Abstract
Background:
Pancreatic ductal adenocarcinoma is a common malignancy with high morbidity. MicroRNAs have been demonstrated to be critical posttranscriptional regulators in tumorigenesis. This study aimed to investigate the effect of microRNA-590 on the proliferation and apoptosis of pancreatic ductal adenocarcinoma.
Material and Methods:
The expression of microRNA-590 and high mobility group AT-hook 2 were examined in clinical pancreatic ductal adenocarcinoma tissues. Pancreatic ductal adenocarcinoma cell line Capan-2 was employed and transfected with microRNA-590 mimics or inhibitor. The correlation between microRNA-590 and high mobility group AT-hook 2 was verified by luciferase reporter assay. Cell viability and apoptosis were detected by MTT and flow cytometry assay. The protein level of high mobility group AT-hook 2, AKT, p-AKT, mTOR, and phosphorylated mTOR were analyzed by Western blotting.
Results:
MicroRNA-590 was found to be negatively correlated with the expression of high mobility group AT-hook 2 in pancreatic ductal adenocarcinoma tissues. Further studies identified high mobility group AT-hook 2 as a direct target of microRNA-590. Moreover, overexpression of microRNA-590 downregulated expression of high mobility group AT-hook 2, reduced cell viability, and promoted cell apoptosis, while knockdown of miR-590 led to an inverse result. MicroRNA-590 also suppressed the phosphorylation of AKT and mTOR without altering total AKT and mTOR levels.
Conclusion:
Our study indicated that microRNA-590 negatively regulates the expression of high mobility group AT-hook 2 in clinical specimens and in vitro. MicroRNA-590 can inhibit cell proliferation and induce cell apoptosis in pancreatic ductal adenocarcinoma cells. This regulatory effect of microRNA-590 may be associated with AKT signaling pathway. Therefore, microRNA-590 has the potential to be used as a biomarker for predicting the progression of pancreatic ductal adenocarcinoma.
Keywords: miR-590, HMGA2, pancreatic cancer, proliferation, apoptosis
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a common malignancy and the fifth cause of cancer-related mortality in developed countries.1,2 Although the treatment improved rapidly, PDAC still remains one of the most malignant cancer with high mortality rate, showing an unsatisfactory status.3 According to the report, nearly 80% of patients with locally advanced or metastatic diseases have poor prognosis.4,5 Therefore, exploring the molecular interactions occurred in the initiation and progression of PDAC will be helpful in developing effective therapies.
High mobility group AT-hook 2 (HMGA2) is a member of HMGA family, which comprises the high mobility group AT-hook1 and high mobility group AT-2 proteins. The HMGA family proteins are characterized by their ability to bind to specific regions of DNA sequences rich in adenine and thymine.6 Among the HMGA family proteins, HMGA2 is reported to be an oncofetal protein that is hardly expressed in the differentiated tissues whereas is highly expressed in a variety of tumors.7,8 Notably, it has been verified that aberrant expression of HMGA2 is highly correlated with malignancies, including cancers of lung, breast, liver, kidney, and colon.9-12 Recent studies showed that the expression of HMGA2 positively related to tumor size and progression of PDAC; moreover, high level of HMGA2 may led to poor prognosis, which implied that HMGA2 may play an important role in the tumorigenesis and progression of PDAC.13
MicroRNAs (miRNAs or miRs) are a group of small noncoding RNA molecules, containing about 20 nucleotides in length,14 which posttranscriptionally regulate the target genes by binding to their 3′untranslated region (3′UTR).15 Accumulating studies have confirmed that miRNAs are widely involved in biological processes. Abnormal expression of miRNAs may contribute to tumorigenesis and malignance through the modulation of tumor suppressor genes. For example, miR-221 promotes metastasis of PDAC by targeting PTEN-Akt,16 miR-200a regulates the proliferation and metastasis of pancreatic cancer through modulating DEK gene,17 miR-543 is downregulated in colorectal cancer samples and acts as tumor suppressor by targeting KRAS, MTA1, and HMGA2.18
In the present study, we observed the negative relationship between the expression of miR-590 and HMGA2 in PDAC tumor tissues and identified HMGA2 as a direct downstream target of miR-590. Our study demonstrated the regulatory effect of miR-590 on the proliferation and apoptosis of PDAC cells through downregulation of HMGA2, suggesting that miR-590 may be used as a potential therapeutic target of PDAC.
