Abstract
High-voltage rhythmic EEG spikes have been recorded in wildtype (WT) rats during periods of light slow-wave sleep and passive wakefulness. The source of this activity is unclear, but has been attributed to either an inherent form of absence epilepsy or a normal feature of rodent sleep EEG. In contrast, little is known about epileptiform spikes in WT mice. We thus characterize and quantify epileptiform discharges in WT mice for the first time. 24-hour wireless telemetry video-EEG recordings in 36 male WT C57 mice were manually scored by blinded reviewers to mark individual spikes and spike trains. Epileptiform spikes were detected in 100% of the recorded WT mice, and spike trains of at least three spikes were recorded in 90% of mice. The spikes were more frequent during the day than at night, and were inversely correlated to each animal’s locomotor activity. However, the discharges were not absent during active nighttime periods. These discharges may indicate a baseline tendency toward epileptic seizures, or perhaps are benign variants of normal rodent background EEG. Regardless, a better understanding of baseline WT EEG activity will aid in differentiating pathological and normal EEG activity in mouse epilepsy models.
Keywords: Epileptiform spikes, wildtype mice, EEG
1. Introduction
Translational epilepsy studies often employ rodent models whose phenotypes recapitulate human disease. In particular, mouse EEG recorded by skull screw electrodes is a valuable tool for phenotypic characterization and for drug discovery [1, 2]. Among the range of signals and metrics that can be derived from mouse EEGs are spikes and sharp waves, occurring individually or in ‘runs’, that closely resemble epileptiform discharges observed in humans with epilepsy. However, healthy rodents without a genetic or acquired predisposition toward epilepsy also have occasional epileptiform discharges that morphologically resemble the spikes and sharp waves attributed to epilepsy[3, 4]. This phenomenon has been described in rats where such discharges, termed high-voltage rhythmic spikes (HVRS), are observed in the EEG of healthy, wildtype (WT) rats [5, 6].
The significance of epileptiform discharges in healthy rodents is unclear – these may indeed be epileptic activity, indicating a baseline rodent tendency toward seizure. Alternatively, they may simply be elements of normal rodent background EEG. Following administration of some anti-seizure drugs, the duration and frequency of these discharges is reduced in rats, suggesting an association with epilepsy [5]. However, one study utilized an operant conditioning paradigm to show that in the presence of reward, rats can alter the duration of these spike bursts, suggesting that these discharges may mark events related to learning and neural plasticity [6]. The level and variability of discharges in the WT population must be understood before this type of activity can be used as an endpoint in characterizing genetic and drug manipulation of seizure phenotypes.
Little work has been done to measure unprovoked epileptiform EEG activity in WT mice. Such investigation is crucial as mouse models of acquired and genetic epilepsy are increasingly prevalent, and EEG distinction between normal and pathologic states can be confounded by epileptifirorm discharges that may be a part of WT mouse EEG background. Accordingly, we describe epileptiform activity in 24-h EEG recordings of WT mice to improve our understanding of normal mouse EEG background features and to facilitate distinctions between pathological and normal EEG activity in mouse epilepsy models.
In an ongoing study, we recorded video EEG in cohorts of autism mouse models and their WT littermate controls [1]. Four sets of WT littermate controls, and naïve C57 mice unrelated to any of those control cohorts, were implanted with wireless telemetry units to test whether and how often epileptiform EEG abnormalities are found in WT mice.
2. Material and Methods
2.1. Animals
Male C57BL/6J WT mice were obtained from Jackson Laboratory (n=30) and the National Cancer Institute (NCI) Mouse Repository (n=6). Mice were either naïve C57 (group 1, n=5), or served as WT (C57 background) littermate controls for mice carrying mutations in Shank3b (group 2, n=13), Gabr3b (group 3, n=6), Cntnap2 (group 4, n=6), and Pten (group 5, n=6) [1, 7–9]. At the time of EEG, the animals were 55.4 ± 7.3 days old and weighed 23.3 ± 1.9g; mean ± SD.
Mice were housed in a temperature-controlled vivarium maintained on a 12-hour light/dark cycle. All procedures were approved by the Animal Care and Use Committee at Boston Children’s Hospital and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. EEG data acquisition
Under isoflurane, mice were intraperitoneally implanted with wireless telemetry transmitters (PhysioTel ETA-F10; DSI, Data Sciences International, St. Paul, MN) using skull screws by threading the electrodes to the cranial cavity per laboratory protocol [1, 2]. After analgesia and a week of recovery, mice were individually housed in transparent home cages in a 12 h light (7am-7pm)/12 h dark (7pm-7am), temperature, and humidity-controlled chamber with ad libitum access to food and water.
