Abstract
BACKGROUND
African Americans (AA) and socioeconomic status (SES) disadvantaged older breast cancer survivors (BCS) are more likely to experience poor functional and health outcomes. However, few studies have evaluated the putative beneficial effects of exercise on these outcomes in older racial minority and SES-disadvantaged BCS.
METHODS
This is a mixed-methods study that includes a randomized-controlled trial, “IMPROVE”, to evaluate a group-based exercise intervention compared to a support group program in older BCS, followed by post-intervention semi-structured interviews to evaluate the intervention. The trial aims to recruit 220 BCS with 55 in each of four strata defined by race (AA versus Non-Hispanic Whites) and SES (disadvantaged vs. non-disadvantaged). Participants are ≥ 65 years old and within five years of treatment completion for stage I-III breast cancer. Participants are randomized to a 52-week, three sessions/week, one-hour/session, moderate intensity aerobic and resistance group exercise intervention, (n=110) or a 52-week, one hour/week, support group intervention [attention-control arm], (n=110). The first 20 weeks of both programs are supervised and the last 32 weeks, unsupervised. The primary outcome is the change in Short Physical Performance Battery (SPPB) Scores at 20 weeks from baseline, between the two arms. Secondary outcomes include change in SPPB scores at 52 weeks, change in body composition and biomarkers, at 20 and 52 weeks from baseline, between arms.
DISCUSSION
Results of the trial may contribute to a better understanding of factors associated with recruitment, and acceptability, and will inform future exercise programs to optimally improve health outcomes for older BCS.
Keywords: Older Breast, Cancer Exercise, African American, Socioeconomic Status-disadvantaged
BACKGROUND
The population of cancer survivors, defined as any person who has been diagnosed with cancer from the time of diagnosis through the remainder of life1, continues to expand largely in the United States because of the exponential growth and aging of the population, and also due to advances in early detection and treatment. As of January 2019, more than 16.9 million cancer survivors were alive in the United States and this number is projected to reach more than 22 million by 2030.2 Breast cancer survivors (BCS), who numbered 3.8 million as of January 1, 2019 (~44%) represent the highest proportion of female cancer survivors in the United States with almost two-thirds (64%) being 65 years and older.2 Due to the aging of the population, the number of breast cancer survivors is projected to further increase to 4.9 million by 2030.2
Despite improving breast cancer survival rates, not all women have benefited equally. Recent data from the American Cancer Society show that, compared with NHW, AA women are 41% more likely to die from breast cancer.3 Furthermore, breast cancer survival rates are significantly lower for older women compared to their younger counterparts4, and are particularly lower for older AA women compared to older NHW.5 Socioeconomic status differences as well as disproportionate burden of comorbidities and health problems among AA such as functional disability6, obesity7, physical inactivity8 and poor health status5 partly contribute to these racial disparities in breast cancer survival. Moreover, with increasing breast cancer survivorship, many women are faced with long-term treatment complications such as lymphedema9, functional decline10,11, cancer-related fatigue12, obesity and physical inactivity which adversely impact the survivorship experience.13 Physical activity has the potential to attenuate many of these problems including functional decline,14–16 may reduce breast cancer mortality by as much as 34% and all-cause mortality by 41%.17, and thus may help to reduce existing racial health disparities among BCS. Nonetheless, only about 34%18 to 70%19 of breast cancer survivors engage in the recommended amount of PA.20 Rates of physical activity are particularly low among AA BCS with only 24% likely to meet recommended physical activity levels.8
While several exercise randomized trials have been conducted among BCS, the inclusion of older women in these trials has been limited.21 In particular, exercise studies involving older AA and SES-disadvantaged BCS, that could inform the design of exercise programs for these underserved population, are lacking.22 Understanding exercise behavior in populations that have high rates of physical inactivity is important for successful physical activity intervention. The Transtheoretical Model (TTM) of behavior change developed by Prochaska et al23–25, serves as a conceptual framework for examining exercise behavior. The TTM is an integrative model of behavior change that involves progressing through five stages of change, including precontemplation, contemplation, preparation, action and maintenance. Key predictors that produce progress through these stages of change include processes of change, self-efficacy and decisional balance (i.e., pros and cons for exercise). These key TTM constructs have been shown to predict exercise behavior in adults26–28, older adults29, individuals with physical disabilities30, cancer patients participating in a supervised exercise31 and older breast cancer survivors32. However, limited work has been focused on older AA or older SES-disadvantaged BCS.
Given that older AA and SES-disadvantaged BCS have been understudied in breast cancer behavioral intervention trials, we are conducting a randomized controlled trial to evaluate the effect of exercise on physical performance, body composition and biomarkers of breast cancer prognosis, in a racially and SES diverse population of older BCS. Secondary aims will examine how race and SES might moderate the intervention effect and will examine for any intersectional relationship of exercise behavior with race and SES, which can be then be targeted for change in future studies. Here we present the rationale, design and methods of this trial, IMPROVE.
METHODS
Study Design and Participants
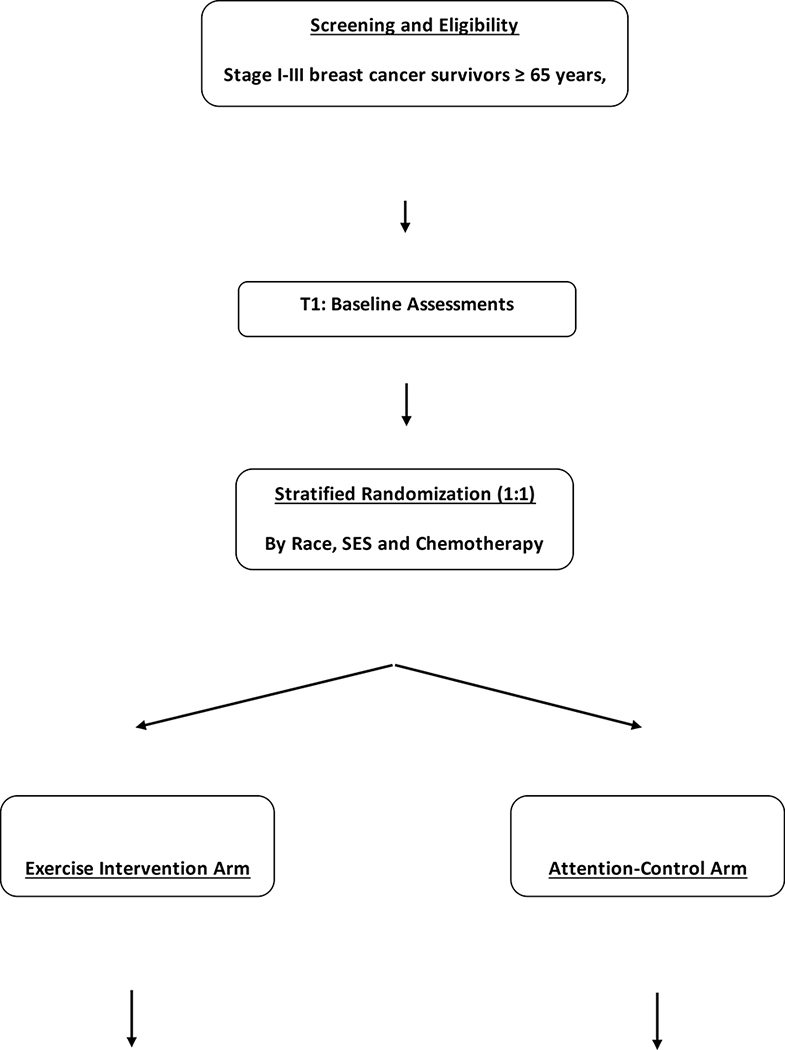
The study design and flow is depicted in Figure 1. This is a mixed-methods study that includes a prospective two-arm randomized-controlled trial testing the effectiveness of a 52-week exercise intervention (n=110) versus support group with activity tracking, [attention-control arm], (n=110), followed by post-intervention semi-structured interviews of participants to better understand and evaluate both the exercise and support group interventions. In brief, women, aged ≥ 65 years, who are no more than five years from treatment completion (surgery, chemotherapy and/or radiation, whichever was last) for pathologically confirmed stage I-III breast cancer, and are African American or Non-Hispanic Whites, are being recruited. We are targeting the inclusion of 55 patients in each race and SES group [African American; Non-Hispanic Whites; SES-disadvantaged; SES non-disadvantaged]. In this trial, we are defining “SES-disadvantaged” as having ≤ high school education and/or median household income ≤$35,000k based on our prior work in older women with breast cancer.6 Because study instruments are in English, participants who are non-English speaking will be excluded. Additionally, participants not able to provide an informed consent will also be excluded. Additional inclusion and exclusion criteria are provided in Table 1. The study was approved by the Institutional Review Boards of the participating hospitals in Cleveland, Ohio.
