Abstract
Macropinocytosis is an important nutrient-scavenging pathway in numerous cancer types, including pancreatic, lung, prostate, and bladder. This Forum highlights recent work identifying the key regulators of macropinocytosis that support tumor cell fitness in different contexts, providing a unique framework for strategies to target macropinocytosis in the treatment of cancer.
Macropinocytosis functions in cancer cells as an endocytic uptake pathway whereby extracellular fluid is engulfed, delivering extracellular proteins, such as serum albumin, and necrotic cell debris to lysosomes for degradation [1–4]. The breakdown of these macropinocytic cargos contributes to the intracellular amino acid pools and to the maintenance of lipid stores, allowing tumor cells to meet their increased nutrient demands and sustain cell survival and proliferation even under the nutrient-poor conditions of the tumor microenvironment. In cancer cells, macropinocytic stimulation can occur in the context of oncogenic transformation (e.g., oncogenic RAS or activated v-Src expression) or tumor suppressor mutations (e.g., loss of PTEN) [5–7]. Enhanced macropinocytosis has also been observed by stimulation with growth factors, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and macrophage colony-stimulating factor (M-CSF) [2]. Macropinocytosis is a process that is governed by actin cytoskeleton remodeling and is initiated by plasma membrane ruffling followed by macropinocytic cup formation, macropinosome closure, and finally macropinosome maturation [8]. There are several key modulators of the actin cytoskeleton that control macropinocytosis, including small GTPases such as Rac, Cdc42, Arf6, and Rab5, p21-activated kinase (Pak), and phosphoinositide signaling regulators such as phosphoinositide 3-kinase (PI3K) and phospholipase C (PLC) [9]. How these various signaling inputs are integrated in cancer cells has not been resolved and the extent of contextual dependence, such as tissue of origin or metastatic versus primary tumor, is important and requires further scrutiny. Here, we highlight evidence from several recent studies that suggest that macropinocytosis in cancer is highly regulated by an intricate network of signals, indicating that we have only begun to appreciate the complexity of macropinocytic control in the context of a tumor.
Oncogenic RAS mutations are common in a variety of solid tumors, including most cases of pancreatic ductal adenocarcinomas (PDACs), about 25–30% of non-small cell lung cancers (NSCLCs), approximately 13% of bladder cancers, and about 40% of colorectal cancers [10]. Ras-triggered macropinocytosis is dependent on both PI3K, which regulates the actin cytoskeleton through Rac1 and promotes macropinosome closure via phosphoinositide signaling, and PLC signaling [11]. Recent studies have revealed that PDAC and bladder cancer cells harboring KRAS or HRAS mutations, respectively, exhibit oncogene-dependent macropinocytosis for the uptake of extracellular proteins [1]. Metabolite analyses showed that protein-derived amino acids were incorporated into several metabolic pathways, including glutamine anaplerosis/oxidation, acetyl-coenzyme A metabolism, reductive carboxylation and serine/glycine cycling. Importantly, the administration of 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), a specific inhibitor of macropinocytosis, reduced macropinocytic uptake in xenograft tumors and significantly suppressed tumor growth. The antineoplastic effects of EIPA seem to be specific to KRAS mutations since EIPA treatment did not suppress the growth of KRAS-wild-type tumors, which intrinsically display low levels of macropinocytosis. These studies set the stage for further exploration of how macropinocytosis and KRas activity might be interlinked and whether such a nutrient supply route is active in other tumor types. KRas signaling and effector pathway output is highly influenced by its association with the plasma membrane. About half of KRAS-mutant NSCLC cell lines are dependent on KRAS oncogene expression to maintain their viability. Under anchorage-independent conditions, integrin αvβ3 supports such oncogenic KRAS addiction by binding to galectin-3 at the plasma membrane and triggering KRas protein nanoclustering [12]. It was observed that in this setting, macropinocytosis was selectively active and contributed to NSCLC cell fitness through the uptake and catabolism of extracellular albumin [13]. Inhibition of galectin-3 activity in KRAS-mutant tumor cells using the investigational inhibitor GCS-100 caused a reduction in albumin degradation both in 3D culture and in xenograft and patient-derived xenograft (PDX) tumor models, thereby suppressing tumor growth. This important work revealed a contextual correlation between oncogenic KRAS dependency and the reliance on macropinocytosis. Moreover, it provided important evidence of integrin αvβ3 as a biomarker to identify tumors that might be susceptible to macropinocytic inhibition.