Materials/Methods
Clinical Tissues
This study was approved by the Institutional Ethics Committee of Yijishan Hospital affiliated to Wannan Medical College and carried out according to the guidelines of the ethical management. A total of 42 cases of PDAC specimens and 28 cases of paired normal tissues were collected from the Yijishan Hospital of Wannan Medical College during 2016 to 2018. Prior written consent was well informed and signed by all participants. Staging and grading were accessed in accordance with the World Health Organization classification and grading system. All patients did not receive chemoradiotherapy before surgery. All tissues were divided into 2 parts, with one half fixed in 4% paraformaldehyde and the other half reserved in liquid nitrogen.
Cell Lines and Cell Culture
Human pancreatic cancer cell lines Capan-2 were purchased from Cell Bank of Type Culture Collection of Chinese Academy of Sciences and cultured in minimal Roswell Parker Memorial Institute 1640 medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), 2 mM l-glutamine, 100 U/mL of penicillin G, and 100 mg/mL of streptomycin (Biofavor Biotech) at 37 °C under normoxic conditions (5% CO2, 95% O2).
Transfection and Plasmid Construction
Capan-2 cells were seeded at a density of 1.0 × 106 cells/mL. After 6 hours of incubation, cells were transfected with miR-590 mimics, miR-590 inhibitors, and their negative controls (Biofavor Biotech) by using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions. Cells were provided a 24-hour starvation for further analyses before reaching a confluence of 90%.
The wild-type sequence of the HMGA2 3′UTR containing predicted miR-590 binding sites was amplified from Capan-2 cells by polymerase chain reaction (PCR). The mutant 3′UTR sequence of HMGA2 was produced using an overlap-extension PCR method. Then, both wild-type and mutant sequences were subcloned into a psiCHECK-2 vector (Promega).
Luciferase Reporter Assays
For luciferase reporter assay, Capan-2 cells were seeded into 24-well plate and then co-transfected with miR-590 mimics and HMGA2-3′UTR-luciferase plasmids. Following culture for 48 hours, cells were collected and lysed. The luciferase activity was measured by a Dual-Luciferase Reporter Assay System (Promega). Each experiment was performed in triplicate.
Western Blotting
Capan-2 cells were collected and lysed in radioimmunoprecipitation buffer (Beyotime). The protein concentration was determined using a bicinchoninic acid assay (Beyotime). Briefly, equivalent weights of protein samples (40 μg/lane) were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subsequently electrotransferred onto polyvinylidene fluoride membranes (Bio-Rad). Subsequently, all membranes were incubated with the following primary antibodies against HMGA2 (ab97276; Abcam), AKT (sc-8312; Santa Cruz), phosphorylated AKT (sc-33437; Santa Cruz), mTOR (sc-8319; Santa Cruz), and phosphorylated mTOR (p-mTOR, sc-101738; Santa Cruz) at 4 °C overnight. After incubation with secondary antibodies for 1 hour at room temperature, all bands were determined using an enhanced chemiluminescence system kit (MultiSciences).
Quantitative Real-Time PCR
Total RNA was extracted from clinical specimens and Capan-2 cells using Trizol Reagent (Invitrogen). After that, all RNAs were reversed transcribed into complementary DNA using reverse transcription reagent kit (Takara Biotechnology). Real-time quantitative PCR was performed via an Applied Biosystems SYBR Green mix kit and the ABI 7900 Real-Time PCR system (Applied Biosystems Life Technologies). Primer sequences are shown in Table 1. Relative miR-590 or HMGA2 mRNA expression was normalized to snRNA U6 (for miRNAs) or GAPDH (for messenger RNA [mRNAs]), respectively. The relative amount of miRNA or mRNA was calculated using the 2−ΔΔCt method.19
Table 1.
RT-PCR Primer Sequences.
| GENE | Primer sequences (5′-3′) |
|---|---|
| HMGA2 | F: CGAAAGGTGCTGGGCAGCTCCGG R: CCATTTCCTAGGTCTGCCTCTTG |
| miR-590 | F: AAAGATTCCAAGAAGCTAAGGGTG R: CCTAACTGGTTTCCTGTGCCTA |
| U6 snRNA | F: CTCGCTTCGGCAGCACATATACT R: ACGCTTCACGAATTTGCGTGTC |
| GAPDH | F: TGAAGGTCGGTGTGAACGGATTTGGTC R: CATGTAGGCCATGAGGTCCACCAC |
Abbreviations: HMGA2, High mobility group AT-hook 2; miR, microRNA.
