Abstract
Background
Dengue virus (DENV) can cause life-threatening disease characterized by endothelial dysfunction and vascular leakage. DENV nonstructural protein 1 (NS1) induces human endothelial hyperpermeability and vascular leak in mice, and NS1 vaccination confers antibody-mediated protective immunity. We evaluated the magnitude, cross-reactivity, and functionality of NS1-specific IgG antibody responses in sera from a phase 2 clinical trial of Takeda’s live-attenuated tetravalent dengue vaccine candidate (TAK-003).
Methods
We developed an enzyme-linked immunosorbent assay to measure anti-DENV NS1 IgG in sera from DENV-naive or preimmune subjects pre- and postvaccination with TAK-003 and evaluated the functionality of this response using in vitro models of endothelial permeability.
Results
TAK-003 significantly increased DENV-2 NS1-specific IgG in naive individuals, which cross-reacted with DENV-1, -3, and -4 NS1 to varying extents. NS1-induced endothelial hyperpermeability was unaffected by prevaccination serum from naive subjects but was variably inhibited by serum from preimmune subjects. After TAK-003 vaccination, all samples from naive and preimmune vaccinees completely abrogated DENV-2 NS1-induced hyperpermeability and cross-inhibited hyperpermeability induced by DENV-1, -3, and -4 NS1. Inhibition of NS1-induced hyperpermeability correlated with NS1-specific IgG concentrations. Postvaccination sera also prevented NS1-induced degradation of endothelial glycocalyx components.
Conclusion
We provide evidence for functional NS1-specific IgG responses elicited by a candidate dengue vaccine.
Clinical Trials Registration
Keywords: Dengue virus, vaccine, nonstructural protein 1, IgG response
(See the Editorial Commentary by Halstead et al, on pages 857–60.)
Dengue is the most prevalent human arboviral disease worldwide, with approximately 390 million annual infections and half the world’s population at risk of infection from 1 of 4 dengue virus serotypes (DENV-1–4) [1]. DENV is a flavivirus transmitted by Aedes aegypti or Aedes albopictus mosquitoes. Outcomes range from asymptomatic infection to dengue fever (DF) to severe dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) [2]. Several DENV vaccine candidates are under development. A chimeric yellow fever virus-tetravalent dengue vaccine (Dengvaxia, Sanofi Pasteur) is approved for age ≥9 years in 20 countries [3–5]. However, Dengvaxia has been associated with increased risk of severe disease in younger and seronegative individuals [4, 6, 7]. Therefore, an urgent need exists for a dengue vaccine that can protect all age groups against all 4 DENV serotypes, irrespective of DENV serostatus. In addition to neutralizing antibodies (NAb) against the viral envelope (E) protein, there is increasing evidence for protective roles of cell-mediated and humoral responses against DENV nonstructural proteins, especially nonstructural protein 1 (NS1) [8].
NS1 is the only viral protein secreted from DENV-infected cells and plays several roles in viral replication and immune evasion [9]. NS1 can act as a viral toxin and contributes to pathogenesis through an endothelial cell-intrinsic route, where the endothelial glycocalyx is degraded by sialidases and the cathepsin L/heparanase pathway [10, 11], and a cytokine-dependent route where NS1 stimulates inflammatory cytokine production from immune cells [12]. DENV infection elicits NS1-specific antibodies, with antibody found in primary infection convalescent sera and in acute and convalescent phases during secondary infection [13–17]. No differences in anti-NS1 antibody titers have been observed between DF and DHF/DSS patients [13–17]; however, antibodies to specific NS1 epitopes are higher in patients with more severe dengue [18]. Furthermore, vaccination with NS1 protects mice from lethal vascular leak, and passive transfer of NS1-specific serum abrogates NS1-induced lethality in vivo [19]. The role of DENV NS1-specific immunity in protection mediated by vaccination in humans has not been investigated.
Takeda’s tetravalent dengue vaccine (TAK-003) consists of an attenuated DENV-2 virus backbone (TDV-2) and 3 chimeric viruses containing the premembrane/membrane and E protein genes of DENV-1, -3, and -4 genetically engineered into TDV-2 [20]. TAK-003 induced NAb responses and seroconversion to all 4 DENV serotypes in phase 1 and 2 studies and was generally safe and well tolerated in children and adults from dengue-endemic and nonendemic countries [21–24]. Here, we determined the magnitude and functionality of NS1-specific IgG responses elicited by TAK-003. Vaccination stimulated strong, sustained, and serotype cross-reactive TDV-2 NS1-specific IgG responses in DENV-naive TAK-003 recipients. Additionally, the DENV-2 NS1 IgG response protected against DENV-2 NS1-induced endothelial hyperpermeability and endothelial glycocalyx-like layer (EGL) degradation in vitro. Cross-reactive IgG also protected against DENV-1, -3, and -4 NS1-induced barrier dysfunction and correlated with the respective IgG concentrations. These results demonstrate that TAK-003 elicits both NAbs to viral structural proteins [25] and NS1-specific humoral responses [25] and that NS1-specific IgG responses can protect against NS1-mediated toxicity in vitro.
Takeda’s live-attenuated tetravalent dengue virus (DENV) vaccine elicits a strong and sustained antibody response against DENV-2 nonstructural protein 1 (NS1), which is cross-reactive against NS1 from DENV-1, -3, and -4 and is protective against DENV NS1-induced endothelial hyperpermeability.
METHODS
Ethics Statement
Study DEN-203 (ClinicalTrials.gov identifier: NCT01511250) was conducted in accordance with Institutional Review Board regulations in the US Code of Federal Regulations and all applicable local regulations. Participants gave written informed consent; children provided verbal or written assent and had written informed consent given by a parent or legal guardian, according to local regulations.
Cell Culture
Human pulmonary microvascular endothelial cell line HPMEC-ST1.6r (HPMEC) was kindly donated by Dr J.C. Kirkpatrick (Johannes Gutenberg University, Germany) and grown as previously described [11].
Recombinant NS1 Proteins
For transendothelial electrical resistance (TEER) assays and confocal microscopy, recombinant NS1 from DENV-1 (Nauru/Western Pacific/1974), DENV-2 (Thailand/16681/84), DENV-3 (Sri Lanka D3/H/IMTSSA-SRI/2000/1266), and DENV-4 (Dominica/814669/1981), greater than 95% purity and certified to be free of endotoxin contaminants, was produced in HEK293 cells by the Native Antigen Company. NS1 preparations were confirmed to be free of bacterial endotoxins using the Endpoint Chromogenic Limulus Amebocyte Lysate QCL-1000TM kit (Lonza) [11].
