Abstract
Interleukin 10 (IL-10) is an anti-inflammatory cytokine that may be protective against coronary atherosclerosis. In an observational study of persons with human immunodeficiency virus (PWH) and uninfected controls, IL-10 was measured in serum samples by means of enzyme-linked immunosorbent assay, and coronary atherosclerosis was assessed using computed tomographic angiography. Among PWH, a 10-fold decrease in IL-10 was associated with a 2.6-fold increase in the odds of coronary plaque (P = .01), after controlling for traditional and nontraditional cardiovascular risk factors. IL-10 was also inversely associated with total coronary plaque (ρ = −0.19; P = .02) and noncalcified coronary plaque (ρ = −0.24; P = .004). Our findings suggest a role for IL-10 in mitigating atherosclerosis in PWH.
Clinical Trials Registration. NCT00455793
Keywords: IL-10, HIV, atherosclerosis, Inflammation
Immune factors that may be atheroprotective in human immunodeficiency virus (HIV) have not been well identified. Our observational study showed an inverse association between anti-inflammatory interleukin 10 and coronary plaque, suggesting that interleukin 10 may help mitigate atherosclerosis in HIV.
(See the Brief Report by Hoel et al, on pages 506–9 and see the Editorial commentary by Currier and Hsue, on pages 495–7.)
Human immunodeficiency virus (HIV) infection is a state of chronic immune activation that occurs as a result of increased intestinal permeability, coinfections, and persistent viremia. In addition to traditional risk factors, inflammation has been increasingly recognized as a key driver of myocardial infarction and coronary atherosclerosis. Thus, it follows that the burden of coronary plaque and the incidence of myocardial infarction are higher in persons with HIV (PWH) than in the general population [1].
There have been intensive efforts to identify novel anti-inflammatory strategies to mitigate plaque formation and progression in both PWH and the general population. As proof of principle, in the general population, the interleukin 1β monoclonal antibody canakinumab has been shown to be effective in the secondary prevention of cardiovascular events [2]. However, in PWH, such strategies must be carefully evaluated because a robust immune response is necessary to maintain virologic suppression. Although proinflammatory markers associated with increased coronary plaque have been well studied in PWH [1, 3], to our knowledge there is a scarcity of evidence surrounding immune factors that may be atheroprotective in this group.
Interleukin 10 (IL-10) is an anti-inflammatory cytokine that is secreted in response to systemic inflammation by monocytes, macrophages, T cells, and dendritic cells [4]. In healthy individuals, IL-10 plays a critical role in limiting the response of the immune system and preventing harm to the host tissue. Given that atherosclerosis is driven by chronic immune activation, the anti-inflammatory nature of IL-10 may confer this cytokine with antiatherogenic properties. Indeed, numerous murine models of atherosclerosis have consistently demonstrated antiatherogenic effects of increased IL-10 expression [5].
Although IL-10 can be helpful in blunting the longstanding consequences of immune activation, it has also been implicated in HIV viral progression and evasion from host immunity. Several in vitro studies in human cell lines have demonstrated that blockade of IL-10 can improve the immune response to HIV [4]. While these studies are varied, in general, increased IL-10 expression is likely to be harmful to HIV virologic control.
In the current study, we examined relationships between IL-10, coronary atherosclerosis, and immune parameters in the setting of chronic HIV infection. We hypothesized that higher levels of IL-10 would be associated with lower coronary plaque prevalence and burden, but also with impaired HIV-related immune indices, including CD4+ T-cell count. We compared our findings in PWH with those in a similar group of uninfected controls.
METHODS
Study Design
This study reports on new analyses from a previously performed observational study of men and women with HIV infection (and comparable HIV-uninfected controls) to assess coronary plaque [3]. Participants between 18 and 65 years of age were recruited from Boston community centers and infectious disease clinics. A total of 144 PWH and 66 HIV-uninfected controls were included in the current analysis, all of whom were asymptomatic, had no known cardiovascular disease, and had measures of coronary plaque and IL-10 available. Controls were recruited from the same communities as the HIV group.