Cell Proliferation Assay
The effect of miR-590 on cell viability was determined using an MTT assay. Capan-2 cells were cultured in 96-well plates (2 × 103 cells per well) for 24, 48, 72, and 96 hours. Then, cells were stained with 10 µL of 5 mg/mL MTT per well (Sigma-Aldrich) for 4 hours at 37 °C. Then culture medium was discarded and 150 µL of dimethyl sulfoxide was added. The absorbance was detected at 490 nm with an ELX-800 spectrometer reader (Bio-Tek Instruments).
Cell Apoptosis Assay
Cell apoptosis was measured by Annexin V-fluorescein isothiocyanate/propidium iodide staining (BD PharMingen) following the manufacturer’s instructions. In brief, Capan-2 cells were collected in 6-well plates at a concentration of 105 cells/ mL. Then, Annexin V-fluorescein isothiocyanate (5 μL) and PI (5 μL) were distributed to each well and the cells were incubated in the dark for 15 minutes to undergo flow cytometry (BD LSRII).
Statistical Analysis
All data were presented as means ± standard deviation. Differences were assessed by 2-tailed Student t test and χ2 test as appropriate. P values of .05 or less were considered as statistically significant. Each experiment was performed in triplicate. Statistical analyses were carried out using SPSS 20.0 (SPSS Inc).
Results
Expression of miR-590 Negatively Correlates With the Expression of HMGA2 in PDAC Samples
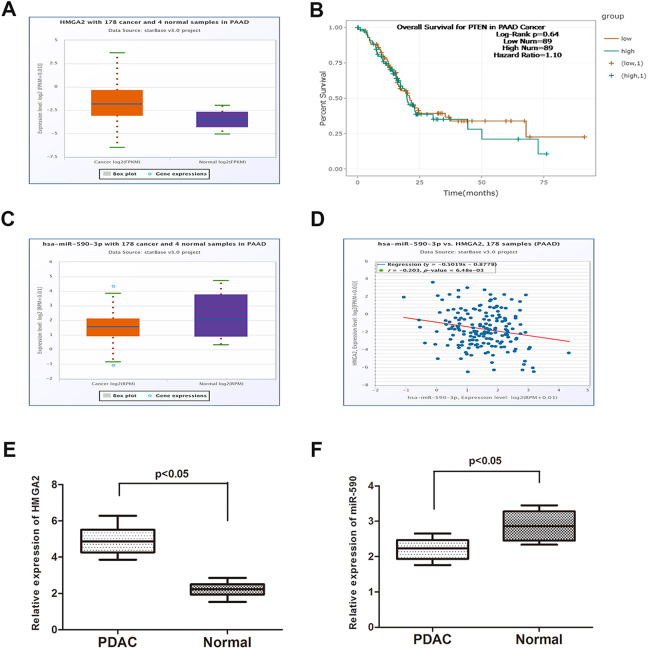
To investigate the expression of HMGA2 in PDAC and normal tissues, we explored the expression of HMGA2 in the TCGA data portal from Starbase version 2.0. As the result showed in Figure 1A, HMGA2 significantly increased in PDAC tissues when compared to normal tissues (P = .034). Moreover, data from Starbase 2.0 showed that patients with high expression of HMGA2 had a poor overall survival time compared to patients with low HMGA2 expression (Figure 1B). Then, we determined the expression of HMGA2 in PDAC and normal tissues collected from our department (Figure 1E; P = .009). The result was in accordance with that from database. On the other hand, database revealed a lower expression of miR-590 in PDAC samples than in paired normal samples (Figure 1C; P = .045). Then, we verified that result using tissues from our department in the same way (Figure 1F; P = .037). A Pearson correlation analysis was performed, and the result showed that the expression of miR-590 was negatively correlated with HMGA2 expression (Figure 1D).
Figure 1.