For enzyme-linked immunosorbent assays (ELISA), recombinant DENV-2 (Thailand/16681/84) NS1 from Abcam; and DENV-1 (Nauru/Western Pacific/1974), DENV-3 (Sri Lanka D3/H/IMTSSA-SRI/2000/1266), and DENV-4 (Dominica/814669/1981) NS1 from the Native Antigen Company were used. Purity and integrity of NS1 was verified by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and concentrations were determined using the bicinchoninic acid assay.
Serum Samples
Individual sera from the phase 2 DEN-203 clinical trial were used throughout this study. DEN-203 enrolled 360 participants to assess safety and immunogenicity of TAK-003 in healthy adults and children living in dengue-endemic countries and was conducted in 2 parts: Part 1—age descending (21–45, 12–20, 6–11, and 1.5–5 years); and Part 2—expansion, ages 1.5 to 11 years. Participants were randomized to receive either 0.5 mL subcutaneous injection of TAK-003 or placebo [24]. Serum samples were collected prevaccination (day 0) and at days 7, 14, 28, 90, 120, 180, 360, and 1080 postvaccination. NAb titers from microneutralization tests (MNT) performed on day 0 samples were used to classify the samples into DENV-naive or preimmune categories [23].
Control DENV-positive sera (Discovery Life Sciences) were tested for NS1-specific IgG levels and pooled to generate the reference standard/positive control (DLS pool). Negative control serum (Bioreclamation IVT) from DENV-negative individuals from nonendemic countries was pooled and tested for DENV reactivity and was negative by reverse transcription polymerase chain reaction, MNT, and binding ELISA.
Monoclonal Antibodies
Wheat germ agglutinin conjugated to Alexa Fluor 647 (WGA-A647, Molecular Probes) and Ab Heparan Sulfate, purified (clone F58-10E4, Amsbio) were used for staining sialic acid and heparan sulfate, respectively. The secondary detection antibody in confocal microscopy experiments was donkey anti-mouse IgM conjugated to Alexa Fluor 488 (Jackson).
Anti-NS1 IgG Enzyme-linked Immunosorbent Assay
Maxisorp 96-well flat bottom microtiter plates (NUNC) were coated with DENV NS1 (1.0 μg/mL) in carbonate/bicarbonate buffer pH 9.6 overnight at 4°C. Plates were washed with 0.01 M phosphate-buffered saline + 0.1% Tween 20 (PBS-T) and blocked with SuperBlock T20 Blocking Buffer (ThermoFisher Scientific) for 1 hour at 37°C. Plates were washed with PBS-T, then 4-fold serially diluted serum samples and controls were added and incubated for 60 minutes at 37°C. Plates were washed with PBS-T and incubated with peroxidase-conjugated goat anti-human IgG-gamma chain (Fitzgerald Inc.) in PBS-T for 60 minutes at 37°C. Plates were washed with PBS-T and color was developed with ABTS Peroxidase Substrate (SeraCare) for 15 minutes at room temperature, stopped with 1× ABTS Peroxidase Stop Solution (KPL), and optical density at 405 nm measured using a Spectramax 384 Plus/306200 plate reader (Molecular Devices) in conjunction with SoftMax Pro software version 7.0.3 (Molecular Devices). The concentration of anti-DENV NS1 IgG in serum samples was determined relative to the reference standard and is reported in relative ELISA units per milliliter (RU/mL).
TEER Assay
NS1-induced endothelial hyperpermeability was evaluated by measuring TEER of HPMEC grown on 24-well Transwell polycarbonate membranes (Transwell permeable support, 0.4 μM, 6.5 mm insert; Corning Inc.) as previously described [11, 19]. Briefly, TEER was measured in Ohms (Ω) every 3 hours following addition of NS1 and serum using an Epithelial Volt Ohm Meter with “chopstick” electrodes (World Precision Instruments). Thirty μL of culture supernatant was removed from the apical chamber and replaced with 30 μL of serum samples immediately preceding addition of DENV NS1 proteins. Untreated cells grown on Transwells were used as negative controls, and Transwells with medium alone were used for blank resistance measurements. Relative TEER represents a ratio of resistance values (Ω) as follows: (Ω experimental condition − Ω medium alone)/ (Ω nontreated endothelial cells − Ω medium alone). Twenty-four hours posttreatment, 50% of upper and lower chamber media was replaced by fresh medium.
Fluorescence Microscopy
Microscopy was performed as previously described [11]. For imaging experiments, HPMEC were grown on coverslips coated with 0.2% gelatin (Sigma). The distribution of sialic acid and heparan sulfate was examined on confluent HPMEC monolayers treated with DENV-2 NS1 (5 µg/mL) and TAK-003 serum or negative or positive control serum (30 µL each) and fixed with 4% paraformaldehyde at 6 hours posttreatment. Primary antibodies were incubated overnight at 4°C, and detection was performed using secondary antibodies conjugated to Alexa fluorophores (488 and 647) using a Zeiss LSM 710 Axio Observer inverted fluorescence microscope equipped with a 34-channel spectral detector. Images acquired using Zen 2010 software (Zeiss) were processed and analyzed with ImageJ [26]. All RGB images were converted to grayscale, then mean grayscale values and integrated density from selected areas were taken, along with adjacent background readings, and plotted as mean fluorescence intensity.
Statistical Analysis
Statistical analyses were performed and all graphs generated using GraphPad Prism 7.0 software. For NS1 IgG concentrations, 1-way ANOVA with Tukey or Sidak multiple comparison test was used. For correlation analyses, the net area under the curve (AUC) was taken from all curves in all TEER experiments using a baseline of Y = 1 while also considering peaks that went below the baseline. For each experiment, the AUC of NS1 + serum was subtracted from the AUC of NS1 alone, providing a reduction in relative TEER for each serum sample, which was then plotted against the log10 IgG concentration, and correlation analysis was performed. The Pearson correlation coefficient and 2-tailed P values are reported within the figures.