Levels of IL-10, high-sensitivity C-reactive protein (hsCRP), high-sensitivity interleukin 6 (hsIL-6), C-C motif chemokine ligand 2 (CCL2, also known as monocyte chemoattractant protein-1), and soluble CD163 (sCD163) were measured in accordance with the manufacturer’s instructions (Invitrogen for IL-10, Roche Diagnostics for hsCRP, R&D Systems for CCL2 and hsIL-6, and Trillium Diagnostics for sCD163). IL-10 concentrations were measured in picograms per milliliter, then log10-transformed. Quantification of coronary plaque and plaque characteristics were obtained by coronary computed tomography angiography, as described elsewhere [3]. CD4+ and CD8+ T-cell counts were assessed by means of flow cytometry. HIV-1 RNA levels was determined by means of ultrasensitive real-time polymerase chain reaction in the Massachusetts General Hospital clinical laboratory (Roche Diagnostics). Values below the detection limit of the assay were reported as being 1 copy/mL below the threshold. HIV testing was performed using enzyme-linked immunosorbent assay and confirmed by means of Western blot in HIV-uninfected participants. All participants provided informed consent before enrollment. This study was approved by the institutional review boards of Massachusetts General Hospital and the Massachusetts Institute of Technology.
Statistical Analysis
Two-group comparisons of continuous, normally distributed variables were analyzed using the Student's t test. Nonnormally distributed variables were analyzed using the Wilcoxon rank-sum test or analyzed after log transformation using the Student's t test. Univariate correlations for relationships with coronary plaque parameters were assessed using the Spearman ρ correlation coefficient because plaque measurements were not normally distributed. Logistic regression analysis was used to assess the relationship of the binary variable of the presence of plaque in relation to IL-10 and other variables. Multivariable logistic regression analyses were performed to control for biologically relevant variables related to HIV infection, inflammation, and traditional cardiovascular risk factors. Although our main analyses included all PWH, we also performed sensitivity analyses. One sensitivity analysis was limited to virally suppressed individuals using antiretroviral therapy (ART), and another excluded individuals using statin therapy. Continuous variables are expressed as means with standard deviations (SDs) unless otherwise specified. Statistical significance was defined as a P value <.05. Statistical analyses were performed using SAS JMP Pro software, version 13.0.
RESULTS
Clinical Characteristics of Study Participants
The PWH and HIV-uninfected control groups were similar in age (mean [SD], 46.8 [7.0] vs 45.6 [7.0] years, respectively; P = .26) and sex (70% vs 62% male; P = .25). Among PWH, the mean (SD) duration of known HIV infection was 14.0 (6.3) years, and 96% of participants were currently using ART. A total of 82% of participants had undetectable HIV RNA. There was no difference in log10 IL-10 values between PWH and HIV-uninfected controls (mean [SD], 0.47 [0.56] vs 0.63 [0.76]; P = .15) (Supplementary Table 1).
IL-10 and Coronary Plaque in PWH
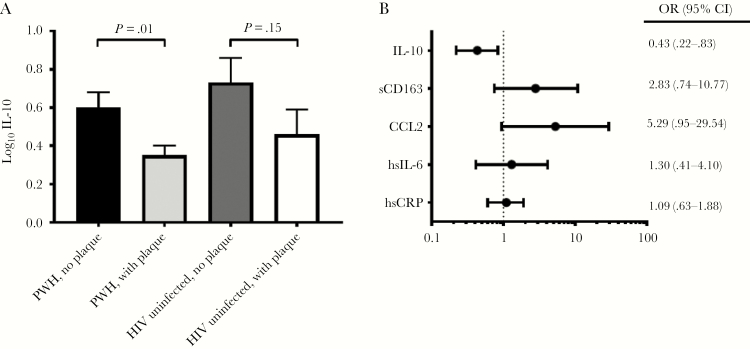
Among PWH, log10 IL-10 was significantly lower in individuals with coronary plaque relative to those without coronary plaque (mean [SD], 0.35 [0.41] vs 0.60 [0.68]; P = .01) (Figure 1A and Supplementary Table 2). Furthermore, a 10-fold decrease in IL-10 levels in PWH was associated with a 2.3-fold increase in the odds of having coronary segments with plaque (P = .007). This finding contrasts with relationships seen between markers associated with increased inflammation (sCD163, CCL2, hsIL-6, and hsCRP) and coronary plaque, in which higher levels tended to correspond to a greater plaque prevalence and burden (Table 1 and Figure 1B). Among PWH, IL-10 inversely correlated with the total number of coronary segments with any plaque (ρ = ˗0.19; P = .02) and the number of coronary segments with noncalcified plaque (ρ = ˗0.24; P = .004). In contrast, IL-10 was not associated with the number of segments with calcified plaque (ρ = ˗0.01; P = .90) (Table 1).