Expression of miR-590 negatively correlates to the expression of HMGA2 in PDAC samples. A, Data from Starbase 2.0 showed that HMGA2 significantly increased in PDAC tissues when compared to normal tissues. B, Data from Starbase 2.0 showed that patients with high expression of HMGA2 had a poor overall survival time compared to patients with low HMGA2 expression. C Database revealed a lower expression of miR-590 in PDAC samples than in paired normal samples. D, The expression of miR-590 was negatively correlated with HMGA2 expression. E, Results from qRT-PCR showed HMGA2 was highly expressed in PDAC tissues than normal group. F, Results from qRT-PCR showed miR-590 was significantly downregulated in PDAC group than normal group. Data are presented as means ± SD of 3 independent experiments (PAAD, Pancreatic adenocarcinoma; *P < .05 compared with control). HMGA2 indicates High mobility group AT-hook 2; miR, microRNA; PDAC, pancreatic ductal adenocarcinoma; qRT-PCR, quantitative real-time polymerase chain reaction; 3′UTR, 3′untranslated region.
MicroRNA-590 Directly Regulates the Expression of HMGA2
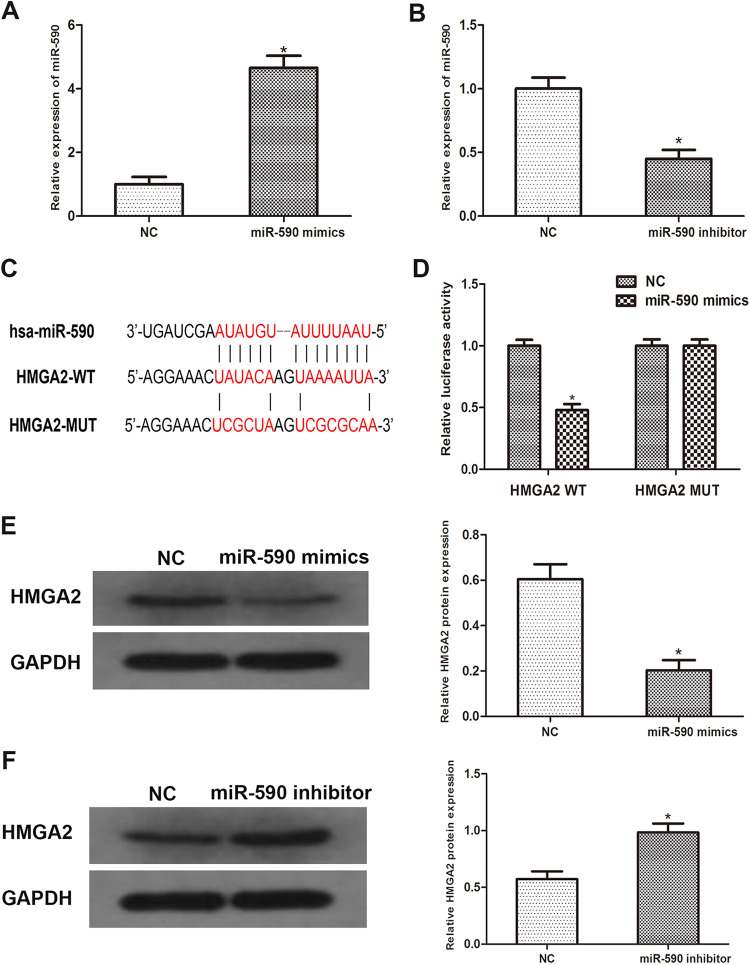
To further investigate the correlation between miR-590 and HMGA2, a PDAC cell line Capan-2 was employed. We transfected Capan-2 cells with miR-590 mimics or inhibitors and then obtained miR-590 overexpressed or knockdown cells (Figure 2A, P = .006; Figure 2B P = .013). By using Western blots, we measured the expression of HMGA2. As the data revealed, the expression of HMGA2 was significantly downregulated in miR-590 overexpressed cells while moderately increased in miR-590 knockdown cells (Figure 2E, P = .017; Figure 2F P = .032). Subsequently, we predicted that HMGA2 was a downstream target of miR-590 by using open access databases (Targetscan, miRanda, and miRwalk 2.0), and a putative binding site in the 3′UTR of HMGA2 for miR-590 was identified (Figure 2C). To confirm this prediction, a luciferase reporter assay was performed. As the result revealed, the reporter activity of HMGA2 3′UTR was significantly abrogated in miR-590 overexpressed cells. However, this effect was reversed when the putative binding site in the 3′UTR of HMGA2 was mutated (Figure 2D; P = .041). Taken together, abovementioned results indicated that miR-590 can negatively regulate the expression of HMGA2 by directly binding to it.
