ability of ouabain to potentiate the in vitro cytotoxicity of vinblastine was assessed. Figure 3e shows that 40 nM ouabain38 is able to significantly enhance the in vitro cytotoxicity of vinblastine towards A549 cells when pretreated for 6 h () and potentially augment the cytotoxicity of cisplatin (Figure S3). The potential mechanisms responsible for the Nrf2 modulatory activity of the cardiac glycosides are discussed further in the supplementary discussion (Supporting Information). Of note is the fact that effects of both ouabain and stattic appear to be relatively specific towards cancer cells since they display considerably less cytotoxicity towards the non-tumorigenic cell lines MCF10a and NIH 3T3 (Figure S5).
siRNA Library Screen
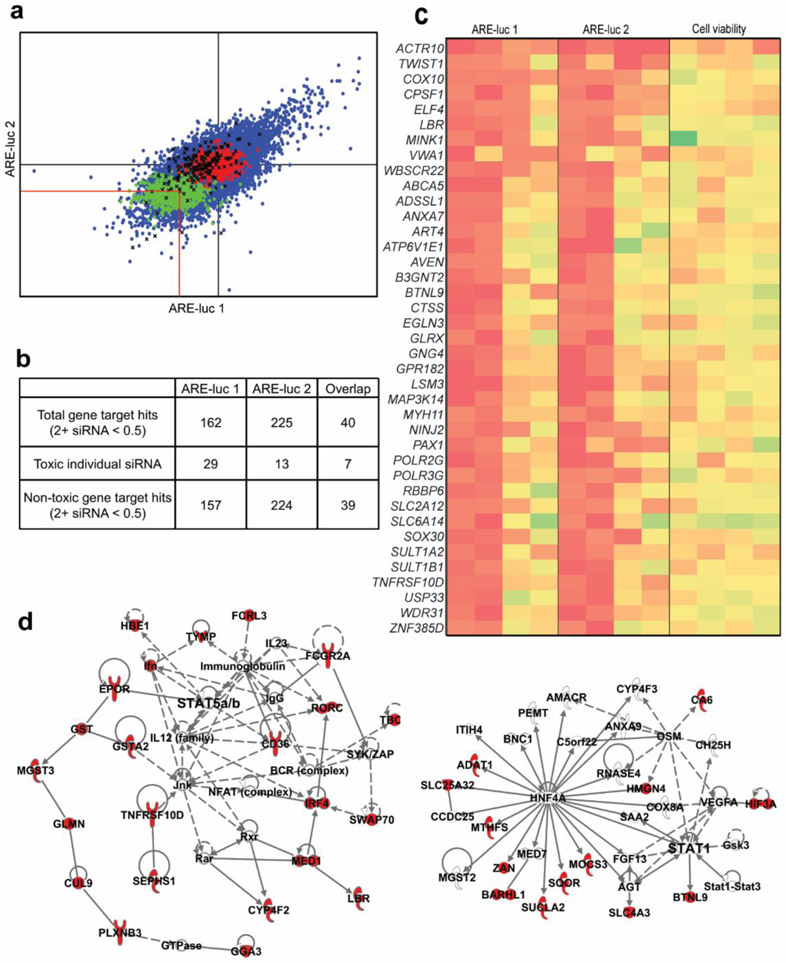
In order to identify potential Nrf2 inhibitor targets neglected by the libraries containing pharmacologically validate compounds, a library of siRNA targeting 7,784 druggable targets, with four independent siRNA per gene, was screened for those that inhibit expression of the ARE-luc reporter in MDA-MB-231 cells. The primary screen was performed by reverse transfection in 384-well plates at a final siRNA concentration of 20 nM (Figure 4a and 4b) and luciferase expression and viability were measured in parallel luminescence assays using the BriteLite and ATPlite plus reagents, respectively, 72 h post transfection (48 h to allow target knockdown plus 24 h to allow the downstream effect to occur). The web application CARD was used to normalize the raw luciferase data and identify likely hit siRNAs as outlined in the methods39. The summary statistics of the normalized luminescence scores were equivalent across all of the plates comprising the siRNA library in both screen replicates (Figure S6). An off-target analysis was also performed using CARD, this is an important step in the analysis of the siRNA library screen data since the library has not been extensively validated with respect to target specificity. This analysis searches the hit siRNA sequences for a common seed sequence. The prevalence of such a sequence among hit siRNA that are supposed to target different genes would indicate their effects are likely mediated through a common, phenotype-relevant off-target gene. Such an effect has been noted in several previously published siRNA library screens, however, no such common seed sequence was found amongst the siRNA hit sequences from either of the screen replicates. In total, 157 and 224 gene targets were identified in the two screen replicates as being potential Nrf2 inhibitor targets in MDA-MB-231 breast cancer cell (Figure 4b), with 40 gene targets common between both replicates (Figure 4b and 4c). The knockdown efficiency of a selection of five of these overlapping hits was assessed and suggests a correlation between the knockdown efficiency of the hit siRNA and the level of ARE-luc inhibition (Figure S7) lending to the validity of the hits from the siRNA primary screen. The only gene hit which was targeted by more than two different siRNAs was ACTR10. There is little literature regarding ACTR10, however this actin related protein has been implicated in axonal mitochondrial transport in zebrafish by constituting part of the dynein-associated dynactin complex40. This indicates a potential role of either the dynactin complex or the actin cytoskeleton itself, in the regulation of Nrf2 activity in MDA-MB-231. Due to the lack of actin targeting compounds in either of the commercial chemical libraries screened this possibility was addressed by screening a proprietary collection of marine natural products of bacterial origin which included two actin disrupting agents dolastatin12 and lyngbyabellin A described in the following section.