RESULTS
TAK-003 Induces a Significant NS1-Specific IgG Response in DENV-Naive Vaccine Recipients
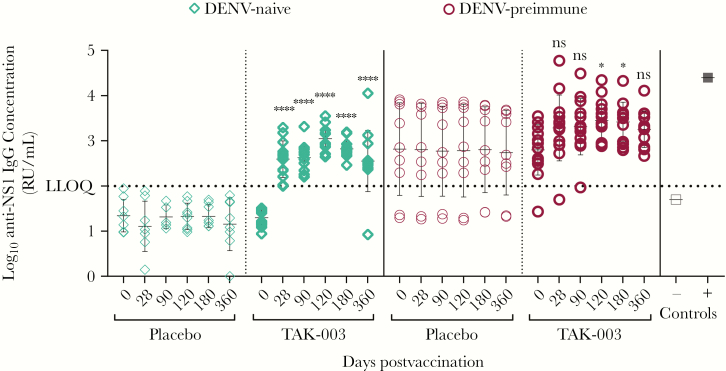
We used an indirect ELISA to measure pre- and postvaccination DENV-2 NS1-specific IgG response in DENV-naive and DENV-preimmune recipients of placebo or TAK-003. In DENV-naive recipients, TAK-003 elicited a significant increase in NS1-specific IgG at all time points postvaccination (Figure 1), compared to no NS1-specific IgG detected in DENV-naive placebo recipients. No significant differences were observed in NS1-specific IgG levels in the DENV-preimmune group between placebo or TAK-003 recipients across the study time course (Figure 1). The anti-NS1 IgG concentration in DENV-naive vaccinees was significantly increased after the first dose (day 0 vs 28) and boosted after the second dose (day 28 and 90 vs 120), and antibodies were sustained through day 360 (Figure 1 and Supplementary Table 1). In DENV-preimmune vaccinees, no significant differences were observed in NS1-specific IgG concentrations pre- and postvaccination through day 90. Anti-NS1 IgG levels increased after the second dose and remained above prevaccination level through day 360 (Figure 1 and Supplementary Table 1). Next, we compared anti-NS1 IgG concentrations between DENV-naive or preimmune TAK-003 recipients at matched time points pre- and postvaccination (Supplementary Figure 1 and Supplementary Table 2). After the first dose, NS1-specific IgG responses were higher in DENV-preimmune vaccinees than in DENV-naive vaccinees (Supplementary Figure 1); however, NS1-specific IgG levels were comparable after the second immunization (day 120 and 180) and then declined by day 360 postvaccination. After 2 vaccinations, NS1-specific IgG levels increased ≥4-fold in 38% of DENV-preimmune vaccine recipients compared to prevaccination levels. In contrast, NS1-specific IgG levels increased ≥4-fold in 100% of DENV-naive vaccine recipients after the second dose, compared to prevaccination levels (Supplementary Table 2).
Figure 1.
Serum NS1 IgG concentrations in recipients of TAK-003. Subject-matched serum samples from placebo or TAK-003 recipients that were either dengue virus (DENV)-naive or DENV-preimmune, obtained at different times pre- or postvaccination, were tested using the DENV-2 NS1 IgG indirect enzyme-linked immunosorbent assay. The OD405 values were plotted against log10 dilution factor. Dilution factors were interpolated for each sample at OD405 of 0.5, and concentration was calculated relative to the assigned EC50 of the reference standard/DENV-positive serum. Statistical analyses were performed using 1-way ANOVA, with Tukey multiple comparison. ****, P < .0001; *, P = .025; ns, not significant. Abbreviations: LLOQ, lower limit of quantitation.
The TAK-003-Induced Anti-NS1 IgG Response is Cross-Reactive With NS1 From DENV-1, -3, and -4
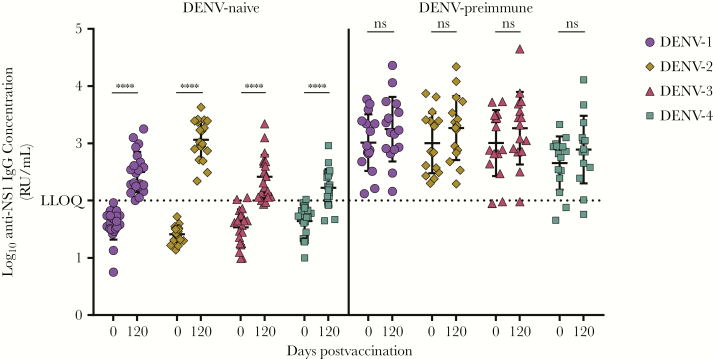
DENV infection elicits both serotype-specific and cross-reactive NS1-specific antibodies [13, 14, 27], and cross-reactive NS1-specific antibodies can confer partial protection against lethal DENV-2 infection in mice [19]. The NS1 component of TAK-003 is encoded by the attenuated DENV-2 backbone, and vaccination elicits IgG against DENV-2 NS1. To evaluate whether the TAK-003–elicited anti-DENV-2 NS1 IgG response was cross-reactive against NS1 from other serotypes, sera from DENV-naive and DENV-preimmune vaccinees were tested for IgG against recombinant DENV-1, -3, and -4 NS1. Vaccination elicited a significant increase in IgG against NS1 from other serotypes in DENV-naive subjects, whereas levels of NS1-specific IgG to the other serotypes were not significantly increased in DENV-preimmune subjects (Figure 2). Following vaccination of naive subjects, seroconversion rates (defined as IgG concentration increase >1.7 log10) to DENV-1, -3, and -4 were 100%, 85%, and 66%, respectively. Seroconversion rates in DENV-preimmune vaccinees against NS1 from all 4 DENV serotypes, pre- and postvaccination, were ≥88%.
Figure 2.
