Abstract
Background
Antimicrobial resistance is a significant concern to public health, and there is a pressing need to develop novel antimicrobial therapeutic modalities.
Methods
In this study, we investigated the capacity for quinine hydrochloride (Q-HCL) to enhance the antimicrobial effects of antimicrobial blue light ([aBL] 405 nm wavelength) against multidrug-resistant (MDR) Gram-negative bacteria in vitro and in vivo.
Results
Our findings demonstrated the significant improvement in the inactivation of MDR Pseudomonas aeruginosa and Acinetobacter baumannii (planktonic cells and biofilms) when aBL was illuminated during Q-HCL exposure. Furthermore, the addition of Q-HCL significantly potentiated the antimicrobial effects of aBL in a mouse skin abrasion infection model. In addition, combined exposure of aBL and Q-HCL did not result in any significant apoptosis when exposed to uninfected mouse skin.
Conclusions
In conclusion, aBL in combination with Q-HCL may offer a novel approach for the treatment of infections caused by MDR bacteria.
Keywords: Acinetobacter baumannii, antimicrobial blue light, antimicrobial resistance, Pseudomonas aeruginosa, quinine
With the rise in antimicrobial resistance, in what is now being referred to as the “post-antibiotic era”, there is a pressing need to develop novel antimicrobial therapeutic modalities [1, 2]. Gram-negative bacterial infections are a particularly important problem, because the presence of their lipopolysaccharide outer membrane layer offers increased protection against traditional antibiotics [3, 4]. In recent years, antimicrobial blue light (aBL) has been emerging as an innovative “nonpharmacological” approach to tackle multidrug-resistant (MDR) infections [5]. The accepted mechanism of aBL inactivation is through photoexcitation of endogenous porphyrins that result in the generation of reactive oxygen species and apoptosis [5]. It is an especially attractive antimicrobial strategy, because previous studies have demonstrated that resistance development to aBL by bacteria through serial exposure is highly unlikely [6, 7]. However, the antimicrobial efficacy of aBL is dependent on the infecting agent, with certain bacterial species being comparatively more tolerant of aBL-mediated killing than others [8]. Therefore, it is paramount that we investigate novel ways to potentiate the efficacy of aBL so that we may harness its full potential. Quinine is a plant-based agent extracted from the bark of the cinchona trees that possesses high activity against malaria [9]. It was discovered that quinine also possesses, at high concentrations, antibacterial properties [10], which suggests it has potential as an antimicrobial adjuvant. In this study, we demonstrated the significant antimicrobial effects elicited when quinine hydrochloride (Q-HCL) is combined with aBL against Gram-negative bacteria.
MATERIALS AND METHODS
Blue Light Source
For aBL irradiation, a light-emitting diode (Thorlabs, Newton, NJ) with a peak emission of 405 nm and a full width at half-maximum of 25 nm was used. The irradiance was regulated by altering the distance of the light source aperture and the target with the use of a PM100D power/energy meter (Thorlabs, Newton, NJ).
Bacterial Strains and Growth Conditions
The bacterial strains used in this study were MDR clinical strains of Pseudomonas aeruginosa and Acinetobacter baumannii that were isolated from soldiers deployed in Afghanistan and found to be resistant to every antibiotic tested (amikacin, ampicillin, aztreonam, cefazolin, cefepime, cefoxitin, ceftazidime, ceftriaxone, cefuroxime, ciprofloxacin, gentamicin, imipenem, and levofloxacin). A bioluminescent variant of P aeruginosa (strain PAO1) containing a chromosomally integrated lux operon from Photorhabdus luminescens [11] was used for the in vivo studies, allowing real-time monitoring of bioluminescence. The bacteria were grown in brain-heart infusion (BHI) medium (agar or broth) at 37°C, 5% CO2, or in an orbital incubator (37°C; 180 rpm), respectively.
Antimicrobial Blue Light + Quinine Hydrochloride Inactivation of Planktonic Bacteria In Vitro
Bacteria were cultured to mid-log phase and adjusted to approximately 109 colony-forming units (CFU)/mL in phosphate-buffered saline (PBS) and transferred to a 35 × 12-mm dish. When necessary, 0.125–1 mg/mL Q-HCL was added to the 35 × 12-mm dish containing the microbial suspension, immediately before aBL irradiation. Antimicrobial blue light (30 mW/cm2) was then delivered to the microbial suspension in the presence or absence of Q-HCL. During aBL irradiation, the bacterial suspension was stirred using a 12-mm magnetic bar (20 rpm) to ensure uniform exposure of cells to aBL. Aliquots (30 μL) of the suspension were then withdrawn at varying time points after the initiation of aBL, and the CFU was determined by serial dilution (10−1 to 10−7 dilution factors) on BHI agar plates as described previously [12]. Experiments were performed in triplicate.
