Abstract
Background
Leptomeningeal inflammation is associated with increased cortical damage and worse clinical outcomes in MS. It may be detected on contrast-enhanced T2-FLAIR imaging as focal leptomeningeal contrast-enhancement (LME).
Objective
To assess the safety of intrathecal (IT) rituximab in progressive MS (PMS) and to assess its effects on LME and CSF biomarkers.
Methods
PMS patients had a screening MRI to detect LME. Participants satisfying eligibility criteria underwent two IT administrations of 25 mg rituximab 2 weeks apart. Follow-up lumbar puncture and MRI were performed at 8 and 24 weeks after the first treatment.
Results
Of 36 patients screened 15 had LME, 11 consented, and 8 received study treatment. Mean age was 56.7 years and number of LME lesions ranged from 1 - 3. No serious adverse effects occurred. We noted profound reductions in peripheral B cells from baseline to week 2 and 8 with some return at week 24. We also observed transient reductions in CSF B cells and CXCL-13 levels with an increase in BAFF levels. However, the number of LME did not change following treatment.
Conclusions
IT rituximab was well tolerated in PMS patients and had transient effects on CSF biomarkers but did not change LME.
Keywords: Intrathecal rituximab, leptomeningeal inflammation, progressive multiple sclerosis, clinical trial
1. INTRODUCTION
Leptomeningeal inflammation was described in patients with secondary progressive multiple sclerosis (SPMS) in 2004 and has subsequently been identified at all stages of the disease (Howell et al., 2011; Lucchinetti et al., 2011; Magliozzi et al., 2007; Serafini et al., 2004). While initial reports described lymphoid follicles in the meninges, subsequent manuscripts suggest a spectrum of changes ranging from unorganized inflammation to tertiary lymphoid tissue (Wicken et al., 2018). Meningeal inflammation has been associated with cortical demyelination and neuro-axonal damage (Choi et al., 2012; Howell et al., 2011; Magliozzi et al., 2010). B cells from MS patients produce molecules that are toxic to oligodendroglia and neurons(Lisak et al., 2017). Since gray matter lesions are linked to disability (Kutzelnigg et al., 2005) and patients with meningeal inflammation have a more severe disease course (Choi et al., 2012), it has been postulated that leptomeningeal inflammation may contribute to disease progression in MS.
Recent work has demonstrated that specific MRI sequences may identify areas of meningeal inflammation(Absinta et al., 2015), which demonstrate leptomeningeal enhancement (LME) on contrast-enhanced T2-FLAIR imaging, which could provide a potential biomarker to assess the effects of interventions on meningeal inflammation. Immune cells in areas of meningeal inflammation include B cells, plasma cells, follicular helper T cells, follicular dendritic cells, and macrophages. Rituximab is a monoclonal antibody targeting CD20 that has been shown in multiple trials to be effective in reducing MS disease activity. Intravenous administration results in very low CSF concentration of rituximab, which may not be sufficient to impact meningeal B cells (Czyzewski et al., 2013). Since B cells are an important component of meningeal inflammation and monoclonal antibodies targeting anti-CD20 are effective therapies in MS, (Hauser et al., 2017; Montalban et al., 2017) we hypothesized that direct intrathecal (IT) administration of rituximab may provide a more effective method of resolving meningeal inflammation (Bonnan et al., 2014).
The goal of this trial was to assess the safety of IT rituximab in progressive MS patients with LME and to assess the effects on both LME and CSF biomarkers.