One-channel video-EEG was recorded differentially between the reference (right olfactory bulb) and active (left occipital lobe) electrodes. EEG (1000 Hz), core-body temperature and locomotor activity (200 Hz) signals were continuously sampled over a period of seven days along with time-registered videos.
2.3. Data analysis and statistics
After 24 h acclimation, the subsequent 24-h period was analyzed by a visual inspection of the EEG utilizing Neuroscore (DSI). EEG was bandpass filtered from 3–70 Hz for scoring purposes. Blinded reviewers marked single epileptiform spikes as well as spike trains defined as a group of ≥ 3 continuous spikes. Individual spike characteristics such as amplitude and duration were used to differentiate spikes from electrical and mechanical artifacts. A spike was defined as a sharp deflection with amplitude ≥ 2x the background activity and 40–60 ms in duration. To minimize bias, each recording was scored by two independent reviewers (HP, SG) and a third reviewer (SD) reconciled disagreements between the two scorings for markers in question. Based on this ascertainment, spike and spike train frequency per animal per 24-h were analyzed, along with actigraphy measures.
Data were analyzed using GraphPad Prism (GraphPad Software Inc., La Jolla, CA). One-way ANOVA was employed to compare the groups for differences in spike and spike train counts in 24 h analysis. Paired t-tests were used to compare spike and spike train counts; locomotor activity between light and dark cycles. Significance is defined at p<0.05. Results are reported as mean ± SEM.
3. Results
3.1. Epileptiform spikes are very common in WT mice
A spike was defined as either single or belonging to a spike train if other spikes were recorded within 500 ms (figure 1A). Epileptiform spikes were recorded in 100% of the 36 implanted mice (figure 1Bi). Total spike frequency varied widely per group (199.5 ± 15.9 per 24 h; 8.3 ± 0.7 per hour). One-way ANOVA revealed a significant difference in the number of spikes per group [F (4, 31) = 7.13, p=0.0003]. The majority of spikes in all groups were isolated (85.7%), rather than components of a spike train (14.3%).
Figure 1. Epileptiform EEG spiking in 24h.
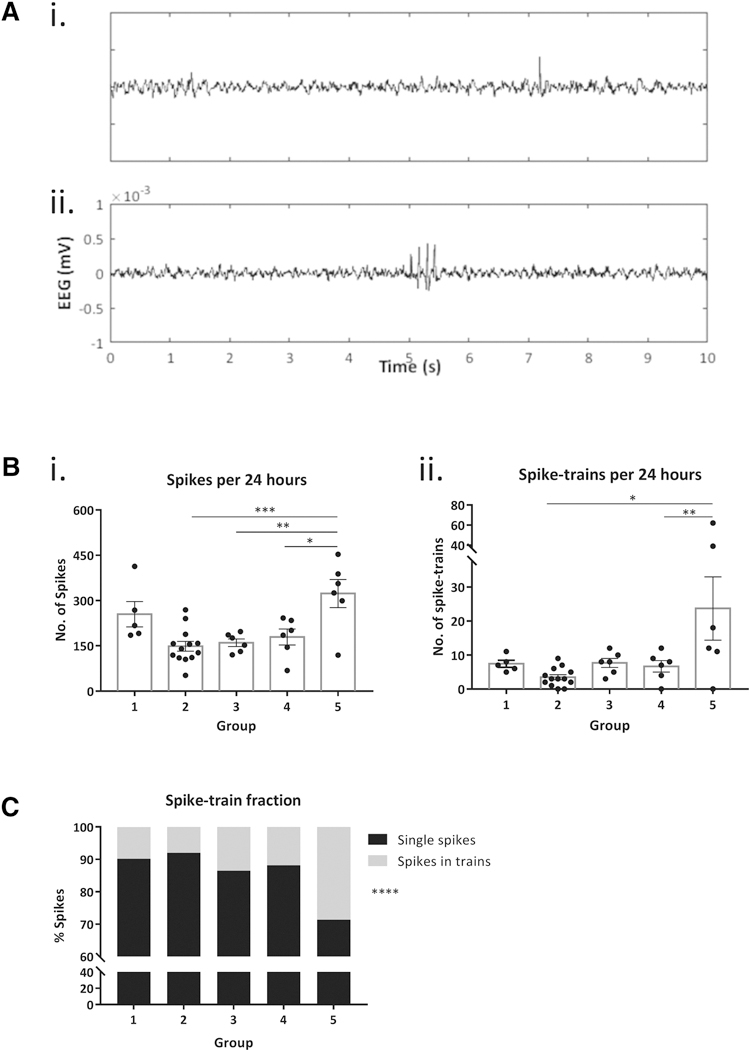
A: Representative traces show background EEG with (i) a single spike and, (ii) a spike train. B: (i) 100% mice exhibited single epileptiform spikes in 24 h EEG. One-way ANOVA demonstrates a significant group effect in the average spike frequency (p=0.003) and Tukey’s post-hoc comparisons show significant differences between group 5 vs groups 2, 3 and 4, respectively. Bars indicate mean ± SEM and scatter dots represent individual values. (ii) 90% mice recorded spike trains comprising at least 3 consecutive spikes in 24-h EEG. The number of spike trains was significantly different among groups (p=0.004) and Tukey’s post-hoc test shows differences in group 5 vs group 2 and 4, respectively. Error bars indicate SEM and scatter dots represent individual values. C: Stacked bar plot shows the fraction of total spiking activity that is composed of single spikes vs spikes in trains, per group. 