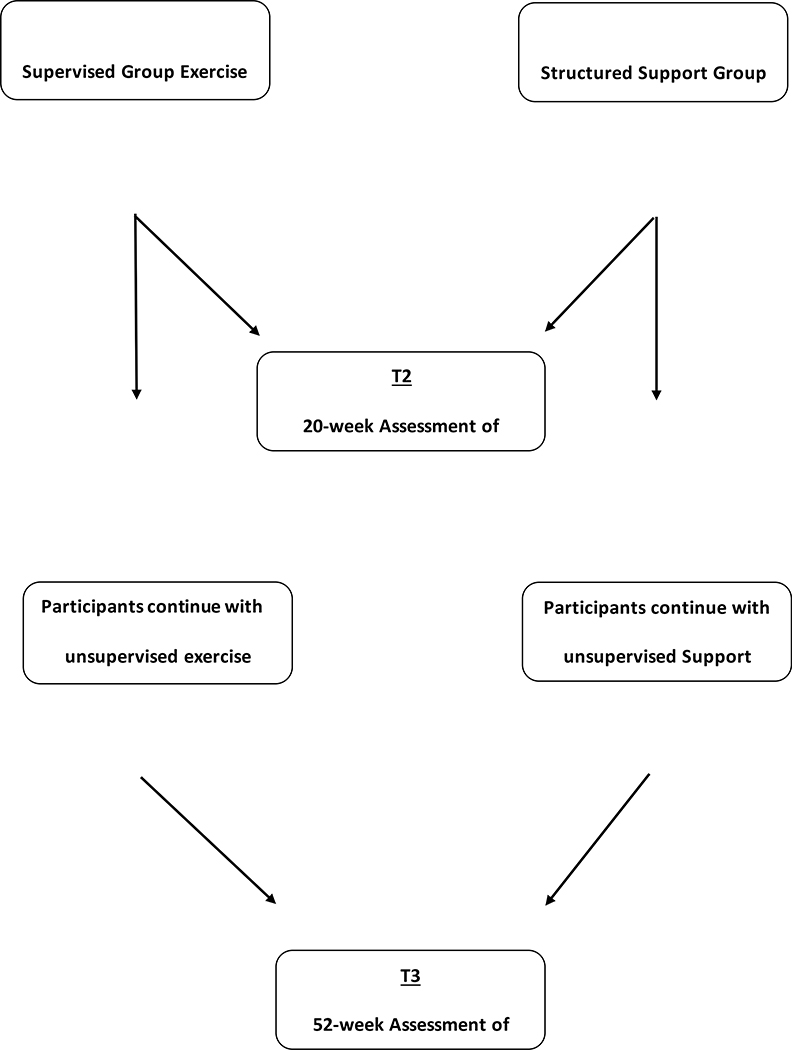
FIGURE 1:
STUDY DESIGN AND FLOW
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| ■ Females with pathologically confirmed breast cancer | ■ Stage IV breast cancer |
| ■ Stage I–III | ■ Patients with end-stage disease, severe dementia and/or life expectancy of less than one year |
| ■ Age: 65 years and older | ■ Inability to understand English as study instruments have not been validated in other languages |
| ■ Have completed primary surgery (Lumpectomy or mastectomy) | ■ Inability to provide informed consent |
| ■ Are within five years of treatment completion (primary surgery, chemotherapy or radiation therapy), whichever was received last | ■ High-riska patients for Cardiovascular Disease per the ASCM/AHA pre-exercise screening questionnaire who do not receive medical clearance from a physician |
| ■ Adjuvant hormonal therapy and targeted therapy are allowed | ■ Other medical or psychological conditions that would make participation unsafe or inhibit our ability to test our primary hypothesis, e.g. Parkinson’s disease, severe arthritis |
| ■ Race: African-Americans and Non-Hispanic Whites |
High risk denotes women with known or signs/symptoms of cardiac, pulmonary and/or metabolic disease such as diabetes
Study Setting
The study is being conducted at a community cancer support center, The Gathering Place (TGP), within the Cleveland Metropolitan area. The mission of the community cancer support center is to support individuals and families touched by cancer through programs and services provided free of charge.
Screening and Recruitment
Potentially eligible participants are identified and recruited using two strategies. First, participants are recruited directly from three participating area hospitals utilizing respective tumor registries or via direct referral from these three hospitals. Patients identified from tumor registries are first mailed a letter and brochure about the study, after obtaining provider permission to contact. They are then contacted by phone two weeks later to further discuss the study. Interested participants, then come to the coordinating site to complete a written informed consent. Written informed consent is obtained by the study principal investigator or a trained research assistant. For participants who are English-speaking but illiterate, an authorized family member is present to witness the oral presentation of the consent form and both participant and authorized family member sign the consent form. Directly referred patients also come to the coordinating site to complete a written informed consent. Second, participants are recruited by utilizing the Ohio Cancer Incidence and Surveillance System/Ohio Department of Health (OCISS/ODH) Database to identify potentially eligible patients from area hospitals including and beyond the three participating hospitals. As described above, potentially eligible participants identified from the OCISS/ODH Database are contacted via mail after provider permission to contact has been obtained. Interested patients then come to the coordinating site to complete a written informed consent.
Immediately after completing the informed consent process, potentially eligible patients are prescreened with the American College of Sports Medicine/American Heart Association (ACSM/AHA) exercise pre-participation questionnaire33 and are risk-stratified into low, moderate and high risk for cardiovascular disease. “High” risk patients require formal physician medical clearance before being fully eligible for study participation. “Low” and “moderate” risk patients are eligible to participate in the study without medical clearance.
Randomization
Following completion of all baseline procedures, participants undergo stratified randomization by SES (SES-disadvantaged versus (vs) SES non-disadvantaged), race (African American vs. Non-Hispanic Whites), and receipt of chemotherapy (Yes vs. No] in a 1:1 ratio to one of two arms; exercise intervention vs. support group with activity tracking. Stratification will help to ensure similar baseline characteristics in each arm and that four strata defined by race (African American / Non-Hispanic Whites) and SES (disadvantaged/non-disadvantaged) are evenly distributed in the two study arms. A computer-generated list of random treatment assignments is generated by the study biostatistician. Group assignments are written on a card, concealed and given to study participants by a research assistant not involved in study enrollment and assessments. All study investigators not involved in delivery of the intervention and research personnel completing study assessments at baseline, 20 and 52 weeks are blinded to group assignment for the duration of the study. In the event of a serious adverse event, blinding is broken due to reporting requirements.
Exercise Intervention
The exercise intervention is informed by a preceding qualitative study of sixty older breast cancer survivors, (30 African American and 30 SES-disadvantaged) who were interviewed for their physical activity beliefs, attitudes and preferences.34 Preferences included group sessions, resistance training to enable them to improve upper body strength and use of electronic tracking devices for monitoring progress.
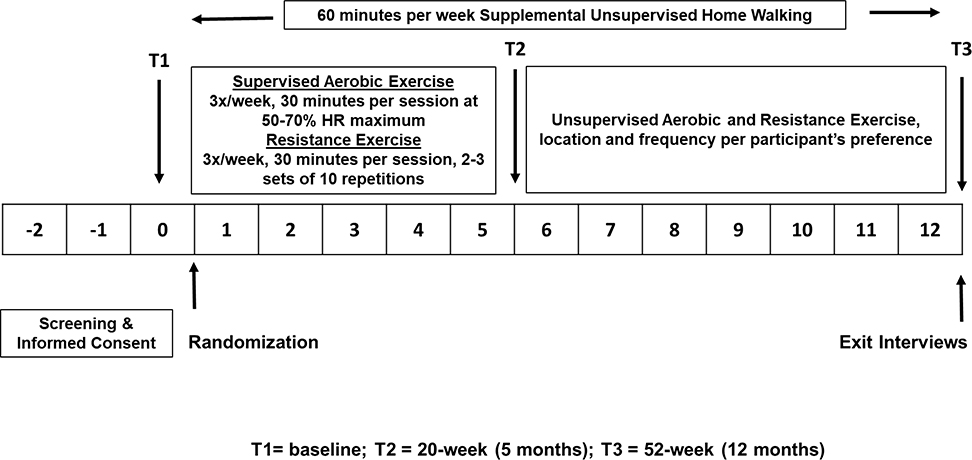
The exercise intervention consists of a 52-week aerobic and resistance group training program designed to achieve 150 minutes of moderate intensity aerobic activity per week and 90 minutes of resistance training per week consistent with recommendations by the US Department of Health and Human Services.22,35 The first 20 weeks are supervised and the last 32 weeks are unsupervised.