2. SUBJECTS/MATERIALS AND METHODS
2.1. Participants and standard protocol approvals, registrations and patient consents
This single-center, open-label trial enrolled adults with a confirmed diagnosis of progressive MS and evidence of LME on contrast-enhanced T2-FLAIR imaging (complete eligibility criteria in Table 1) between August 2014 and May 2017. Study procedures were approved by the Johns Hopkins Institutional Review Board. The trial was registered on clinicaltrials.gov (NCT02253264).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| 1. Diagnosis of PPMS by 2010 McDonald criteria or SPMS by Lublin and Reingold criteria 2. Age ≥ 18 years 3. MRI Brain demonstrating evidence of leptomeningeal enhancement on contrast enhanced FLAIR images within the past 12 months 4. Patients may be on no MS treatment or should have been on the same treatment for at least 6 months and are not expected to switch therapy in the next 6 months 5. Must have completed a 3-month washout of Dimethyl fumarate or Fingolimod, or undergone a 11-day cholestyramine/ charcoal washout if on Teriflunomide |
| Exclusion criteria |
| 1. Severe intolerance of lumbar puncture in the past 2. Treatment with a chemotherapeutic agent in the past year or chronic infectious disease 3. Peripheral CD19 counts below lower limit of normal in patients previously treated with rituximab 4. Calculated creatinine clearance ≥ 70 ml/min calculated using Cockroft-Gault equation 5. Female patients of childbearing potential not willing to use contraception (IUD, OCP or double barrier) 6. Corticosteroid treatment within the past 30 days 7. Known history of other neuroinflammatory or systemic autoimmune disease 8. Known bleeding diathesis or ongoing anticoagulation (oral/ injectable) 9. Receipt of live vaccination within 1 month prior to scheduled study drug dosing 10. Hemoglobin < 10 mg/dL, or Platelet count < 100,000 /mm3 or WBC count < 2,000 or > 15,000 /mm3 11. Alanine transaminase and/or aspartate aminotransferase > 2.5 x ULN or Total bilirubin > 2.5 x ULN 12. Positive for Hepatitis B surface antigen (HBsAg) or Positive for Hepatitis C antibody (HCV Ab) 13. Moderate or severe acute illness with or without fever 14. Current use (or use within the past 3 months) of natalizumab as MS therapy |
2.2. Study drug
We selected the dose of 25 mg of rituximab, since it was the highest dose previously well-tolerated in studies in CNS lymphoma (Rubenstein et al., 2013). Similar to intravenous dosing protocols, we re-dosed at two weeks to improve the likelihood of CNS and meningeal B cell depletion. We obtained an investigational new drug approval (IND122360) from the FDA.
2.3. Trial visits and safety monitoring
The overall design of the trial is depicted in Figure 1A. Participants eligible for the trial at screening underwent repeat imaging to confirm LME. Eligibility and the Expanded Disability Status Scale (EDSS) score were confirmed at the baseline visit. Participants returned for the first study treatment, which involved oral pre-medication (diphenhydramine 50 mg, ranitidine 150 mg, and acetaminophen 650 mg) followed 30 minutes later by lumbar puncture; 15 ml of spinal fluid was withdrawn, and then 25 mg of rituximab (in 10 ml of normal saline) was injected over 10 minutes. Patients were placed in Trendelenburg position for 90 minutes and observed for adverse events. The procedure was repeated two weeks later. Follow up visits occurred 8 and 24 weeks after the first treatment visit. At each of these follow up visits, patients underwent contrast-enhanced brain MRI, lumbar puncture, phlebotomy, EDSS, and adverse-event assessment. The final follow up visit occurred at 48 weeks and involved phlebotomy for safety labs, EDSS and adverse-event assessment.
Figure 1. Trial design and the effect of IT rituximab on B cell populations and biomarkers in CSF.
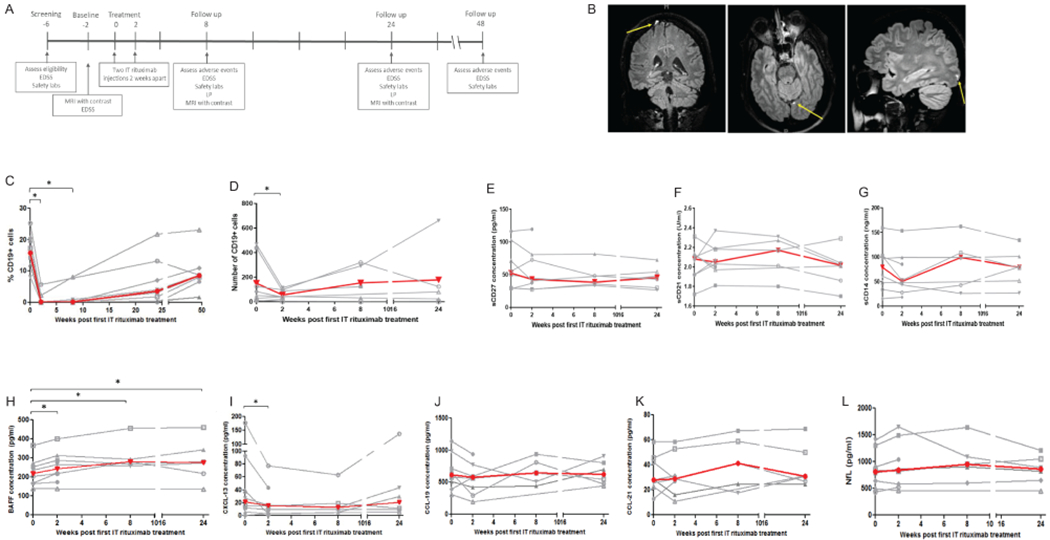
The design of the trial is depicted in panel A; timeline is in weeks. Panel B depicts three examples of leptomeningeal contrast enhancing lesions noted in participants in the trial (yellow arrows). Panel C shows change in peripheral B cell counts over the course of the trial (individual participant data in gray, median across participants in red), while panel D shows CSF B cell counts. We also assessed change in CSF levels of markers of various immune cell populations: E (sCD27), F (sCD21), and G (sCD14). The changes in CSF levels of cytokines/chemokines are depicted in panel H (BAFF), I (CXCL-13), J (CCL-19), and K (CCL-21). Lastly, we measured levels of neurofilament-light chain (NfL) in the CSF (panel L). Comparisons were made with either paired t-test or Wilcoxon sign rank test. * p<0.05
2.4. Sample size
A sample size of 10 would enable us to conclude with 90% confidence that the rate of occurrence of serious toxicity with IT rituximab was less than 20% (Rubenstein et al., 2007) if none of the patients experienced a serious adverse event during the study (Carter and Woolson, 2004). We planned to enroll 12 participants to account for potential dropouts.