Figure 1.
Relationships of anti-inflammatory interleukin 10 (IL-10) and proinflammatory markers with coronary plaque. A, IL-10 levels among persons with human immunodeficiency virus (HIV) (PWH) and HIV-uninfected controls, with or without coronary plaque. IL-10 concentrations were measured in picograms per milliliter, then log10-transformed. Shaded bars represent means, and error bars standard errors of the mean. B, Odds ratios (OR) for the presence of plaque for every 10-fold increase in the inflammatory marker of interest among all PWH, with 95% confidence intervals (CIs). Abbreviations: CCL2, C-C motif chemokine ligand 2; hsCRP, high-sensitivity C-reactive protein; hsIL-6, high-sensitivity interleukin 6; sCD163, soluble CD163.
Table 1.
Relationships Between Anti-inflammatory and Proinflammatory Markers and Coronary Plaque in Persons With Human Immunodeficiency Virus
| All PWH (n = 144) | Virally Suppressed PWH Receiving ART (n = 120) | |||
|---|---|---|---|---|
| Relationships of Anti-inflammatory and Proinflammatory Markers with Number of Coronary Segments Containing Plaque | Spearman ρ | P Value | Spearman ρ | P Value |
| Anti-inflammatory markers | ||||
| IL-10 | ||||
| Noncalcified plaque | −0.24 | .004a | −0.20 | .03a |
| Calcified plaque | −0.01 | .90 | −0.01 | .90 |
| Total plaque | −0.19 | .02a | −0.18 | .06 |
| Proinflammatory markers | ||||
| sCD163 | ||||
| Noncalcified plaque | 0.19 | .02a | 0.24 | .009a |
| Calcified plaque | 0.08 | .35 | 0.06 | .50 |
| Total plaque | 0.11 | .17 | 0.18 | .06 |
| CCL2 | ||||
| Noncalcified plaque | 0.10 | .23 | 0.11 | .24 |
| Calcified plaque | 0.12 | .16 | 0.10 | .27 |
| Total plaque | 0.22 | .01a | 0.21 | .02a |
| hsIL-6 | ||||
| Noncalcified plaque | 0.09 | .31 | 0.18 | .07 |
| Calcified plaque | ˗0.06 | .52 | −0.03 | .77 |
| Total plaque | 0.06 | .51 | 0.18 | .07 |
| hsCRP | ||||
| Noncalcified plaque | −0.06 | .51 | 0.02 | .86 |
| Calcified plaque | −0.04 | .67 | 0.002 | .98 |
| Total plaque | 0.01 | .88 | 0.09 | .33 |
Abbreviations: ART, antiretroviral therapy; CCL2, C-C motif chemokine ligand 2; hsCRP, high-sensitivity C-reactive protein; hsIL-6, high-sensitivity interleukin 6; IL-10, interleukin 10; PWH, persons with human immunodeficiency virus; sCD163, soluble CD163.
aSignificant at P <.05.
IL-10 and Coronary Plaque in HIV-Uninfected Controls
Among HIV-uninfected controls, log10 IL-10 values did not differ significantly between individuals with and those without plaque (mean [SD], 0.46 [0.65] vs 0.73 [0.82]; P = .15) (Figure 1A and Supplementary Table 2). There were no statistically significant relationships between IL-10 and plaque parameters (any type of plaque, ρ = −0.15 and P = .23; noncalcified plaque, ρ = −0.16 and P = .20; and calcified plaque, ρ = −0.10 and P = .40).