Phase I: Supervised 20-Week Exercise Program
Figure 2 depicts the design of the exercise intervention. During the first 20 weeks, patients randomized to the exercise arm are asked to complete three, one-hour exercise sessions per week in groups of 6–12 participants, totaling 60 sessions. Exercise is supervised by ACSM certified exercise trainers. During each exercise session, participants warm up for five minutes and then complete 25-minutes of aerobic exercise on a treadmill, elliptical or stationary recumbent bicycle. The aerobic exercise is performed at moderate intensity (50%−70% of their maximum heart rate (HRmax), which is estimated using the six-minute walk test.33,36,37). Heart rate is recorded using Fitbit (details below). Participants also use a Rating of Perceived Exertion (RPE) chart38 to help ensure their level of exertion is in the moderate intensity range. The 90 minutes per week of supervised aerobic exercise is supplemented with at least 60 minutes per week of unsupervised home walking to achieve the goal of 150 minutes or more of moderate intensity aerobic exercise per week.
FIGURE 2:
EXERCISE INTERVENTION
Resistance training is prescribed according to ACSM guidelines and follows the Physical Activity and Lymphedema Trial protocol, developed by Schmitz, et al.39,40 During the first 20 weeks of supervised training, patients are asked to complete three 30-minute resistance training sessions per week, which are conducted before or immediately following the aerobic exercise. The program is designed to increase muscle strength in all major groups, with each session two days apart.
Table 2 outlines the progression of resistance exercise. During the introduction sessions participants complete 1–2 sets of 10 repetitions of six common strength-training exercises (chest press, leg press, dead lift, shoulder scaption, leg curls and seated rows) using variable resistance machines and free weights for muscles of the chest, back, shoulders, quadriceps, hamstrings, gluteals, biceps and triceps. Due to the need for lymphedema precautions, different protocols are used in determining resistance for upper and lower body strength training. For upper body strength training, participants start with 1lb weight for each exercise and progress by 1/2 or 1 lb increments by the next week, if there is no new or increase in lymphedema-related symptoms. In the event of new-onset or worsening of pre-existing lymphedema, the exercise identified to be associated with the symptoms is skipped or a lower weight is used. For lower body resistance training, a standard progressive strength training approach is adopted. During sessions participants start by performing one to two sets of 10 repetitions, gradually increasing to three sets of 10 repetitions by session five. When three sets of 10 repetitions have been performed for a given weight and no adverse events are noted after two consecutive sessions participants increase the resistance by the smallest possible weight in the next session.
Table 2.
Resistance exercise progression.
| Week | Sessions | Sets | Repetitions | Exercise | Weight |
|---|---|---|---|---|---|
| 1–2 | 1–2 | 1 | 10 | ■ Chest press | ■ Chest Press 10lbs |
| ■ Leg press | ■ Leg press 20lbs | ||||
| ■ Dead lift | ■ Dead Lift 3lbs | ||||
| 3–4 | 2 | 10 | ■ Shoulder scaption | ■ Shoulder Scaption 2lbs | |
| 5–6 | 3 | 10 | ■ Leg curls | ■ Leg curls 20lbs | |
| ■ Row | ■ Row 10lbs | ||||
| 3–6 | 7–18 | 3 | 10 | ■ Chest press | 50% of 1RM chest press, 60% of 1RM leg press, resistance increases by smallest increment (2.5lbs) every 2–3 sessions |
| ■ Leg press | |||||
| ■ Dead lift | |||||
| ■ Shoulder scaption | |||||
| ■ Leg curls | |||||
| ■ Row | |||||
| 7–20 | 19–60 | 3 | 10 | ■ Chest press | 50% of 1RM chest press, 60% of 1RM leg press, resistance increases by smallest increment (2.5lbs) every 2–3 sessions |
| ■ Leg press | |||||
| ■ Dead lift | |||||
| ■ Shoulder scaption | |||||
| ■ Leg curls | |||||
| ■ Row |
Phase II: Unsupervised 32-Week Exercise Program
During the 32-week unsupervised program participants randomized to the exercise intervention arm are asked to continue to exercise for at least 150 minutes at moderate intensity and continue strength training 2–3 days/week. Patients can choose to attend regular exercise classes offered at TGP for all cancer patients, at any other exercise facility of their choice or at home. The regular exercise classes at TGP are led by ACSM certified exercise trainers with varying class size of between 8–15 participants per class. During this phase, study participants in the exercise arm are contacted by the study’s exercise trainer via phone or have an in-person meeting every three weeks where they get feedback and synchronize their Fitbits (see below).
Home Walking Program
During the entire 52-week period all participants in the exercise arm are encouraged to supplement their exercise training with an unsupervised brisk home walking program for 1–3 days to ensure they achieve a total of at least 150 minutes of moderate intensity exercise per week.
Physical Activity Tracking for Participants in Exercise Group
Exercise participants are provided with, and asked to wear a Fitbit Blaze/Versa (Fitbit Inc., San Francisco, CA, USA) on their wrist during all supervised and unsupervised exercise sessions and are encouraged to also wear it throughout the day, during normal daily activities. Each participant’s Fitbit is linked to the Fitabase analytics system (Small Steps Labs, San Diego, CA, USA), a cloud-based data aggregation platform which aggregates daily totals for step count, heart rate, and intensity of physical activity, allowing investigators to monitor participants’ physical activity and to extract Fitbit data for analysis at the end of the study. In addition, participants are asked to maintain exercise logs daily that include information on the mode, frequency and duration of all purposeful physical activity.
During the first 20 weeks, exercise logs and Fitbit data are reviewed by the exercise trainer with brief weekly feedback and goal setting discussions. In addition, the exercise trainers meet with each participant at baseline, six and 16 weeks to outline and re-adjust goals, and provide in-depth feed-back and a personalized report. As mentioned above, during the 32 weeks of unsupervised activity, participants are asked to return to TGP to synchronize their Fitbit every 3 weeks and are provided with a brief feed-back based on their personalized Fitbit report.
Support Group with Activity Tracking (Attention-Control Group)
Participants in the support group attend a Successful Survivorship Health Education and Support Group Program that is matched to the exercise intervention with regards to location and environment (The Gathering Place), social interaction (group sessions) and duration (52 weeks). Sessions are one hour, once a week in the first 20 weeks, and flexible in the last 32 weeks.
Group discussions during the first 20 weeks center on, but are not limited to, the impact of the cancer diagnosis on participant lives, family dynamics and aging. In addition, group members have the opportunity to experience programs aimed at self-care such as guided meditation, expressive arts, journaling and nutrition. Other activities include Labyrinth Walk, Create-a Personal Labyrinth, Self-Care Art Activity, Gratitude Art Activity, Wisdom Cards, Drumming and Hands on Cooking Class. During the last 32 weeks, support group participants have the same flexibility as PA participants to attend programs, other than exercise, that are regularly offered to cancer survivors at TGP.
Participants in the support group arm are asked to maintain their current level of physical activity when they first enroll in the study and not to increase it or enroll in any new physical activity or exercise program for the duration of the study. All participants are given a Fitbit Tracker (Blaze/Versa) and asked to wear it during any purposeful exercise and during normal daily activities. Fitbits for participants in the support group are also linked to Fitabase. Participants are also asked to return to TGP to synchronize their Fitbits every 3 weeks.
Retention and Adherence Consideration
Retention and adherence strategies regardless of intervention arm include: 1) free transportation to and from the facility for those with transportation needs; 2) weekly follow-up phone calls after missed sessions; 3) weekly automated telephone prompts reminding participants of upcoming sessions; 4) for exercise participants, in-depth 6- and 16-week face-to-face review of exercise logs and Fitbit data with feed-back and goal-setting during the 20-week supervised phase of the intervention; and 5) 3-weekly visits by participants to TGP during the maintenance phase to synchronize their Fitbit and, and for exercise participants, to obtain brief feed-back based on their personalized Fitbit report.