2.5. CSF processing and analyses
CSF was immediately subjected to centrifugation, and cells were utilized for flow-cytometry. The remaining supernatant was aliquoted and stored at −80°C until the end of the study. Antibodies for flow-cytometry included: CD45, CD3, CD19, CD27, and IgD. Data were analyzed using Flowjo software to identify B cells and memory subsets.
2.6. Measurement of CSF biomarkers
The levels of B-cell activating factor (BAFF), C-X-C motif chemokine binding ligand-13 (CXCL-13), chitinase-3 like-1 (CHI3-L1), C-C motif chemokine ligand-19 (CCL19), C-C motif chemokine ligand-21 (CCL21), and soluble-CD14 were measured using Luminex kits (R&D), while levels of neurofilament-light (NfL) (Uman diagnostics), soluble-CD21 (Abcam), and soluble-CD27 (R&D) were measured by ELISA kits per manufacturer’s instructions.
2.7. MRI acquisition and analysis
MRI scans were performed on a 3-tesla Phillips Intera scanner. Scans included pre- and post-contrast T1-weighted images and pre- and post-contrast 3-D T2-FLAIR, the latter at least 10 minutes following IV administration of 0.1 mmol/kg of gadopentetate dimeglumine. Images were analyzed by the study radiologist (DSR) to identify the number and location of focal LME, according to previously proposed criteria(Absinta et al., 2015).
2.8. Statistical analyses
We compared levels of biomarkers and cell counts at various time points using paired t-tests or Wilcoxon signed rank tests based on normality of distribution of variables.
3. RESULTS
3.1. Study participants
Of the 36 participants screened for the trial, 15 (42%) demonstrated the presence of LME (Figure 1B). Eleven consented to participate in the trial, and eight fulfilled eligibility criteria and received the intervention. Mean age was 56.7 years, most participants had SPMS (n=7) and median baseline EDSS was 6.0. Demographic and disease characteristics of the study participants are in Table 2.
Table 2.
Participant characteristics
| Characteristic | Value |
|---|---|
| Age (mean ± SD), years | 56.7 ± 7.3 |
| Sex (F:M) | 5:3 |
| Disease duration (mean ± SD), years | 18.8 ± 7.1 |
| Diagnosis | |
| Primary Progressive | 1 |
| Secondary Progressive | 7 |
| Baseline EDSS, median (range) | 6.0 (4.0 – 7.0) |
| No. of LME lesions, median (range) | 1 (1 - 3) |
3.2. Adverse Events
None of the 8 participants experienced a serious adverse event related to IT rituximab. The most common adverse event was infusion-related and resulted in transient paresthesias involving the groin and lower extremities lasting about 30-45 minutes following IT rituximab administration. This occurred in five participants and resolved without residual neurological sequela. Other adverse effects are listed in Table 3. There were no infectious complications over the 48 weeks of follow-up.
Table 3.