IL-10 and Cardiovascular Risk Factors in PWH and HIV-Uninfected Controls
Among PWH, no significant relationships were observed between IL-10 and several proinflammatory markers known to be related to atherosclerosis in HIV (sCD163, ρ = 0.03 and P = .68; CCL2, ρ = 0.05 and P = .55; hsIL-6, ρ = −0.09 and P = .31; hsCRP, ρ = 0.05 and P = .55). Similarly, among HIV-uninfected controls, no significant relationships were observed between IL-10 and these proinflammatory markers.
In PWH, IL-10 also had no relationship to traditional cardiovascular risk factors, such as hemoglobin A1c (ρ = −0.07; P = .43), total cholesterol (ρ = −0.10; P = .22), low-density lipoprotein (ρ = −0.11; P = .18), triglycerides (ρ = −0.02; P = .86), high-density lipoprotein (ρ = −0.02; P = .82), and body mass index (BMI) (ρ = −0.06; P = .45). Similarly, no relationships between IL-10 and these cardiovascular risk factors were observed in HIV-uninfected controls. IL-10 was inversely related to CD4+ T-cell count (ρ = −0.20; P = .02). In contrast, there was no relationship between IL-10 and HIV RNA (ρ = 0.08; P = .37).
Multivariate Analysis in PWH
In PWH, log10 IL-10 was inversely associated with the presence of coronary plaque (odds ratio = 0.39; P = .01) in a model that controlled for total Framingham point score, BMI, CD4+ T-cell count, log10 HIV RNA, log10 sCD163, log10 CCL2, log10 C-reactive protein (CRP), log10 hsIL-6 values, pack-years, and race.
Sensitivity Analysis of ART-Treated and Virally Suppressed PWH
We performed a sensitivity analysis that was limited to virally suppressed participants with current ART use (n = 120). Among this subgroup of PWH, IL-10 remained significantly lower in those with plaque relative to those without plaque (mean [SD], 0.36 [0.41] vs 0.60 [0.69]; P = .03). IL-10 also remained inversely correlated with the number of coronary segments with noncalcified plaque (ρ = −0.20; P = .03) (Supplementary Table 2). Similarly, in this group, log10 IL-10 was independently and inversely associated with the presence of plaque (odds ratio = 0.42; P = .04) in a model that controlled for total Framingham point score, BMI, CD4+ T-cell count, log10 HIV RNA, log10 sCD163, log10 CCL2, log10 CRP, log10 hsIL-6 values, pack-years, and race.
Sensitivity Analysis in PWH Excluding Individuals Using Statin Therapy
We performed a sensitivity analysis that was limited to PWH who were not currently using statin therapy (n = 125). Among this subgroup of PWH, IL-10 levels remained inversely correlated with noncalcified plaque segments (ρ = −0.22; P = .02). PWH with plaque had lower log10 IL-10 levels than those without plaque (P = .01). Moreover, in PWH, log10 IL-10 was inversely associated with the presence of coronary plaque (odds ratio, 0.41; P = .04) in a model that controlled for total Framingham point score, BMI, CD4+ T-cell count, log10 HIV RNA, log10 sCD163, log10 CCL2, log10 CRP, log10 hsIL-6 values, pack-years, and race.
Discussion
In PWH, higher IL-10 was associated with a lower burden of total coronary plaque and, more specifically, a lower burden of coronary segments with noncalcified plaque. Furthermore, a 10-fold decrease in IL-10 levels among all PWH was associated with a >2-fold increase in the odds of having coronary plaque. These relationships remained significant when controlling for traditional cardiovascular risk factors (eg, Framingham risk score and BMI), HIV-specific factors (eg, HIV RNA and CD4+ T cells), and proinflammatory markers known to be associated with plaque development (eg, sCD163 and CCL2). These novel findings may implicate an antiatherogenic role for IL-10 in PWH.
Chronic immune activation is a consequence of HIV infection. Inflammation, in turn, has been shown to drive the pathogenesis of coronary atherosclerotic disease at every step of plaque formation [1]. Furthermore, the plaques that are most inflamed, as characterized by the infiltration of activated macrophages and T cells, are most prone to rupture [1]. Accordingly, developing strategies to mitigate inflammation in order to slow plaque formation and progression is an important goal of cardiovascular disease research. Indeed, statins, the mainstay of both primary and secondary cardiovascular disease prevention, have been shown to have pleiotropic anti-inflammatory effects that extend beyond their ability to lower cholesterol levels.