Figure 4.
Screen for siRNA modulators of Nrf2 activity. (a) Primary screens for siRNA Nrf2 modulators using the MDA-MB-231 ARE-luc reporter cell line. Raw luciferase output for each siRNA was expressed relative to the median luciferase values of the negative control present on each plate and the standardized scores of the two replicate screens are plotted against each other. The impact on viability of each siRNA was assessed in parallel and siRNA that caused greater than 50% toxicity were excluded from the downstream analyses. Red lines indicate the cutoff for hit siRNAs that decrease luciferase output by greater than 50%. Blue dots: test siRNA. Green dots: positive control siRNA (NFE2L2 SMARTpool). Red dots: negative control siRNA. Black X: toxic test siRNA. (b) Hit summary of the two siRNA ARE-luc inhibitor screens. The total number of gene hits is the number of genes targeted by more than two different siRNA from each screen. The total number of toxic individual siRNA is the number of single siRNA ARE-luc inhibitor hits that cause a greater than 50% decrease in viability. The number of non-toxic gene target hits is the number of genes targeted by more than two different siRNAs after the removal of the toxic individual siRNA hits. (c) Heatmap representing the standardized ARE-luc and viability scores for the overlapping set of siRNA ARE-luc inhibitor hits from the two replicate siRNA modulator screens. Each column represents a single siRNA targeting the indicated gene (d) Two examples of enriched networks resulting from the IPA analysis of the combined list of ARE-luc inhibitor gene hits from both ARE-luc screens. Red nodes are genes present in the combined list of ARE-luc inhibitor genes hits, colorless nodes are genes that interact with the ARE-luc inhibitor gene hits. Solid edges represent physical interaction between the protein products of the indicated genes. Dashed edges represent non-physical, functional interactions between the indicated genes or their protein products.
The result of the siRNA screens were analyzed for canonical pathway and network enrichment using Ingenuity Pathway Analysis (IPA). The list of overlapping hits from both the screen replicates was not enriched for any one particular pathway, however, the network analysis revealed the enrichment of two networks both of which are centered on known regulators of Nrf2. The most significantly enriched network contains hit genes linked to peroxisome proliferator receptor gamma (PPARG) and BRCA1 and the next most significant network contains the well-characterized post-translational Nrf2 regulatory kinases Akt, Erk and p38/MAPK as well as NF-κB (Figure S8 and Table S3). Given the modest but encouraging overlap between the two screen replicates, the hit lists were combined and analyzed using IPA. The results of this analysis show the most significantly enriched canonical pathway was the protein kinase A signaling pathway (P = 0.000138) however this was due to the presence of only 17 of the 384 pathway components in the hit list (Table S4 and Figure S9). The enrichment in this case is likely driven by the presence of siRNAs that target PRKACG, which encodes the gamma regulatory subunit of protein kinase A, which is a component of the central hub of this pathway. The role of this pathway is discussed further in the supplementary material however since this pathway is known to regulate Nrf2 activity in various different cell types and lends confidence to the primary screen results.