Antimicrobial Blue Light + Quinine Hydrochloride Inactivation of Bacterial Biofilms
Bacterial biofilms were grown for 24 hours in 96-well microtiter plates as described previously [13, 14]. The wells were washed 3 times with PBS to remove any medium or planktonic cells. Before aBL irradiation, aliquots of 200 μL fresh PBS (with or without 1 mg/mL Q-HCL) were added to the wells. The biofilms were then irradiated with aBL at an irradiance of 60 mW/cm2 until radiant exposures of 108 J/cm2 were delivered. The adhered cells within the biofilms were then isolated through a thorough agitation with a pipette tip in the appropriate wells, transferring the 200-μL cell/biofilm suspension to a 1.5-mL microcentrifuge tube. A further 200 μL PBS were then added to the wells, and the biofilm isolation was repeated twice more, to ensure adequate removal of bacterial biofilms. The collected cells were subsequently sonicated using a branson 2510 water bath sonicator (Marshall Scientific, LLC), before CFU quantification. Experiments were conducted in triplicate.
Transmission Electron Microscopy to Detect Ultrastructural Changes to Bacteria Treated With Antimicrobial Blue Light + Quinine Hydrochloride
Planktonic bacteria that were treated with aBL + Q-HCL (81J/cm2; 1 mg/mL), aBL alone (81 J/cm2), Q-HCL (1 mg/mL) alone, or untreated were immediately fixed in a 2.5% glutaraldehyde and 2% paraformaldehyde solution and prepared from transmission electron microscopy (TEM) as described previously [14]. The sections were examined on a Philips CM-10 TEM (Eindhoven, The Netherlands). Multiple bacterial sections were analyzed microscopically with images that were most representative being presented in this study.
Scanning Electron Microscopy for Visualization of Bacterial Biofilms
Biofilms were grown for 24 hours on sterile 13-mm circles of ACLAR 33oC and washed with sterile PBS before fixing at 4°C for 24 hours in a 0.1 M cacodylate buffer containing 2.5% glutaraldehyde, 0.15% safranin O, and 0.15% alcian blue. After fixation, the biofilms were washed with 0.1 M cacodylate buffer and fixed with 1% osmium tetroxide for 2 hours, before dehydrating in 100% ethanol. The biofilms were then dried with the use of a critical-point drying, mounted on specimen stubs, and sputter coated with platinum (10 nm). The biofilms were then observed using a scanning electron microscope (Hitachi S-4800; Hitachi Medical Corporation, Tokyo, Japan) and acquisition of micrographs occurred under high vacuum using a voltage of 3.0 kV.
Single-Cell Raman Spectroscopy to Detect the Presence of Quinine Hydrochloride Within Bacterial Cells
Bacteria were cultured to mid-log phase before treatment with aBL (27–108 J/cm2) combined with Q-HCL (1 mg/mL). After aBL + Q-HCL treatment, 1.5 μL of the bacterial suspension from each group (ie, aBL + Q-HCL, Q-HCL, aBL, and untreated bacterial cells) was sandwiched between 2 cover slides (No. 1 slides; VWR) before imaging on a Raman microscope (HORIBA). Single cells were identified on the microscope, and the center of the bacterial cells were excited at 532 nm with the ×40 air objective. Experiments were conducted at least in triplicate.
Antimicrobial Blue Light-Quinine Hydrochloride Inactivation of Bacteria in a Mouse Abrasion Wound
Female BALB/c mice aged 6–8 weeks and weighing 17–19 grams were purchased from Charles River Laboratories (Wilmington, MA). All animal procedures were approved by the Institutional Animal Care and Use Committees of Massachusetts General Hospital (protocol number: 2015N000187) in accordance with National Institute of Health guidelines. Before producing the abrasion wounds in mice, mice were given intraperitoneal injections with the use of a ketamine/xylazine cocktail (20–100 mg/kg). The mice were then shaved, and the tissue was carefully abraded within a defined 1.0-cm × 1.0-cm area using a #15 sterile scalpel blade. The scraped area did not produce any blood. Within 5 minutes of producing the abrasion, 50 µL bacterial suspension containing approximately 108 CFU in PBS was inoculated onto the wound and uniformly applied gently using the side of a pipette tip. A bioluminescent P aeruginosa strain (PAO1) was used as the model organism for this study. Three hours after inoculating the bacterial suspension, aBL was applied at 60 mW/cm2 (with or without 0.2 mg/cm2 Q-HCL), until a total radiant exposure of 135 J/cm2 was delivered. Bioluminescence imaging was carried out after each aliquot of aBL. For each condition, a group of 6 mice were used.
Bioluminescence Imaging In Vivo
The bioluminescence imaging of bacteria in mouse abrasion wounds was performed by using an IVIS Lumina II In Vivo Imaging System (PerkinElmer, Inc., Hopkinton, MA). The luminescence intensity, which is the consequence of the photon flux emitted by the bioluminescent bacterial cells, directly correlates to the bacterial viability and can be measured at the site of infection in mice using a region of interest tool. The system was operated using the Living Image software, which provides image acquisition tools including photon counting to permit real-time quantification of the relative luminescence units.
TUNEL Assay to Detect Apoptotic Cells in Mouse Skin Treated With Antimicrobial Blue Light + Quinine Hydrochloride
The presence of apoptotic cells that resulted from aBL + Q-HCL therapy was determined in healthy mouse skin as described previously [15]. Mouse skin was initially exposed to a combination of 135 J/cm2 aBL and 0.2 mg/cm2 Q-HCL. An untreated skin sample was also included as the control. Fluorescence images were visualized with the use of NanoZoomer S60 Digital slide scanner where a fluorescein isothiocyanate was used as the fluor and 4’,6-diamidino-2-phenylindole was used as the nuclear counterstain. In addition, a DNase I (RQ1 RNasefree DNase; Promega) treated section (which induces significant DNA damage) served as a positive control.