Figure 2.
MiR-590 directly binds to HMGA2 in PDAC samples. A and B, PCR results showed miR-590 mimics or inhibitors were transfected into Capan-2 cells successfully. C, Sequence alignment of predicted miR-590 binding sites with the HMGA2 3′UTR and the mutated sequence of miR-590. D, Luciferase reporter assay was performed in Capan-2 cells that were co-transfected with miR-590 mimics and reporter vectors that containing HMGA2 3′UTR or mutated HMGA2 3′UTR. Relative luciferase activities are shown. E and F, Western blot analysis reveals that the protein level of HMGA2 increased significantly in miR-590 overexpressed cells than miR-590 knockdown cells. Data are presented as means ± SD of 3 independent experiments (*P < .05 compared to control). HMGA2 indicates High mobility group AT-hook 2; miR, microRNA; PDAC, pancreatic ductal adenocarcinoma; PCR, polymerase chain reaction; 3′UTR, 3′untranslated region.
MicroRNA-590 Regulates the Proliferation and Promotes Apoptosis of PDAC Cells
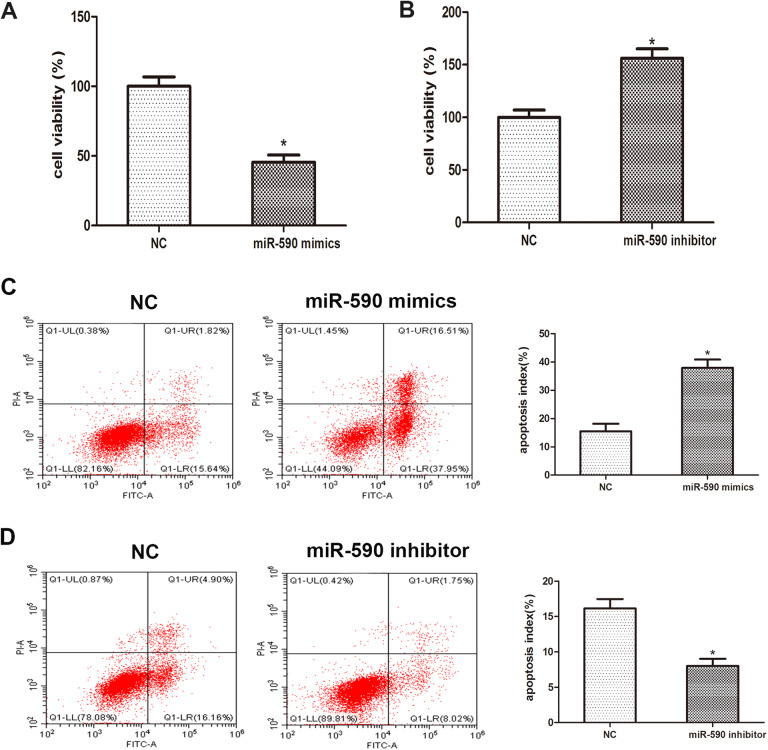
To delineate the role of miR-590 in the proliferation of PDAC cells, MTT assay was performed and the result revealed that the viability of Capan-2 cells transfected with miR-590 mimics were remarkably inhibited when compared with control group, while transfection with miR-590 inhibitors strongly promoted cell viability (Figure 3A, P = .029; Figure 3B P = .044). Moreover, we investigated the role of miR-590 in the apoptosis of PDAC cells. Data from flow cytometry showed that overexpression of miR-590 caused a significant apoptotic rate compared to the control group and that promotive effect was abrogated by the usage of miR-590 inhibitors (Figure 3C, P = .017; Figure 3D, P = .036). Taken together, abovementioned results implied that miR-590 may regulate the proliferation and apoptosis of PDAC cells.
Figure 3.