Despite a lack of pathway enrichment amongst the siRNA inhibitors, the IPA analysis hinted at the involvement of the Stat1/3/5 pathway in ARE-luc expression (Figure 4d). The Stat3 inhibitor stattic was a hit in the small molecule screen, and showed a significant ARE-luc inhibition window in MDA-MB-231 cells and decreased the expression of Nrf2 target genes in A549 cells (Figures 2c-e). A regulatory interaction between Stat3 and Nrf2 was predicted by an in silico network analysis that combined genome-wide data regarding predicted and known physical interactions of Nrf2 with its list of target genes, transcription factors that control NFE2L2 transcription and miRNA data41. Furthermore, it has been recently proposed that the Nrf2 inhibitor, wogonin, reverses adriamycin resistance in myelogenous leukemia cells and decreases NFE2L2 gene expression by inhibiting Stat3 and NF-κB signaling42. These results are further evidence that despite the lack of enrichment of many canonical pathways’ central components, enrichment of a peripheral pathway component is enough to implicate the involvement of the pathway in Nrf2 activity.
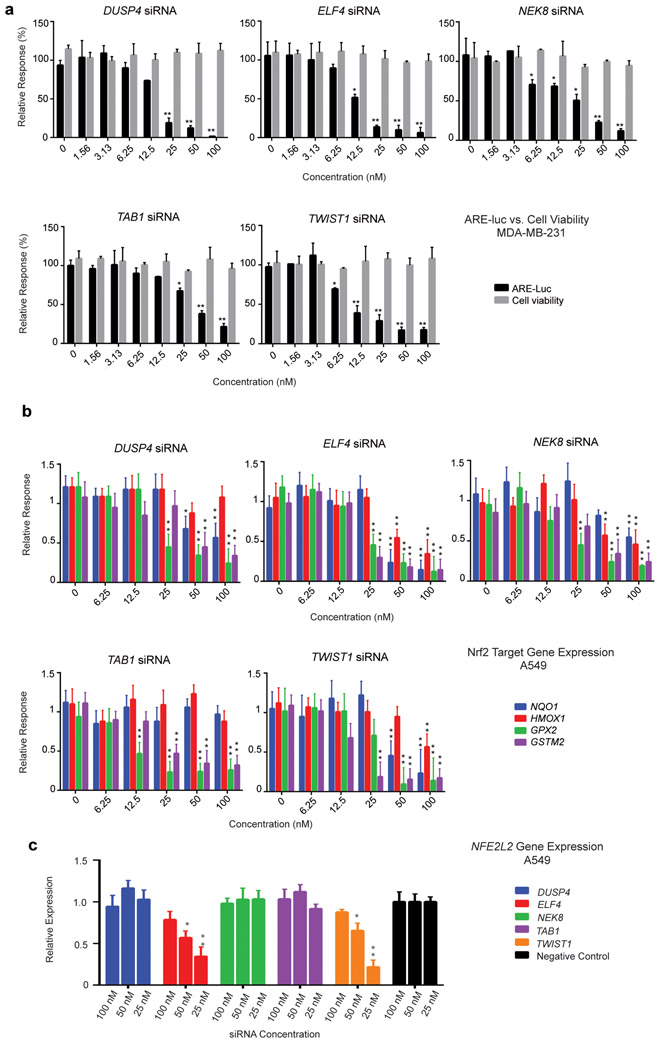
Selected potential novel Nrf2-inhibitor targets from the siRNA screen were chosen for individual validation. Two independent siRNA for each putative target gene, differing in sequence from those present in the siRNA library, were tested in a dose-response parallel luciferase and viability assay. Of the siRNAs tested in this manner, six potential Nrf2-inhibitor targets (TWIST1, ELF4, TAB1, NEK8, DUSP4 and NDUFS4) were identified by virtue of their ability to inhibit expression of the ARE-luc reporter without a significant effect on cell viability in the MDA-MB-231 ARE-luc reporter cell line (Figure 5a). The high degree knockdown efficiency of these independent siRNAs indicates that the inhibitory effect on the ARE-luc reporter is due to knockdown of the specific target gene expression and not an off target effect (Figure S10). The siRNA of targeting five of the six putative Nrf2 inhibitor genes (TWIST1, ELF4, TAB1, NEK8 and DUSP4) also decreased Nrf2 target-gene expression in A549 cells (Figure 5b), suggesting that these Nrf2-inhibitor targets are conserved between at least these two cell types. To date none of these genes have been empirically determined to modulate Nrf2 activity however there is substantial circumstantial evidence for their role and is discussed further in the supplementary discussion however we suggest that the transcription factors TWIST1 and ELF4 could directly modulate NFE2L2 gene expression as well as the expression of its target genes (Figure 5c).
Figure 5.