Lymphedema Assessments and Adverse Events
The following is undertaken to minimize new onset or exacerbation of pre-existing lymphedema for all study participants regardless of intervention arm: 1) lymphedema education at enrollment; 2) lymphedema assessment at three time points (baseline, 20 and 52 weeks) using the validated Norman Lymphedema Survey.41; 3) Arm girth measurements at baseline, 20 and 52 weeks using a spring loaded Gulick tape measure. In addition, exercise intervention arm participants complete monthly arm girth measurements and a brief self-reported lymphedema symptom questionnaire during the first 20 weeks. Geometric volume is calculated from girth measurements using formulas for a cylinder and a frustum.42 Participants who develop new onset or exacerbation of pre-existing lymphedema as measured by a volume increase of >3% during girth measurements are: 1) referred to the lymphedema clinic in their respective hospitals; 2) provided with a compression garment by the study; and, 3) require use of compression garment on affected arm with lymphedema during all exercise sessions, based on the lymphedema risk-reduction guidelines from the National Lymphedema Network.43 Adverse events will be participant self-reported and will be assessed and recorded by the interventionist or facilitator during exercise or support group sessions. Any adverse events, whether or not related to study intervention, are reported to the IRB and Data and Toxicity Management Committee.
Intervention Standardization, Training and Quality Control
Treatment fidelity is considered with regard to study design, training, delivery of intervention, and receipt of intervention. Prior to study initiation, study investigators ensured prescribed intervention and study procedures are clearly outlined and delineated in the study protocol and Manual of Operation. Training of the interventionists are completed as delineated in a procedure manual, and retraining are ongoing through monthly meetings between the interventionists and study investigators to monitor and refine intervention activities, and clarify procedures. Direct observation of intervention delivery is undertaken periodically by investigators to monitor intervention quality. Participants are also assessed for the degree to which they have understood the intervention by direct observation of participants during exercise sessions and a review of exercise log books.
Criteria for Study Withdrawal
Participation in the study is voluntary and study participants may withdraw for any reason. In addition, study investigators may withdraw participants in the event of a serious adverse event that makes continued participation in the study unsafe for the patient, breast cancer recurrence or a new diagnosis of any cancer.
Study Outcomes and Measures
Table 3 outlines study calendar of procedures and assessments that are completed at baseline, 20 and 52 weeks. In brief, following the completion of the written informed consent process, study participants are scheduled for a visit to complete baseline assessments in the following order, all on the same day: (1) 8-hour fasting blood draw for biomarker testing; (2) Comprehensive Geriatric Assessment (CGA) and other questionnaires listed in Table 3; and (3) Body Composition measurements. Strength and Fitness Testing are completed at TGP within one to three days prior to randomization and start of exercise and support group programs and include the following (see details below): 1) a six-minute walk test37; 2) One Repetition Maximum (1-RM) Chest Press; and 3) 1-RM Lower extremity strength test. All baseline assessments are repeated at 20 weeks (end of supervised exercise training) and at 52 weeks (end of unsupervised exercise training) in the same order and with the same equipment. Participants who withdraw or dropout of the study for any reason are asked to complete an optional end of study assessment at the time of withdrawal or dropout.
Table 3.
Assessments and Measures.
| Outcomes | Measure or procedure | T1 | T2 | T3 |
|---|---|---|---|---|
| Primary outcome | ||||
| Physical performance | Short physical performance battery | X | X | X |
| Secondary outcomes | ||||
| Functional status | Basic and instrumental activities of daily living | X | X | X |
| Body composition | DEXA (e.g. % body fat, lean muscle mass) weight, height, BMI | X | X | X |
| Cardiopulmonary fitness | Six minute walk test | X | X | X |
| Biomarkers of functional disability and breast cancer prognosis | Biomarkers of inflammation, insulin pathway, sex hormones and coagulation | X | X | X |
| Physical activity | Fitbit data (Daily step count, Heart rate, MVPA) | DAILY | ||
| Self-report physical activity | Minnesota leisure time activity | X | X | X |
| Quality of life | Functional assessment of cancer therapy-breast (FACT-B) | X | X | X |
| Tertiary outcomes | ||||
| Performance status and physical function | ECOG-PS, KPS, Grip strength, SF-36 physical Function subscale | X | X | X |
| Lymphedema assessment | Norman lymphedema survey, arm measurements | X | X | X |
| Fitness testing | Leg and chest press | X | X | X |
| Risk of functional decline | Vulnerable elders survey | X | X | X |
| Fatigue | FACT-F | X | X | X |
| Sleep | Pittsburgh sleep quality index | X | X | X |
| Cognitive status | MMSE, Clock drawing test | X | X | X |
| Falls | Number of falls in the last 6 months | X | X | X |
| Depression | Geriatric depression scale | X | X | X |
| Social support and activities | Medical outcomes social support survey; social activity limitations measure | X | X | X |
| Medication count | Medical records review | X | X | X |
| Comorbidity | Charlson’s comorbidity index, Comorbidity count | X | X | X |
| Exercise behavior | Stages of exercise behavior, Self-efficacy, processes of change questionnaire | X | X | X |
| Attitudes and beliefs | Perceived barriers of PA questionnaire, OPAPAEQ, Social influence and attitudes toward physical activity questionnaire | X | X | X |
| Nutrition | NCI Fruit and vegetable screener | X | X | X |
| Others | ||||
| Breast cancer and treatment characteristics | Medical records abstraction | X | ||
| Socio-demographic, Smoking/Alcohol history | Socio-demographic, smoking and alcohol questionnaire | X | X | X |
Primary Outcome: Physical Performance
The change in Short Physical Performance Battery (SPPB) score at 20 weeks compared to baseline, between arms, is the primary outcome. Changes are evaluated at 52 weeks as a secondary outcome. The SPPB is a performance-based assessment that evaluates three lower extremity performance measures to assess lower extremity strength: a hierarchical test of standing balance, five consecutive chair rises, and usual gait speed. A 4-point scale is used for each task, summary scores range from 0–12 and higher scores denote higher physical performance. The SPPB is simple to administer44,45, has excellent test-retest reliability and sensitivity to change.46
Secondary Outcomes
Activities of Daily Living/ Instrumental Activities of Daily Living (ADL/IADL)
Changes in Activities of Daily Living/ Instrumental Activities of Daily Living (ADL/IADL) score at 20 weeks, and at 52 weeks compared with baseline, between arms, are examined as secondary outcomes. Katz and Lawton’s ADL/IADL measure self-reported dependence/non-dependence with bathing, transfer, dressing, continence, toileting, feeding, shopping, using telephone, managing medications, housekeeping, laundry, transportation, ability to manage finances and preparing meals.47,48 ADL and IADL scores range from 0–6 and 0–8, respectively, with lower scores denoting higher dependence. Both instruments are widely used in clinical practice and well validated for ascertaining functional disability/decline in research.49
Body Composition
Height is measured to the nearest 0.1 cm and weight measured to the nearest 0.01 kg to compute Body Mass Index (BMI). Waist and hip circumference are measured to the nearest 0.1 cm to compute waist to hip ratio. Percentage body fat, Total Mass (Kg), Fat Body Mass (g), Lean Body Mass (g) Bone Mineral Content (g) and Fat Free Mass (g) are measured using a dual-energy x-ray absorptiometry (Lunar model iDXA; Core Scan Software, GE Healthcare, Madison, WI). Scans are completed with subjects placed in a supine position with arms and legs close to their body for a whole body scan following a standardized protocol (Lunar Corp., Model DPX-L, Madison, WI). All scans and QC measures are performed by the same technologist on the same equipment in the same location in a semi-automatic manner according to standardized procedures.