Statistical Analyses
Data were presented as the mean ± standard error, and differences between means were compared for significance by either an unpaired t test or a one-way analysis of variance. P < .05 were considered significant.
RESULTS
Quinine Hydrochloride Enhanced the Antimicrobial Effects of Antimicrobial Blue Light in a Dose-Dependent Manner Against Planktonic Bacteria
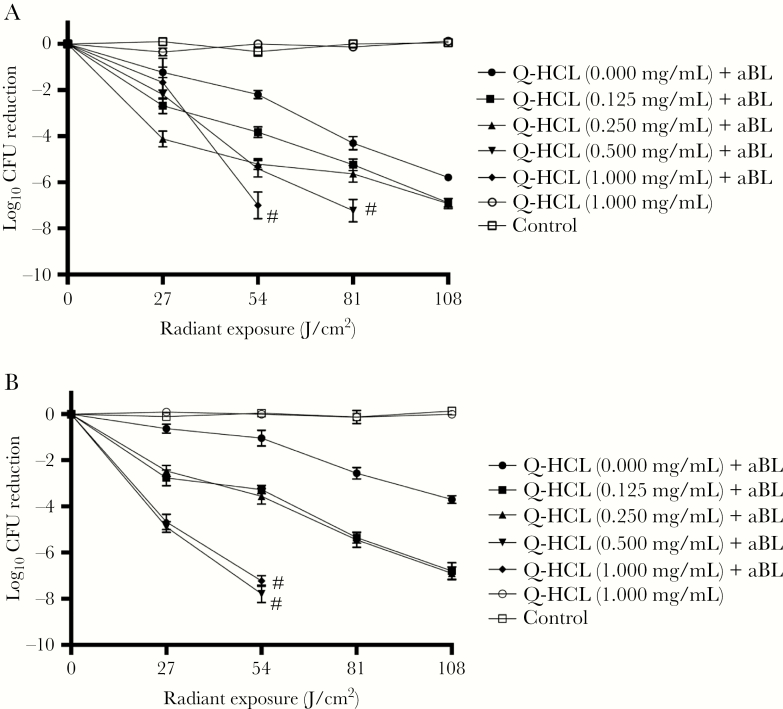
In this study, we initially investigated the potential of Q-HCL to enhance the antimicrobial effects of aBL in planktonic Gram-negative bacteria. We found that the addition of Q-HCL at nonbactericidal concentrations (0.125–1.000 mg/mL) during aBL inactivation of bacteria resulted in a significant potentiation of antimicrobial effects relative to aBL alone. For both bacterial species, 1 mg/mL Q-HCL was the most effective concentration when combined with aBL. After 54 J/cm2 aBL, in the presence of 1 mg/mL Q-HCL, an inactivation of ≥7-log10 CFU was achieved (Figure 1A and B) (P < .001). Antimicrobial blue light alone, at an equivalent dose, inactivated 1.05 and 2.19 log10 CFU, in P aeruginosa and A baumannii, respectively. Quinine HCL, at an equivalent dose, did not influence bacterial viability in either bacterial species.
Figure 1.
Dose-response curves illustrating the log10 colony-forming unit (CFU) reductions of (A) Pseudomonas aeruginosa and (B) Acinetobacter baumannii after treatment with 1 mg/mL quinine hydrochloride (Q-HCL) and increasing radiant exposures of antimicrobial blue light (aBL) (27–108 J/cm2). Error bars: standard error of the mean (SEM). # denotes the serial dilution reached maximum threshold for detection (ie, no colonies observed at a 10–1 dilution factor).
For both bacteria, combining Q-HCL at a concentration of 0.125 mg/mL with aBL at 81 J/cm2 resulted in a >5-log10 CFU reduction, compared with aBL alone, at an equivalent dose, which inactivated 2.57-log10 CFU and 4.30-log10 CFU, in P aeruginosa and A baumannii, respectively (P < .01) (Figure 1A and B). When the Q-HCL concentration was increased to 0.250 mg/mL in the presence of 81 J/cm2, a similar inactivation was achieved (>5-log10 CFU) in both bacteria. Furthermore, the Q-HCL concentration of 0.500 mg/mL in the presence of aBL (81 J/cm2), however, resulted in a >7-log10 in both species (P < .001) (Figure 1A and B). Findings suggest that Q-HCL potentiated aBL inactivation in a dose-dependent manner.