MicroRNA-590 regulates the proliferation and apoptosis of PDAC cell line. A, MTT assay was performed to analyze cell viability after the transfection of miR-590 mimics, miR-590 inhibitors. The absorbance value was examined at 24, 48, 72, and 96 hours after transfection. B and C, Flow cytometry was performed 48 hours post-transfection. Apoptic cell rate was showed in histogram. Data are presented as means ± SD of 3 independent experiments (*P < .05 compared to control). PDAC indicates pancreatic ductal adenocarcinoma.
Influence of miR-590 on AKT Signaling Pathway
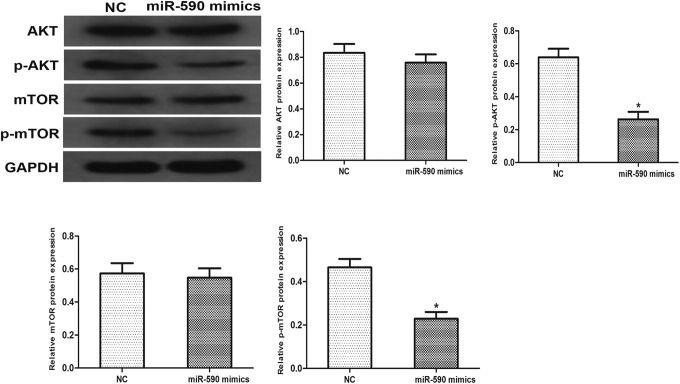
To verify whether miR-590 was involved in the regulation of AKT signaling pathway, we analyzed the phosphorylation level of AKT and mTOR. Western blotting was performed, and results showed that overexpression of miR-590 markedly reduced the phosphorylation of AKT (P = .025) and mTOR (P = .039). The total level of AKT (P = .14) and mTOR (P = .54) remain the same related to the expression of miR-590. These results demonstrated that miR-590 involves in the regulation of AKT signaling pathway, which may be an important factor to the turmorigenesis of Capan-2 cells (Figure 4).
Figure 4.
Effects of miR-590 on AKT signaling pathway. The protein level of AKT, p-AKT, mTOR, and p-mTOR were examined by western blotting. GAPDH was used as an internal control. Data are presented as means ± SD of 3 independent experiments (*P < .05 compared to control).
Discussion
Pancreatic ductal adenocarcinoma is the fourth cause of cancer-related death among all cancers. The 5-year survival rate of PDAC is just 7% to 8%. Due to its early metastasis and invasion, PDAC is difficult to be diagnosed early until it has developed into advanced stages or distant metastasis. The underlying mechanisms of invasion and metastasis remain to be unfolded. Previous studies have indicated that miRNAs involve in multiple biological processes, and they can regulate tumor proliferation and apoptosis in cancer cells by targeting specific genetic markers.20 Based on these discoveries, we have therefore analyzed and identified miRNAs that regulate the progression of PDAC.
It has been well-known that miRNA regulates gene expression at the posttranscriptional level in the way of translational inhibition and mRNA destabilization. Accumulating studies have reported that miRNAs act as oncogene or tumor suppressor in different cancers.21,22 MicroRNA-590 has been reported to be downregulated in breast cancer and suppresses cell survival by targeting sirtuin-1 and deacetylation of P53.23 It has also been reported that miR-590 acts as a tumor suppressor in osteosarcoma by targeting SOX9.24 Latest research unfolded that miR-590 can be used as a prognostic biomarker for glioma.25 However, the role of miR-590 on PDAC and the molecular mechanism have not been investigated. In the present study, we found a negative correlation between miR-590 and HMGA2 in PDAC tissues. MicroRNA-590 was significant downregulated in PDAC tissues compared to paired normal tissues, while HMGA2 was remarkably increased in tumors. Subsequently, we verified a binding correlation between miR-590 and HMGA2 via open access bioinformatic databases and identified HMGA2 as a direct downstream target of miR-590 by luciferase reporter assay.
High mobility group AT-hook 2 has been reported to be aberrantly expressed in a variety of cancers and plays a critical role in the regulation of cell growth.11,26 Its regulatory effect on cell proliferation and metastasis of tumors may be ascribed to the activation of by tumor growth factor β/Smad3 signaling pathway.27,28 In our in vitro studies, we observed that overexpression of miR-590 caused a reduction in HMGA2 expression, while knockdown of miR-590 led to a significant increase in HMGA2. These data indicated that miR-590 negatively regulates the expression of HMGA2 in PDAC cells, which is in accordance with the results from clinical specimens.