Cross-reactivity of the TDV-2 NS1 IgG response. Pre- and postvaccination (day 0 and day 120, respectively) serum samples from dengue virus (DENV)-naive or DENV-preimmune TAK-003 recipients were tested in the NS1 IgG indirect enzyme-linked immunosorbent assay using NS1 antigen from DENV-1, -2, -3, or -4. The concentration of NS1 IgG calculated relative to the DENV-positive control serum is plotted against times postvaccination for each serotype. Statistics were performed using 1-way ANOVA, with Sidak multiple comparison. ****, P < .0001; ns, not significant. Abbreviations: LLOQ: lower limit of quantitation
NS1-Specific IgG Responses in TAK-003–Vaccinated Individuals Abrogate DENV-2 NS1-Induced Endothelial Hyperpermeability In Vitro
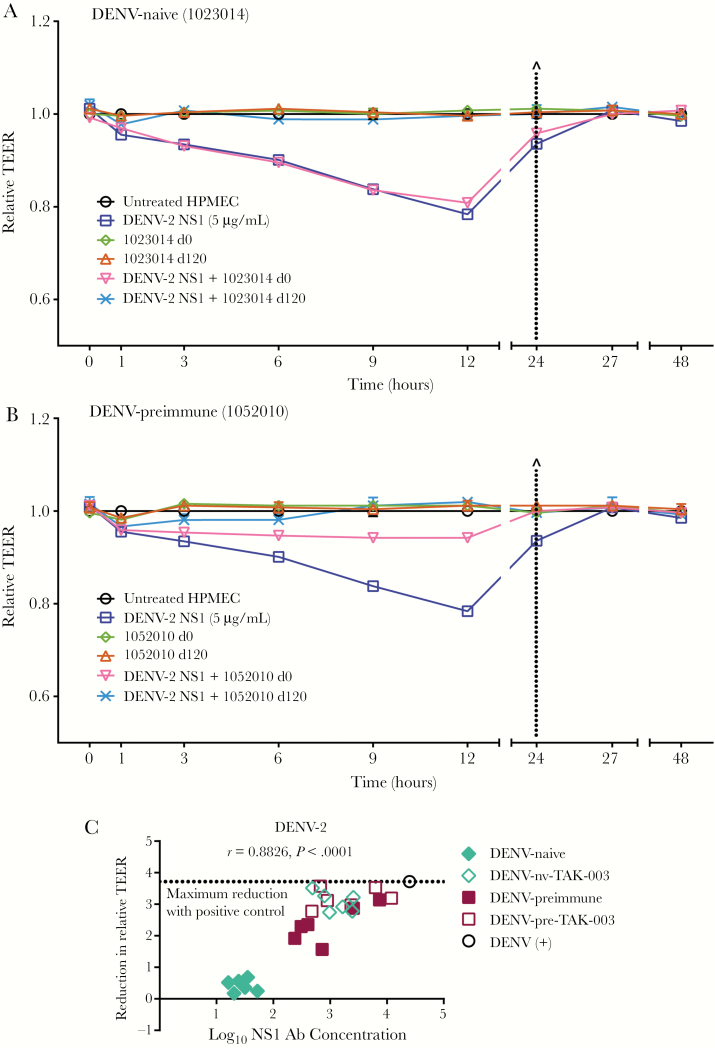
NS1 from all 4 DENV serotypes can induce hyperpermeability of HPMEC as measured by reduction in TEER [11], and this can be blocked using NS1-immune sera [19]. Sera from 12 subjects (6 DENV-naive, 6 DENV-preimmune) at day 0 prevaccination and day 120 postvaccination were evaluated by TEER to assess the effect of TAK-003–induced NS1-specific IgG responses on NS1-mediated endothelial hyperpermeability. DENV-naive prevaccination serum samples did not prevent NS1-mediated barrier dysfunction, but day 120 postvaccination samples from all naive vaccinees blocked decreases in TEER (Figure 3A and Supplementary Figure 3). Preimmune day 0 samples protected to varying degrees, while all day 120 postvaccination samples completely abrogated NS1-induced hyperpermeability (Figure 3B and Supplementary Figure 4). Pooled positive control serum also completely prevented DENV-2 NS1-induced hyperpermeability, while negative control serum did not (Supplementary Figure 2). The reduction in NS1-mediated hyperpermeability mediated by TAK-003 sera, measured by AUC from each TEER analysis, strongly correlated with NS1-specific antibody levels (r = 0.8826; P < .0001) (Figure 3C). Taken together, these results indicate that immunization with TAK-003 stimulates a protective anti-DENV-2 NS1 antibody response capable of blocking NS1-mediated hyperpermeability in vitro.
Figure 3.
Post-TAK-003 vaccination sera protect against dengue virus (DENV)-2 NS1-induced hyperpermeability, and protection correlates with IgG concentration. The effect of pre- or postvaccination sera on DENV-2 NS1-induced endothelial hyperpermeability was evaluated by TEER. Representative graphs are shown for (A) DENV-naive (subject ID: 1023014) and (B) DENV-preimmune (subject ID: 1052010) samples. HPMEC were grown on Transwell semipermeable membranes (0.4 µm pore size), and serum samples (30 µL) were added to the apical chamber in the presence or absence of 5 µg/mL DENV-2 NS1 (DENV-2 NS1, blue squares; day 0 serum alone, green diamonds; day 120 serum alone, red triangles; day 0 serum + DENV-2 NS1, inverted pink triangles; day 120 serum + DENV-2 NS1, light blue ×). Endothelial permeability was measured at indicated time points over 48 hours. Dotted line indicates change of medium. Relative TEER values from 1 independent experiment performed in duplicate are plotted. Error bars indicate SEM. C, Correlation analysis of 6 DENV-naive and 6 preimmune samples from both day 0 and day 120 (24 total samples). Area under the curve (AUC) values were calculated for each curve in each TEER experiment. The absolute reduction in relative TEER for each serum sample (A, B, and Supplementary Figures 3 and 4) was calculated by subtracting the AUC of NS1 + serum from the AUC of NS1 alone. Reduction was plotted against the log10 of IgG concentrations for each sample, and a correlation analysis was performed. The dotted line represents the reduction of TEER by DENV-positive (+) control serum. Naive subjects, day 0 (filled) and day 120 (open) diamonds; preimmune subjects, day 0 (filled) and day 120 (open) squares; positive control, open black circle. Abbreviations: Ab, antibody; DENV-nv-TAK-003, DENV-naive TAK-003 recipients; DENV-pre-TAK-003, DENV-preimmune TAK-003 recipients; HPMEC, human pulmonary microvascular endothelial cells; TEER, transendothelial electrical resistance.