Quinine Hydrochloride Enhanced the Antimicrobial Effects of Antimicrobial Blue Light in Bacterial Biofilms
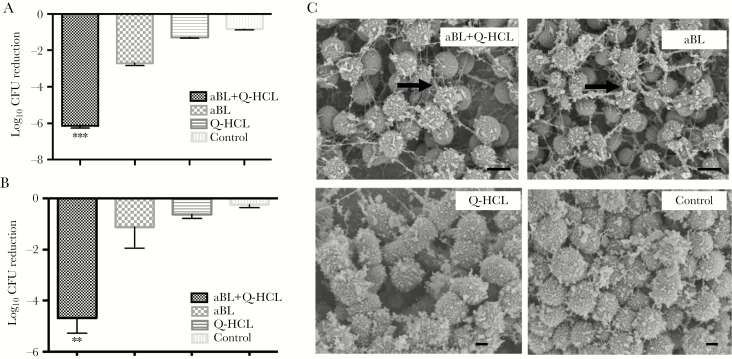
We next wanted to determine whether Q-HCL enhanced the antimicrobial effects of aBL when exposed to bacterial biofilms. For both bacterial species, 108 J/cm2 aBL in combination with 1 mg/mL Q-HCL was enough to inactivate 6.15-log10 CFU and 5.67-log10 CFU, in P aeruginosa and A baumannii, respectively. Antimicrobial blue light alone, at an equivalent dose, inactivated 2.71-log10 CFU and 1.11-log10 CFU (P < .003) (Figure 2A and B). Unexpectedly, Q-HCL (1 mg/mL) alone did have a significant effect on the viability on both bacterial species (P < .03).
Figure 2.
(A) Bar graph illustrating the log10 colony-forming unit (CFU) reductions of Pseudomonas aeruginosa and (B) Acinetobacter baumannii, after irradiation with 108 J/cm2 antimicrobial blue light (aBL) in combination with 1 mg/mL quinine hydrochloride (Q-HCL). Treatment with 108 J/cm2 aBL alone and 1 mg/mL Q-HCL alone and an untreated control were also included. Error bars: SEM. **, P < .01; ***, P < .001. (C) The SEM images show the morphology and ultrastructure of the bacterial biofilms after treatment with aBL + Q-HCL, aBL, Q-HCL, or untreated control. Black arrows: biofilm matrix. Bars: 500 nm.
Scanning electron microscopy was also performed on the biofilms of representative Gram-negative bacterium, in which 24-hour bacterial biofilms were treated with 108 J/cm2 aBL and 1 mg/mL Q-HCL. An important and unexpected finding was the apparent degradation of the EPS after aBL + Q-HCL (Figure 2C, black arrow), particularly when compared with the aBL group, where the matrix is thicker and more defined (Figure 2C, black arrow). Both the untreated control and Q-HCL groups were morphologically similar with no visibility of the EPS matrix (Figure 2C).
Quinine Hydrochloride Induced Cytoplasmic and Cell Wall Damage When Combined With Antimicrobial Blue Light
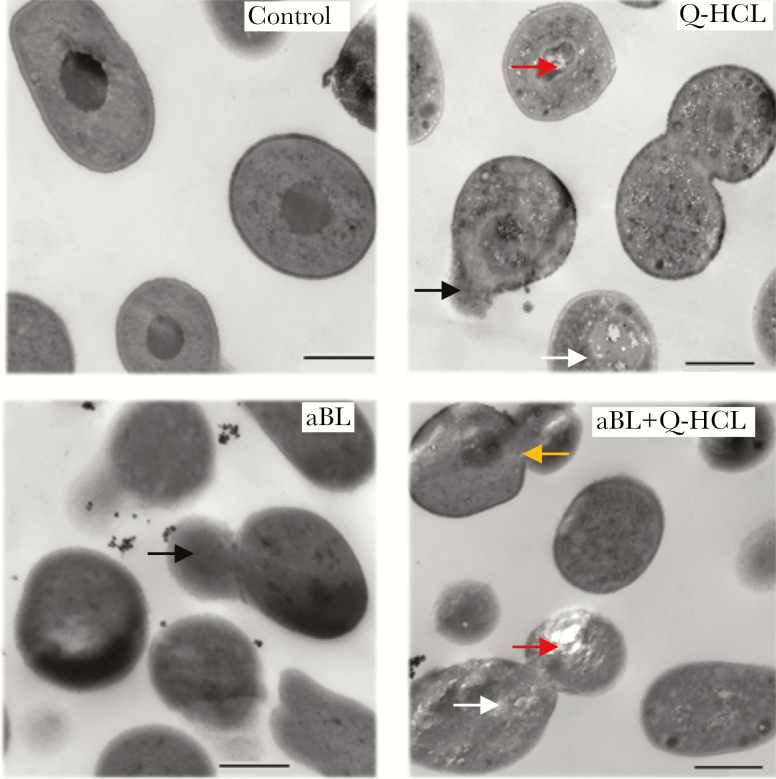
The untreated control illustrated an intact cell wall, membrane, and cytoplasm (Figure 3). Quinine HCL alone resulted in cellular damage, with spillage of intracellular contents (Figure 3, black arrows) as well as possible internalization of the Q-HCL molecule (Figure 3, white arrows). The damage elicited by Q-HCL, although not previously shown to induce antimicrobial effects, may play a role in enhancing aBL activity through reducing the integrity of the internal structures within the bacterial cell. Antimicrobial blue light-treated cells resulted in spillage of intracellular contents (Figure 3, black arrows) with no obvious indication of specific damage to the cell wall or cytoplasm. Antimicrobial blue light + Q-HCL exposure resulted in obvious spillage of intracellular contents (Figure 3, black arrows) and intracellular vacuole formation (Figure 3, red arrows), which is indicative of cytoplasmic damage [16], possible internalization of the Q-HCL molecule (Figure 3, white arrow), and damage to the cell wall (Figure 3, yellow arrow).
Figure 3.