Validation of selected ARE-luc inhibitor gene hits using two different siRNAs targeting genes identified in the high-throughput MDA-MB-231 ARE-luc modulator screen. (a) Dose-response ARE-luc and cell viability assay. The two different siRNA targeting the indicated genes were transfected at the indicated concentrations into the MDA-MB-231 ARE-luc reporter cell line. ARE-luc output and cell viability was quantified 72 h post-transfection using the BriteLite reagent and the MTT assay. The assay was performed in triplicate with two different siRNA, raw luciferase and MTT assay values are expressed relative to the non-targeting siRNA negative control. The mean and standard deviation of the relative luciferase and MTT assay values were calculated. (b) Nrf2 target gene expression in response to transfection with two different siRNA targeting the indicated genes. The expression of four Nrf2 target genes (NQO1, HMOX1, GPX2 and GSTM2) was quantified by RT-qPCR using GAPDH as the reference gene. Error bars represent ± SEM, * P ≤ 0.05, ** P ≤ 0.01 (One way ANOVA followed by Dunnett’s test) relative to negative control. (c) NFE2L2 gene expression in A549 cells in response hit siRNA treatment. The effect of treatment with three concentrations of two independent siRNA for each putative ARE inhibitor gene on NFE2L2 gene expression was quantified by RT-qPCR. NFE2L2 expression in response to hit siRNA is expressed relative to that of the negative control siRNA treatment and the average of the two independent siRNA is depicted. GAPDH was used as the endogenous control gene. Error bars represent ± SEM, * P ≤ 0.05, ** P ≤ 0.01 (One way ANOVA followed by Dunnett’s test) relative to negative control siRNA.
Identification of Marine Natural Products as Nrf2 Inhibitors
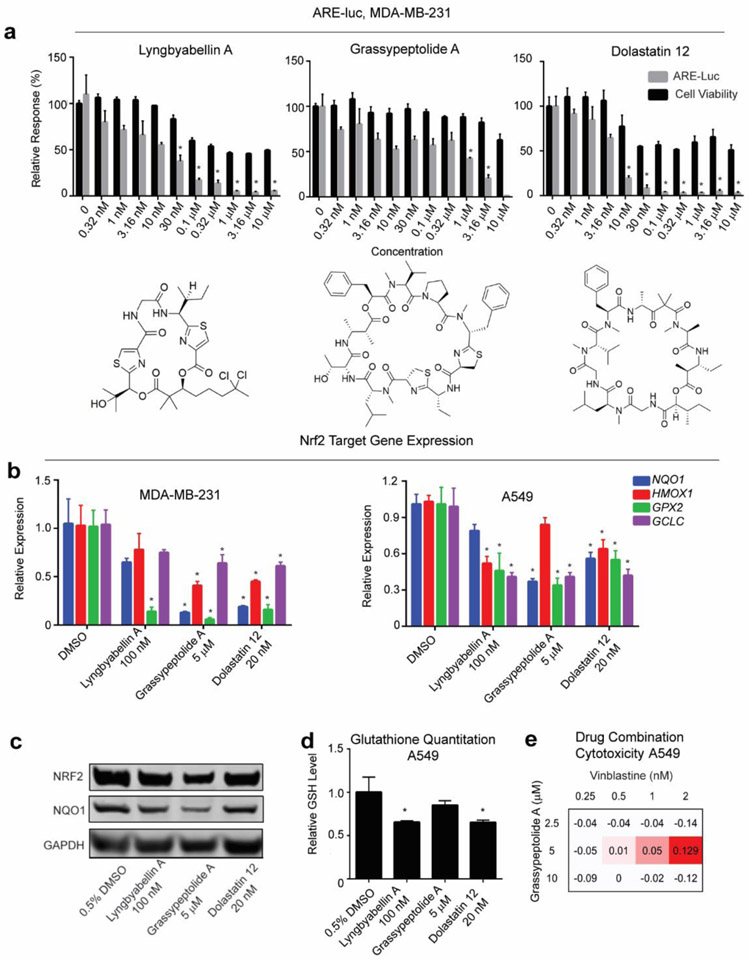
We then screened a focused library of natural products from marine cyanobacteria. Since there is an absence of actin targeting compounds in either of the compound libraries screened, we also included two known cyanobacteria-derived actin disruptors, using the MDA-MB-231 ARE-luc reporter cell line. As a result, three known natural products, lyngbyabellin A43, grassypeptolide A44, 45 and dolastatin 1246 were identified to display a specific window of ARE-luc inhibitory activity at 24 h without significant cytotoxicity at the same time point (Figure 6a). To validate the Nrf2 inhibitory effect of these compounds, Nrf2 target gene expression was measured in MDA-MB-231 cells after 24 h exposure. Cells were treated with a concentration of each compound which suppressed luciferase activity to less than 20% of the solvent treated control, whilst maintaining cell viability at more than 70% based on MDA-MB-231 ARE-luc reporter assay results. To establish whether the Nrf2 inhibitory effect observed with the three marine natural products can be translated into another cell type, the effect of these compounds on Nrf2 activity in the human lung carcinoma cell line A549 was also tested. Considering the similarity of the dose-response patterns of these natural product hits on MDA-MB-231 and A549 cells after 24 h treatment (Figure S11), A549 cells were treated with the same doses as MDA-MB-231 cells. All the natural products tested effectively decreased the transcription of Nrf2 target genes NQO1, HMOX1, GPX2 and GCLC (Figure 6b) in both cancer cells. Moreover, lyngbyabellin A, grassypeptolide A and dolastatin 12 reduced protein expression level of Nrf2 and its target NQO1 (Figure 6c) and the total cellular levels of glutathione in A549 cells after 24 h exposure (Figure 6d) as well, which is also consistent with reported Nrf2 inhibitor activities20,33 and is discussed further in the supplementary material.