TAK-003-Elicited Anti-NS1 IgG Responses Are Cross-Protective Against Endothelial Hyperpermeability Induced by NS1 From Other DENV Serotypes, and Protection Correlates With Cross-Reactive Antibody Concentration
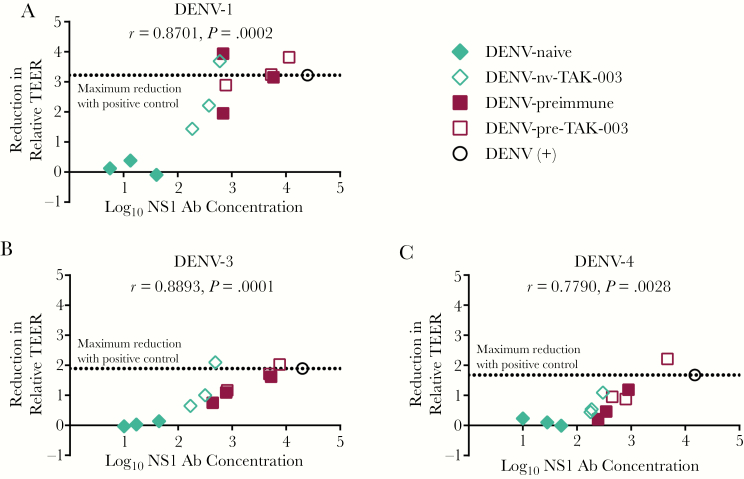
We evaluated whether the TAK-003-elicited, cross-reactive anti-DENV-2 NS1 IgG response was cross-protective against DENV-1, -3, and -4 NS1-induced endothelial hyperpermeability. We analyzed a subset of DENV-naive and preimmune day 0 and day 120 postvaccination samples against recombinant DENV-1, -3, and -4 NS1 using TEER and found that sera from TAK-003 recipients could cross-protect against NS1 from other DENV serotypes (Supplementary Figures 6, 7, and 8). DENV-positive control serum fully blocked hyperpermeability induced by DENV-1–4 NS1 while negative control serum did not. As observed with the anti-DENV-2 NS1 IgG response, the ability to prevent DENV-1, -3, and -4 NS1-induced hyperpermeability correlated with the magnitude of cross-reactive NS1-specific antibodies in each sample (Figure 4). Sera from DENV-preimmune vaccinees reduced DENV-1, -3, and -4 NS1-mediated hyperpermeability, both pre- and postvaccination (Supplementary Figures 6, 7, and 8). These results suggest that vaccination of naive recipients with TAK-003 elicits a protective, cross-reactive, NS1-specific IgG response capable of blocking NS1-mediated hyperpermeability.
Figure 4.
A–C, Post-TAK-003 vaccination sera cross-protect against dengue virus (DENV)-1, -3, and -4 NS1-induced hyperpermeability, and protection correlates with IgG concentration. The effect of pre- or postvaccination sera on DENV-1, -3, or -4 NS1-induced endothelial hyperpermeability was evaluated by TEER. Correlation analysis of 3 DENV-naive and 3 preimmune samples pre- (day 0) and post- (day 120) vaccination was performed as follows. Area under the curve (AUC) values were calculated for each curve in each TEER experiment. The absolute reduction in relative TEER for each serum sample (A, B, and Supplementary Figures 6, 7, and 8) was calculated by subtracting the AUC of NS1 + serum from the AUC of NS1 alone. Reduction was plotted against the log10 of IgG concentrations for each sample, and a correlation analysis was performed. The dotted line represents the reduction of TEER by DENV-positive (+) control serum. Naive subjects, day 0 (filled) and day 120 (open) diamonds; preimmune subjects, day 0 (filled) and day 120 (open) squares; positive control, open black circle. Abbreviations: Ab, antibody; DENV-nv-TAK-003, DENV-naive TAK-003 recipients; DENV-pre-TAK-003, DENV-pre-immune TAK-003 recipients; TEER, transendothelial electrical resistance.
The NS1-Specific IgG Response to TAK-003 is Comparable to DENV Infection
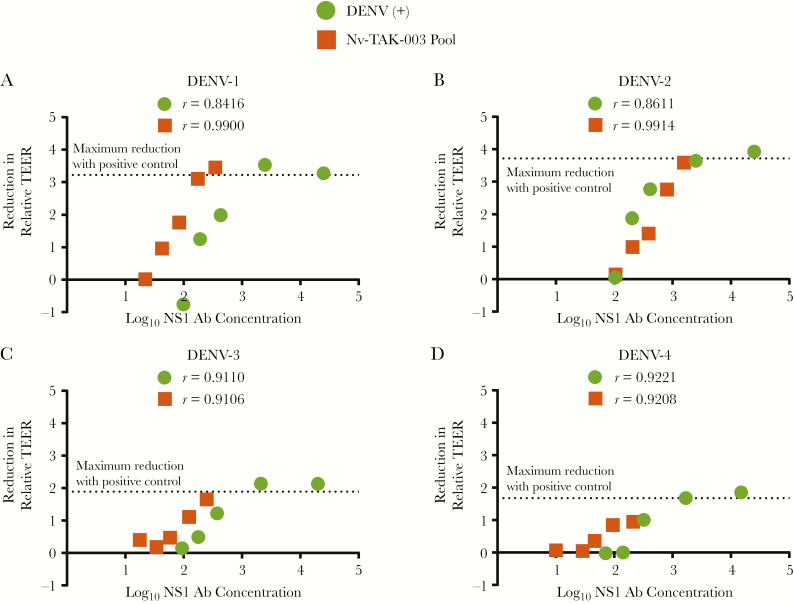
Serial dilutions of pooled serum from DENV-infected individuals or DENV-naive vaccinees at day 120 postvaccination were evaluated by TEER and ELISA, and the reduction in relative TEER was correlated with NS1 IgG concentrations against each DENV serotype. There was a dose-dependent correlation between TEER reduction and anti-NS1 IgG level against all DENV serotypes from both DENV-naive vaccinees and DENV-infected serum pools (Figure 5). An anti-DENV-2 NS1 IgG concentration of approximately 2000 relative ELISA units correlated with maximal protection against NS1-mediated endothelial hyperpermeability across DENV-1–4 for both postinfection and postvaccination sera, and the correlation curves almost completely overlapped for the TAK-003-vaccinated and DENV-infected sera for DENV-2 (Figure 5B). The highest NS1 IgG concentrations in DENV-naive vaccinees completely inhibited hyperpermeability mediated by DENV-1, -2, and -3 NS1 (Figure 5A, 5C, and 5D), comparable to the immune response from DENV-infected individuals. However, the correlation curves were offset between vaccinated and infected serum in the cross-reactive response to DENV-1, -3, and -4 NS1. Additionally, DENV-4 NS1-mediated endothelial hyperpermeability was not completely inhibited by postvaccination serum from DENV-naive vaccinees. Therefore, cross-reactive antibodies may be qualitatively different after infection and vaccination.
Figure 5.