85.6% of spikes across all mice were isolated. Groups include naïve C57 WT mice (group 1, n=5), WT littermate controls of autism mouse models of Shank3b (group 2, n=13), Gabr3b (group 3, n=6), Cntnap2 (group 4, n=6), and Pten (group 5, n=6). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
3.2. Trains of epileptiform spikes are common, but not universal, in WT mice
Spike trains containing at least 3 spikes were recorded in 90% (32/36) of mice. The total number of spike trains (8.6 ± 1.9 per 24 h) was significantly different among groups as computed by one-way ANOVA [F (4, 31) = 4.75, p=0.004] (figure 1Bii). Spike trains on average contained 3.6 ± 0.07 spikes, and average spike train duration was 0.42 ± 0.02 seconds. 65.2% of the total trains marked across all groups were composed of only 3 spikes, the most common train length observed. 19.4% of marked trains included 4 consecutive spikes, 7.7% (5 spikes), and 5.5% (≥6 spikes). The fraction of spikes occurring in spike trains was significantly lower relative to single spikes that were not part of spike trains (Chi-square test, χ2= 389.4; p<0.0001; figure 1C).
3.3. Circadian variation of epileptiform spike activity in WT mice
Hourly analysis of 24-h EEG revealed a circadian pattern of epileptiform spike and spike train frequencies in mice during the light (7am-7pm) and dark (7pm-7am) cycles as seen in figures 2Ai and 2Bi. The hourly spike count was significantly higher (10.7 ± 0.8 spikes) during the daytime versus in the nighttime (5.9 ± 0.6 spikes; paired t-test, p<0.0001; figure 2Aii). Likewise, the hourly frequency of spike trains, figure 2Bii, in the daytime was significantly greater (0.4 ± 0.08) than in the nighttime (0.3 ± 0.08; paired t-test, p = 0.003). Figure 2Ci captures the circadian variations in the locomotor activity of these mice across the day and night cycles. The spike counts were inversely correlated with animal’s locomotion during these periods with higher hourly activity counts reported in dark (641.7 ± 57 AU), as compared to light cycle (1717 ± 157.4 AU) using paired t-test (p<0.0001). By qualitative analysis of the video recording, EEG spikes occurred most frequently while mice were in a state of physical arrest, corresponding to this quantification.
Figure 2. Hourly circadian variations in epileptiform spiking and locomotor activity.