Adverse events
| Possibly related to study drug | |
|---|---|
| Event | No. of participants (%) |
| Transient paresthesias in groin and lower extremities | 5 (62.5) |
| Transient increase in lower extremity spasticity | 1 (12.5) |
| Transient lower extremity weakness | 1 (12.5) |
| Worsened fatigue | 2 (25) |
| Fever and rigors | 1 (12.5) |
| Unrelated to study drug | |
| Event | No. of participants (%) |
| Post-lumbar puncture headache | 2 (25) |
| Disorientation and drowsiness# | 1 (12.5) |
Following administration of lorazepam for transient worsening of lower extremity spasticity
3.3. Change in Peripheral and CSF B cells
There was a profound reduction in peripheral-blood B cells in all participants from baseline (median: 18.6%, IQR: 7.2) to week 2 (median: 0.1%, IQR: 1.2) and week 8 (median: 0.1%, IQR: 0.9) with some return at 24 weeks (median: 3.6%, IQR: 9.0) (Figure 1C). The duration of B cell depletion was variable but was generally sustained for several weeks in the majority of participants. Interestingly, the reduction in the CSF B cell population was more transient (Fig 1D).
3.4. CSF and Imaging biomarkers
With respect to CSF cytokines and chemokines, there was a small increase in BAFF and a transient reduction in CXCL-13 (Fig 1H–I). We noted no change in markers of CNS immune cells (sCD14, sCD21, and sCD27 [Fig 1E–G]) or the T-cell chemokines CCL-19 and CCL-21 (Fig 1J–K). CSF NfL levels, which have been linked to inflammatory disease activity, did not change over the course of the study (Fig 1L).
We noted no change in the number or appearance of LME lesions during the 24-week imaging follow-up period. There was no development of new LME lesions over the course of the study.
4. DISCUSSION
In this open-label trial of IT rituximab treatment in progressive MS patients with imaging evidence of meningeal inflammation, we noted no significant adverse effects attributable to the intervention. Intrathecal administration of rituximab resulted in significant and sustained reduction in circulating B cells and a transient drop in CSF B cells, but this did not translate to a change in the number or appearance of LME on imaging.
Few studies have utilized an intrathecal approach to administer rituximab with the goal of targeting compartmentalized inflammation in progressive MS (Bergman et al., 2018; Komori et al., 2016; Topping et al., 2016). Our trial was unique in the inclusion of imaging to identify a subset of participants with LME for inclusion in the trial. We hypothesized that this would potentially identify MS patients most likely to derive a benefit from the intervention (for definitive trials) and provide an additional secondary outcome for this pilot trial. We noted a lack of significant adverse effects during the study related to IT rituximab. The most common adverse effect, transient paresthesia in the lower extremities, has been previously described in other trials of IT rituximab.
We utilized a similar dosing strategy for IT rituximab as the RIVITALISE trial, which additionally utilized IV rituximab (Komori et al., 2016). Other IT rituximab trials were different due to use of more doses of IT rituximab or an Omaya reservoir to deliver rituximab, as opposed to lumbar puncture (Bergman et al., 2018; Topping et al., 2016). Similar to these trials, we noted a marked and sustained peripheral B cell depletion, which suggests that even small doses of rituximab are sufficient to produce peripheral B cell depletion. However, we did not note a sustained depletion of CSF B cells either by flow cytometry or using sCD21 as a surrogate for intrathecal B cells. This may suggest the need for repeat dosing of rituximab for sustained intrathecal B cell depletion or could be related to inadequate efficacy of rituximab in eliminating intrathecal B cells due to lower CSF complement levels and insufficient antibody-mediated NK cell-mediated cytotoxicity (Komori et al., 2016). Rituximab administered through a lumbar puncture can access the cerebral sulci based on previous data from isotope encephalography and from MRI following IT gadolinium administration (Bannister, 1970; Eide and Ringstad, 2015). Type I anti-CD20 antibodies such as rituximab can lead to internalization of the antibody-CD20 complex which can also play a role in reduced ability to deplete B cells. Novel type II anti-CD20 agents have demonstrated slower rate of internalization as well as increased ability to induce direct cell death independent of the mechanisms mentioned above and hence may be attractive targets for future studies targeting leptomeningeal inflammation (Beers et al., 2010; Herter et al., 2013).
The lack of change in LME on MRI could be related to the transiency of CSF B cell depletion, which could suggest a lack of complete B cell depletion from areas of meningeal inflammation. The lack of complete B cell depletion in areas of leptomeningeal inflammation could potentially be related to resistance to the effect of rituximab due to the presence of high levels of B cell survival factors such as BAFF within these niches (Magliozzi et al., 2004; Thaunat et al., 2008). Alternatively, the lack of change on imaging could relate to persistence of other immune cell population in areas of meningeal inflammation or the presence of chronic anatomical alterations (fibrosis and/or incomplete repair of inflammation-mediated changes in the blood-CSF barrier), that are not significantly affected by IT rituximab treatment. The anatomical basis for leakage of intravenous contrast into these areas of leptomeningeal inflammation is unknown, and since no intervention has been shown to produce a sustained effect on areas of LME, whether this will be a suitable marker of response to treatments targeting meningeal inflammation remains unclear.