Circulating Biomarkers
The selection of specific biomarkers to include in this study has been guided by literature supporting association between certain biomarkers and functional and mobility disability50–52, and biomarkers implicated in energy balance in relation to cancer53,54. Biomarkers will largely focus on inflammation, insulin pathway, sex hormones and coagulation. Specific biomarkers include C-peptide, insulin, leptin, adiponectin, resistin, IGF-1, IGFBP-1, IGFBP-3, interleukin 6, Tumor Necrosis Factor Alpha, C-Reactive Protein, estradiol and estrone. Participants come in for blood draw after an overnight fast. Approximately 20mls of blood are drawn at each time point (baseline, 20 and 52 weeks) after a 12-hour fast. Samples are sent to the Translational Research Core (TRC) Laboratory at the Case Comprehensive Cancer Center where they are stored at −80° C until analysis. At study completion, all samples will be batch tested. Cytokines will be determined using commercially available multi-plex enzyme-linked immunosorbent assay (ELISA) kits (Meso-Scale Discovery, Gaithersburg, MD). Insulin and IGF-1 will be determined using commercially available ELISA kits from Beckman Coulter (Brea, California) using a one-step enzymatically amplified sandwich-type immunoassays. High sensitivity CRP was determined using Nephelometry Siemens (Dade Behring) BNII. Estradiol, and estrone will be measured by radioimmunoassay with preceding organic solvent extraction and Celite column partition chromatography steps. All samples will be run in a blinded fashion, in duplicate with internal controls. Insulin resistance will be calculated by the Homeostasis Model Assessment (HOMA-IR) formula, (insulin [microunits/ml] x glucose [MG/DL/405)55–57 that utilizes fasting glucose and insulin levels.
Cardiopulmonary Fitness
The Six-Minute Walk Test is used to estimate cardiopulmonary fitness.37 This is a sub-maximal measure of aerobic capacity where the goal is to walk as far as possible in six minutes. Participants walk back and forth between two cones, using ambulation aids if they do so normally and taking breaks from walking as needed, but not sitting down. At the end of six minutes, participants are asked to stop where they are and a chair is provided. The total distance ambulated is measured with a measuring wheel and recorded. Participants are given as much time as needed to recover before proceeding to the one-rep maximum testing.
Muscle Strength Testing
Participants test for their one-rep maximum (1RM) on a leg press and a chest press machine to assess lower body and upper body strength, respectively. Both tests are symptom-limited, so if participants have restricting orthopedic or lymphedema issues, these tests can be modified. Before beginning, participants engage in a brief stretching routine and the correct form for the lifts are demonstrated. For leg press, participants perform 4–6 repetitions with 20 lbs. of resistance. Once completed, the research assistant asks the participant for a rating of perceived exertion (RPE) on a scale of 1–10 and records it. Based on the ease of the lift, lifting form, and RPE, between 2.5lbs and 10 lbs are added. The participant then completes a single repetition and gives a new RPE. The research assistant continues to add a small amount of weight, have the participant perform a single repetition, and get an RPE until either the participant says that they do not wish to continue or the lifting form becomes inadequate. At this point the research assistant records the greatest amount of weight lifted in a single repetition as the 1RM. The same process is repeated for chest press, except the starting weight is 10 lbs and weight increases of between 2.5 and 5 lbs are made using supplemental weights.
Other Functional Measures
Risk of functional decline is assessed with the Vulnerable Elders Survey (VES-13) which is a 13-item self-administered instrument, validated in community dwelling elders to predict functional decline and mortality.49,58,59 Grip strength is measured with a Jamar hand dynamometer (Sammons Preston Rolyan, Bolingbrook, Illinois).60
Physical Activity
Self-reported physical activity levels are ascertained using the Minnesota Leisure Time Physical Activity Questionnaire, a well validated instrument61 and the 7-day PA recall.62,63
Comprehensive Geriatric Assessment
Domains that are evaluated include functional assessment (ADL/IADLs, falls, grip strength)) cognitive status (Mini-Mental State Examination64,65) psychosocial status (Geriatric Depression Scale66,67), comorbidity (Charlson’s Comorbidity Index68) and medication review for polypharmacy.
Patient Reported Outcomes (PROs) and other Covariates
PROs include Quality of Life (Functional Assessment of Cancer Therapy [FACT-B]69), Fatigue (FACT-Fatigue)70,and sleep disturbance (The Pittsburgh Sleep Index).71,72 A self-report demographic questionnaire captures data on race, education, marital status, income and other variables. Other measures include Perceived Barriers of Physical Activity (modified version of the Barriers to Health Activities Scale73–75); Attitudes toward physical activity (The Questions on Attitudes Toward Physical Activity measure76); Exercise Behavior (the stages of change in exercise behavior questionnaire25,77); Self-efficacy (a self-report 5-point Likert scale developed by Marcus et al,78); ten-item Temptations Scale which assesses how tempted an individual is not to be physically active79; and the pros and cons of physical activity using a five-point scale (1=not important to 5=extremely important).80 Medical records abstraction is completed to obtain data on tumor and treatment characteristics.
Intervention Evaluation and Acceptability: Exit Interviews
Using a mixed-methods approach, both exercise and support group participants complete exit interviews within one to two weeks of study completion (52 weeks). The purpose of exit interviews is to evaluate the exercise and support group programs and to use information gleaned from these interviews to further refine both programs for improved acceptability in future studies and programs. Exit interviews are conducted “one-on-one” by a research assistant not involved in the exercise or support group activities. Interviews include both structured and open-ended questions and utilize a standardized guide adapted from our prior work34 and modified by study investigators to specifically evaluate both interventions. Specifically, questions focus on; (a) reasons for enrolling in the program; (b) concerns prior to enrollment; (c) most helpful and least helpful parts of the program; (d) facilitators and barriers for adhering to the program; and (e) recommendations for improvement. All interviews are audiotaped by the research coordinator conducting the interviews. Audio-taped interviews are transcribed by a professional transcriptionist, who has no patient contact and has no access to Protected Health Information (PHI). Participants who withdraw from the study are given the option to complete end of study procedures including exit interviews.
Data Management and Quality Assurance
Study data is collected and managed using REDCap (Research Electronic Data Capture), which is a secure web-based electronic data capture tool, designed to support data capture for research studies. Following the completion of the informed consent process, participants are assigned an encrypted patient identifier number. The key to the identifiers is maintained in a computerized file which is password protected with PI access only. This de-identifier is used on all standardized patient data forms, and for data entry purposes. As a result, the study database (REDCap) holds strictly de-identified data using an arbitrary participant ID. Hard copies of research documents are maintained in folders kept in a locked cabinet under the PI’s supervision. Data from the Fitbit trackers are de-identified, downloaded and extracted from Fitabase (Small Steps Labs, San Diego, California). This process described above ensures that participant’s protected health information collected before, during and after the study are maintained in a confidential manner in compliance with regulations enacted pursuant to the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
All data is entered into REDCap by a research assistant, and are double-checked by the data manager. Discrepancies are corrected based on source documents. At study conclusion, all data entered into REDCap will be converted to SAS software for statistical analysis by the study biostatistician. Data quality are monitored once a month by random inspection of the completed forms by the study PI.
Data Safety and Toxicity Committee
The coordinating center’s Data and Safety Toxicity Committee, which is independent of the study sponsor, oversees the study to ensure the safety of study participants, through review of adverse events. Other responsibilities include: 1) reviewing and analyzing the progress of the study; 2) approving amendments to the trial protocol, if warranted; 3) monitoring the safety of the study interventions and diagnostic procedures; and 4) ensuring data quality. The Data Safety and Toxicity Committee include members with expertise in clinical research, including but not limited to, intervention research, oncology, biostatistics and ethics.
Statistical Analyses and Power
The study examines the exercise intervention effect on changes in primary and secondary outcomes from baseline to weeks 20 and 52. For the primary outcome SPPB, a mixed linear model is fit to the changes from baseline to 20 weeks with baseline SPPB as a covariate, and contrast statements are used to test hypotheses of interest. The primary comparison compares the change from baseline in SPPB at week 20 between treatment groups using a 2-sided test with significance level 0.05. Secondary analysis will compare changes at 52 weeks. Assuming the estimated standard deviation of change in SPPB from baseline to weeks 20 and 52 to be 2.7081,82 and allowing for up to 15% dropout, with 220 women randomized, the study will have 80% power to detect a difference in mean change of SPPB of 1.12 corresponding to an effect size of 0.41.83 Similar analysis is carried out to compare changes in the secondary outcome ADL/IADL, but using a generalized estimating equations model more appropriate due to non-normality of this outcome. Prior to the mixed model analysis, demographic and clinical characteristics are compared between the treatment arms using appropriate tests for continuous and categorical data. The primary analysis adjusts for the randomization factors SES, race, and chemotherapy in addition to baseline level of the outcome. Secondary analyses in addition adjust for baseline covariates or treatments found to be imbalanced between groups. With regards to missing data, the proposed mixed model for longitudinal analysis of SPPB efficiently uses all available data for each subject, and is valid when there are missing values due to missed visits/dropout, provided the data is missing at random, i.e. ignorable.84 Simulation studies have shown it to be less biased than ad-hoc methods such as last-value-carried forward.85 As a secondary sensitivity analysis, if dropout is greater than 10%, changes in SPPB will be estimated assuming a non-ignorable shared parameter model,86 and compared to results obtained from the primary analysis.