Correlation of cross-reactive magnitude and functionality: TAK-003-vaccinated versus dengue virus (DENV)-infected sera. Postvaccination (day 120) serum from 4 DENV-naive recipients of TAK-003 were pooled (Nv-TAK-003 pool). DENV (+) serum or Nv-TAK-003 pool serum (neat and 2-fold serially diluted sera) were evaluated by (A) DENV-1, (B) -2, (C) -3, and (D) -4 NS1 TEER and enzyme-linked immunosorbent assays, and reduction in relative TEER was correlated with NS1 IgG concentration against each DENV serotype. A dose-dependent correlation was observed between serum-driven protection in TEER and corresponding NS1 IgG concentration across all 4 serotypes, in both DENV-naive TAK-003 recipients and DENV-infected serum pools. The dotted line represents the reduction of TEER by neat DENV-positive (+) control serum. r values are indicated for each serum type, across each serotype. Abbreviations: Ab, antibody; TEER, transendothelial electrical resistance.
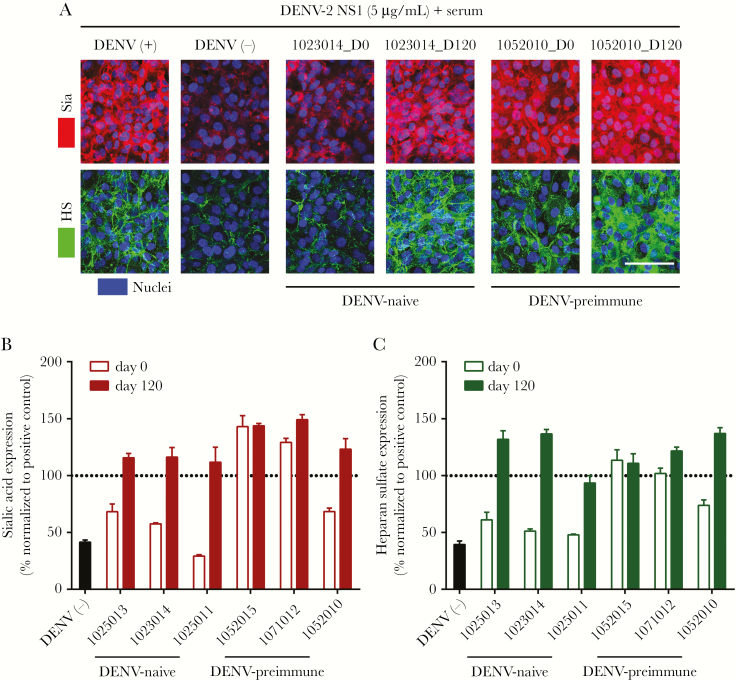
Post-TAK-003 Vaccination Serum Prevents DENV-2 NS1-Induced Degradation of Glycocalyx Components In Vitro
The endothelial glycocalyx is a major determinant of endothelial barrier function. We previously demonstrated that NS1 from DENV-1–4 triggers degradation of endothelial cell surface glycocalyx components in vitro [10, 11]. Using a subset of serum samples evaluated by TEER, we used confocal microscopy to visualize the effect of pre- and postvaccination sera from DENV-naive and preimmune subjects on NS1-induced disruption of sialic acid and heparan sulfate, 2 key components of the glycocalyx (Figure 6 and Supplementary Figure 5). Positive control serum was used as a baseline for protection, and negative control serum represented maximum NS1-mediated disruption. Sialic acid and heparan sulfate expression levels in the presence of NS1 and control or TAK-003 recipient serum were imaged (Figure 6A) and quantitated (Figure 6B and 6C). Reflecting TEER results, we found that day 0 sera from DENV-naive patients had no substantial protective effect, while DENV-naive day 120 postvaccination sera completely blocked NS1-induced degradation of both sialic acid and heparan sulfate (Figure 6A–6C and Supplementary Figure 5). Similarly, day 0 samples from DENV-preimmune patients exhibited varying levels of protection, and preimmune day 120 postvaccination sera were completely protective (Figure 6A–6C and Supplementary Figure 5). Taken together, these results suggest that the NS1-specific IgG response stimulated by TAK-003 can protect against NS1-induced hyperpermeability by preventing the degradation of key glycocalyx components.
Figure 6.
Sera from TAK-003-vaccinated patients prevents dengue virus (DENV)-2 NS1-induced sialic acid and heparan sulfate degradation on human pulmonary microvascular endothelial cells (HPMEC). The effect of DENV-naive (1023014) or preimmune (1052010) sera on DENV-2 NS1-induced disruption of sialic acid (Sia) and heparan sulfate (HS). A, The integrity of the endothelial glycocalyx-like layer on HPMEC was assessed by the surface expression of sialic acid (red, top row) and heparan sulfate surface expression (green, bottom row) at 6 hours posttreatment with serum (as indicated) + DENV-2 NS1 (5 µg/mL) at 37°C, as visualized via confocal microscopy. Nuclei were stained with Hoechst (blue). Images are representative of 1 independent experiment performed in duplicate (×20; scale bar, 50 μm). B and C, Quantification of mean fluorescence intensity (MFI) of (B) sialic acid and (C) heparan sulfate expression from (A) and Supplementary Figure 5 from 1 independent experiment. Values normalized to MFI from the NS1 + positive control serum group (represented by dotted line at 100%) and expressed as percentage of control. Error bars indicate SEM.
DISCUSSION
Several lines of evidence highlight the importance of humoral and cell-mediated immune responses to nonstructural proteins, including NS1, in protection elicited by dengue vaccines [28]. The currently licensed live-attenuated dengue vaccine (Dengvaxia) does not include DENV NS1 [29]; live-attenuated DENV-based vaccine candidates, including TAK-003, encode DENV NS1. While TAK-003 elicits cross-reactive NS1-specific CD8+ T-cell responses [30], the humoral response to NS1 following vaccination had not been previously characterized.
Here, we evaluated the magnitude and functionality of the NS1-specific IgG response elicited by TAK-003 and found that TAK-003 vaccination stimulates a robust anti-NS1 IgG response in DENV-naive vaccinees. Although levels of anti-NS1 IgG detected in DENV-naive vaccinees was significantly lower than levels in DENV-preimmune vaccinees after the first TAK-003 dose, NS1-specific IgG levels were comparable between the 2 groups after the second vaccine dose and persisted through 1 year postvaccination. Vaccination had a greater effect on NS1-specific IgG responses across all postvaccination time points in DENV-naive compared to DENV-preimmune recipients, likely due to high levels of anti-NS1 antibodies in many preimmune subjects prior to vaccination.