Representative transmission electron microscopy images illustrating the damage to bacterial cells elicited after no treatment (control), quinine hydrochloride (Q-HCL) (1 mg/mL), antimicrobial blue light (aBL) (81 J/cm2), or aBL + Q-HCL (81 J/cm2 + 1 mg/mL) treatment. Black arrows, intracellular spillage; red arrow, vacuole formation; white arrow, possible Q-HCL molecule; and yellow arrow, cell wall breakage. Bars: 500 nm.
Antimicrobial Blue Light Improved Uptake of Quinine Hydrochloride Into the Bacterial Cell
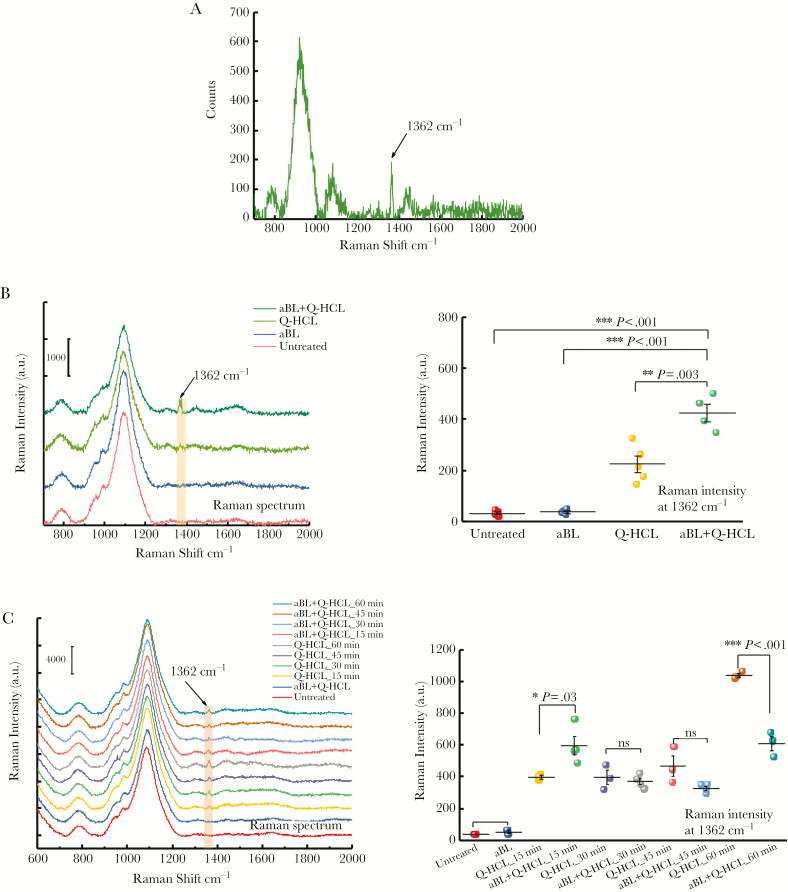
In this study, we wanted to determine whether Q-HCL was internalized by the bacteria. Therefore, we performed Raman spectroscopy on single bacterial cells of A baumannii and P aeruginosa that were exposed to Q-HCL (with or without aBL). Antimicrobial blue light alone-treated and untreated samples served as controls. The Raman spectrum of the Q-HCL molecule was also determined to facilitate identification of the molecule within the cell (Figure 4A). For A baumannii, exposure to Q-HCL alone for 30 minutes (equivalent to the duration of aBL treatment) resulted in a very small peak at 1362 cm−1 (Figure 4B), which is consistent with the location of the peak from the Q-HCL spectrum, which indicated the presence of Q-HCL within the cell. However, unexpectedly, when A baumannii was simultaneously exposed to Q-HCL and aBL, the peak was considerably increased (Figure 4B), suggesting a significant improvement in the uptake of the molecule. When the Raman intensities at 1362 cm−1 of Q-HCL- and aBL + Q-HCL-treated groups were quantified and compared, a 2-fold increase was observed, indicating that aBL illumination had improved the uptake of Q-HCL into the cell (P = .003) (Figure 4B).
Figure 4.
Raman spectra illustrating the spectrum of (A) quinine hydrochloride (Q-HCL), (B) Acinetobacter baumannii exposed to 54 J/cm2 antimicrobial blue light (aBL), 1 mg/mL Q-HCL, aBL + Q-HCL (54 J/cm2; 1 mg/mL), or untreated, and A baumannii-quantitative Raman intensity analyses at 1362 cm−1 following: aBL, Q-HCL, or aBL + Q-HCL exposure or untreated. (C) Raman spectra of Pseudomonas aeruginosa after aBL (15–60 minutes; 30 mW/cm2), 1 mg/mL Q-HCL, or aBL + Q-HCL (15–60 minutes; 30 mW/cm2; 1 mg/mL) exposure or untreated, and P aeruginosa quantitative Raman intensity analyses at 1362 cm−1 after aBL (15–60 minutes; 30 mW/cm2), 1 mg/mL Q-HCL, or aBL + Q-HCL (15–60 minutes; 30 mW/cm2; 1 mg/mL) exposure or untreated were also included. Error bars: SEM.