In HIV, we show for the first time that the anti-inflammatory cytokine IL-10 was inversely related to total coronary plaque and noncalcified coronary plaque. Compared with calcified plaque, noncalcified plaque is more prone to rupture and more likely to cause cardiovascular events [6]. Noncalcified plaque also has a higher macrophage count and is more proinflammatory than calcified plaque [6]. Our findings are consistent with those in a study by Desvarieux et al [7], which showed that a low anti-inflammatory profile (a composite measure of 4 anti-inflammatory markers that included IL-10) was associated with a greater carotid intima media thickness in PWH. Of note, that study did not independently assess IL-10 concentrations and focused on carotid intima media thickness rather than coronary plaque. Our human data are also supported by findings in prior animal studies, which have suggested that IL-10 may reduce plaque formation. In atherosclerosis-prone mouse models, increased IL-10 expression led to a reduction in plaque size [8].
There are several plausible mechanisms by which IL-10 might impede the formation of atherosclerotic lesions. Differentiation of circulating monocytes into macrophages in the vascular wall requires a confluence of inflammatory signals. IL-10 has been shown to inhibit the release of several proinflammatory cytokines from monocytes, such as interleukin 1β, tumor necrosis factor α, and interleukin 8 [5]. IL-10 has also been shown to inhibit the macrophage secretion of matrix metalloproteinase 9, a metalloproteinase that has been linked to the weakening of the fibrous caps that are disrupted during plaque rupture [9]. In cultured macrophages, IL-10 has been shown to have antiapoptotic properties, such as a decrease in the expression of activated caspase-3 [9]. By preventing cell death and lipid release in the necrotic core, IL-10 may improve plaque stability [9].
Finally, IL-10 may be atheroprotective owing to its ability to increase cholesterol efflux from macrophages by up-regulating the transporters ABCA1 and ABCG1 [10]. Macrophage lipid accumulation is a critical step in atherogenesis, and cholesterol efflux through ABCA1 has been shown to be inhibited by the HIV protein Nef [11]. The role of IL-10 in facilitating the removal of lipids from the macrophage may underlie the unique association between IL-10 and noncalcified (rather than calcified) plaque. Compared with calcified plaque, evidence suggests that noncalcified plaque is characterized by more dynamic lipid metabolism. In a study of the general population, plasma lipid species were shown to predict noncalcified but not calcified plaque burden [12]. Furthermore, in PWH, statin therapy has been shown to reduce noncalcified rather than calcified plaque [13].
Despite the beneficial role of IL-10 in atherosclerosis progression, its function in PWH may be more nuanced. Although IL-10 may dampen immune activation to protect against atherosclerosis, it may also blunt the host response to the HIV virus and promote persistent viremia. Thus, blockade of IL-10 signaling has been considered as a strategy to improve HIV virologic control. In particular, in viremic patients, blockade of the IL-10 receptor (interleukin 10Rα) resulted in an enhancement of HIV-specific CD4+ T-cell responses [14]. In keeping with this trend, we showed an inverse relationship between IL-10 and CD4+ T-cell count in PWH, as has been reported elsewhere [15]. Taken together, these findings suggest that strategies to augment IL-10 for the prevention or treatment of atherosclerosis must be carefully considered with respect to the maintenance of HIV viral suppression. Moreover, efforts to antagonize IL-10 signaling in order to improve virologic control could potentially exacerbate coronary atherosclerosis in a population already at high risk for cardiovascular disease.
Strengths of our analysis include its large sample of participants with and without HIV infection. In addition, our models controlled for traditional and nontraditional cardiovascular risk factors, which adds to the robustness of our findings. Limitations of our study include its cross-sectional design from which we cannot determine causality. We also report the concentration of IL-10 in serum, rather than in the plaque or at the level of the macrophage, where IL-10 is most likely to exert its antiatherogenic effects. We also lack flow cytometry data and are therefore unable to relate IL-10 levels to immune cell subsets of interest.