Our evaluation of biomarkers in the CSF revealed no sustained change in several markers for various intrathecal immune cell populations. We did note an increase in CSF BAFF levels, as reported in the RIVITALISE trial (Komori et al., 2016), and a reduction in CXCL-13 levels, a chemokine important for the formation of B cell follicles, but this change was not sustained. CSF NfL levels, thought to reflect ongoing axonal damage, did not change over the course of the study, likely due to the short duration of the trial and lack of relapses in this cohort of progressive MS patients (Gunnarsson et al., 2011).
The limitations of the study included a small sample size, relatively short duration of follow up, and lack of untreated controls. Additionally, we did not reach the target sample size of ten participants completing IT rituximab treatment due to slow recruitment, which related to both protocol requirements (including multiple lumbar punctures and scans) and the stringent eligibility criteria.
Future studies could consider using a combination approach to target multiple cell types found in meningeal inflammatory aggregates or utilize a CNS-penetrant B cell targeting agent that does not require additional functional mechanisms (such as complement) for B cell elimination. Additionally, identification of further targets from pre-clinical models may help develop effective methods to eliminate meningeal inflammation and potentially slow progression.
ACKNOWLEDGEMENTS
We would like to thank Dr. John Laterra, Dr. Michael Levy and Dr. Matthias Holdhoff for serving on the Data Safety Monitoring Board.
This study was supported by a pilot award from the Race to Erase MS and a grant from the International Progressive MS Alliance to PAC. PB was supported by a Career Transition Award from the National MS Society, the John F Kurtzke Clinician Scientist development award from the American Academy of Neurology and a Young Investigator Award from the Race to Erase MS. DSR and IC are supported by the Intramural Research Program of NINDS.
REFERENCES
- Absinta M, Vuolo L, Rao A, Nair G, Sati P, Cortese ICM, Ohayon J, Fenton K, Reyes-Mantilla MI, Maric D, Calabresi PA, Butman JA, Pardo CA, Reich DS, 2015. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 10.1212/WNL.0000000000001587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister R, 1970. The place of isotope encephalography by the lumbar route in neurological diagnosis. Proc. R. Soc. Med 63, 921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers SA, French RR, Chan HTC, Lim SH, Jarrett TC, Vidal RM, Wijayaweera SS, Dixon SV, Kim H, Cox KL, Kerr JP, Johnston DA, Johnson PWM, Verbeek JS, Glennie MJ, Cragg MS, 2010. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood 115, 5191–5201. 10.1182/blood-2010-01-263533 [DOI] [PubMed] [Google Scholar]
- Bergman J, Burman J, Gilthorpe JD, Zetterberg H, Jiltsova E, Bergenheim T, Svenningsson A, 2018. Intrathecal treatment trial of rituximab in progressive MS. Neurology 1 10.1212/WNL.0000000000006500 [DOI] [PubMed] [Google Scholar]
- Bonnan M, Ferrari S, Bertandeau E, Demasles S, Krim E, Miquel M, Barroso B, 2014. Intrathecal rituximab therapy in multiple sclerosis: review of evidence supporting the need for future trials. Curr. Drug Targets 15, 1205–14. [DOI] [PubMed] [Google Scholar]
- Carter RE, Woolson RF, 2004. Statistical design considerations for pilot studies transitioning therapies from the bench to the bedside. J. Transl. Med 2, 37 10.1186/1479-5876-2-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SR, Howell OW, Carassiti D, Magliozzi R, Gveric D, Muraro P. a, Nicholas R, Roncaroli F, Reynolds R, 2012. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain 135, 2925–37. 10.1093/brain/aws189 [DOI] [PubMed] [Google Scholar]
- Czyzewski K, Styczynski J, Krenska A, Debski R, Zajac-Spychala O, Wachowiak J, Wysocki M, 2013. Intrathecal therapy with rituximab in central nervous system involvement of post-transplant lymphoproliferative disorder. Leuk. Lymphoma 54, 503–6. 10.3109/10428194.2012.718342 [DOI] [PubMed] [Google Scholar]
- Eide PK, Ringstad G, 2015. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol. open 4, 2058460115609635 10.1177/2058460115609635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson M, Malmeström C, Axelsson M, Sundström P, Dahle C, Vrethem M, Olsson T, Piehl F, Norgren N, Rosengren L, Svenningsson A, Lycke J, 2011. Axonal damage in relapsing multiple sclerosis is markedly reduced by natalizumab. Ann. Neurol 69, 83–89. 10.1002/ana.22247 [DOI] [PubMed] [Google Scholar]