For P aeruginosa, exposure to Q-HCL alone also resulted in uptake into the cell, with exposure for 60 minutes resulting in the highest Raman intensity (indicating that more Q-HCL was present within the cell) (Figure 4C). When the mixture of P aeruginosa and Q-HCL was exposed with 27 J/cm2 aBL, a significant increase in the Raman intensity was observed, suggesting increased uptake of Q-HCL by P aeruginosa (P = .03) (Figure 4C), when compared with Q-HCL alone. However, with higher radiant exposures of aBL (54–108 J/cm2), a decrease in the Raman intensity at 1362 cm−1 was observed, which suggests that Q-HCL was lost from the cell, because of damage to the cell membrane/wall. Furthermore, at 60 minutes incubation (reflecting the duration of 108 J/cm2 aBL exposure) of Q-HCL treatment (with or without aBL), there was a significantly lower Raman intensity at 1362 cm−1 detectable in the bacterial cell simultaneously treated with Q-HCL and aBL, which further suggests an inability to accumulate the molecule within the cell because of significant cell wall damage
Quinine Hydrochloride Enhanced the Antimicrobial Effects of Antimicrobial Blue Light In Vivo
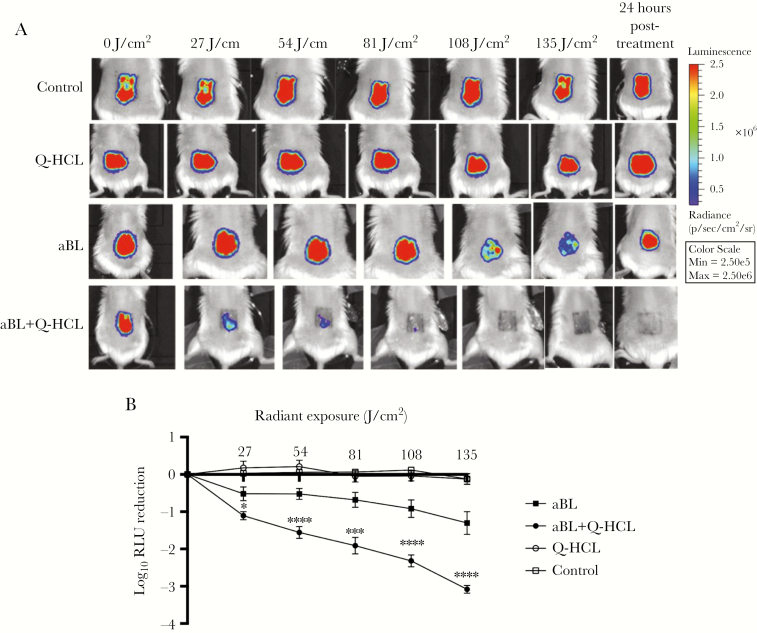
To predict the clinical translatability of combining aBL and Q-HCL, we investigated the effect of aBL + Q-HCL in a mouse abrasion infection model [14]. Treatment of Q-HCL alone at 0.2 mg/cm2 did not significantly influence bacterial bioluminescence relative to the untreated control (P > .09) (Figure 5A and B). When aBL (135 J/cm2) alone was delivered, a strong bioluminescence signal remained, reflecting a 1.30-log10 relative light unit (RLU) reduction (Figure 5A and B). However, when the wound infection was treated with aBL in the presence of Q-HCL (0.2 mg/cm2), the signal was lost completely from the wound (Figure 5A), with a 3.08-log10 RLU reduction (Figure 5B), thus qualitatively and quantitatively demonstrating the vast improvement in the efficacy of aBL when in the presence of 0.2 mg/cm2 Q-HCL. Even more important, 24 hours after exposure to aBL + Q-HCL, there was no obvious recurrence of the bioluminescence signal (Figure 5A), suggesting that PAO1 was inactivated within the wound after treatment.
Figure 5.
Representative bioluminescence images of mouse abrasions infected with a bioluminescent variant of Pseudomonas aeruginosa PAO1: (A) untreated, 0.2 mg/cm2 quinine hydrochloride (Q-HCL) alone, different radiant exposures of antimicrobial blue light (aBL) alone, or aBL + 0.2 mg/cm2 Q-HCL. (B) Dose-response of mean bacterial luminescence from mouse wounds infected with PAO1 and treated with 27, 54, 81, 108, or 135 J/cm2 aBL with or without 0.2 mg/cm2 Q-HCL and 24 hours posttreatment (n = 6). Error bars: SEM. *, P < .05; ***, P < .001; ****, P < .0001. Pseudo-color scale semiquantitatively shows the relative light units based on red or blue color.
Combined Antimicrobial Blue Light + Quinine Hydrochloride Exposure Did Not Result in Apoptosis of Mouse Skin Cells
To determine the genotoxic potential arising through the combination of aBL and Q-HCL on mammalian tissue, the therapeutic dose of aBL + Q-HCL that resulted in elimination of PAO1 (135 J/cm2 aBL; 0.2 mg/cm2 Q-HCL) was administered to uninfected mouse skin tissue before assessing genotoxicity using the TUNEL assay [17]. Results illustrated no increased number in apoptotic cells when compared with the untreated control immediately after combined aBL + Q-HCL treatment or 24 hours posttreatment (data not shown).