Figure 6.
Inhibitor hits from natural product library screening. (a) Structure of lyngbyabellin A, grassypeptolide A and dolastatin 12 with their differential ARE inhibitory effects versus cell viability from MDA-MB-231 ARE-luc reporter assay. Error bars represent ± SEM. (b) Transcriptional expression levels of NQO1, HMOX1, GPX2 and GCLC decreased in MDA-MB-231 and A549 cells upon treatment of natural product inhibitor hits for 24 h, with GAPDH as endogenous control. Error bars represent ± SEM. (c) Quantification of Nrf2 and its target NQO1 protein expression by western blot with GAPDH as endogenous control after 24 h treatment with natural products Nrf2 inhibitors in A549 cells. (d) A549 cellular reduced glutathione (GSH) content level decreased after 24 h natural product Nrf2 inhibitor hits treatment. In each case error bars represent ± SEM * P < 0.05 relative to vehicle control (One way ANOVA followed by Dunnett’s test). (e) Δ Bliss independence calculations for A549 cells co-treated with grassypeptolide A and vinblastine. A549 cells were treated with grassypeptolide A in serial concentrations for 24 h, followed by treatment with vinblastine in serial concentrations for 24h. “Δ Bliss independence” is the difference between observed growth inhibition and Bliss expectation. Values greater than zero represent a synergistic response. Bliss expectation equals to C=(A+B)-(A×B), where A and B are the growth inhibition fractions of two compounds at a given dose. Cell viability was quantified using MTT assay.
Grassypeptolide A has been reported to have moderate cell cytotoxicity toward HT29 cells, cause G0/G1 phase cell cycle arrest at lower concentrations while induce G2/M arrest at higher concentration and exhibit apoptosis-inducing activity47. Interestingly, grassypeptolide A was found to preferentially inhibit dipeptidyl peptidase 8 (DPP8) over dipeptidyl peptidase 4 (DPP4)44, 48. As mentioned in the introduction, DPP3 protein can compete with Nrf2 for binding to KEAP1 and prevent ubiquitination of Nrf2, which increases its cellular concentration and triggers the expression of Nrf2 target genes11. Given the functionality of DPP3, it is tempting to speculate that DPP8 may also be able to compete with Nrf2 to associate with KEAP1. And by inhibiting the KEAP1 binding activity with DPP8, grassypeptolide A may be able to stimulate the degradation of Nrf2, thus inhibiting the expression of Nrf2 target genes and sensitizing cancer cells.
To verify the practical effect of natural product inhibitor hits, a combination study was carried out in A549 cells line. Due to the potential novelty of grassypeptolide A target and mode-of-action, this assay was performed with grassypeptolide A. As shown in Figure 6e, co-treatment of A549 cells with grassypeptolide A and vinblastine proved to be synergistic at certain dose as determined by changes in Bliss dependence calculations. . Moreover, co-treatment of A549 cells with grassypeptolide A and cisplatin also show potential synergy (Figure S12).
CONCLUSION
Through our compound screening efforts we have identified promising Nrf2-inhibitors of diverse mechanism. Likewise, by screening a library of siRNA that target the druggable genome, we have also identified novel Nrf2-inhibitor targets for which there are currently no inhibitors. The combination of these screening strategies has enabled us to define a broader set of Nrf2-inhibitor targets than either in isolation would be capable of. Screening of a novel natural product library focused on marine cyanobacterial compounds revealed novel activities for compounds with known and unknown targets. The breadth of diversity of the inhibitors and targets identified herein reflects the multifaceted aspects of Nrf2 activity regulation, which will facilitate the mechanistic delineation of novel Nrf2 inhibitors in the future and enable the development of specific Nrf2 targeted drug screens as well.