In addition to Ras, other signaling pathways have also been demonstrated to control macropinocytic uptake in tumor cells. Redelman-Sidi et al. performed a genome-wide shRNA screen aimed at identifying negative regulators of macropinocytic uptake in RAS-wild-type bladder cancer cells [14]. They identified five negative regulators of the Wnt pathway, including DKK2, KREMEN1, NKD1, SMAD4, and MAPK9. Knockdown of these genes concomitantly activated macropinocytosis and canonical Wnt signaling through β-catenin. Treatment of bladder cancer cells with recombinant Wnt3A protein or the expression of a constitutively activated form of β-catenin induced robust macropinocytosis and supported albumin-dependent proliferation under nutrient starvation conditions. Using the Pak1 inhibitor IPA-3, it was demonstrated that the Wnt pathway induced macropinocytosis in a Pak1-dependent manner. Furthermore, activation of the Wnt pathway was also required for oncogenic Ras-driven macropinocytosis in multiple tumor cell types. It would be interesting to scrutinize whether exogenous expression or activation of the Wnt pathway antagonists identified in the screen has the capacity to suppress macropinocytosis in vivo and modulate bladder tumor growth.
PTEN phosphatase acts as a tumor suppressor by antagonizing the PI3K signaling pathway via the conversion of PI(3,4,5)P3 to PI(4,5)P2. Up to 60% of prostate cancers have monoallelic or complete loss of PTEN, and PTEN-deficient prostate cancer cells exhibit enhanced macropinocytosis [4]. Although loss of PTEN activity is inversely correlated with activation of the PI3K pathway, PTEN loss on its own is insufficient to trigger macropinocytosis. In PTEN-deficient prostate cancer cells, both pharmacological inhibition of AMP-activated protein kinase (AMPK) and expression of dominant-negative AMPKα resulted in blockade of macropinocytosis [4]. These effects were attributed to the AMPK-dependent activation of Rac1. Hence, both PTEN loss and AMPK activation are essential for macropinocytosis in prostate cancer cells. AMPK is best characterized as a sensor for cellular energy homeostasis, but in this case AMPK-mediated macropinocytosis in prostate cancer cells was nutrient independent. Interestingly, PTEN-deficient prostate cancer cells utilize macropinocytosis to engulf necrotic cell debris, which is then digested in lysosomes to produce amino acids, fatty acids, and cholesterol. Macropinocytosis might be a new vulnerability in prostate tumors, as EIPA treatment in vivo impeded macropinocytosis and tumor growth.