DISCUSSION
In this study, we demonstrated that the addition of a nonlethal concentration of Q-HCL during aBL illumination significantly potentiated inactivation by >103-fold in both MDR P aeruginosa and A baumannii, planktonic cells, and those within 24-hour biofilms. There have been limited studies that have investigated the antimicrobial effects of quinine against bacteria [10, 18]. A study by Wolf et al [18] demonstrated that quinine sulfate reduces the ability for Escherichia coli to invade mammalian cells. However, they found that at the highest concentration tested (100 μM), no antibacterial action was elicited. In our study, we found that 1 mg/mL Q-HCL (reflecting a 2.5-mM concentration) did not elicit any antimicrobial effects, suggesting that quinine does not possess significant bactericidal properties.
Kharal et al [10] investigated minimum inhibitory concentrations (MICs) of quinine dihydrochloride on a panel of pathogenic Gram-negative and Gram-positive bacteria. They found the MICs to be high, ranging between 31.25 and 125 g/mL. A recent study revealed that in fungal organisms, perturbation of the cell wall sensitized them to the antimicrobial effects of chloroquine [19]. It has also been demonstrated previously that aBL has the capacity to elicit damage to the cell wall or membrane [20]. Therefore, it is possible that any damage to the cell wall induced by aBL or Q-HCL may have primed the enhanced antimicrobial effects. However, further work looking at how aBL and Q-HCL influence the bacterial membrane potential would be necessary to evaluate the relationship between cell permeabilization and antimicrobial effect.
Several studies found that quinine is capable of binding heme [21, 22]. In Plasmodium spp, it is hypothesized that quinine-heme complex formation is responsible for the associated antimalarial effects because this interaction blocks heme polymerization in the host, preventing the formation of the hemozoin [21]. A more recent study by Martins et al found that quinine aids in bacterial host clearance by blocking the interaction of heme with DOCK8. This further provides evidence on how the interaction between heme and quinine can influence biological mechanisms [22]. Porphyrin production is tightly regulated within the heme biosynthesis pathway, through the presence of heme [23]. When heme is in abundance, it results in destabilization of glutamyl-tRNA reductase, thus resulting in cessation of porphyrin production [24]. Therefore, we hypothesize that the interaction between quinine and heme limits or prevents this destabilization of glutamyl-tRNA reductase, resulting in the overproduction of intermediate porphyrins, and thus increasing the numbers of porphyrins that may be excited by aBL. Further work looking into the capacity for Q-HCL to perturb the heme biosynthesis pathway in this way is warranted to corroborate this hypothesis.
Scanning electron microscopy performed on A baumannii bacterial biofilms (Figure 2C–F) suggested that the combination of aBL and Q-HCL may reduce the biofilm matrix and may in turn increase the permeability of antimicrobial molecules (such as Q-HCL), thus explaining the significant potential in antimicrobial effects that was observed. Further work quantifying this reduction of the EPS matrix is warranted to corroborate this hypothesis.
Raman spectroscopy revealed that Q-HCL is taken up by bacteria in the absence of aBL. However, the addition of aBL significantly potentiated the uptake of Q-HCL into the cell. It has been shown that aBL exposure results in a loss in cell membrane integrity [20], which might explain this phenomenon of increased uptake through facilitating entry of the molecule into the cell. Another possible explanation for the increased uptake of Q-HCL that was induced by aBL could be a result of increased expression of transmembrane porins such as OmpX and OmpF, which have been shown to become overexpressed because of antibiotic or environmental stressors [25, 26]. However, further work would be required to validate these hypotheses.
To predict the clinical translatability of aBL + Q-HCL therapy, we sought to determine whether improvements in the antimicrobial effects of aBL may be enhanced by aBL in vivo. We established that after 135 J/cm2 aBL (37.5 minutes at 60 mW/cm2), the bacterial burden was eliminated from the wound, without recurrence of infection 24 hours posttreatment. Antimicrobial blue light or Q-HCL alone were insufficient to eliminate the infection. In addition, we found that at an exposure of 135 J/cm2 and 0.2 mg/cm2 Q-HCL (therapeutic ratio of 1), there was no increased number in apoptotic cells, when compared with the untreated control. These findings suggest that at the therapeutic dose required to eliminate P aeruginosa within mouse abrasion wounds, aBL may be safely administered in combination with Q-HCL.
CONCLUSIONS
In conclusion, aBL is a potential therapeutic modality for the treatment of localized infections, such as those we have established a novel, effective, and safe antimicrobial combination therapy that involves the use of 2 nontraditional antimicrobials. However, an important limitation of aBL is that the penetration depth is low, which might limit its effectiveness against deeper infections. Furthermore, we demonstrated that aBL can enhance the uptake of an antimicrobial molecule into bacterial cells. Further studies are needed to determine whether more mature wound infections may be successfully treated with aBL + Q-HCL and to investigate the mechanism of aBL enhancement by Q-HCL.
Notes
Acknowledgments. We thank Dr. Joanna B. Goldberg (Emory University School of Medicine) for providing the bioluminescent Pseudomonas aeruginosa PAO1 strain. In addition, we thank Dr. Clinton Murray (Brooke Army Medical Center, Fort Sam Houston, TX) for providing the multidrug-resistant Acinetobacter baumannii and P aeruginosa strains.