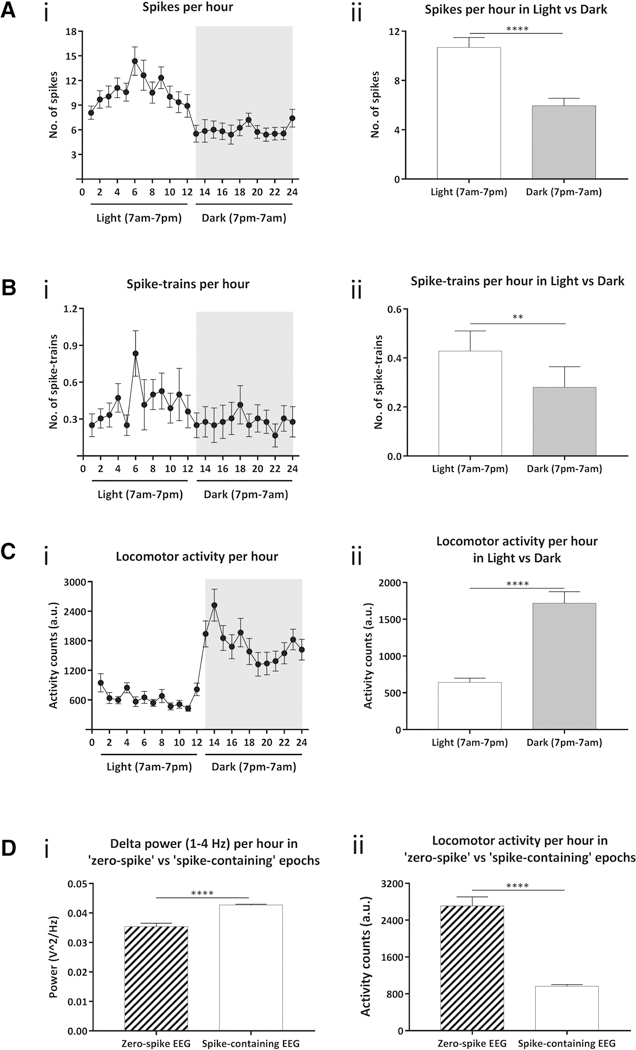
A: (i) Hourly spikes in 24h EEG show a circadian pattern to the epileptiform spiking (ii) Total number of single spikes during day, 12h of light cycle (7am-7pm) was significantly higher than at night, 12h of dark cycle (7pm-7am). B: (i) Average hourly spike trains in 24h EEG also exhibit a circadian variation. (ii) Quantification of average spike trains showed a significantly higher frequency during light versus dark cycle. C: (i) Hourly locomotion shows a significant increase in the locomotor activity of mice during nighttime. (ii) The mice are significantly hyperactive during the dark cycle relative to light cycle. D: (i) Spectral power analysis comparing hour-long epochs containing either zero spikes or one or more spikes from all mice demonstrates a significant increase in power in the delta frequency band during ‘spike-containing’ epochs over ‘zero-spike’ epochs (ii) Locomotor activity during the same epochs was found to be inversely correlated to delta power, with significantly less locomotion during ‘spike-containing’ epochs than in those with zero spikes. Error bars indicate SEM. ***p<0.01, ****p<0.0001.
For further analysis, we dichotomized all EEG data into 60-minute bins that were sorted as either spike-free, or contained at least one EEG spike. Spectral analysis of the EEG computed via fast-Fourier transform revealed significantly lower power per hour in the delta frequency band (1–4 Hz) during ‘zero spike’ EEG epochs as compared to delta power during epochs that contained at least one spike (unpaired t-test, p<0.0001; figure 2Di). This was inversely correlated with locomotor activity during these periods, with significant hyperactivity during ‘zero spike’ epochs versus ‘spike-containing’ epochs (unpaired t-test, p<0.0001; figure 2Dii). Further segmentation of hours with EEG spikes into 1-minute bins revealed that 88% of these periods showed no locomotor activity, indicating that the majority, but not the entirety, of epileptiform spiking occurred during sleep or inactivity.
4. Discussion
We demonstrate for the first time that epileptiform spikes are common in WT mice, and universal in the studied strains derived from the C57 background. We anticipate that these data will guide future EEG analyses, by providing an expectation of a non-zero spike rate in mouse EEG.
Notably, while all groups had spikes on EEG, there was significant variability in spike frequency between groups of mice with one group (littermate controls of Pten mutants) having appreciably higher spike frequency than the others. This indicates that genetic background factors likely contribute to the common spikes in mice. Although all mice in this study were WT, groups 2–5 were derived as littermate controls from different mutant backgrounds. While mutant lines of groups 2–5 were extensively backcrossed to the C57BL/6J strain – the most common strain used in neuroscience research, genetic drift and analogous factors that may govern background genetics within a mouse colony may explain the observed variability. Supporting this conclusion, the C57 mice used for backcrossing in group 5 stood out from others, as they were sourced from the NCI Mouse Repository where the Pten line was maintained in isolation as compared to Jackson Laboratories for other groups. An effect on spike frequency due to strain differences in parenting is another intriguing possibility that cannot be excluded. The differences observed here emphasize the importance of proper selection and characterization of controls when conducting mouse EEG experiments where epileptiform spike frequency is among the outcome measures.
The biological substrate of the recorded spikes remains in question. Plausibly, these discharges are benign epileptiform transients seen in sleep [3]. Our data demonstrating increased spikes during daytime (sleep periods for mice) support this possibility. Spectral analysis indicating increased power in the delta frequency band during periods of spiking further corroborates this finding, as increased delta power is a feature of sleep EEG [10]. Yet since not all spikes in our recordings were recorded from sleep, the likelihood is that these are benign epileptic transients analogous to those recorded in humans [11–14].