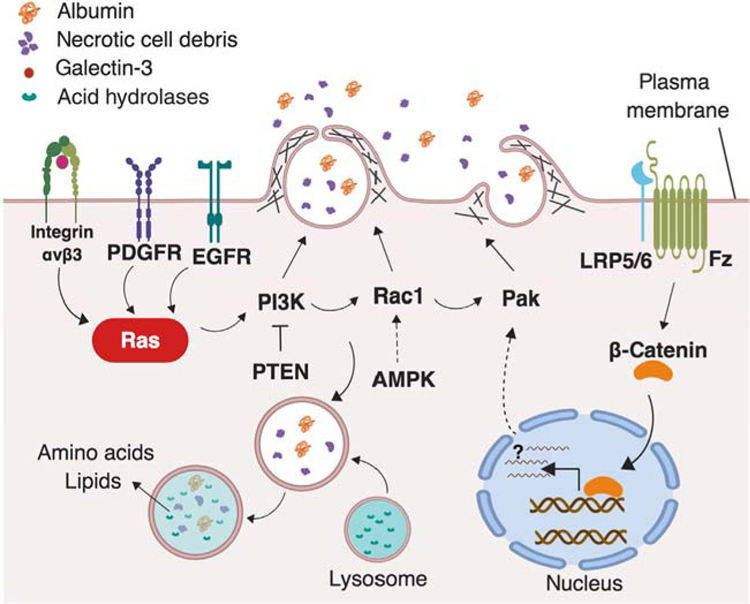
These recent studies paint an elaborate picture of the complex signaling network that regulates macropinocytosis in tumor cells (Figure 1). While it is daunting to think that our current knowledge may represent the tip of the iceberg in terms of the mechanistic underpinnings driving this uptake process, it is encouraging to uncover multiple dependencies and vulnerabilities to target therapeutically. It would be beneficial to scrutinize how fine modulation of Ras signaling translates to macropinocytic output and the extent to which macropinocytosis is influenced by context, such as tissue of origin, nutrient and oxygen availability, differentiation status, and clonal evolution. From a therapeutics perspective, further exploration is required to discern whether macropinocytosis inhibition can be effectively combined with other targeted approaches or with established chemotherapies. While the associations between macropinocytosis and oncogene-driven proliferation are well established, it took more than 30 years to finally make the functional connections between this uptake pathway and cancer metabolism. With this information in hand, we are now much better positioned to examine the intricate details of macropinocytosis with the end goal of developing means to cut off the fuel supply to tumors.
Figure 1. The Complex Signaling Network Driving Macropinocytosis in Cancer.
Context-specific key drivers of macropinocytosis have been identified in multiple cancer types. The activation of the Ras pathway, by oncogenic mutation, integrin nanoclustering, or receptor tyrosine kinases, including EGFR and PDGFR, activates downstream effectors such as PI3K, Rac1, and Pak, which are well-known modulators of macropinocytosis. PTEN loss and activation of AMPK can activate PI3K and Rac1 to trigger macropinocytosis in a KRas-independent manner. In addition, the activation of Frizzled (Fz) and LRP5/6 receptors activates the canonical Wnt pathway through inducing the accumulation and nuclear translocation of β-catenin, which in turn activates Pak in a transcription-dependent manner.
Acknowledgments
This work was supported by NIH grant CA207189 to C.C. The figure in this Forum was created using BioRender. We are grateful to the many scientists who have contributed to this field but whose work was not cited due to space limitations.
References
- 1.Commisso C et al. (2013) Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Recouvreux MV and Commisso C (2017) Macropinocytosis: a metabolic adaptation to nutrient stress in cancer. Front. Endocrinol. (Lausanne) 8, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palm W et al. (2015) The utilization of extracellular proteins as nutrients is suppressed by mTORC1. Cell 162, 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SM et al. (2018) PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov 8, 866–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veithen A et al. (1996) v-Src induces constitutive macropinocytosis in rat fibroblasts. J. Cell Sci 109, 2005–2012 [DOI] [PubMed] [Google Scholar]
- 6.Bar-Sagi D and Feramisco JR (1986) Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068 [DOI] [PubMed] [Google Scholar]
- 7.Redelman-Sidi G et al. (2013) Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res 73, 1156–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerr MC and Teasdale RD (2009) Defining macropinocytosis. Traffic 10, 364–371 [DOI] [PubMed] [Google Scholar]
- 9.Egami Y et al. (2014) Small GTPases and phosphoinositides in the regulatory mechanisms of macropinosome formation and maturation. Front. Physiol 5, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbs GA et al. (2016) RAS isoforms and mutations in cancer at a glance. J. Cell Sci 129, 1287–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amyere M et al. (2000) Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell 11, 3453–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seguin L et al. (2014) An integrin β3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol 16, 457–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seguin L et al. (2017) Galectin-3, a druggable vulnerability for KRAS-addicted cancers. Cancer Discov 7, 1464–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redelman-Sidi G et al. (2018) The canonical Wnt pathway drives macropinocytosis in cancer. Cancer Res 78, 4658–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]