MATERIALS AND METHODS
Details of the experimental procedures are provided in the Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (grant R01CA172310), Shenzhen Peacock Plan (KQTD2015071714043444) and the Debbie and Sylvia DeSantis Chair professorship (H.L.). We thank D. D. Zhang for supplying the MDA-MB-231 ARE-luc reporter cell line and N. C. H. Quek for help with figure preparation.
Footnotes
The Supporting Information is available free of charge on the ACS Publications website.
Supplementary discussion; methods, including compound library screen, siRNA library screen, screen validation, marine natural products screen, cell viability assay, RT-qPCR and glutathione assay; supplementary references; supporting figures S1-S15 and tables S1-S4 (pdf).
REFERENCES
- [1].Jaramillo MC, and Zhang DD (2013) The emerging role of the Nrf2-KEAP1 signaling pathway in cancer, Genes Dev. 27, 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hayes JD, and Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism, Trends Biochem. Sci 39, 199–218. [DOI] [PubMed] [Google Scholar]
- [3].Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, Chiba T, Igarashi K, and Yamamoto M (2004) Oxidative stress sensor KEAP1 functions as an adaptor for Cul3-based E3 ligase to regulate for proteasomal degradation of Nrf2, Mol. Cell. Biology 24, 7130–7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ma Q (2013) Role of Nrf2 in Oxidative Stress and Toxicity, Ann. Rev. Pharmacol 53, 401–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Taguchi K, and Yamamoto M (2017) The KEAP1-NRF2 System in Cancer, Front. Oncol 7, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hayes JD, and McMahon M (2006) The double-edged sword of Nrf2: Subversion of redox homeostasis during the evolution of cancer, Mol. Cell 21, 732–734. [DOI] [PubMed] [Google Scholar]
- [7].Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, Yamamoto M, and Hirohashi S (2008) Genetic alteration of KEAP1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer, Gastroenterology 135, 1358–1368. [DOI] [PubMed] [Google Scholar]
- [8].Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, and Yamamoto M (2006) Structural basis for defects of KEAP1 activity provoked by its point mutations in lung cancer, Mol. Cell 21, 689–700. [DOI] [PubMed] [Google Scholar]
- [9].Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N, Itoh K, and Yamamoto M (2009) Nrf2 Enhances Cell Proliferation and Resistance to Anticancer Drugs in Human Lung Cancer, Clin. Cancer Res 15, 3423–3432. [DOI] [PubMed] [Google Scholar]
- [10].Liu Y, Kern JT, Walker JR, Johnson JA, Schultz PG, and Luesch H (2007) A genomic screen for activators of the antioxidant response element. Proc. Natl. Acad. Sci. USA, 104, 5205–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hast BE, Goldfarb D, Mulvaney KM, Hast MA, Siesser PF, Yan F, Hayes DN, and Major MB (2013) Proteomic analysis of ubiquitin ligase KEAP1 reveals associated proteins that inhibit NRF2 ubiquitination, Cancer Res. 73, 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Huang HC, Nguyen T, and Pickett CB (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription, J. Biol. Chem 277, 42769–42774. [DOI] [PubMed] [Google Scholar]
- [13].Yu R, Mandlekar S, Lei W, Fahl WE, Tan TH, and Kong ANT (2000) p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens, J. Biol. Chem 275, 2322–2327. [DOI] [PubMed] [Google Scholar]
- [14].Sun Z, Huang ZP, and Zhang DD (2009) Phosphorylation of Nrf2 at Multiple Sites by MAP Kinases Has a Limited Contribution in Modulating the Nrf2-Dependent Antioxidant Response, PLoS One 4, e6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, Toppin HA, and Zhang DD (2011) Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism, Proc. Natl. Acad. of Sci. USA 108, 1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harder B, Tian W, La Clair JJ, Tan AC, Ooi A, Chapman E, and Zhang DD (2017) Brusatol overcomes chemoresistance through inhibition of protein translation, Mol. Carcinog 56, 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun XH, Wang Q, Wang Y, Du LQ, Xu C, and Liu Q (2016) Brusatol Enhances the Radiosensitivity of A549 Cells by Promoting ROS Production and Enhancing DNA Damage, Int. J. Mol. Sci 17, E997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, and Wang XJ (2011) Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs, Free Rad. Biol. and Med 50, 1599–1609. [DOI] [PubMed] [Google Scholar]