Another possibility is that the recorded spikes in mice reflect a predisposition to seizures. Published studies indicate that spike-wave discharges in rats peak in light slow-wave sleep, passive wakefulness, drowsiness, and transition from sleep to wakefulness [3, 15], which correlates to occurrence of myoclonic and other primary generalized seizures in humans [16]. We observed spikes in mice during periods of inactivity and sleep, which is in line with these findings in rats. Future studies will be needed to determine how the prevalence of mouse epileptiform changes as a function of sleep phase. Among other valuable experiments will be those aimed to investigate the pharmacology and physiology of EEG spikes in mice and those aimed to test explicitly whether these discharges differ from those recorded in rats [4].
Last, we note that frequent epileptiform discharges in healthy WT mice mark an important confound in translational epilepsy research: If epileptiform discharges are an element of normal rodent physiology, then binary distinction between epilepsy and exaggeration of normal background EEG features is not possible when EEG is analyzed by either visual inspection or by digital spike detection. Moreover, our results underscore that the field would benefit from a refined definition of seizure in rodent models. A quantitative EEG analysis or assessment of behavioral seizure features by video-EEG may be needed to conclude that increased background spike frequency or spike train duration corresponds to epilepsy in a rodent model.
Acknowledgements
The study is originally a component of the Autism Speaks’ Preclinical Autism Consortium for Therapeutics (PACT). The authors thank Dr. Crawley for her insights on mouse models of autism for the PACT studies and for reading and providing feedback on this manuscript. The authors also acknowledge Boston Children’s Hospital’s Neurodevelopmental Behavioral Core (CHB IDDRC, 1U54HD090255) for their assistance.
Funding
This work was supported by NIH NINDS (R01NS088583; AR) and Autism Speaks (8073; MS, AR) awards, the Translational Research Program (TRP) at Boston Children’s Hospital (AR), and the services of Boston Children’s Hospital’s Experimental Neurophysiology Core (ENC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
None of the authors has any conflict of interest to disclose.
References
- 1.Dhamne SC, et al. , Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism, 2017. 8: p. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly E, et al. , mGluR5 Modulation of Behavioral and Epileptic Phenotypes in a Mouse Model of Tuberous Sclerosis Complex. Neuropsychopharmacology, 2018. 43(6): p. 14571465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coenen AM, et al. , Absence epilepsy and the level of vigilance in rats of the WAG/Rij strain. Neurosci Biobehav Rev, 1991. 15(2): p. 259–63. [DOI] [PubMed] [Google Scholar]
- 4.Danober L, et al. , Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol, 1998. 55(1): p. 27–57. [DOI] [PubMed] [Google Scholar]
- 5.Shaw FZ, 7–12 Hz high-voltage rhythmic spike discharges in rats evaluated by antiepileptic drugs and flicker stimulation. J Neurophysiol, 2007. 97(1): p. 238–47. [DOI] [PubMed] [Google Scholar]
- 6.Taylor JA, et al. , Voluntary Control of Epileptiform Spike-Wave Discharges in Awake Rats. J Neurosci, 2017. 37(24): p. 5861–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLorey TM, et al. , Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res, 2008. 187(2): p. 207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penagarikano O, et al. , Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell, 2011. 147(1): p. 235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page DT, et al. , Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci U S A, 2009. 106(6): p. 1989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Church MW, Changes in frequency and amplitude of delta activity during sleep. Electroencephalogr Clin Neurophysiol, 1975. 39(1): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 11.Santoshkumar B, et al. , Prevalence of benign epileptiform variants. Clin Neurophysiol, 2009. 120(5): p. 856–61. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan K, Santoshkumar B, and Venugopal A, Prevalence of benign epileptiform variants observed in an EEG laboratory from South India. Clin Neurophysiol, 1999. 110(2): p. 280–5. [DOI] [PubMed] [Google Scholar]
- 13.Monin J, et al. , Prevalence of benign epileptiform variants during initial EEG examination in French military aircrew. Neurophysiol Clin, 2018. 48(3): p. 171–179. [DOI] [PubMed] [Google Scholar]
- 14.Tatum WO, Normal “suspicious” EEG. Neurology, 2013. 80(1 Suppl 1): p. S4–11. [DOI] [PubMed] [Google Scholar]
- 15.Shaw FZ, Is spontaneous high-voltage rhythmic spike discharge in Long Evans rats an absence-like seizure activity? J Neurophysiol, 2004. 91(1): p. 63–77. [DOI] [PubMed] [Google Scholar]
- 16.Bagshaw AP, et al. , Multimodal neuroimaging investigations of alterations to consciousness: the relationship between absence epilepsy and sleep. Epilepsy Behav, 2014. 30: p. 33–7. [DOI] [PubMed] [Google Scholar]