Financial support. The study was funded in part by the National Institutes Health (R01AI123312; to T. D.) and the US Department of Defense (FA9550-17-1-0277; to T. D.). L. G. L. was supported by an American Society for Laser Medicine and Surgery Research Grant (BS.F04.18).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jackson N, Czaplewski L, Piddock LJV. Discovery and development of new antibacterial drugs: learning from experience? J Antimicrob Chemother 2018; 73:1452–9. [DOI] [PubMed] [Google Scholar]
- 2. Kmietowicz Z. Few novel antibiotics in the pipeline, WHO warns. BMJ 2017; 358:j4339. [DOI] [PubMed] [Google Scholar]
- 3. Delcour AH. Outer membrane permeability and antibiotic resistance. Biochimica et Biophysica Acta 2008; 1794:808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. May KL, Grabowicz M. The bacterial outer membrane is an evolving antibiotic barrier. Proc Natl Acad Sci U S A 2018; 115:8852–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Wang Y, Wang Y, et al. Antimicrobial blue light inactivation of pathogenic microbes: state of the art. Drug Resist Updat 2017; 33-35:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leanse LG, Harrington OD, Fang Y, Ahmed I, Goh XS, Dai T. Evaluating the potential for resistance development to antimicrobial blue light (at 405 nm) in Gram-negative bacteria: in vitro and in vivo studies. Front Microbiol 2018; 9:2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tomb RM, Maclean M, Coia JE, MacGregor SJ, Anderson JG. Assessment of the potential for resistance to antimicrobial violet-blue light in Staphylococcus aureus. Antimicrob Resist Infect Control 2017; 6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gupta S, Maclean M, Anderson JG, MacGregor SJ, Meek RM, Grant MH. Inactivation of micro-organisms isolated from infected lower limb arthroplasties using high-intensity narrow-spectrum (HINS) light. Bone Joint J 2015; 97-B:283–8. [DOI] [PubMed] [Google Scholar]
- 9. Achan J, Talisuna A, Erhart O, et al. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J 2010; 144:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kharal SA, Hussain Q, Ali S, Fakhuruddin. Quinine is bactericidal. J Pak Med Assoc 2009; 59:208–12. [PubMed] [Google Scholar]
- 11. Damron FH, McKenney ES, Barbier M, Liechti GW, Schweizer HP, Goldberg JB. Construction of mobilizable mini-Tn7 vectors for bioluminescent detection of gram-negative bacteria and single-copy promoter lux reporter analysis. Appl Environ Microbiol 2013; 79:4149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jett BD, Hatter KL, Huycke MM, Gilmore MS. Simplified agar plate method for quantifying viable bacteria. Biotechniques 1997; 23:648–50. [DOI] [PubMed] [Google Scholar]
- 13. O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp 2011; 47:2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Wu X, Chen J, et al. Antimicrobial blue light inactivation of Gram-negative pathogens in biofilms: in vitro and in vivo studies. J Infect Dis 2016; 213:1380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Zhu Y, Chen J, et al. Antimicrobial blue light inactivation of Candida albicans: in vitro and in vivo studies. Virulence 2016; 7:536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Yang D, Wang S, et al. The detailed bactericidal process of ferric oxide nanoparticles on E. coli. Qiu Molecules 2018; 23:E606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Majtnerová P, Roušar T. An overview of apoptosis assays detecting DNA fragmentation. Mol Biol Rep 2018; 45:1467–78. [DOI] [PubMed] [Google Scholar]
- 18. Wolf R, Baroni A, Greco R, et al. Quinine sulfate and bacterial invasion. Ann Clin Microbiol Antimicrob 2002; 22:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Islahudin F, Khozoie C, Bates S, Ting KN, Pleass RJ, Avery SV. Cell wall perturbation sensitizes fungi to the antimalarial drug chloroquine. Antimicrob Agents Chemother 2013; 57:3889–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKenzie K, Maclean M, Grant MH, Ramakrishnan P, MacGregor SJ, Anderson JG. The effects of 405 nm light on bacterial membrane integrity determined by salt and bile tolerance assays, leakage of UV-absorbing material and SYTOX green labelling. Microbiology 2016; 162:1680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alumasa JN, Gorka AP, Casabianca LB, Comstock E, de Dios AC, Roepe PD. The hydroxyl functionality and a rigid proximal N are required for forming a novel non-covalent quinine-heme complex. J Inorg Biochem 2011; 105:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins R, Maier J, Gorki AD, et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat Immunol 2016; 17:1361–72. [DOI] [PubMed] [Google Scholar]
- 23. Dailey HA, Dailey TA, Gerdes S, et al. Prokaryotic heme biosynthesis: multiple pathways to a common essential product. Mirobiol Mol Biol Rev 2017; 81:e00048–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choby JE, Grunenwald CM, Celis AI, Gerdes SY, DuBois JL, Skaar EP. Staphylococcus aureus HemX modulates glutamyl-tRNA reductase abundance to regulate heme biosynthesis. mBio 2018; 9:e02287–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pomposiello PJ, Bennik MH, Demple B. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J Bacteriol 2001; 183:3890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dupont M, James CE, Chevalier J, Pagès JM. An early response to environmental stress involves regulation of OmpX and OmpF, two enterobacterial outer membrane pore-forming proteins. Antimicrob Agents Chemother 2007; 51:3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]