- [19].Zhu JY, Wang HH, Chen F, Fu JQ, Xu YY, Hou YY, Kou HH, Zhai C, Nelson B, Zhang Q, Andersen ME, and Pi JB (2016) An overview of chemical inhibitors of the Nrf2-ARE signaling pathway and their potential applications in cancer therapy, Free Rad. Biol. and Med 99, 544–556. [DOI] [PubMed] [Google Scholar]
- [20].Bollong MJ, Yun H, Sherwood L, Woods AK, Lairson LL, and Schultz PG (2015) A Small Molecule Inhibits Deregulated NRF2 Transcriptional Activity in Cancer, ACS Chem. Biol 10, 2193–2198. [DOI] [PubMed] [Google Scholar]
- [21].Singh A, Venkannagari S, Oh KH, Zhang Y-Q, Rohde JM, Liu L, Nimmagadda S, Sudini K, Brimacombe KR, Gajghate S, Ma J, Wang A, Xu X, Shahane SA, Xia M, Woo J, Mensah GA, Wang Z, Ferrer M, Gabrielson E, Li Z, Rastinejad F, Shen M, Boxer MB, and Biswal S (2016) Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors, ACS Chem. Biol 11, 3214–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Du Y, Villeneuve NF, Wang XJ, Sun Z, Chen WM, Li J, Lou HX, Wong PK, and Zhang DD (2008) Oridonin confers protection against arsenic-induced toxicity through activation of the Nrf2-mediated defensive response, Environ. Health Perspect 116, 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bousquet MS, Ma JJ, Ratnayake R, Havre PA, Yao J, Dang NH, Paul VJ, Carney TJ, Dang LH, and Luesch H (2016) Multidimensional Screening Platform for Simultaneously Targeting Oncogenic KRAS and Hypoxia-Inducible Factors Pathways in Colorectal Cancer, ACS Chem. Biol 11, 1322–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang X-J, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, and Zhang DD (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2, Carcinogenesis 29, 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, and Biswal S (2006) Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer, PLoS Medicine 3, 1865–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, and Hirohashi S (2008) Loss of KEAP1 function activates Nrf2 and provides advantages for lung cancer cell growth, Cancer Res. 68, 1303–1309. [DOI] [PubMed] [Google Scholar]
- [27].Taguchi K, Shimada M, Fujii S, Sumi D, Pan X, Yamano S, Nishiyama T, Hiratsuka A, Yamamoto M, Cho AK, Froines JR, and Kumagai Y (2008) Redox cycling of 9,10-phenanthraquinone to cause oxidative stress is terminated through its monoglucuronide conjugation in human pulmonary epithelial A549 cells, Free Rad. Biol. and Med 44, 1645–1655. [DOI] [PubMed] [Google Scholar]
- [28].Wang R, An J, Ji FQ, Jiao HQ, Sun HM, and Zhou DS (2008) Hypermethylation of the KEAP1 gene in human lung cancer cell lines and lung cancer tissues, Biochem. Biophys. Res. Commun 373, 151–154. [DOI] [PubMed] [Google Scholar]
- [29].Skowron MA, Niegisch G, Albrecht P, van Koeveringe G, Romano A, Albers P, Schulz WA, and Hoffmann MJ (2017) Various Mechanisms Involve the Nuclear Factor (Erythroid-Derived 2)-Like (NRF2) to Achieve Cytoprotection in Long-Term Cisplatin-Treated Urothelial Carcinoma Cell Lines, Int. J. Mol. Sci. 18 E1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Olayanju A, Copple IM, Bryan HK, Edge GT, Sison RL, Wong MW, Lai Z-Q, Lin Z-X, Dunn K, Sanderson CM, Alghanem AF, Cross MJ, Ellis EC, Ingelman-Sundberg M, Malik HZ, Kitteringham NR, Goldring CE, and Park BK (2015) Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2, Free Rad. Biol. Med 78, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vartanian S, Ma TP, Lee J, Haverty PM, Kirkpatrick DS, Yu K, and Stokoe D (2016) Application of Mass Spectrometry Profiling to Establish Brusatol as an Inhibitor of Global Protein Synthesis, Mol. Cell. Proteomics 15, 1220–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jiménez A, Carrasco L, and Vázquez D (1977) Enzymic and nonenzymic translocation by yeast polysomes. Site of action of a number of inhibitors, Biochemistry 16, 4727–30. [DOI] [PubMed] [Google Scholar]