In conclusion, our data highlight a compelling inverse relationship between IL-10 and atherosclerosis in PWH. Our findings suggest that IL-10 may be atheroprotective in HIV, which is supported by animal models and mechanistic studies. We also find that IL-10 is inversely related to CD4+ T-cell counts, in keeping with studies demonstrating that IL-10 can impair the immune response to HIV. In sum, though IL-10 may be an attractive target for therapeutic interventions to treat atherosclerosis in HIV, potential strategies will require a nuanced approach that carefully balances immune activation and immunosuppression.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The funding sources had no direct role in the design of the study, the data analysis, or the writing of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grant 1KL2TR002542-01 to L. T. F., RO1HL123351 and K23HL092792 to J. L. and 5T32DK007028-44 to L. C.), Bristol-Myers Squibb, (grant 1 UL1 RR025758-04), the Harvard Clinical and Translational Science Center, and the National Center for Research Resources.
Potential conflicts of interest. U. H. received grant support from MedImmune, Kowa, and HeartFlow, unrelated to this work. M. T. L. received consulting fees from PQBypass, research support from NVIDIA, grant support from MedImmune, and research funding from Kowa, unrelated to this work. J. L. has served on a medical affairs advisory board for Gilead Sciences and as a consultant for ViiV Healthcare, unrelated to this work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 2019 Conference on Retroviruses and Opportunistic Infections 4–7 March, 2019; Seattle, WA.
References
- 1. Lo J, Plutzky J. The biology of atherosclerosis: general paradigms and distinct pathogenic mechanisms among HIV-infected patients. J Infect Dis 2012; 205(suppl 3):S368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–31. [DOI] [PubMed] [Google Scholar]
- 3. Fitch KV, Srinivasa S, Abbara S, et al. . Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208:1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwon DS, Kaufmann DE. Protective and detrimental roles of IL-10 in HIV pathogenesis. Eur Cytokine Netw 2010; 21:208–14. [DOI] [PubMed] [Google Scholar]
- 5. Han X, Boisvert WA. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb Haemost 2015; 113:505–12. [DOI] [PubMed] [Google Scholar]
- 6. Wahlgren CM, Zheng W, Shaalan W, Tang J, Bassiouny HS. Human carotid plaque calcification and vulnerability. Relationship between degree of plaque calcification, fibrous cap inflammatory gene expression and symptomatology. Cerebrovasc Dis 2009; 27:193–200. [DOI] [PubMed] [Google Scholar]
- 7. Desvarieux M, Boccara F, Meynard JL, et al. . Infection duration and inflammatory imbalance are associated with atherosclerotic risk in HIV-infected never-smokers independent of antiretroviral therapy. AIDS 2013; 27:2603–14. [DOI] [PubMed] [Google Scholar]
- 8. Namiki M, Kawashima S, Yamashita T, et al. . Intramuscular gene transfer of interleukin-10 cDNA reduces atherosclerosis in apolipoprotein E-knockout mice. Atherosclerosis 2004; 172:21–9. [DOI] [PubMed] [Google Scholar]
- 9. Han X, Kitamoto S, Lian Q, Boisvert WA. Interleukin-10 facilitates both cholesterol uptake and efflux in macrophages. J Biol Chem 2009; 284:32950–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubic T, Lorenz RL. Downregulated CD36 and oxLDL uptake and stimulated ABCA1/G1 and cholesterol efflux as anti-atherosclerotic mechanisms of interleukin-10. Cardiovasc Res 2006; 69:527–35. [DOI] [PubMed] [Google Scholar]
- 11. Mujawar Z, Rose H, Morrow MP, et al. . Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol 2006; 4:e365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellims AH, Wong G, Weir JM, Lew P, Meikle PJ, Taylor AJ. Plasma lipidomic analysis predicts non-calcified coronary artery plaque in asymptomatic patients at intermediate risk of coronary artery disease. Eur Heart J Cardiovasc Imaging 2014; 15:908–16. [DOI] [PubMed] [Google Scholar]
- 13. Lo J, Lu MT, Ihenachor EJ, et al. . Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015; 2:e52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brockman MA, Kwon DS, Tighe DP, et al. . IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 2009; 114:346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan RC, Landay AL, Hodis HN, et al. . Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr 2012; 60:359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.