Previous study has reported that HMGA2 promotes cell proliferation by activating AKT pathway.16,29 Based on these findings, we investigated whether AKT signaling pathway mediated tumor suppression that induced by miR-590. As results showed, overexpressed miR-590 markedly stagnated the phosphorylation of AKT and mTOR rather than total AKT and mTOR expression. Collectively, our data suggested that miR-590 may somehow involve in the regulation of AKT signaling. Moreover, this regulation might be initiated after the modulation of the expression of HMGA2 by miR-590.
Conclusion
In conclusion, our present study indicated that miR-590 may play a critical role in the tumorigenesis of PDAC cells by inducing cell apoptosis and restraining cell proliferation, and this regulatory effect may, at least in part, be ascribed to the modulation of HMGA2 through AKT signaling pathway. These results implied that miR-590 has a potential to be used as a diagnostic biomarker in the progression of PDAC.
Abbreviations
- mRNA
messenger RNA
- PDAC
pancreatic ductal adenocarcinoma
- HMGA2
High mobility group AT-hook 2
- miR
microRNA
- 3′UTR
3′untranslated region
- PCR
polymerase chain reaction
- 3′UTR
3′untranslated region.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by An’hui Youth Science and Technology funding (2018050304010281).
ORCID iD: Jia-Ding Mao  https://orcid.org/0000-0001-7710-6063
https://orcid.org/0000-0001-7710-6063
References
- 1. Sclafani F, Iyer R, Cunningham D, Starling N. Management of metastatic pancreatic cancer: current treatment options and potential new therapeutic targets. Crit Rev Oncol Hematol. 2015;95(3):318–336. doi:10.1016/j.critrevonc.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi:10.3322/caac.21254 [DOI] [PubMed] [Google Scholar]
- 3. Yang CM, Ji S, Li Y, Fu L-Y, Jiang T, Meng F-D. Beta-Catenin promotes cell proliferation, migration, and invasion but induces apoptosis in renal cell carcinoma. Onco Targets Ther. 2017;10:711–724. doi:10.2147/OTT.S117933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burris HR, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi:10.1200/JCO.1997.15.6.2403 [DOI] [PubMed] [Google Scholar]
- 5. Yang E, Cisowski J, Nguyen N, et al. Dysregulated protease activated receptor 1 (PAR1) promotes metastatic phenotype in breast cancer through HMGA2. Oncogene. 2016;35(12):1529–1540. doi:10.1038/onc.2015.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J, Zhang S, Shan J, et al. Elevated HMGA2 expression is associated with cancer aggressiveness and predicts poor outcome in breast cancer. Cancer Lett. 2016. a;376(2):284–292. doi:10.1016/j.canlet.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 7. Parameswaran S, Xia X, Hegde G, Ahmad I. Hmga2 regulates self-renewal of retinal progenitors. Development. 2014;141(21):4087–4097. doi:10.1242/dev.107326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piscuoglio S, Zlobec I, Pallante P, et al. HMGA1 and HMGA2 protein expression correlates with advanced tumour grade and lymph node metastasis in pancreatic adenocarcinoma. Histopathology. 2012;60(3):397–404. doi:10.1111/j.1365-2559.2011.04121.x [DOI] [PubMed] [Google Scholar]
- 9. Huang W, Li J, Guo X, Zhao Y, Yuan X. miR-663a inhibits hepatocellular carcinoma cell proliferation and invasion by targeting HMGA2. Biomed Pharmacother. 2016;81:431–438. doi:10.1016/j.biopha.2016.04.034 [DOI] [PubMed] [Google Scholar]