All analysis will be conducted at study conclusion using SAS software version 9.4. No interim analyses are planned.
Feasibility and Adherence Analysis
Three outcomes will be used to evaluate adherence to, and feasibility of the exercise and support group programs: 1) Exercise and support session adherence is the total number of sessions attended divided by the expected number of sessions for the 20-week period. Session adherent (Yes or No) is defined as attending ≥ 70% of expected sessions over the 20-week period; 2) Sustainability is the proportion of participants who complete the 52-week program divided by the total number of participants who enrolled in the program. The program is deemed sustainable if ≥70% of participants remain in the program throughout the 52-week period; 3) Exercise dose adherence, is determined by the proportion of weeks during which participants in the exercise arm meet their prescribed exercise intensity of 150 minutes per week of moderate intensity aerobic exercise. Participants are deemed exercise dose adherent (Yes or No) if they meet their prescribed exercise intensity of 150 minutes of moderate intensity aerobic exercise per week for at least 70% of the 52-week period. We expect exercise session adherence, sustainability, and exercise dose adherence to the exercise program to be 70%. We will calculate one sided upper 95% confidence limits for each rate and will conclude that feasibility is not met if the upper limit is less than 70%. With n=110, this corresponds to 69 (63%) or fewer women meeting the feasibility requirement. If in fact the true feasibility rate is 70%, the chance of concluding non-feasibility is 0.036. The probability of concluding non-feasibility is 0.81 or more when the true rate is ≤ 60%.
DISCUSSION
Breast cancer mortality rates remain significantly higher for older women compared with younger women. Compared to rates in 1990, the breast cancer mortality rate in the general population has decreased by 2.5%/year (yr.) for women age 20 to 49 years, 2.1%/yr. for women age 50 to 64 years, 2.0%/yr. for women age 65 to 74 years, and only 1.1%/yr. for women age ≥ 75 years.4
Breast cancer survival rates are particularly lower for older African American women compared with older Non-Hispanic Whites. A SEER-Medicare database analysis of more than 28,000 older women with breast cancer showed that five- year survival rates are almost 13% lower in older African American women compared with older Non-Hispanic Whites women5 and suggests that the racial disparity in breast cancer survival among older women is partly due to the poorer health status of African American (vs. Non-Hispanic Whites ) at breast cancer diagnosis.5 This poorer health status is hypothesized to blunt the long-term survival benefit derived from cancer treatment.5
Functional status, a measure of the ability to perform Basic and Instrumental Activities of Daily Living (ADL/IADLs), is a key summary measure of health.87 Functional status predicts many outcomes in older persons, including total mortality.88,89 Functional disability rates are disproportionately higher among African American and women of lower SES. Our previous work showed that among older women with non-metastatic breast cancer, African American (vs. Non-Hispanic Whites ) and women of lower SES (vs. higher SES) were four times more likely to have functional disability.6 Additionally, we found that household income and educational status explained 54% and 17% of the excess odds of this disparate functional disability due to race among older breast cancer survivors.6 Furthermore, women of lower SES (vs higher SES) were two times more likely to develop functional decline within 12 months of a new breast cancer diagnosis.90
Importantly, older women with breast cancer who have functional decline have been shown to have an increased risk of death.91,92 As the number of older breast cancer survivors continues to rise, attention must be given to the corresponding potential increases in functional limitations in the aging and breast cancer survivors populations.
Observational studies have shown that regular physical activity can reduce the risk of functional limitations.93 Furthermore, observational studies show that regular physical activity after breast cancer diagnosis is associated with reduced breast cancer-specific and all-cause mortality.17,94 The most active compared to the least active survivors in the Women’s Healthy Eating and Living study had a 53% decreased risk of mortality.95
However, approximately 65% of breast cancer survivors do not meet the national recommended levels of physical activity.8 Furthermore, compared with Non-Hispanic Whites, African American breast cancer survivors are about 1.5 times more likely not to meet national recommended levels of physical activity with rates as low as 24%.8 Promotion of opportunities to engage in healthy behaviors is therefore critical to addressing health disparities among African Americans.96 Randomized controlled trials have demonstrated that physical activity attenuates functional decline among older cancer survivors.21,97 While several physical activity and exercise studies have been conducted among younger breast cancer survivors, few have been conducted among older breast cancer survivors21 and none have targeted the specific needs of older African American and SES disadvantaged breast cancer survivors.22
Our trial, “IMPROVE”, will be the first trial to evaluate the effects of exercise on functional outcomes, body composition, geriatric assessment variables and patient reported outcomes such as quality of life and fatigue in older breast cancer survivors using a program that has been targeted to be conducive to older, African American and SES-disadvantaged survivors. The study population includes three disparate and underserved populations, (older breast cancer survivors in general, older African American breast cancer survivors and older SES-disadvantaged breast cancer survivors) in one study, making this approach efficient, pragmatic and innovative for addressing research gaps in behavioral health research. These three populations have been identified as understudied groups in behavioral research.22 Our study examines how race and SES are interactive and interdependent in shaping exercise behavior, and functional outcomes. This will provide new information that can be used to enhance and promote our efforts to reduce functional health disparities among older breast cancer survivors.
Our study is also significant because it addresses a major health issue, functional disability, which has enormous public health consequences including increased health care utilization and costs, morbidity and mortality.98,99 An effective intervention for functional disability will therefore have benefits at both the individual and societal level. Over the long term, improved functional and health status could potentially translate to longer life expectancy, allowing older breast cancer survivors to derive the long-term benefits from breast cancer treatment.
Additional strengths and unique aspects of this study include the formative work34 that was done in the year prior to the conduct of this current exercise study. That formative work aimed to examine the knowledge, attitudes, beliefs and pbe of older breast cancer survivors toward physical activity, and included a patient population that is similar in racial and SES characteristics to our current exercise study. Our intervention program is informed by this formative work and, includes facilitators of exercise such as paid transportation for those in need, multiple modes (aerobic, strength training) and types (bike, treadmill, elliptical, resistance machines, free weights, resistance bands) of exercise and inclusion of music and dance aspects. In our formative work, we found that 80% of breast cancer survivors interviewed preferred strength training exercises (mainly due to concerns with decreased upper body strength after breast cancer treatment)34. Many of these survivors expressed the desire to increase their strength using free weights, resistance bands and strength/weight machines34. Nearly all older breast cancer survivors indicated they preferred a group exercise program and over 90% stated that a tracking device would serve as a motivator to exercise. Therefore, we provided activity trackers to all women who participated in the IMPROVE trial, recognizing this may serve to motivate physical activity in women in the support group as well as the exercise intervention arm. Thus, the support group arm will not serve as a true “control” arm but provide the ability to determine if and how much activity increases with providing just a tracker and, will also provide insights on whether the tracker can help sustain activity during the follow-up period in the exercise intervention arm. Thus, information gleaned from our earlier formative work informed the design of our intervention, increasing the likelihood of high acceptability.
Furthermore, the IMPROVE trial will be examining putative mediators and moderators of change including self-efficacy. The study design which includes both an initial supervised phase and an unsupervised maintenance phase, should allow us to examine and identify factors associated with sustainability. Additionally, we are conducting exit interviews of all study participants at the conclusion of the 52-week period for program evaluation. Thus, the results of the IMPROVE trial will help inform future exercise trials in our growing population of older breast cancer survivors, and should give us a better understanding of the pattern of exercise behavior that can then be targeted for change and improved intervention development.
This study will also examine biomarkers of functional decline and breast cancer prognosis. The identification of bio-behavioral pathways through which exercise may affect functional decline and the potential effect of exercise on putative pathways linking lifestyle and breast cancer recurrence and prognosis is significant.100,101 This will enhance our understanding of the mechanism by which the proposed intervention is effective and will strengthen the scientific validity of study findings. Moreover, biomarkers will inform risk stratification, prognostication, and help identify new targets for therapeutic intervention.