NS1 is the site of one of the attenuating mutations (an invariant glycine at position 53 to aspartate) in the DENV-2 backbone used for TAK-003 [31]. Using a panel of sera with high, medium, and low NS1-specific IgG concentrations, we observed no differences in ELISA concentrations to recombinant wild-type DENV-2 NS1 and TAK-003 NS1 encoding G53D (data not shown).
TAK-003 is a tetravalent formulation, with structural proteins from DENV-1–4, eliciting NAb responses against each serotype. The attenuated backbone is derived from DENV-2; thus, vaccination elicits IgG to DENV-2 NS1 in both DENV-naive and DENV-preimmune TAK-003 recipients, which cross-reacts with NS1 from DENV-1, -3, and -4. Cross-reactivity of anti-DENV-2 NS1 IgG in DENV-naive recipients was highest to DENV-1, followed by DENV-3 and DENV-4. Postvaccination sera from DENV-preimmune individuals were similarly cross-reactive across DENV-1–4. This trend may reflect inherent differences between cross-reactive antibodies in response to multiple infections in the preimmune group versus the first exposure to DENV NS1 postvaccination in naive subjects, especially because amino acid identity and homology between TDV-2 and DENV-1, -3, and -4 (Supplementary Table 3) does not exactly mirror the differences in anti-NS1 IgG cross-reactivity across the serotypes in naive vaccinees.
Recently, substantial progress has been made towards understanding the role of NS1 in dengue pathogenesis, and evidence suggests that NS1 is a mediator of vascular leak during DENV infection. NS1 has previously been explored as a vaccine candidate, and both NS1 recombinant protein and DNA vaccines are highly immunogenic and protective in mouse models [19, 32–34]. Naive mice that received adjuvanted recombinant NS1 or passive transfer of NS1-immune mouse sera or NS1-specific monoclonal antibodies (MAbs) were protected against DENV vascular leak disease, likely by preventing NS1-mediated pathogenesis and/or mediating lysis of DENV-infected cells [19, 35, 36]. Additionally, several immunodominant B cell NS1 epitopes conserved across DENV-1–4 have been identified in DENV-infected mice [37], suggesting that an NS1 subunit or live DENV vaccine may protect against other DENV serotypes. We found that the TDV-2 NS1 IgG response in both DENV-naive and preimmune TAK-003 recipients is functional as it protects against DENV-2 NS1-induced endothelial hyperpermeability and EGL disruption, and this response correlates with the anti-TDV-2 NS1 antibody concentration. Blocking of DENV-2 NS1-mediated endothelial hyperpermeability by serum from DENV-naive vaccinees, DENV-preimmune vaccinees, or DENV-infected individuals was similar; however, the correlation trends varied among DENV serotypes. This suggests potential mechanistic differences in cross-reactivity driven by tetravalent vaccination versus natural infection. Comparison of the quality and quantity of the NS1-specific antibody repertoire after vaccination and natural infection could identify determinants of DENV-2–specific and cross-reactive NS1 immune responses elicited by vaccination, and this is the focus of future studies.
Previous work has shown that anti-NS1 antibodies can activate complement (C′), most likely to lyse DENV-infected cells [38]. Though this may eliminate infected cells [39], it could also contribute to pathogenesis if anti-NS1 antibodies recognize surface-bound soluble NS1 on noninfected cells, particularly endothelial cells. As such, further investigation into the repertoire of anti-NS1 antibodies elicited by TAK-003 vaccination in the context of complement activation is important, especially in light of recent work that identified an anti-NS1 MAb that triggers complement-mediated lysis of DENV-infected cells, presumably through antibody-dependent cell-mediated cytotoxicity [40].
Because NS1 plays multiple roles in pathogenesis, including activation of immune cells leading to inflammatory cytokine production [12] and complement interactions [39, 41], it will be interesting to evaluate the effects of TAK-003 vaccination sera on other aspects of NS1 pathogenesis in vitro and in vivo. Overall, this is the first indication that vaccination stimulates an NS1-specific IgG response that is cross-reactive, functional, and may contribute to protection against severe dengue disease.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the clinical study investigators and volunteers, and Derek Wallace, Gilad Gordon, and Dan Stinchcomb for clinical study design and conduct, Kelley Moss and Jen Kilbury for clinical sample logistics, and Ralph Braun for assistance in anti-NS1 IgG ELISA method development.
Financial support. This work was supported by Takeda Vaccines, Inc. and National Institutes of Health, National Institute of Allergy and Infectious Diseases (grant number R01 AI24493 to E. H.).
Potential conflicts of interest. M. S., H. W., Y. K., M. E., and H. D. are employees of Takeda Vaccines, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Pan American Dengue Research Network Meeting, Galveston, Texas, 9–11 April 2018 and American Society for Tropical Medicine and Hygiene Annual Meeting, New Orleans, Louisiana, 28 October–1 November 2018.