- 10. Na N, Si T, Huang Z, et al. High expression of HMGA2 predicts poor survival in patients with clear cell renal cell carcinoma. Onco Targets Ther. 2016;9:7199–7205. doi:10.2147/OTT.S116953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pallante P, Sepe R, Puca F, Fusco A. High mobility group a proteins as tumor markers. Front Med (Lausanne). 2015;2:15 doi:10.3389/fmed.2015.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu J, Zhang S, Shan J, et al. Elevated HMGA2 expression is associated with cancer aggressiveness and predicts poor outcome in breast cancer. Cancer Lett. 2016. b;376(2):284–292. doi:10.1016/j.canlet.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 13. Chiou SH, Dorsch M, Kusch E, et al. Hmga2 is dispensable for pancreatic cancer development, metastasis, and therapy resistance. Sci Rep. 2018;8(1):14008 doi:10.1038/s41598-018-32159-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bushati N, Cohen SM, microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi:10.1146/annurev.cellbio.23.090506.123406 [DOI] [PubMed] [Google Scholar]
- 15. Kala R, Peek GW, Hardy TM, Tollefsbol TO. MicroRNAs: an emerging science in cancer epigenetics. J Clin Bioinforma. 2013;3(1):6 doi:10.1186/2043-9113-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang W, Yang Y, Xia L, et al. MiR-221 promotes capan-2 pancreatic ductal adenocarcinoma cells proliferation by targeting PTEN-Akt. Cell Physiol Biochem. 2016;38(6):2366–2374. doi:10.1159/000445589 [DOI] [PubMed] [Google Scholar]
- 17. Wu X, Wu G, Wu Z, Yao X, Li G. MiR-200a suppresses the proliferation and metastasis in pancreatic ductal adenocarcinoma through downregulation of DEK gene. Transl Oncol. 2016;9(1):25–31. doi:10.1016/j.tranon.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fan C, Lin Y, Mao Y, et al. MicroRNA-543 suppresses colorectal cancer growth and metastasis by targeting KRAS, MTA1 and HMGA2. Oncotarget. 2016;7(16):21825–21839. doi:10.18632/oncotarget.7989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3(3):71–85. [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang T, Li M, Li Q, et al. MicroRNA-98-5p inhibits cell proliferation and induces cell apoptosis in hepatocellular carcinoma via targeting IGF2BP1. Oncol Res. 2017;25(7): 1117–1127. doi:10.3727/096504016X14821952695683 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Lin Y, Liu AY, Fan C, et al. MicroRNA-33b inhibits breast cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci Rep. 2015;5:9995 doi:10.1038/srep09995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okato A, Goto Y, Kurozumi A, et al. Direct regulation of LAMP1 by tumor-suppressive microRNA-320a in prostate cancer. Int J Oncol. 2016;49(1):111–122. doi:10.3892/ijo.2016.3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abdolvahabi Z, Nourbakhsh M, Hosseinkhani S, et al. MicroRNA-590-3P suppresses cell survival and triggers breast cancer cell apoptosis via targeting sirtuin-1 and deacetylation of p53. J Cell Biochem. 2019;120(6):9356–9368. doi:10.1002/jcb.28211 [DOI] [PubMed] [Google Scholar]
- 24. Wang WT, Qi Q, Zhao P, Li C-Y, Yin X-Y, Yan R-B. miR-590-3p is a novel microRNA which suppresses osteosarcoma progression by targeting SOX9. Biomed Pharmacother. 2018;107:1763–1769. doi:10.1016/j.biopha.2018.06.124 [DOI] [PubMed] [Google Scholar]
- 25. Bobowicz M, Skrzypski M, Czapiewski P, et al. Prognostic value of 5-microRNA based signature in T2-T3N0 colon cancer. Clin Exp Metastasis. 2016;33(8):765–773. doi:10.1007/s10585-016-9810 -1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takaha N, Sowa Y, Takeuchi I, Hongo F, Kawauchi A, Miki T. Expression and role of HMGA1 in renal cell carcinoma. J Urol. 2012;187(6):2215–2222. doi:10.1016/j.juro.2012.01.069 [DOI] [PubMed] [Google Scholar]
- 27. Lee AW, Ma BBY, Ng WT, Chan ATC. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. 2015;33(29):3356–3364. doi:10.1200/JCO.2015.60.9347 [DOI] [PubMed] [Google Scholar]
- 28. Morishita A, Zaidi MR, Mitoro A, et al. HMGA2 is a driver of tumor metastasis. Cancer Res. 2013;73(14):4289–4299. doi:10.1158/0008-5472.CAN-12-3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan L, Wei X, Zheng L, et al. Amplified HMGA2 promotes cell growth by regulating Akt pathway in AML. J Cancer Res Clin Oncol. 2016;142(2):389–399. doi:10.1007/s00432-015-2036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]