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung H-P, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L, OPERA I and OPERA II Clinical Investigators, 2017. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med 376, 221–234. 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- Herter S, Herting F, Mundigl O, Waldhauer I, Weinzierl T, Fauti T, Muth G, Ziegler-Landesberger D, Van Puijenbroek E, Lang S, Duong MN, Reslan L, Gerdes CA, Friess T, Baer U, Burtscher H, Weidner M, Dumontet C, Umana P, Niederfellner G, Bacac M, Klein C, 2013. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol. Cancer Ther 12, 2031–42. 10.1158/1535-7163.MCT-12-1182 [DOI] [PubMed] [Google Scholar]
- Howell OW, Reeves C. a, Nicholas R, Carassiti D, Radotra B, Gentleman SM, Serafini B, Aloisi F, Roncaroli F, Magliozzi R, Reynolds R, 2011. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134, 2755–71. 10.1093/brain/awr182 [DOI] [PubMed] [Google Scholar]
- Komori M, Lin YC, Cortese I, Blake A, Ohayon J, Cherup J, Maric D, Kosa P, Wu T, Bielekova B, 2016. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann. Clin. Transl. Neurol 3, 166–179. 10.1002/acn3.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H, 2005. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128, 2705–2712. 10.1093/brain/awh641 [DOI] [PubMed] [Google Scholar]
- Lisak RP, Nedelkoska L, Benjamins JA, Schalk D, Bealmear B, Touil H, Li R, Muirhead G, Bar-Or A, 2017. B cells from patients with multiple sclerosis induce cell death via apoptosis in neurons in vitro. J. Neuroimmunol 309, 88–99. 10.1016/j.jneuroim.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BFG, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Brück W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM, 2011. Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med 365, 2188–97. 10.1056/NEJMoa1100648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliozzi R, Columba-Cabezas S, Serafini B, Aloisi F, 2004. Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol 148, 11–23. 10.1016/j.jneuroim.2003.10.056 [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F, 2007. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130, 1089–104. 10.1093/brain/awm038 [DOI] [PubMed] [Google Scholar]
- Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R, 2010. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol 68, 477–93. 10.1002/ana.22230 [DOI] [PubMed] [Google Scholar]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung H-P, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, ORATORIO Clinical Investigators, 2017. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl. J. Med 376, 209–220. 10.1056/NEJMoa1606468 [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Fridlyand J, Abrey L, Shen A, Karch J, Wang E, Issa S, Damon L, Prados M, McDermott M, O’Brien J, Haqq C, Shuman M, 2007. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J. Clin. Oncol 25, 1350–6. 10.1200/JCO.2006.09.7311 [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Li J, Chen L, Advani R, Drappatz J, Gerstner E, Batchelor T, Krouwer H, Hwang J, Auerback G, Kadoch C, Lowell C, Munster P, Cha S, Shuman M. a, Damon LE, 2013. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood 121, 745–51. 10.1182/blood-2012-07-440974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F, 2004. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 14, 164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaunat O, Patey N, Gautreau C, Lechaton S, Fremeaux-Bacchi V, Dieu-Nosjean M-C, Cassuto-Viguier E, Legendre C, Delahousse M, Lang P, Michel J-B, Nicoletti A, 2008. B Cell Survival in Intragraft Tertiary Lymphoid Organs After Rituximab Therapy. Transplantation 85, 1648–1653. 10.1097/TP.0b013e3181735723 [DOI] [PubMed] [Google Scholar]
- Topping J, Dobson R, Lapin S, Maslyanskiy A, Kropshofer H, Leppert D, Giovannoni G, Evdoshenko E, 2016. The effects of intrathecal rituximab on biomarkers in multiple sclerosis. Mult. Scler. Relat. Disord 6, 49–53. 10.1016/j.msard.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Wicken C, Nguyen J, Karna R, Bhargava P, 2018. Leptomeningeal inflammation in multiple sclerosis: insights from animal and human studies. Mult. Scler. Relat. Disord. 26, 173–182. 10.1016/j.msard.2018.09.025 [DOI] [PubMed] [Google Scholar]