References
- 1. Bhatt S, Gething PW, Brady OJ, et al. . The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention, and control. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- 3. Capeding MR, Tran NH, Hadinegoro SR, et al. ; CYD14 Study Group Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014; 384:1358–65. [DOI] [PubMed] [Google Scholar]
- 4. Hadinegoro SR, Arredondo-García JL, Capeding MR, et al. ; CYD-TDV Dengue Vaccine Working Group Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195–206. [DOI] [PubMed] [Google Scholar]
- 5. Villar L, Dayan GH, Arredondo-García JL, et al. ; CYD15 Study Group Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113–23. [DOI] [PubMed] [Google Scholar]
- 6. Halstead SB. Licensed dengue vaccine: public health conundrum and scientific challenge. Am J Trop Med Hyg 2016; 95:741–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katzelnick LC, Gresh L, Halloran ME, et al. . Antibody-dependent enhancement of severe dengue disease in humans. Science 2017; 358:929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glasner DR, Puerta-Guardo H, Beatty PR, Harris E. The good, the bad, and the shocking: the multiple roles of dengue virus nonstructural protein 1 in protection and pathogenesis. Annu Rev Virol 2018; 5:227–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res 2013; 98:192–208. [DOI] [PubMed] [Google Scholar]
- 10. Glasner DR, Ratnasiri K, Puerta-Guardo H, Espinosa DA, Beatty PR, Harris E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog 2017; 13:e1006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puerta-Guardo H, Glasner DR, Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog 2016; 12:e1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Modhiran N, Watterson D, Muller DA, et al. . Dengue virus NS1 protein activates cells via Toll-like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 2015; 7:304ra142. [DOI] [PubMed] [Google Scholar]
- 13. Shu PY, Chen LK, Chang SF, et al. . Dengue NS1-specific antibody responses: isotype distribution and serotyping in patients with dengue fever and dengue hemorrhagic fever. J Med Virol 2000; 62:224–32. [DOI] [PubMed] [Google Scholar]
- 14. Hertz T, Beatty PR, MacMillen Z, Killingbeck SS, Wang C, Harris E. Antibody epitopes identified in critical regions of dengue virus nonstructural 1 protein in mouse vaccination and natural human infections. J Immunol 2017; 198:4025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuno G, Vorndam AV, Gubler DJ, Gómez I. Study of anti-dengue NS1 antibody by western blot. J Med Virol 1990; 32:102–8. [DOI] [PubMed] [Google Scholar]
- 16. Churdboonchart V, Bhamarapravati N, Peampramprecha S, Sirinavin S. Antibodies against dengue viral proteins in primary and secondary dengue hemorrhagic fever. Am J Trop Med Hyg 1991; 44:481–93. [DOI] [PubMed] [Google Scholar]
- 17. Valdés K, Alvarez M, Pupo M, Vázquez S, Rodríguez R, Guzmán MG. Human dengue antibodies against structural and nonstructural proteins. Clin Diagn Lab Immunol 2000; 7:856–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai YC, Chuang YC, Liu CC, et al. . Antibodies against modified NS1 wing domain peptide protect against dengue virus infection. Sci Rep 2017; 7:6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 2015; 7:304ra141. [DOI] [PubMed] [Google Scholar]
- 20. Osorio JE, Partidos CD, Wallace D, Stinchcomb DT. Development of a recombinant, chimeric tetravalent dengue vaccine candidate. Vaccine 2015; 33:7112–20. [DOI] [PubMed] [Google Scholar]
- 21. George SL, Wong MA, Dube TJ, et al. . Safety and immunogenicity of a live attenuated tetravalent dengue vaccine candidate in flavivirus-naive adults: a randomized, double-blinded phase 1 clinical trial. J Infect Dis 2015; 212:1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Osorio JE, Velez ID, Thomson C, et al. . Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rupp R, Luckasen GJ, Kirstein JL, et al. . Safety and immunogenicity of different doses and schedules of a live attenuated tetravalent dengue vaccine (TDV) in healthy adults: a phase 1b randomized study. Vaccine 2015; 33:6351–9. [DOI] [PubMed] [Google Scholar]
- 24. Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, et al. . Safety and immunogenicity of a tetravalent dengue vaccine candidate in healthy children and adults in dengue-endemic regions: a randomized, placebo-controlled phase 2 study. J Infect Dis 2016; 213:1562–72. [DOI] [PubMed] [Google Scholar]
- 25. Swanstrom JA, Henein S, Plante JA, et al. . Analyzing the human serum antibody responses to a live attenuated tetravalent dengue vaccine candidate. J Infect Dis 2018; 217:1932–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shu PY, Chen LK, Chang SF, et al. . Potential application of nonstructural protein NS1 serotype-specific immunoglobulin G enzyme-linked immunosorbent assay in the seroepidemiologic study of dengue virus infection: correlation of results with those of the plaque reduction neutralization test. J Clin Microbiol 2002; 40:1840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halstead SB. Which dengue vaccine approach is the most promising, and should we be concerned about enhanced disease after vaccination? There is only one true winner. Cold Spring Harb Perspect Biol 2018; 10:pii: a030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011; 29:7229–41. [DOI] [PubMed] [Google Scholar]
- 30. Chu H, George SL, Stinchcomb DT, Osorio JE, Partidos CD. CD8+ T-cell responses in flavivirus-naive individuals following immunization with a live-attenuated tetravalent dengue vaccine candidate. J Infect Dis 2015; 212:1618–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butrapet S, Huang CY, Pierro DJ, Bhamarapravati N, Gubler DJ, Kinney RM. Attenuation markers of a candidate dengue type 2 vaccine virus, strain 16681 (PDK-53), are defined by mutations in the 5’ noncoding region and nonstructural proteins 1 and 3. J Virol 2000; 74:3011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Falgout B, Bray M, Schlesinger JJ, Lai CJ. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J Virol 1990; 64:4356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amorim JH, Diniz MO, Cariri FA, et al. . Protective immunity to DENV2 after immunization with a recombinant NS1 protein using a genetically detoxified heat-labile toxin as an adjuvant. Vaccine 2012; 30:837–45. [DOI] [PubMed] [Google Scholar]
- 34. Lu H, Xu XF, Gao N, Fan DY, Wang J, An J. Preliminary evaluation of DNA vaccine candidates encoding dengue-2 prM/E and NS1: their immunity and protective efficacy in mice. Mol Immunol 2013; 54:109–14. [DOI] [PubMed] [Google Scholar]
- 35. Henchal EA, Henchal LS, Schlesinger JJ. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J Gen Virol 1988; 69:2101–7. [DOI] [PubMed] [Google Scholar]
- 36. Wan SW, Lu YT, Huang CH, et al. . Protection against dengue virus infection in mice by administration of antibodies against modified nonstructural protein 1. PLoS One 2014; 9:e92495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen Y, Pan Y, Guo Y, Qiu L, Ding X, Che X. Comprehensive mapping of immunodominant and conserved serotype- and group-specific B-cell epitopes of nonstructural protein 1 from dengue virus type 1. Virology 2010; 398:290–8. [DOI] [PubMed] [Google Scholar]
- 38. Schlesinger JJ, Brandriss MW, Walsh EE. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus non-structural glycoprotein NS1. J Gen Virol 1987; 68:853–7. [DOI] [PubMed] [Google Scholar]
- 39. Avirutnan P, Punyadee N, Noisakran S, et al. . Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 2006; 193:1078–88. [DOI] [PubMed] [Google Scholar]
- 40. Wan SW, Chen PW, Chen CY, et al. . Therapeutic effects of monoclonal antibody against dengue virus NS1 in a STAT1 knockout mouse model of dengue infection. J Immunol 2017; 199:2834–44. [DOI] [PubMed] [Google Scholar]
- 41. Nascimento EJ, Silva AM, Cordeiro MT, et al. . Alternative complement pathway deregulation is correlated with dengue severity. PLoS One 2009; 4:e6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.