- [33].Schust J, Sperl B, Hollis A, Mayer TU and Berg T (2006) Stattic: a small-molecule inhibitor of STAT3 activation and dimerization, Chem. Biol 13, 1235–42. [DOI] [PubMed] [Google Scholar]
- [34].Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, Wakabayashi N, Fujii J, Myers A, and Biswal S (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress, Free Radi. Biol. Med 46, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kensler TW, Wakabayash N, and Biswal S (2007) Cell survival responses to environmental stresses via the KEAP1-Nrf2-ARE pathway, Annu. Rev. Pharmacol 89–116. [DOI] [PubMed] [Google Scholar]
- [36].Akera T, and Brody TM (1976) Inotropic action of digitalis and ion-transport, Life Sciences 18, 135–142. [DOI] [PubMed] [Google Scholar]
- [37].Zhang ZB, Li ZC, Tian J, Jiang W, Wang Y, Zhang XJ, Li ZR, You QD, Shapiro JI, Si SY, and Xie ZJ (2010) Identification of Hydroxyxanthones as Na/K-ATPase Ligands, Mol. Pharmacol 77, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schatzmann HJ and Räss B (1965) Inhibition of the active Na-K-transport and Na-K-activated membrane ATP-ase of erythrocyte stroma by ouabain, Helv. Physiol. Pharmacol. Acta 65, C47–9 [PubMed] [Google Scholar]
- [39].Dutta B, Azhir A, Merino LH, Guo YJ, Revanur S, Madhamshettiwar PB, Germain RN, Smith JA, Simpson KJ, Martin SE, Beuhler E, and Fraser IDC (2016) An interactive web-based application for Comprehensive Analysis of RNAi-screen Data, Nat. Comm s7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Herbert AL, Fu MM, Drerup CM, Gray RS, Harty BL, Ackerman SD, O’Reilly-Pol T, Johnson SL, Nechiporuk AV, Barres BA, and Monk KR (2017) Dynein/dynactin is necessary for anterograde transport of Mbp mRNA in oligodendrocytes and for myelination in vivo, Proc. Natl. Acad. Sci. USA 114, E9153–E9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Turei D, Papp D, Fazekas D, Foldvari-Nagy L, Modos D, Lenti K, Csermely P, and Korcsmaros T (2013) NRF2-ome: An Integrated Web Resource to Discover Protein Interaction and Regulatory Networks of NRF2, Oxid. Med. and Cell. Longev 2013, 737591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu XF, Zhang XB, Zhang Y, Yang L, Liu YC, Huang SL, Lu L, Kong LY, Li ZY, Guo QL, and Zhao L (2017) Wogonin reversed resistant human myelogenous leukemia cells via inhibiting Nrf2 signaling by Stat3/NF-kappa B inactivation, Sci. Rep 7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [43].Luesch H, Yoshida WY, Moore RE, Paul VJ, and Mooberry SL (2000) Isolation, structure determination, and biological activity of lyngbyabellin A from the marine cyanobacterium Lyngbya majuscula, J. Nat. Prod 63, 611–615. [DOI] [PubMed] [Google Scholar]
- [44].Kwan JC, Rocca JR, Abboud KA, Paul VJ, and Luesch H (2008) Total structure determination of grassypeptolide, a new marine cyanobacterial cytotoxin, Org. Lett 10, 789–792. [DOI] [PubMed] [Google Scholar]
- [45].Kwan JC, Ratnayake R, Abboud KA, Paul VJ, and Luesch H (2010) Grassypeptolides A-C, cytotoxic bis-thiazoline containing marine cyclodepsipeptides, J. Org. Chem 75, 8012–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Harrigan GG, Yoshida WY, Moore RE, Nagle DG, Park PU, Biggs J, Paul VJ, Mooberry SL, Corbett TH, and Valeriote FA (1998) Isolation, structure determination, and biological activity of dolastatin 12 and lyngbyastatin 1 from Lyngbya majuscula/Schizothrix calcicola cyanobacterial assemblages, J. Nat. Prod 61, 1221–1225. [DOI] [PubMed] [Google Scholar]
- [47].Liu H, Liu Y, Wang Z, Xing X, Maguire AR, Luesch H, Zhang H, Xu Z, and Ye T (2013) Total synthesis and biological evaluation of grassypeptolide A, Chem. Eur. J 19, 6774–6784. [DOI] [PubMed] [Google Scholar]
- [48].Kwan JC, Liu Y, Ratnayake R, Hatano R, Kuribara A, Morimoto C, Ohnuma K, Paul VJ, Ye T, and Luesch H (2014) Grassypeptolides as natural inhibitors of dipeptidyl peptidase 8 and T-cell activation, ChemBiochem 15, 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.