In conclusion, to the best of our knowledge, this will be the first trial to evaluate the putative beneficial effects of exercise on functional and health outcomes in the underserved population of older breast cancer survivors from minority and SES disadvantaged backgrounds. Results of the trial may contribute to a better understanding of factors associated with recruitment, sustained participation and acceptability, and will inform future exercise programs to optimally improve the functional and health outcomes for older women during breast cancer survivorship.
Acknowledgements
The authors would like to thank mediUSA for providing compression garments free of charge to study participants, volunteers for assisting with study assessments and medical oncologists and nurse practitioners for referring patients for study participation.
Funding
This work was supported by the National Institute on Minority Health and Health Disparities (R01 MD009699 to Cynthia Owusu, M.D.).
ABBREVIATIONS
- SES
Socioeconomic Status
- BCS
Breast cancer survivors
- SPPB
Short Physical Performance Battery
- PA
Physical Activity
- TGP
The Gathering Place
- OCISS/ODH
Ohio Cancer Incidence and Surveillance System/Ohio Department of Health
- ACSM/AHA
American College of Sports Medicine/American Heart Association
- PI
Principal Investigator
- AE
Adverse Events
- SAE
Serious Adverse Events
- CGA
Comprehensive Geriatric Assessment
- 1-RM
One Repetition Maximum
- ADL/IADL
Activities of Daily Living/Instrumental Activities of Daily Living
- RPE
Rating of Perceived Exertion
- VES-13
Vulnerable Elders Survey
- FACT
Functional Assessment of Cancer Therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Berry LL, Davis SW, Godfrey Flynn A, et al. : Is it time to reconsider the term “cancer survivor”? J Psychosoc Oncol 37:413–426, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Nogueira L, Mariotto AB, et al. : Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 69:363–385, 2019 [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Miller KD, Goding Sauer A, et al. : Cancer statistics for African Americans, 2019. CA Cancer J Clin 69:211–233, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Smith BD, Jiang J, McLaughlin SS, et al. : Improvement in breast cancer outcomes over time: are older women missing out? J Clin Oncol 29:4647–53, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Silber JH, Rosenbaum PR, Clark AS, et al. : Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 310:389–97, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Owusu C, Schluchter M, Koroukian SM, et al. : Racial disparities in functional disability among older women with newly diagnosed nonmetastatic breast cancer. Cancer 119:3839–46, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-prevention-and-early-detection-facts-andfigures/cancer-prevention-and-early-detection-facts-and-figures-2015-2016.pdf. Last accessed 07/14/2019.
- 8.Hair BY, Hayes S, Tse CK, et al. : Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer 120:2174–82, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armer JM, Stewart BR: Post-breast cancer lymphedema: incidence increases from 12 to 30 to 60 months. Lymphology 43:118–27, 2010 [PMC free article] [PubMed] [Google Scholar]
- 10.Hurria A, Soto-Perez-de-Celis E, Allred JB, et al. : Functional Decline and Resilience in Older Women Receiving Adjuvant Chemotherapy for Breast Cancer. J Am Geriatr Soc 67:920–927, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deimling GT, Arendt JA, Kypriotakis G, et al. : Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc 57 Suppl 2:S289–92, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt ME, Chang-Claude J, Seibold P, et al. : Determinants of long-term fatigue in breast cancer survivors: results of a prospective patient cohort study. Psychooncology 24:40–6, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Runowicz CD, Leach CR, Henry NL, et al. : American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol 34:611–35, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SS, Yoo W, Whitehead MS, et al. : Advancing breast cancer survivorship among African-American women. Breast Cancer Res Treat 153:253–61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder DC, Morey MC, Sloane R, et al. : Reach out to ENhancE Wellness in Older Cancer Survivors (RENEW): design, methods and recruitment challenges of a home-based exercise and diet intervention to improve physical function among long-term survivors of breast, prostate, and colorectal cancer. Psychooncology 18:429–39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demark-Wahnefried W, Morey MC, Sloane R, et al. : Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol 30:2354–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim EM, Al-Homaidh A: Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol 28:753–65, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Mason C, Alfano CM, Smith AW, et al. : Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev 22:1153–61, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irwin ML, McTiernan A, Bernstein L, et al. : Physical activity levels among breast cancer survivors. Med Sci Sports Exerc 36:1484–91, 2004 [PMC free article] [PubMed] [Google Scholar]
- 20.Adult participation in aerobic and muscle-strengthening physical activities--United States, 2011. MMWR Morb Mortal Wkly Rep 62:326–30, 2011 [PMC free article] [PubMed] [Google Scholar]
- 21.Morey MC, Snyder DC, Sloane R, et al. : Effects of home-based diet and exercise on functional outcomes among older, overweight long-term cancer survivors: RENEW: a randomized controlled trial. Jama 301:1883–91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz KH, Courneya KS, Matthews C, et al. : American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 42:1409–26, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Prochaska JO, DiClemente CC: Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 51:390–5, 1983 [DOI] [PubMed] [Google Scholar]
- 24.Prochaska JO, DiClemente CC, Norcross JC: In search of how people change. Applications to addictive behaviors. Am Psychol 47:1102–14, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Prochaska JO, Velicer WF: The transtheoretical model of health behavior change. Am J Health Promot 12:38–48, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Cardinal BJ, Kosma M: Self-efficacy and the stages and processes of change associated with adopting and maintaining muscular fitness-promoting behaviors. Res Q Exerc Sport 75:186–96, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Cardinal BJ, Sachs ML: Prospective analysis of stage-of-exercise movement following mail-delivered, self-instructional exercise packets. Am J Health Promot 9:430–2, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Cardinal BJ, Tuominen KJ, Rintala P: Cross-cultural comparison of American and Finnish college students’ exercise behavior using transtheoretical model constructs. Res Q Exerc Sport 75:92–101, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Cardinal BJ: Facilitating physical activity and health behavior change among older adults. Contemporary Psychology, APA Review of Books; 49:15–17., 2002 [Google Scholar]
- 30.Cardinal BJ, Kosma M, McCubbin JA: Factors influencing the exercise behavior of adults with physical disabilities. Med Sci Sports Exerc 36:868–75, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Courneya KS, Segal RJ, Reid RD, et al. : Three independent factors predicted adherence in a randomized controlled trial of resistance exercise training among prostate cancer survivors. J Clin Epidemiol 57:571–9, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Loprinzi PD, Cardinal BJ, Si Q, et al. : Theory-based predictors of follow-up exercise behavior after a supervised exercise intervention in older breast cancer survivors. Support Care Cancer 20:2511–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American College of Sports Medicine (ACSM) – Guidelines for exercise testing and prescription. 8th ed. Philadelphia: Lippincott, Williams and Wilkines; 2009. [Google Scholar]
- 34.Owusu C, Antognoli E, Nock N, et al. : Perspective of older African-American and Non-Hispanic white breast cancer survivors from diverse socioeconomic backgrounds toward physical activity: A qualitative study. J Geriatr Oncol 9:235–242, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Department of Health and Human Services; https://health.gov/paguidelines/2008/. Last accessed on 07/14/2019 2008 [Google Scholar]
- 36.Cahalin LP, Mathier MA, Semigran MJ, et al. : The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 110:325–32, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Laboratories ATSCoPSfCPF: ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166:111–7, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Borg GA: Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–81, 1982 [PubMed] [Google Scholar]
- 39.Schmitz KH, Troxel AB, Cheville A, et al. : Physical Activity and Lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials 30:233–45, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitz KH, Ahmed RL, Troxel AB, et al. : Weight lifting for women at risk for breast cancer-related lymphedema: a randomized trial. JAMA 304:2699–705, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Norman SA, Miller LT, Erikson HB, et al. : Development and validation of a telephone questionnaire to characterize lymphedema in women treated for breast cancer. Phys Ther 81:1192–205, 2001 [PubMed] [Google Scholar]
- 42.Briele HA Jr., Schneebaum S, Barnicle M, et al. : Method of measurement for volume of an extremity. Surg Gynecol Obstet 169:349–51, 1989 [PubMed] [Google Scholar]
- 43.Position Statement of the National Lymphedema Network. Lymphedema Risk Reduction Practices. By the National Lymphedema Network Medical Advisory Committee; May 2012. https://lymphnet.org/position-papers. Last accessed 08/14/2019. [Google Scholar]
- 44.Guralnik JM, Ferrucci L, Pieper CF, et al. : Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 55:M221–31, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Studenski S, Perera S, Wallace D, et al. : Physical performance measures in the clinical setting. J Am Geriatr Soc 51:314–22, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Ostir GV, Volpato S, Fried LP, et al. : Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women’s Health and Aging Study. J Clin Epidemiol 55:916–21, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Katz S, Ford AB, Moskowitz RW, et al. : Studies Of Illness In The Aged. The Index Of Adl: A Standardized Measure Of Biological And Psychosocial Function. Jama 185:914–9, 1963 [DOI] [PubMed] [Google Scholar]
- 48.Lawton MP: Scales to measure competence in everyday activities. Psychopharmacol Bull 24:609–14, 1988 [PubMed] [Google Scholar]
- 49.Min L, Yoon W, Mariano J, et al. : The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc 57:2070–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nicklas BJ, Brinkley TE: Exercise training as a treatment for chronic inflammation in the elderly. Exerc Sport Sci Rev 37:165–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicklas BJ, Hsu FC, Brinkley TJ, et al. : Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc 56:2045–52, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen HJ, Harris T, Pieper CF: Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med 114:180–7, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Winters-Stone KM, Wood LJ, Stoyles S, et al. : The Effects of Resistance Exercise on Biomarkers of Breast Cancer Prognosis: A Pooled Analysis of Three Randomized Trials. Cancer Epidemiol Biomarkers Prev 27:146–153, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, et al. : Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 67:378–397, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace TM, Matthews DR: The assessment of insulin resistance in man. Diabet Med 19:527–34, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Bonora E, Kiechl S, Willeit J, et al. : Insulin resistance as estimated by homeostasis model assessment predicts incident symptomatic cardiovascular disease in caucasian subjects from the general population: the Bruneck study. Diabetes Care 30:318–24, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Bonora E, Targher G, Alberiche M, et al. : Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23:57–63, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Saliba D, Orlando M, Wenger NS, et al. : Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci 55:M750–6, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Min LC, Elliott MN, Wenger NS, et al. : Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc 54:507–11, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Bohannon RW, Schaubert KL: Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther 18:426–7, quiz 428, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Pereira MA, FitzGerald SJ, Gregg EW, et al. : A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc 1997. June;29(6 Suppl):S1–205. 1997 [PubMed] [Google Scholar]
- 62.Blair SN, Haskell WL, Ho P, et al. : Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 122:794–804, 1985 [DOI] [PubMed] [Google Scholar]
- 63.Sallis JF, Haskell WL, Wood PD, et al. : Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121:91–106, 1985 [DOI] [PubMed] [Google Scholar]
- 64.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–98, 1975 [DOI] [PubMed] [Google Scholar]
- 65.Crum RM, Anthony JC, Bassett SS, et al. : Population-based norms for the Mini-Mental State Examination by age and educational level. Jama 269:2386–91, 1993 [PubMed] [Google Scholar]
- 66.Fountoulakis KN, Tsolaki M, Iacovides A, et al. : The validation of the short form of the Geriatric Depression Scale (GDS) in Greece . Aging (Milano) 11:367–72, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Yesavage JA: Geriatric Depression Scale. Psychopharmacol Bull 24:709–11, 1988 [PubMed] [Google Scholar]
- 68.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–83, 1987 [DOI] [PubMed] [Google Scholar]
- 69.Brady MJ, Cella DF, Mo F, et al. : Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 15:974–86, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Yellen SB, Cella DF, Webster K, et al. : Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage 13:63–74, 1997 [DOI] [PubMed] [Google Scholar]
- 71.Carpenter JS, Andrykowski MA: Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 45:5–13, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Beaudreau SA, Spira AP, Stewart A, et al. : Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med 13:36–42, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stuifbergen AK, Becker HA: Predictors of health-promoting lifestyles in persons with disabilities. Res Nurs Health 17:3–13, 1994 [DOI] [PubMed] [Google Scholar]
- 74.Im EO, Chee W, Lim HJ, et al. : Midlife women’s attitudes toward physical activity. J Obstet Gynecol Neonatal Nurs 37:203–13, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Im EO, Chang SJ, Ko Y, et al. : A national internet survey on midlife women’s attitudes toward physical activity. Nurs Res 61:342–52, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Armitage CJ: Can the theory of planned behavior predict the maintenance of physical activity? Health Psychol 24:235–45, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Marshall SJ, Biddle SJ: The transtheoretical model of behavior change: a meta-analysis of applications to physical activity and exercise. Ann Behav Med 23:229–46, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Marcus BH, Selby VC, Niaura RS, et al. : Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport 63:60–6, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Marcus BH, Rossi JS, Selby VC, et al. : The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychol 11:386–95, 1992 [DOI] [PubMed] [Google Scholar]
- 80.Nigg CR, Rossi JS, Norman GJ, et al. : Structure of decisional balance for exercise adoption. Ann Behav Med;20:S211, 1998 [Google Scholar]
- 81.Owusu C, Schluchter M, Koroukian SM, et al. : Black-white disparity in physical performance among older women with newly diagnosed non-metastatic breast cancer: Exploring the role of inflammation and physical activity. J Geriatr Oncol 9:613–619, 2018 [DOI] [PubMed] [Google Scholar]
- 82.Owusu C, Margevicius S, Schluchter M, et al. : Short Physical Performance Battery, usual gait speed, grip strength and Vulnerable Elders Survey each predict functional decline among older women with breast cancer. J Geriatr Oncol 8:356–362, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd edition. Lawrence Erlbaum, Hillsdale, New Jersey, 2008. [Google Scholar]
- 84.Rubin DB: Inference and missing data. Biometrika 63:581–592, 1976 [Google Scholar]
- 85.Mallinckrodt CH, Clark WS, David SR: Accounting for dropout bias using mixed-effects models. J Biopharm Stat 11:9–21, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Vonesh EF, Greene T, Schluchter MD: Shared parameter models for the joint analysis of longitudinal data and event times. Stat Med 25:143–63, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Fried TR, Bradley EH, Williams CS, et al. : Functional disability and health care expenditures for older persons. Arch Intern Med 161:2602–7, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Manton KG: A longitudinal study of functional change and mortality in the United States. J Gerontol 43:S153–61, 1988 [DOI] [PubMed] [Google Scholar]
- 89.Wolinsky FD, Callahan CM, Fitzgerald JF, et al. : Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol 48:S94–101, 1993 [PubMed] [Google Scholar]
- 90.Owusu C, Margevicius S, Schluchter M, et al. : Vulnerable elders survey and socioeconomic status predict functional decline and death among older women with newly diagnosed nonmetastatic breast cancer. Cancer 122:2579–86, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sehl M, Lu X, Silliman R, et al. : Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv 7:20–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang V, Zhao S, Boscardin J, et al. : Functional Status and Survival After Breast Cancer Surgery in Nursing Home Residents. JAMA Surg 153:1090–1096, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Visser M, Simonsick EM, Colbert LH, et al. : Type and intensity of activity and risk of mobility limitation: the mediating role of muscle parameters. J Am Geriatr Soc 53:762–70, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Beasley JM, Kwan ML, Chen WY, et al. : Meeting the physical activity guidelines and survival after breast cancer: findings from the after breast cancer pooling project. Breast Cancer Res Treat 131:637–43, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bertram LA, Stefanick ML, Saquib N, et al. : Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control 22:427–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Satcher DM: Securing the Right to Healthcare and Well-Being, The Covenant with Black America, (Chicago: The Third World Press; ). 2006 [Google Scholar]
- 97.Blair CK, Morey MC, Desmond RA, et al. : Light-intensity activity attenuates functional decline in older cancer survivors. Med Sci Sports Exerc 46:1375–83, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guralnik JM, Alecxih L, Branch LG, et al. : Medical and long-term care costs when older persons become more dependent. Am J Public Health 92:1244–5, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reuben DB, Seeman TE, Keeler E, et al. : The effect of self-reported and performance-based functional impairment on future hospital costs of community-dwelling older persons. Gerontologist 44:401–7, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Irwin ML, McTiernan A, Manson JE, et al. : Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer Prev Res (Phila) 4:522–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. : Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 104:815–40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]